Impact of Dietary Sugars on Gut Microbiota and Metabolic Health
Abstract
1. Introduction
2. Dietary Sugars—An Overview
3. Insights of Gut Microbiota Composition and Function
4. Impact of Dietary Sugars on Gut Microbiota and Metabolic Health
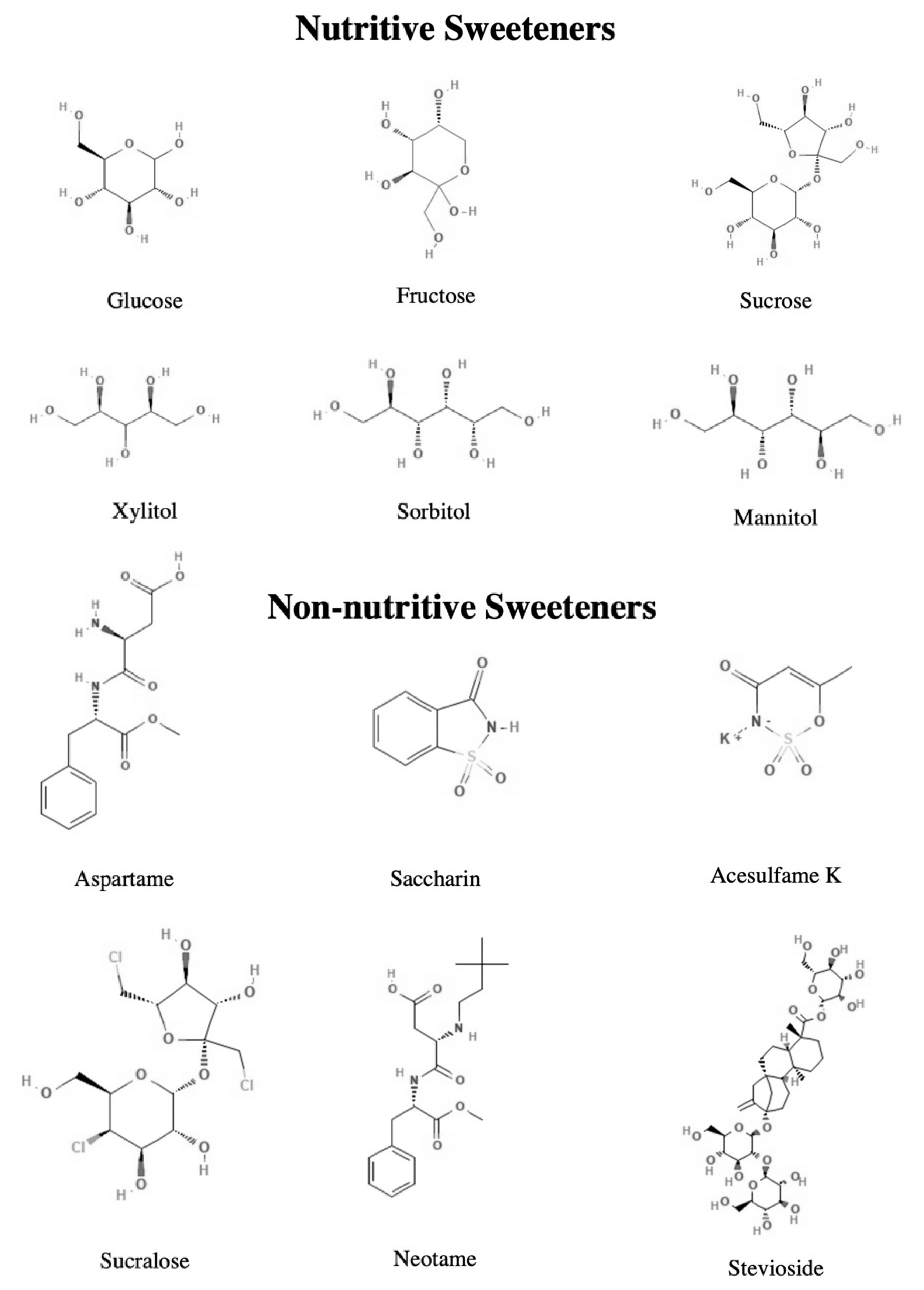
4.1. Nutritive Sweeteners
4.1.1. Glucose, Fructose, Sucrose
4.1.2. Polyols
| Intervention | Animal Model | Outcomes | Ref. |
|---|---|---|---|
| Administration of high-glucose and high-fructose diet (65.0% of calories in carbohydrate: 85% from glucose or fructose and 15% from sucrose) (12 weeks) | Male C57BL/6J mice | ↑ Glucose intolerance and fasting blood glucose concentration ↑ Total and LDL cholesterol ↑ Serum endotoxin levels ↑ Proteobacteria, in particular Desulfovibrio vulgaris ↓ Bacteroidetes (Muribaculum intestinale) ↑ Akkermansia muciniphila ↓ ZO-1 and occludin expression in the colon ↑ Inflammatory cytokines, TNF-α and IL-1β, in the colon | [83] |
| Administration of fructose at low dose (Fru-L), (2.6 g/kg/day), moderate dose (Fru-M), (5.3 g/kg/day), high dose (Fru-H), (10.5 g/kg/day) (20 weeks) | Male Sprague Dawley rats | No significant differences in body weight and fasting blood glucose Fru-H ↑ Hepatic lipid accumulation and inflammatory cell infiltration in pancreas and colon ↑ Expression of lipid accumulation proteins (perilipin-1, ADRP, and Tip-47) in the colon ↑ Uric acid levels ↓ TJ proteins including ZO-1 and occludin ↑ Parasutterella and Blantia ↓ Intestinimonas Fru-L, Fru-M, Fru-H ↑ IL-6, TNF-α, and MIP-2 ↓ IL-10 ↓ isobutyric acid | [84] |
| High-sucrose diet (5.3 g sucrose/kg/day) (4 weeks) | Male Wistar rats | ↑ Liver organ weight ↑ Serum triglycerides and cholesterol levels ↑ Hepatic lipids levels ↑ Bacteroidetes and Verrucomicriobia, Erysipelotrichaceae, Turicibacteraceae, Bacteroidaceae ↓ Firmicutes, Ruminococcaceae, Clostridiales, and Lactobacillacae | [85] |
| 2% (2.17 g/kg/day), or 5% (5.42 g/kg/day) (w/w) xylitol (3 months) | Male C57BL/6 wild-type mice | No significant changes in brain, pancreas, colon and liver organ weights ↑ SCFA’s, especially butyrate in the mucosa and propionate in the lumen 5% xylitol ↑ Bifidobacterium, Lactobacillus, and Erysipelotrichaceae ↓ Blautia and Staphylococcus | [86] |
| Approach | Animal Model | Outcomes | Ref. |
| Xylitol solution of 40 mg/kg and 200 mg/kg body weight/day (16 weeks) | Male C57B1/6J mice | Body composition, hepatic and serum lipid parameters, oral glucose tolerance were unaffected ↓ Bacteroidetes phylum and genus Barnesiella | [90] |
| 1.0 g/100 kcal or 2.0 g/100 kcal of xylitol in the diet (8 weeks) | Diet-induced obese male Sprague Dawley rats | ↓ Visceral fat mass, plasmatic insulin and lipid profile ↑ Fatty acid oxidation-related genes GM assessment was not evaluated | [91] |
| 10% sorbitol (2.07 g/day) in water (16 days) | Male Wistar rats | Colonic and cecal wall weights ↓ Serum lipid levels, triglycerides, total cholesterol, HDL-cholesterol and LDL-cholesterol ↑ Butyrate level in the cecum and colon ↑ Lactobacillus in feces, colon, cecum | [88] |
| 2% (w/w) lactilol or 2% (w/w) polydextrose and lactilol (3 weeks) | Male Wistar rats | No differences in body weight No changes in the crypt:villus ratio ↑ IgA (lack of mucosal inflammation) ↑ Production of butyrate ↓ pH | [89] |
4.2. Non-Nutritive Sweeteners
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzi, S.C.; Britton, R.A. Adaptation of the Gut Microbiota to Modern Dietary Sugars and Sweeteners. Adv. Nutr. 2020, 11, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Heidari-Beni, M.; Kelishadi, R. The Role of Dietary Sugars and Sweeteners in Metabolic Disorders and Diabetes. In Sweeteners: Pharmacology, Biotechnology, and Applications; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 225–243. [Google Scholar]
- Hattori, K.; Akiyama, M.; Seki, N.; Yakabe, K.; Hase, K.; Kim, Y.G. Gut Microbiota Prevents Sugar Alcohol-Induced Diarrhea. Nutrients 2021, 13, 2029. [Google Scholar] [CrossRef]
- Shankar, P.; Ahuja, S.; Sriram, K. Non-nutritive sweeteners: Review and update. Nutrition 2013, 29, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Liauchonak, I.; Qorri, B.; Dawoud, F.; Riat, Y.; Szewczuk, M.R. Non-Nutritive Sweeteners and Their Implications on the Development of Metabolic Syndrome. Nutrients 2019, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ramirez, S.; Martinez-Tapia, B.; Gonzalez-Castell, D.; Cuevas-Nasu, L.; Shamah-Levy, T. Westernized and Diverse Dietary Patterns Are Associated with Overweight-Obesity and Abdominal Obesity in Mexican Adult Men. Front. Nutr. 2022, 9, 891609. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z. Gut Microbiota: An Important Link between Western Diet and Chronic Diseases. Nutrients 2019, 11, 2287. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, E.; Sayavedra, L. 3.07—Diet, Microbiota and the Gut-Brain Axis. In Comprehensive Gut Microbiota; Glibetic, M., Ed.; Elsevier: Oxford, UK, 2022; pp. 69–83. [Google Scholar]
- Monteiro-Alfredo, T.; Caramelo, B.; Arbelaez, D.; Amaro, A.; Barra, C.; Silva, D.; Oliveira, S.; Seica, R.; Matafome, P. Distinct Impact of Natural Sugars from Fruit Juices and Added Sugars on Caloric Intake, Body Weight, Glycaemia, Oxidative Stress and Glycation in Diabetic Rats. Nutrients 2021, 13, 2956. [Google Scholar] [CrossRef] [PubMed]
- Paglia, L. The sweet danger of added sugars. Eur. J. Paediatr. Dent. 2019, 20, 89. [Google Scholar] [CrossRef]
- Schiano, C.; Grimaldi, V.; Scognamiglio, M.; Costa, D.; Soricelli, A.; Nicoletti, G.F.; Napoli, C. Soft drinks and sweeteners intake: Possible contribution to the development of metabolic syndrome and cardiovascular diseases. Beneficial or detrimental action of alternative sweeteners? Food Res. Int. 2021, 142, 110220. [Google Scholar] [CrossRef]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012, 346, e7492. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Jin, Y.; Chen, G.; Ma, X.; Zhang, L. Gut Microbiota Dysbiosis Drives the Development of Colorectal Cancer. Digestion 2021, 102, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.L.; Hall, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhou, Z. Alterations of the gut microbiome and metabolome in alcoholic liver disease. World J. Gastrointest. Pathophysiol. 2014, 5, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, S.; Gomez-Martinez, S.; Diaz, L.E.; Nova, E.; Urrialde, R.; Marcos, A. Potential Effects of Sucralose and Saccharin on Gut Microbiota: A Review. Nutrients 2022, 14, 1682. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef]
- Adithya, K.K.; Rajeev, R.; Selvin, J.; Seghal Kiran, G. Dietary Influence on the Dynamics of the Human Gut Microbiome: Prospective Implications in Interventional Therapies. ACS Food Sci. Technol. 2021, 1, 717–736. [Google Scholar] [CrossRef]
- Freeman, C.R.; Zehra, A.; Ramirez, V.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Impact of sugar on the body, brain, and behavior. Front. Biosci. 2018, 23, 2255–2266. [Google Scholar] [CrossRef]
- Shi, Y.N.; Liu, Y.J.; Xie, Z.; Zhang, W.J. Fructose and metabolic diseases: Too much to be good. Chin. Med. J. 2021, 134, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Viana, S.D.; Nunes, S.; Reis, F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 1876–1897. [Google Scholar] [CrossRef] [PubMed]
- Viana, S.D.; Nunes, S.; Reis, F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities—Role of gut microbiota dysbiosis. Ageing Res. Rev. 2020, 62, 101123. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, A.; Labonte, M.E.; Brassard, D.; Bedard, A.; Laramee, C.; Robitaille, J.; Desroches, S.; Provencher, V.; Couillard, C.; Vohl, M.C.; et al. Intakes of Total, Free, and Naturally Occurring Sugars in the French-Speaking Adult Population of the Province of Quebec, Canada: The PREDISE Study. Nutrients 2019, 11, 2317. [Google Scholar] [CrossRef] [PubMed]
- van Loveren, C. Sugar Restriction for Caries Prevention: Amount and Frequency. Which Is More Important? Caries Res. 2019, 53, 168–175. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Fidler Mis, N.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellof, M.; Embleton, N.D.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; et al. Sugar in Infants, Children and Adolescents: A Position Paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 681–696. [Google Scholar] [CrossRef]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J.; American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism and the Council on Epidemiology and Prevention. Dietary sugars intake and cardiovascular health: A scientific statement from the American Heart Association. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Gil, A. Sucrose: Dietary Importance. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 199–204. [Google Scholar] [CrossRef]
- Amarra, M.S.; Khor, G.L.; Chan, P. Intake of added sugar in Malaysia: A review. Asia Pac. J. Clin. Nutr. 2016, 25, 227–240. [Google Scholar] [CrossRef]
- Piekara, A.; Krzywonos, M.; Szymańska, A. Sweetening Agents and Sweeteners in Dietary Supplements for Children-Analysis of the Polish Market. Nutrients 2020, 12, 2387. [Google Scholar] [CrossRef]
- Fitch, C.; Keim, K.S. Position of the Academy of Nutrition and Dietetics: Use of nutritive and nonnutritive sweeteners. Acad. Nutr. Diet. 2012, 112, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Grembecka, M. Natural sweeteners in a human diet. Rocz. Panstw. Zakl. Hig. 2015, 66, 195–202. [Google Scholar] [PubMed]
- MC, Y.-B. Sweeteners. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 562–572. [Google Scholar]
- Blekas, G.A. Food Additives: Classification, Uses and Regulation. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 731–736. [Google Scholar] [CrossRef]
- Hess, J.; Latulippe, M.E.; Ayoob, K.; Slavin, J. The confusing world of dietary sugars: Definitions, intakes, food sources and international dietary recommendations. Food Funct. 2012, 3, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Chakraborty, R. Sweeteners: Classification, Sensory and Health Effects. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar] [CrossRef]
- Lenhart, A.; Chey, W.D. A Systematic Review of the Effects of Polyols on Gastrointestinal Health and Irritable Bowel Syndrome. Adv. Nutr. 2017, 8, 587–596. [Google Scholar] [CrossRef]
- Msomi, N.Z.; Erukainure, O.L.; Islam, M.S. Suitability of sugar alcohols as antidiabetic supplements: A review. J. Food Drug Anal. 2021, 29, 1–14. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Pastor-Villaescusa, B.; Rueda-Robles, A.; Abadia-Molina, F.; Ruiz-Ojeda, F.J. Plausible Biological Interactions of Low- and Non-Calorie Sweeteners with the Intestinal Microbiota: An Update of Recent Studies. Nutrients 2020, 12, 1153. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I. Sweeteners as food additives in the XXI century: A review of what is known, and what is to come. Food Chem. Toxicol. 2017, 107, 302–317. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Saez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.; Upreti, M.; Prakash, I. Diterpene glycosides from Stevia rebaudiana. Molecules 2011, 16, 3552–3562. [Google Scholar] [CrossRef]
- Baker-Smith, C.M.; de Ferranti, S.D.; Cochran, W.J.; Abrams, S.A.; Fuchs, G.J.; Kim, J.H.; Lindsey, C.W.; Magge, S.N.; Rome, E.S.; Schwarzenberg, S.J.; et al. The Use of Nonnutritive Sweeteners in Children. Pediatrics 2019, 144, e20192765. [Google Scholar] [CrossRef]
- Walbolt, J.; Koh, Y. Non-nutritive Sweeteners and Their Associations with Obesity and Type 2 Diabetes. J. Obes. Metab. Syndr. 2020, 29, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Microbiome. Yale J. Biol. Med. 2016, 89, 275–276. [Google Scholar]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Intestinal microbiota and their metabolic contribution to type 2 diabetes and obesity. J. Diabetes Metab. Disord. 2021, 20, 1855–1870. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Borse, S.P.; Chhipa, A.S.; Sharma, V.; Singh, D.P.; Nivsarkar, M. Management of Type 2 Diabetes: Current Strategies, Unfocussed Aspects, Challenges, and Alternatives. Med. Princ. Pract. 2021, 30, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Doumatey, A.P.; Adeyemo, A.; Zhou, J.; Lei, L.; Adebamowo, S.N.; Adebamowo, C.; Rotimi, C.N. Gut Microbiome Profiles Are Associated With Type 2 Diabetes in Urban Africans. Front. Cell Infect. Microbiol. 2020, 10, 63. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sorensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol 2020, 11, 25. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.; Kotlo, K.U.; Dudeja, P.K.; Layden, B.T. Role of Short Chain Fatty Acid Receptors in Intestinal Physiology and Pathophysiology. Compr. Physiol. 2018, 8, 1091–1115. [Google Scholar] [CrossRef] [PubMed]
- Correa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Carretta, M.D.; Quiroga, J.; Lopez, R.; Hidalgo, M.A.; Burgos, R.A. Participation of Short-Chain Fatty Acids and Their Receptors in Gut Inflammation and Colon Cancer. Front. Physiol. 2021, 12, 662739. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Wang, Q.; Wu, Q.; Mao-Draayer, Y.; Kim, C.H. Author Correction: Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci. Rep. 2019, 9, 17511. [Google Scholar] [CrossRef] [PubMed]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Tazoe, H.; Otomo, Y.; Kaji, I.; Tanaka, R.; Karaki, S.I.; Kuwahara, A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J. Physiol. Pharmacol. 2008, 59 (Suppl. S2), 251–262. [Google Scholar] [PubMed]
- Hernandez, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Trent, C.M.; Blaser, M.J. Microbially Produced Acetate: A “Missing Link” in Understanding Obesity? Cell Metab. 2016, 24, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Ji, J.; Shu, D.; Zheng, M.; Wang, J.; Luo, C.; Wang, Y.; Guo, F.; Zou, X.; Lv, X.; Li, Y.; et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 2016, 6, 24838. [Google Scholar] [CrossRef]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-Talk between PPARgamma and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shi, L.; Xin, W.; Xu, J.; Xu, J.; Li, Q.; Xu, Z.; Wang, J.; Wang, G.; Yao, W.; et al. Activation of PPARgamma inhibits pro-inflammatory cytokines production by upregulation of miR-124 in vitro and in vivo. Biochem. Biophys. Res. Commun. 2017, 486, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; He, Z.; Chen, W.; Holzman, I.R.; Lin, J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr. Res. 2007, 61, 37–41. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Kubota, T.; Takada, E.; Takai, K.; Fujiwara, N.; Arimitsu, N.; Murayama, M.A.; Ueda, Y.; Wakisaka, S.; Suzuki, T.; et al. Propionate-producing bacteria in the intestine may associate with skewed responses of IL10-producing regulatory T cells in patients with relapsing polychondritis. PLoS ONE 2018, 13, e0203657. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Hu, F.B.; Tappy, L.; Brand-Miller, J. Dietary carbohydrates: Role of quality and quantity in chronic disease. BMJ 2018, 361, k2340. [Google Scholar] [CrossRef]
- Seo, Y.S.; Lee, H.B.; Kim, Y.; Park, H.Y. Dietary Carbohydrate Constituents Related to Gut Dysbiosis and Health. Microorganisms 2020, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Goodman, B.E. Insights into digestion and absorption of major nutrients in humans. Adv. Physiol. Educ. 2010, 34, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, W.; Song, G.; Pang, S.; Peng, Z.; Li, Y.; Wang, P. High-Fructose Diet Increases Inflammatory Cytokines and Alters Gut Microbiota Composition in Rats. Mediat. Inflamm. 2020, 2020, 6672636. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Araki, Y.; Hanzawa, F.; Umeki, M.; Kojima, T.; Nishimura, N.; Ikeda, S.; Mochizuki, S.; Oda, H. High sucrose diet-induced dysbiosis of gut microbiota promotes fatty liver and hyperlipidemia in rats. J. Nutr. Biochem. 2021, 93, 108621. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Ye, K.; Li, M.; Ying, J.; Wang, H.; Han, J.; Shi, L.; Xiao, J.; Shen, Y.; Feng, X.; et al. Xylitol enhances synthesis of propionate in the colon via cross-feeding of gut microbiota. Microbiome 2021, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.-L.; Cai, X.; Zheng, X.-Y.; Chen, D.-S.; Li, M.; Liu, Z.-Q.; Chen, K.-Q.; Han, F.-F.; Zhu, X. Influences of Xylitol Consumption at Different Dosages on Intestinal Tissues and Gut Microbiota in Rats. J. Agric. Food Chem. 2021, 69, 12002–12011. [Google Scholar] [CrossRef]
- Sarmiento-Rubiano, L.A.; Zuniga, M.; Perez-Martinez, G.; Yebra, M.J. Dietary supplementation with sorbitol results in selective enrichment of lactobacilli in rat intestine. Res. Microbiol. 2007, 158, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Peuranen, S.; Tiihonen, K.; Apajalahti, J.; Kettunen, A.; Saarinen, M.; Rautonen, N. Combination of polydextrose and lactitol affects microbial ecosystem and immune responses in rat gastrointestinal tract. Br. J. Nutr. 2004, 91, 905–914. [Google Scholar] [CrossRef]
- Uebanso, T.; Kano, S.; Yoshimoto, A.; Naito, C.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of Consuming Xylitol on Gut Microbiota and Lipid Metabolism in Mice. Nutrients 2017, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Amo, K.; Arai, H.; Uebanso, T.; Fukaya, M.; Koganei, M.; Sasaki, H.; Yamamoto, H.; Taketani, Y.; Takeda, E. Effects of xylitol on metabolic parameters and visceral fat accumulation. J. Clin. Biochem. Nutr. 2011, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Tu, P.; Chi, L.; Gao, B.; Ru, H.; Lu, K. Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food Chem. Toxicol. 2017, 107 Pt B, 530–539. [Google Scholar] [CrossRef]
- Daly, K.; Darby, A.C.; Hall, N.; Nau, A.; Bravo, D.; Shirazi-Beechey, S.P. Dietary supplementation with lactose or artificial sweetener enhances swine gut Lactobacillus population abundance. Br. J. Nutr. 2014, 111 (Suppl. S1), S30–S35. [Google Scholar] [CrossRef]
- Abou-Donia, M.B.; El-Masry, E.M.; Abdel-Rahman, A.A.; McLendon, R.E.; Schiffman, S.S. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats. J. Toxicol. Environ. Health A 2008, 71, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Uebanso, T.; Ohnishi, A.; Kitayama, R.; Yoshimoto, A.; Nakahashi, M.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of Low-Dose Non-Caloric Sweetener Consumption on Gut Microbiota in Mice. Nutrients 2017, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLoS ONE 2017, 12, e0178426. [Google Scholar] [CrossRef]
- De la Garza, A.L.; Romero-Delgado, B.; Martinez-Tamez, A.M.; Cardenas-Tueme, M.; Camacho-Zamora, B.D.; Matta-Yee-Chig, D.; Sanchez-Tapia, M.; Torres, N.; Camacho-Morales, A. Maternal Sweeteners Intake Modulates Gut Microbiota and Exacerbates Learning and Memory Processes in Adult Male Offspring. Front. Pediatr. 2021, 9, 746437. [Google Scholar] [CrossRef]
- Palmnas, M.S.; Cowan, T.E.; Bomhof, M.R.; Su, J.; Reimer, R.A.; Vogel, H.J.; Hittel, D.S.; Shearer, J. Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PLoS ONE 2014, 9, e109841. [Google Scholar] [CrossRef]
- Lu, J.F.; Zhu, M.Q.; Zhang, H.; Liu, H.; Xia, B.; Wang, Y.L.; Shi, X.; Peng, L.; Wu, J.W. Neohesperidin attenuates obesity by altering the composition of the gut microbiota in high-fat diet-fed mice. FASEB J. 2020, 34, 12053–12071. [Google Scholar] [CrossRef]
- Chi, L.; Bian, X.; Gao, B.; Tu, P.; Lai, Y.; Ru, H.; Lu, K. Effects of the Artificial Sweetener Neotame on the Gut Microbiome and Fecal Metabolites in Mice. Molecules 2018, 23, 367. [Google Scholar] [CrossRef]
- Li, X.Y.; He, C.; Zhu, Y.; Lu, N.H. Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J. Gastroenterol. 2020, 26, 2187–2193. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Zaky, A.; Glastras, S.J.; Wong, M.Y.W.; Pollock, C.A.; Saad, S. The Role of the Gut Microbiome in Diabetes and Obesity-Related Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9641. [Google Scholar] [CrossRef]
- Gardner, C.; Wylie-Rosett, J.; Gidding, S.S.; Steffen, L.M.; Johnson, R.K.; Reader, D.; Lichtenstein, A.H.; American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Disease in the Young, and the American Diabetes Association. Nonnutritive sweeteners: Current use and health perspectives: A scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2012, 35, 1798–1808. [Google Scholar] [CrossRef]
- Narayanan, S.; Bhutiani, N.; Adamson, D.T.; Jones, C.M. Pancreatectomy, Islet Cell Transplantation, and Nutrition Considerations. Nutr. Clin. Pract. 2021, 36, 385–397. [Google Scholar] [CrossRef]
| Approach | Animal Model | Outcomes | Ref. |
|---|---|---|---|
| 0.1 mg/mL saccharin in drinking water (10 weeks) | Diet-induced obese male C57Bl/6 Mice | Impaired glucose tolerance ↑ Bacteroides genus and Clostridiales order ↓ Lactobacillus reuteri | [92] |
| Oral dosing of Splenda (gavage) at 1.1, 3.3, 5.5 or 11 mg/kg/day sucralose (12 weeks) | Male Sprague Dawley rats | Body weight ↓ Bacteroides, bifidobacterium, lactobacilli and Clostridium ↑ pH | [95] |
| Group 1: Administration of a high dose of sucralose (HS, 15 mg/kg body weight per day Group 2: Administration of Acesulfame K solution of 15 mg/kg body weight per day (8 weeks) | Male C57B1/6J mice | Group 1 ↑ Hepatic cholesterol concentration ↓ Clostridium cluster XIVa ↓ Butyrate concentration in cecal contents Group 2 GM was found unchanged | [96] |
| Oral dosing of Acesulfame K (gavage) at 37.5 mg/kg body weigh/day (4 weeks) | Male and female CD-1 mice | ↑ Body weight (male mice only) ↑ Bacteroides (male mice group) ↓ Lactobacillus and Clostridium (female mice group) | [97] |
| High stevia diet (2.5% steviol glycosides) (Gestation and lactation period) | Female Wistar rats Male offspring (with standard diet) | ↑ Fasting glucose levels of male offspring ↓ Bacteroides, Cyanobacteria ↑ Firmicutes, Elusimicrobia, Lactobacillus | [98] |
| Low-dose aspartame (5–7 mg/kg/day) in drinking water (8 weeks) | Diet-induced obese male Sprague Dawley rats | ↓ Body fat percentage, insulin levels Fasting hyperglycemia and impaired insulin tolerance ↑ Enterobacteriaceae, Clostridium leptum Serum propionate | [99] |
| 50 mg/kg/day of neohesperidin by gavage (4 groups: normal diet; normal diet + neo; High fat diet (HFD); HFD + neo) (12 weeks) | Male C57BL/6J mice | ↓ Weight gain, dysfunctional glucose homeostasis, fatty liver, and systemic inflammation in HFD-fed mice ↑ Firmicutes Bacteroidetes (neo group) | [100] |
| 0.75 mg/kg/day of neotame in drinking water (4 weeks) | Male CD-1 mice | No differences in body weight ↑ concentration of lipids and fatty acids in feces (linoleic acid, stearic acid, 1-monopalmitin and 1,3-dipalmitate) ↑ Bacteroidetes phylum ↓ Firmicutes, Blautia, Dorea, Oscillospira and Ruminococcus Microbial dysbiosis index | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, K.; Ferreira, G.; Reis, F.; Viana, S. Impact of Dietary Sugars on Gut Microbiota and Metabolic Health. Diabetology 2022, 3, 549-560. https://doi.org/10.3390/diabetology3040042
Garcia K, Ferreira G, Reis F, Viana S. Impact of Dietary Sugars on Gut Microbiota and Metabolic Health. Diabetology. 2022; 3(4):549-560. https://doi.org/10.3390/diabetology3040042
Chicago/Turabian StyleGarcia, Karina, Gonçalo Ferreira, Flávio Reis, and Sofia Viana. 2022. "Impact of Dietary Sugars on Gut Microbiota and Metabolic Health" Diabetology 3, no. 4: 549-560. https://doi.org/10.3390/diabetology3040042
APA StyleGarcia, K., Ferreira, G., Reis, F., & Viana, S. (2022). Impact of Dietary Sugars on Gut Microbiota and Metabolic Health. Diabetology, 3(4), 549-560. https://doi.org/10.3390/diabetology3040042








