Verapamil and Its Role in Diabetes
Abstract
:1. Introduction
2. Method Section
Scientific Research
3. Insulin Secretion in Pancreatic β-Cells and the Role of TXNIP—Influence of Verapamil and Clinical Implications
3.1. The Role of Pancreatic β-Cells in T1D and T2D
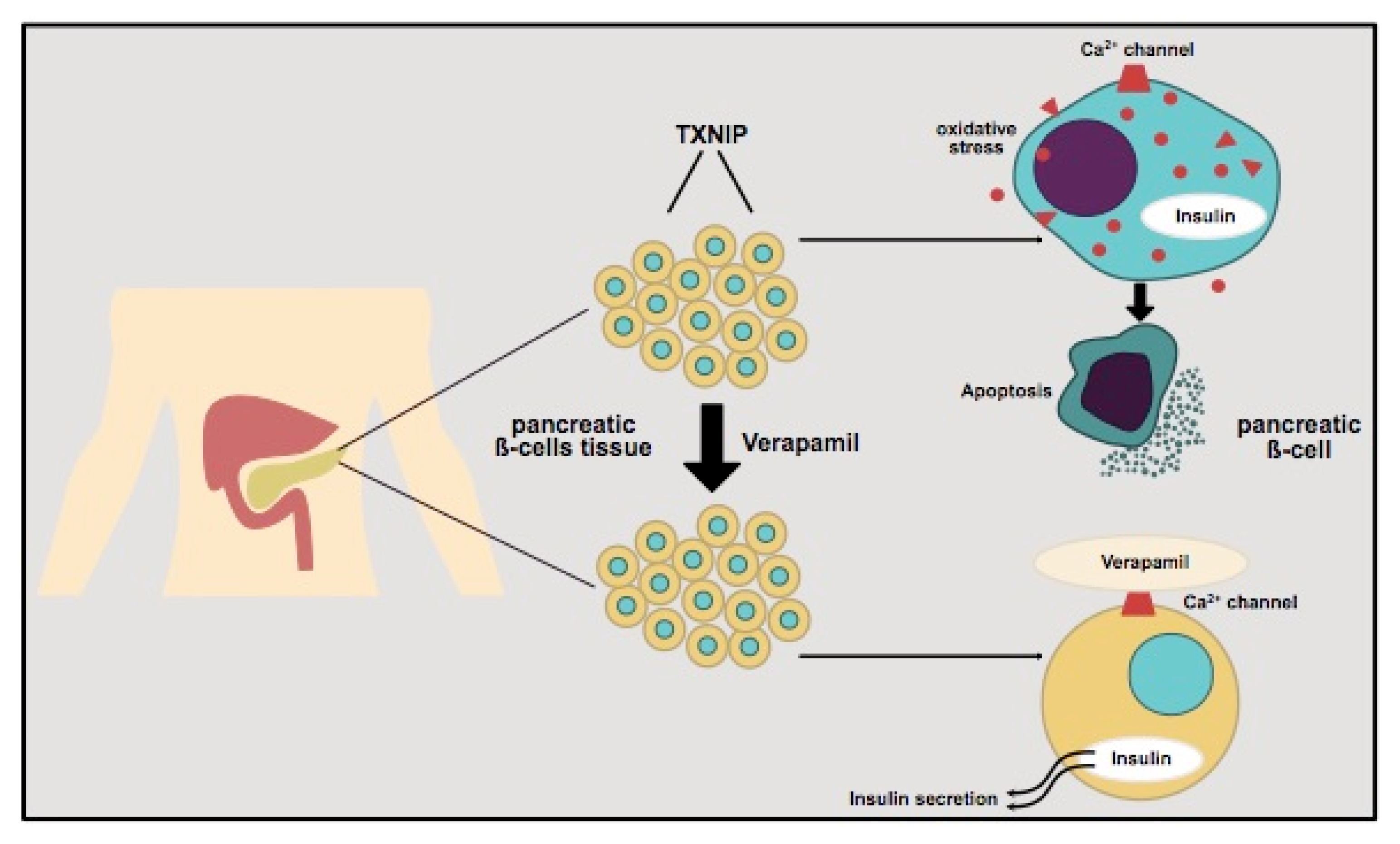
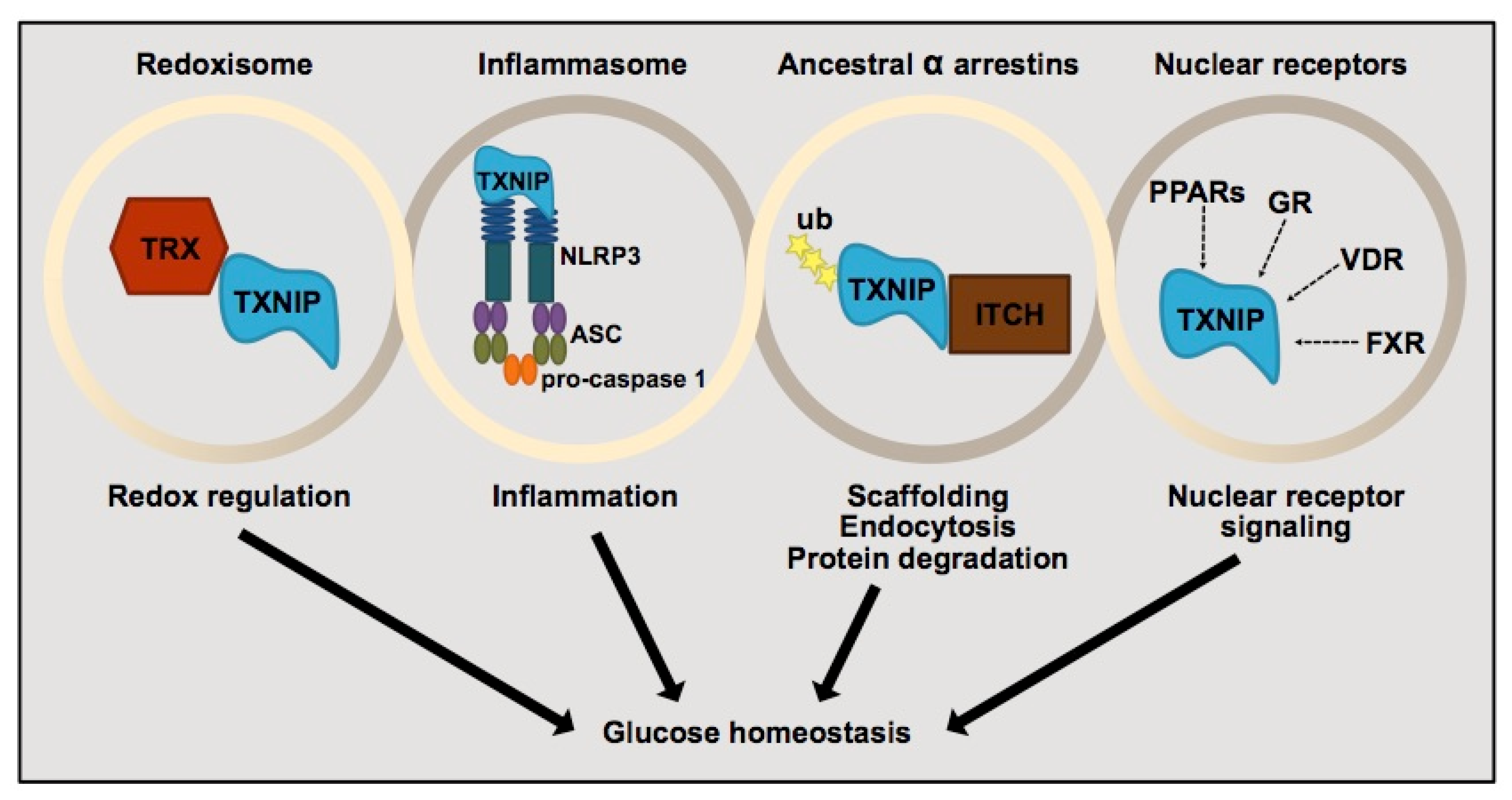
3.2. Thioredoxin-Interacting Protein (TXNIP) and Its Regulation of Pancreatic β-Cells
3.3. Verapamil and Its Impact on Diabetes
3.3.1. Verapamil Administration and β-Cell Mass in Mouse Model
3.3.2. Clinical Implications in Type 1 Diabetes (T1D)
3.3.3. Clinical Implications in Type 2 Diabetes (T2D)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyko, E.J.; Magliano, D.G.; Karuranga, S.; Piemonte, L.; Riley, P.; Saeedi, P.; Sun, H. IDF Diabetes Atlas, 10 th Edition Committee. 2021. Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 19 June 2022).
- Lu, J.; Xia, Q.; Zhou, Q. How to make insulin-producing pancreatic β cells for diabetes treatment. Sci. China Life Sci. 2017, 60, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuluc, P.; Theiner, T.; Jacobo-Piqueras, N.; Geisler, S. Role of High Voltage-Gated Ca2+ Channel Subunits in Pancreatic β-Cell Insulin Release. From Structure to Function. Cells 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 2021, 22, 142–158. [Google Scholar] [CrossRef]
- Yin, T.; Kuo, S.-C.; Chang, Y.-Y.; Chen, Y.-T.; Wang, K.-W.K. Verapamil Use Is Associated With Reduction of Newly Diagnosed Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2017, 102, 2604–2610. [Google Scholar] [CrossRef] [Green Version]
- Rickels, M.R.; Evans-Molina, C.; Bahnson, H.T.; Ylescupidez, A.; Nadeau, K.J.; Hao, W.; Clements, M.A.; Sherr, J.L.; Pratley, R.E.; Hannon, T.S.; et al. High residual C-peptide likely contributes to glycemic control in type 1 diabetes. J. Clin. Investig. 2020, 130, 1850–1862. [Google Scholar] [CrossRef]
- Carr, A.L.J.; Oram, R.A.; Marren, S.M.; McDonald, T.J.; Narendran, P.; Andrews, R.C. Measurement of Peak C-Peptide at Diagnosis Informs Glycemic Control but not Hypoglycemia in Adults With Type 1 Diabetes. J. Endocr. Soc. 2021, 5, bvab127. [Google Scholar] [CrossRef]
- Zenz, S.; Mader, J.K.; Regittnig, W.; Brunner, M.; Korsatko, S.; Boulgaropoulos, B.; Magnes, C.; Raml, R.; Narath, S.H.; Eller, P.; et al. Impact of C-Peptide Status on the Response of Glucagon and Endogenous Glucose Production to Induced Hypoglycemia in T1DM. J. Clin. Endocrinol. Metab. 2018, 103, 1408–1417. [Google Scholar] [CrossRef] [Green Version]
- Narendran, P.; Jackson, N.; Daley, A.; Thompson, D.; Stokes, K.; Greenfield, S.; Charlton, M.; Curran, M.; Solomon, T.; Nouwen, A.; et al. Exercise to preserve β-cell function in recent-onset Type 1 diabetes mellitus (EXTOD)a randomized controlled pilot trial. Diabet. Med. 2017, 34, 1521–1531. [Google Scholar] [CrossRef] [Green Version]
- Chetan, M.R.; Charlton, M.H.; Thompson, C.; Dias, R.P.; Andrews, R.C.; Narendran, P. The Type 1 diabetes ‘honeymoon’ period is five times longer in men who exercise: A case-control study. Diabet. Med. 2019, 36, 127–128. [Google Scholar] [CrossRef] [Green Version]
- Pastore, M.-R.; Bazzigaluppi, E.; Belloni, C.; Arcovio, C.; Bonifacio, E.; Bosi, E. Six Months of Gluten-Free Diet Do Not Influence Autoantibody Titers, but Improve Insulin Secretion in Subjects at High Risk for Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2003, 88, 162–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, K.S.; Buyken, A.E.; Nowotny, B.; Strassburger, K.; Simon, M.-C.; Pacini, G.; Szendroedi, J.; Müssig, K.; Roden, M. The Impact of Dietary Factors on Glycemic Control, Insulin Sensitivity and Secretion in the First Years after Diagnosis of Diabetes. Exp. Clin. Endocrinol. Diabetes 2016, 124, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Gentile, L.; Castiglione, A.; Prandi, V.; Canil, S.; Ghigo, E.; Ciccone, G. C-peptide and the risk for incident complications and mortality in type 2 diabetic patients: A retrospective cohort study after a 14-year follow-up. Eur. J. Endocrinol. 2012, 167, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffes, M.W.; Sibley, S.; Jackson, M.; Thomas, W. β-Cell Function and the Development of Diabetes-Related Complications in the Diabetes Control and Complications Trial. Diabetes Care 2003, 26, 832–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.K.; DuBose, S.N.; Haller, M.J.; Miller, K.M.; DiMeglio, L.A.; Bethin, K.E.; Goland, R.S.; Greenberg, E.M.; Liljenquist, D.R.; Ahmann, A.J.; et al. Prevalence of Detectable C-Peptide according to Age at Diagnosis and Duration of Type 1 Diabetes. Diabetes Care 2015, 38, 476–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Scholten, B.J.; Kreiner, F.F.; Gough, S.C.L.; von Herrath, M. Current and future therapies for type 1 diabetes. Diabetologia 2021, 64, 1037–1048. [Google Scholar] [CrossRef]
- Ovalle, F.; Grimes, T.; Xu, G.; Patel, A.J.; Grayson, T.B.; Thielen, L.A.; Li, P.; Shalev, A. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat. Med. 2018, 24, 1108–1112. [Google Scholar] [CrossRef]
- Xu, G.; Chen, J.; Jing, G.; Shalev, A. Preventing β-Cell Loss and Diabetes With Calcium Channel Blockers. Diabetes 2012, 61, 848–856. [Google Scholar] [CrossRef] [Green Version]
- Pathak, V.; Pathak, N.M.; O’Neill, C.L.; Guduric-Fuchs, J.; Medina, R.J. Therapies for Type 1 Diabetes: Current Scenario and Future Perspectives. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 117955141984452. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Weng, S.-J.; Chang, S.-H.; Li, R.-Y.; Shane, G.-T.; Hsu, J.-P.; Yeh, S.-W.; Chang, A.-C.; Lee, M.-J. Evaluating the antidiabetic effects of R-verapamil in type 1 and type 2 diabetes mellitus mouse models. PLoS ONE 2021, 16, e0255405. [Google Scholar] [CrossRef]
- Bergson, P.; Lipkind, G.; Lee, S.P.; Duban, M.-E.; Hanck, R.A. Verapamil Block of T-Type Calcium Channels. Mol. Pharmacol. 2011, 79, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, N.A.; Wagstaff, A.J.; Keam, S.J. Trandolapril/Verapamil Sustained Release. Drugs 2005, 65, 1893–1914. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.E.; Weyer, C. The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia 2001, 44, 929–945. [Google Scholar] [CrossRef] [Green Version]
- Cooper-DeHoff, R.; Cohen, J.D.; Bakris, G.L.; Messerli, F.H.; Erdine, S.; Hewkin, A.C.; Kupfer, S.; Pepine, C.J. Predictors of Development of Diabetes Mellitus in Patients With Coronary Artery Disease Taking Antihypertensive Medications (Findings from the INternational VErapamil SR-Trandolapril STudy [INVEST]). Am. J. Cardiol. 2006, 98, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Cooper-DeHoff, R.M.; Aranda, J.M., Jr.; Gaxiola, E.; Cangiano, J.L.; Garcia-Barreto, D.; Conti, C.R.; Hewkin, A.; Pepine, C.J. Blood pressure control and cardiovascular outcomes in high-risk Hispanic patients—Findings From the International Verapamil SR/Trandolapril Study (INVEST). Am. Heart J. 2006, 151, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Khodneva, Y.; Shalev, A.; Frank, S.J.; Carson, A.P.; Safford, M.M. Calcium channel blocker use is associated with lower fasting serum glucose among adults with diabetes from the REGARDS study. Diabetes Res. Clin. Pract. 2016, 115, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.-Y.; Jung, C.-H.; Mok, J.-O.; Kang, S.-K.; Kim, C.-H. Association between serum C-peptide levels and chronic microvascular complications in Korean type 2 diabetic patients. Geol. Rundsch. 2011, 49, 9–15. [Google Scholar] [CrossRef]
- Sari, R.; Balci, M.K. Relationship between C peptide and chronic complications in type-2 diabetes mellitus. J. Natl. Med. Assoc. 2005, 97, 1113–1118. [Google Scholar]
- Juntti-Berggren, L.; Larsson, O.; Rorsman, P.; Ammala, C.; Bokvist, K.; Wahlander, K.; Nicotera, P.; Dypbukt, J.; Orrenius, S.; Hallberg, A.; et al. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science 1993, 261, 86–90. [Google Scholar] [CrossRef]
- Lam, T.K.T.; Cherney, D.Z.I. Beta cell preservation in patients with type 1 diabetes. Nat. Med. 2018, 24, 1089–1090. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Liu, D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr. Diabetes Rev. 2012, 9, 25–53. [Google Scholar] [CrossRef]
- Malayeri, A.; Zakerkish, M.; Ramesh, F.; Galehdari, H.; Hemmati, A.A.; Angali, K.A. The Effect of Verapamil on TXNIP Gene Expression, GLP1R mRNA, FBS, HbA1c, and Lipid Profile in T2DM Patients Receiving Metformin and Sitagliptin. Diabetes Ther. 2021, 12, 2701–2713. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.C.; Gaglia, J.; Bonner-Weir, S. Inadequate β-cell mass is essential for the pathogenesis of type 2 diabetes. Lancet Diabetes Endocrinol. 2020, 8, 249–256. [Google Scholar] [CrossRef]
- Hara, M.; Fowler, J.; Bell, G.; Philipson, L. Resting beta-cells—A functional reserve? Diabetes Metab. 2016, 42, 157–161. [Google Scholar] [CrossRef]
- Halban, P.A.; Polonsky, K.S.; Bowden, D.W.; Hawkins, M.A.; Ling, C.; Mather, K.J.; Powers, A.C.; Rhodes, C.J.; Sussel, L.; Weir, G.C. β-Cell Failure in Type 2 Diabetes: Postulated Mechanisms and Prospects for Prevention and Treatment. Diabetes Care 2014, 37, 1751–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrannini, E. The Stunned β Cell: A Brief History. Cell Metab. 2010, 11, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dai, C.; Guo, M.; Taylor, B.; Harmon, J.S.; Sander, M.; Robertson, R.P.; Powers, A.C.; Stein, R. Inactivation of specific β cell transcription factors in type 2 diabetes. J. Clin. Investig. 2013, 123, 3305–3316. [Google Scholar] [CrossRef] [Green Version]
- Ferrannini, E.; Natali, A.; Muscelli, E.; Nilsson, P.M.; Golay, A.; Laakso, M.; Beck-Nielsen, H.; Mari, A. Natural history and physiological determinants of changes in glucose tolerance in a non-diabetic population: The RISC Study. Diabetologia 2011, 54, 1507–1516. [Google Scholar] [CrossRef] [Green Version]
- Ward, W.K.; Bolgiano, D.C.; McKnight, B.; Halter, J.B.; Porte, D. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J. Clin. Investig. 1984, 74, 1318–1328. [Google Scholar] [CrossRef]
- Yoshihara, E. TXNIP/TBP-2: A Master Regulator for Glucose Homeostasis. Antioxidants 2020, 9, 765. [Google Scholar] [CrossRef]
- Thielen, L.; Shalev, A. Diabetes pathogenic mechanisms and potential new therapies based upon a novel target called TXNIP. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 75–80. [Google Scholar] [CrossRef]
- Chen, J.; Hui, S.T.; Couto, F.M.; Mungrue, I.; Davis, D.B.; Attie, A.D.; Lusis, A.J.; Davis, R.A.; Shalev, A. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J. 2008, 22, 3581–3594. [Google Scholar] [CrossRef] [PubMed]
- Muoio, D.M. TXNIP Links Redox Circuitry to Glucose Control. Cell Metab. 2007, 5, 412–414. [Google Scholar] [CrossRef] [Green Version]
- Minn, A.H.; Hafele, C.; Shalev, A. Thioredoxin-Interacting Protein Is Stimulated by Glucose through a Carbohydrate Response Element and Induces β-Cell Apoptosis. Endocrinology 2005, 146, 2397–2405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalev, A.; Pise-Masison, C.A.; Radonovich, M.; Hoffmann, S.C.; Hirshberg, B.; Brady, J.N.; Harlan, D.M. Oligonucleotide Microarray Analysis of Intact Human Pancreatic Islets: Identification of Glucose-Responsive Genes and a Highly Regulated TGFβ Signaling Pathway. Endocrinology 2002, 143, 3695–3698. [Google Scholar] [CrossRef] [Green Version]
- Shalev, A. Lack of TXNIP protects β-cells against glucotoxicity. Biochem. Soc. Trans. 2008, 36, 963–965. [Google Scholar] [CrossRef]
- Parikh, H.; Carlsson, E.; Chutkow, W.A.; Johansson, L.E.; Storgaard, H.; Poulsen, P.; Saxena, R.; Ladd, C.; Schulze, P.C.; Mazzini, M.J.; et al. TXNIP Regulates Peripheral Glucose Metabolism in Humans. PLOS Med. 2007, 4, e158. [Google Scholar] [CrossRef] [Green Version]
- Yoshihara, E.; Fujimoto, S.; Inagaki, N.; Okawa, K.; Masaki, S.; Yodoi, J.; Masutani, H. Disruption of TBP-2 ameliorates insulin sensitivity and secretion without affecting obesity. Nat. Commun. 2010, 1, 127. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.; Xu, G.; Grayson, T.B.; Shalev, A. Cytokines Regulate β-Cell Thioredoxin-interacting Protein (TXNIP) via Distinct Mechanisms and Pathways. J. Biol. Chem. 2016, 291, 8428–8439. [Google Scholar] [CrossRef] [Green Version]
- Karunakaran, U.; Moon, J.S.; Lee, H.W.; Won, K.C. CD36 initiated signaling mediates ceramide-induced TXNIP expression in pancreatic beta-cells. Biochim. Biophys. Acta-Mol. Basis Dis. 2015, 1852, 2414–2422. [Google Scholar] [CrossRef] [Green Version]
- Reich, E.; Tamary, A.; Sionov, R.V.; Melloul, D. Involvement of thioredoxin-interacting protein (TXNIP) in glucocorticoid-mediated beta cell death. Diabetologia 2012, 55, 1048–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, J.; Wang, J.-J.; Zhang, W.-F.; Jiao, X.-Y. Role of autophagy in TXNIP overexpression-induced apoptosis of INS-1 islet cells. Sheng Li Xue Bao Acta Physiol. Sin. 2017, 69, 445–451. [Google Scholar]
- Muoio, D.M.; Newgard, C.B. Obesity-Related Derangements in Metabolic Regulation. Annu. Rev. Biochem. 2006, 75, 367–401. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, H.; LaRose, C.; Fisk, C.; Waldhart, A.; Meng, X.; Zhao, G.; Wu, N. TXNIP interaction with GLUT1 depends on PI(4,5)P2. Biochim. Biophys. Acta-Biomembr. 2021, 1863, 183757. [Google Scholar] [CrossRef]
- Tang, L.; El-Din, T.M.G.; Swanson, T.M.; Pryde, D.C.; Scheuer, T.; Zheng, L.; Catterall, W.A. Structural basis for inhibition of a voltage-gated Ca2+ channel by Ca2+ antagonist drugs. Nature 2016, 537, 117–121. [Google Scholar] [CrossRef]
- Xu, G.; Grimes, T.D.; Grayson, T.B.; Chen, J.; Thielen, L.A.; Tse, H.M.; Li, P.; Kanke, M.; Lin, T.-T.; Schepmoes, A.A.; et al. Exploratory study reveals far reaching systemic and cellular effects of verapamil treatment in subjects with type 1 diabetes. Nat. Commun. 2022, 13, 1159. [Google Scholar] [CrossRef]
- Wondafrash, D.Z.; Nire’A, A.T.; Tafere, G.G.; Desta, D.M.; Berhe, D.A.; Zewdie, K.A. Thioredoxin-Interacting Protein as a Novel Potential Therapeutic Target in Diabetes Mellitus and Its Underlying Complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Lido, P.; Romanello, D.; Tesauro, M.; Bei, A.; Perrone, M.A.; Palazzetti, D.; Noce, A.; di Lullo, L.; Calò, L.; Cice, G. Verapamil: Prevention and treatment of cardio-renal syndromes in diabetic hypertensive patients? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1524–1534. [Google Scholar] [CrossRef]
- Afzal, N.; Ganguly, P.K.; Dhalla, K.S.; Pierce, G.N.; Singal, P.K.; Dhalla, N.S. Beneficial Effects of Verapamil in Diabetic Cardiomyopathy. Diabetes 1988, 37, 936–942. [Google Scholar] [CrossRef]
- Chen, J.; Cha-Molstad, H.; Szabo, A.; Shalev, A. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am. J. Physiol. Metab. 2009, 296, E1133–E1139. [Google Scholar] [CrossRef] [Green Version]
- Thrane, M.T.; Holst, J.J.; Busch-Sørensen, M.; Sjøstrand, H.; Sengelov, H.; Lyngsøe, J. Influence of short term verapamil treatment on glucose metabolism in patients with non-insulin dependent diabetes mellitus. Eur. J. Clin. Pharmacol. 1991, 41, 401–404. [Google Scholar] [CrossRef]
- Carnovale, C.; Dassano, A.; Mosini, G.; Mazhar, F.; D’Addio, F.; Pozzi, M.; Radice, S.; Fiorina, P.; Clementi, E. The β-cell effect of verapamil-based treatment in patients with type 2 diabetes: A systematic review. Geol. Rundsch. 2020, 57, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gong, H.; Hu, X.; Shi, P.; Cen, H.; Li, C. Effect of verapamil on bone mass, microstructure and mechanical properties in type 2 diabetes mellitus rats. BMC Musculoskelet. Disord. 2022, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Osipovich, A.B.; Stancill, J.S.; Cartailler, J.-P.; Dudek, K.D.; Magnuson, M.A. Excitotoxicity and Overnutrition Additively Impair Metabolic Function and Identity of Pancreatic β-Cells. Diabetes 2020, 69, 1476–1491. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [Green Version]
- Mendes, R.; Sousa, N.; Almeida, A.; Subtil, P.; Guedes-Marques, F.; Reis, V.; Themudo-Barata, J.L. Exercise prescription for patients with type 2 diabetes—a synthesis of international recommendations: Narrative review: Table 1. Br. J. Sports Med. 2016, 50, 1379–1381. [Google Scholar] [CrossRef] [Green Version]
- Thielen, L.A.; Chen, J.; Jing, G.; Moukha-Chafiq, O.; Xu, G.; Jo, S.; Grayson, T.B.; Lu, B.; Li, P.; Augelli-Szafran, C.E.; et al. Identification of an Anti-diabetic, Orally Available Small Molecule that Regulates TXNIP Expression and Glucagon Action. Cell Metab. 2020, 32, 353–365.e8. [Google Scholar] [CrossRef]
- Pham, V.T.; Ciccaglione, M.; Ramirez, D.G.; Benninger, R.K.P. Ultrasound Imaging of Pancreatic Perfusion Dynamics Predicts Therapeutic Prevention of Diabetes in Preclinical Models of Type 1 Diabetes. Ultrasound Med. Biol. 2022, 48, 1336–1347. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, P.; Aberer, F.; Eckstein, M.L.; Haupt, S.; Erlmann, M.P.; Moser, O. Verapamil and Its Role in Diabetes. Diabetology 2022, 3, 393-406. https://doi.org/10.3390/diabetology3030030
Zimmermann P, Aberer F, Eckstein ML, Haupt S, Erlmann MP, Moser O. Verapamil and Its Role in Diabetes. Diabetology. 2022; 3(3):393-406. https://doi.org/10.3390/diabetology3030030
Chicago/Turabian StyleZimmermann, Paul, Felix Aberer, Max L. Eckstein, Sandra Haupt, Maximilian P. Erlmann, and Othmar Moser. 2022. "Verapamil and Its Role in Diabetes" Diabetology 3, no. 3: 393-406. https://doi.org/10.3390/diabetology3030030
APA StyleZimmermann, P., Aberer, F., Eckstein, M. L., Haupt, S., Erlmann, M. P., & Moser, O. (2022). Verapamil and Its Role in Diabetes. Diabetology, 3(3), 393-406. https://doi.org/10.3390/diabetology3030030





