Surface Modification of Poly(butyl methacrylate) with Sulfomethylated Resorcinarenes for the Selective Extraction of Dichromate Ion in Aqueous Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Resorcinarenes
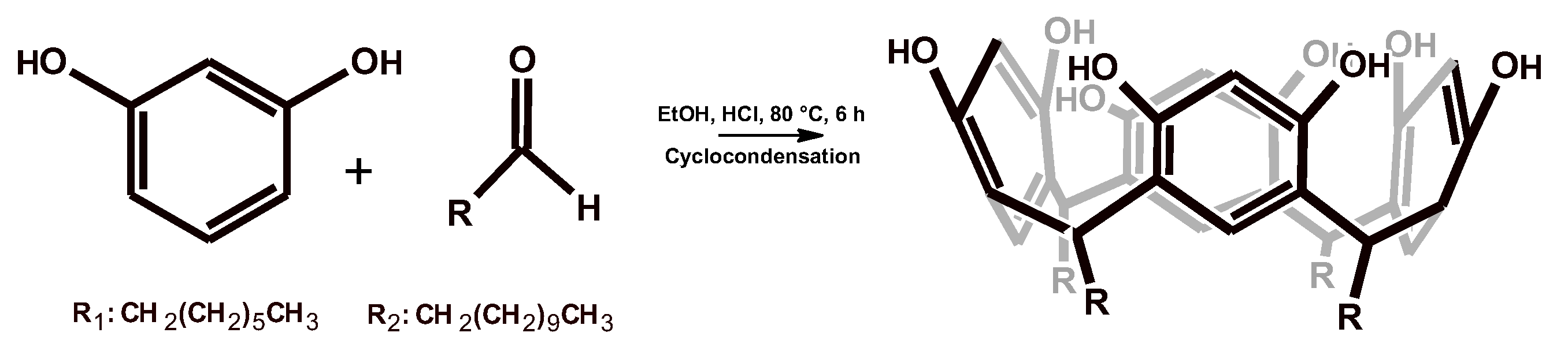
2.1.1. Synthesis of Resorcinarenes
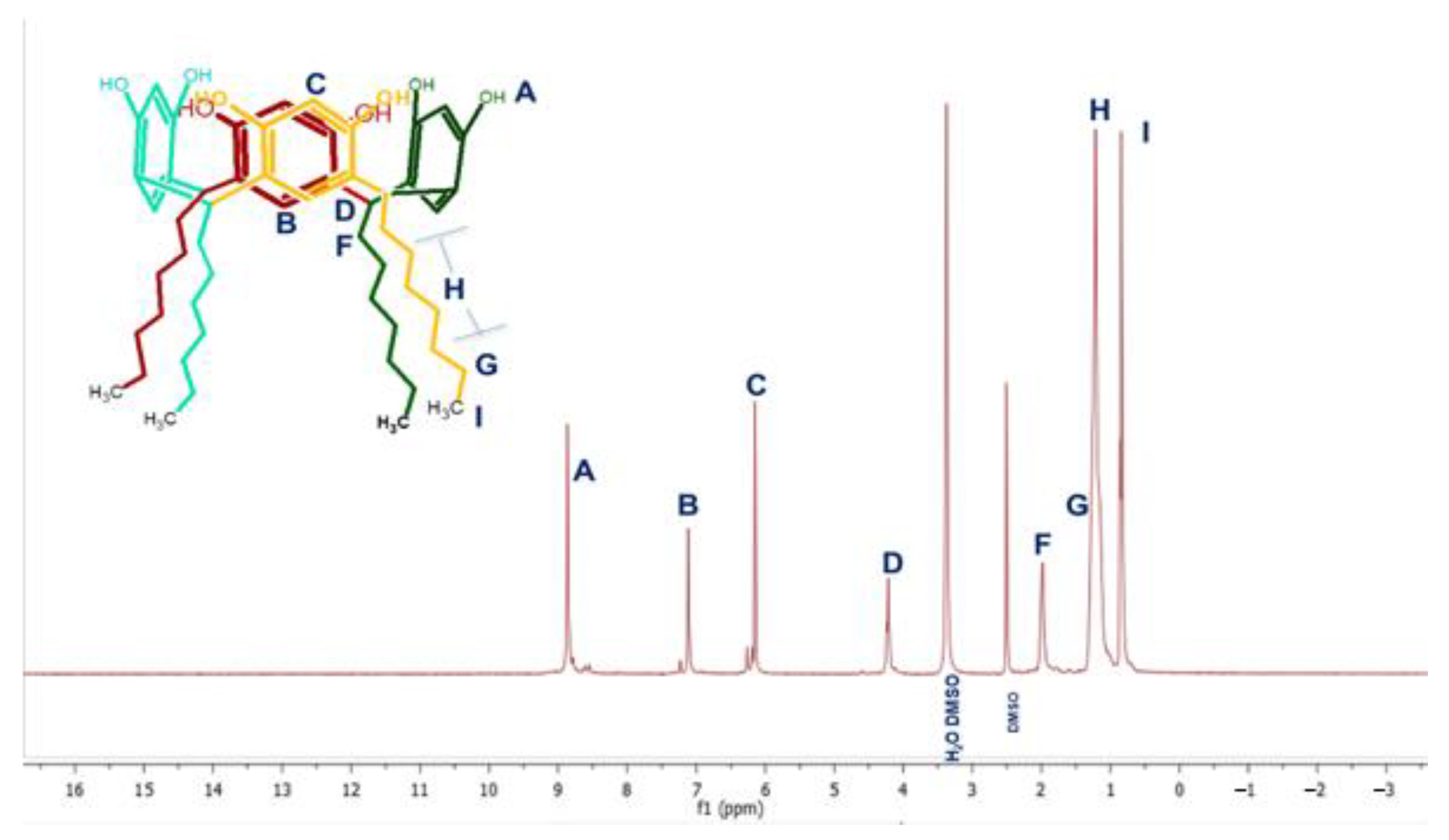
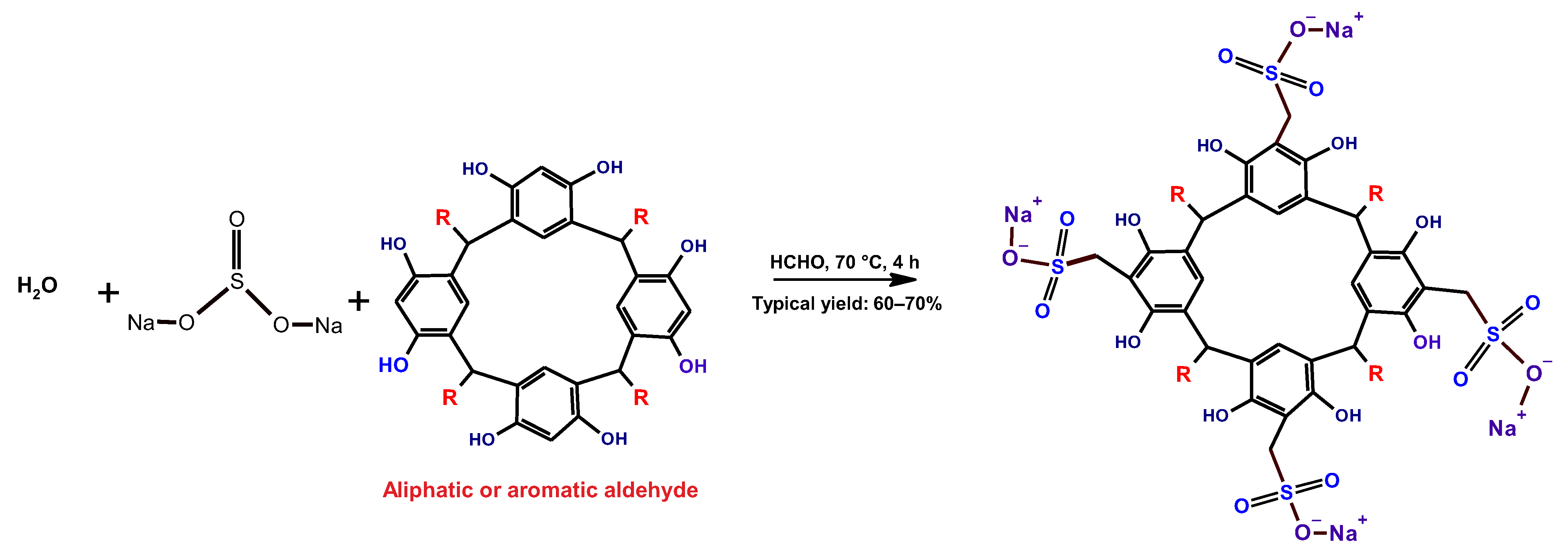
2.1.2. Synthesis of the Sulfometilated Resorcinarenes
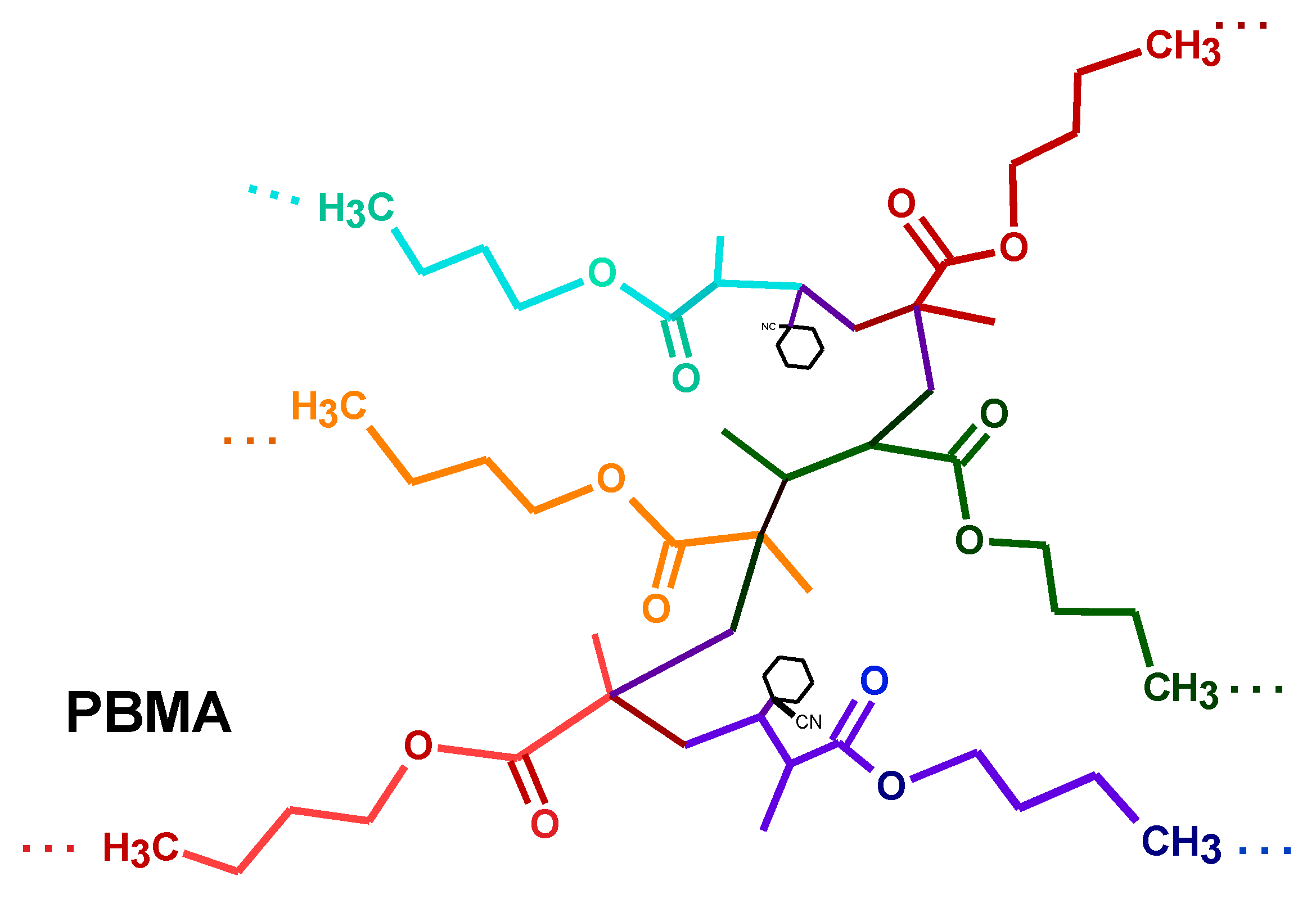
2.2. Impregnation of PBMA
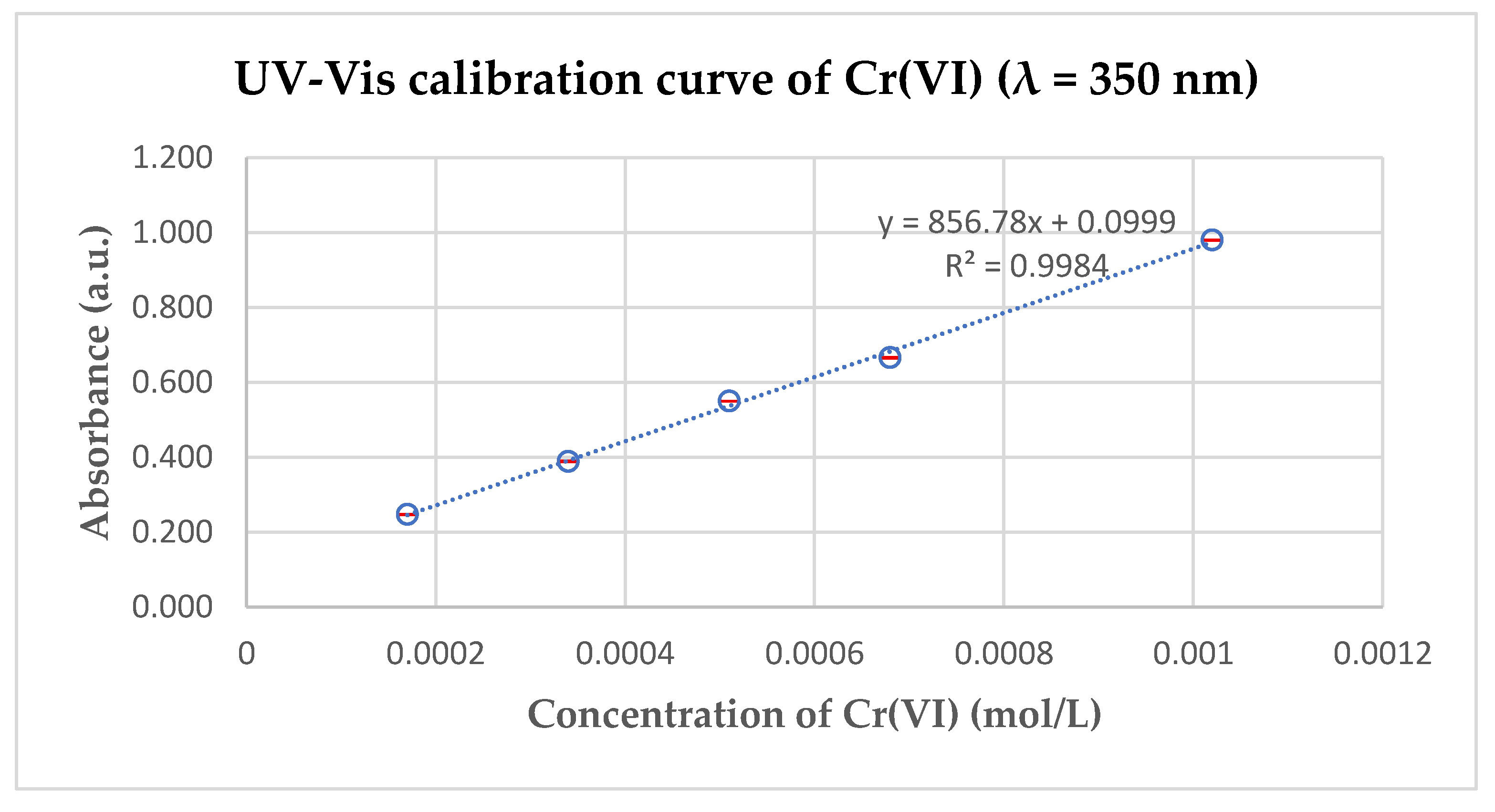
2.3. Dichromate Calibration Curve
3. Results and Discussion
3.1. Synthesis of C-Tetra(alkyl)calix[4]resorcinarene
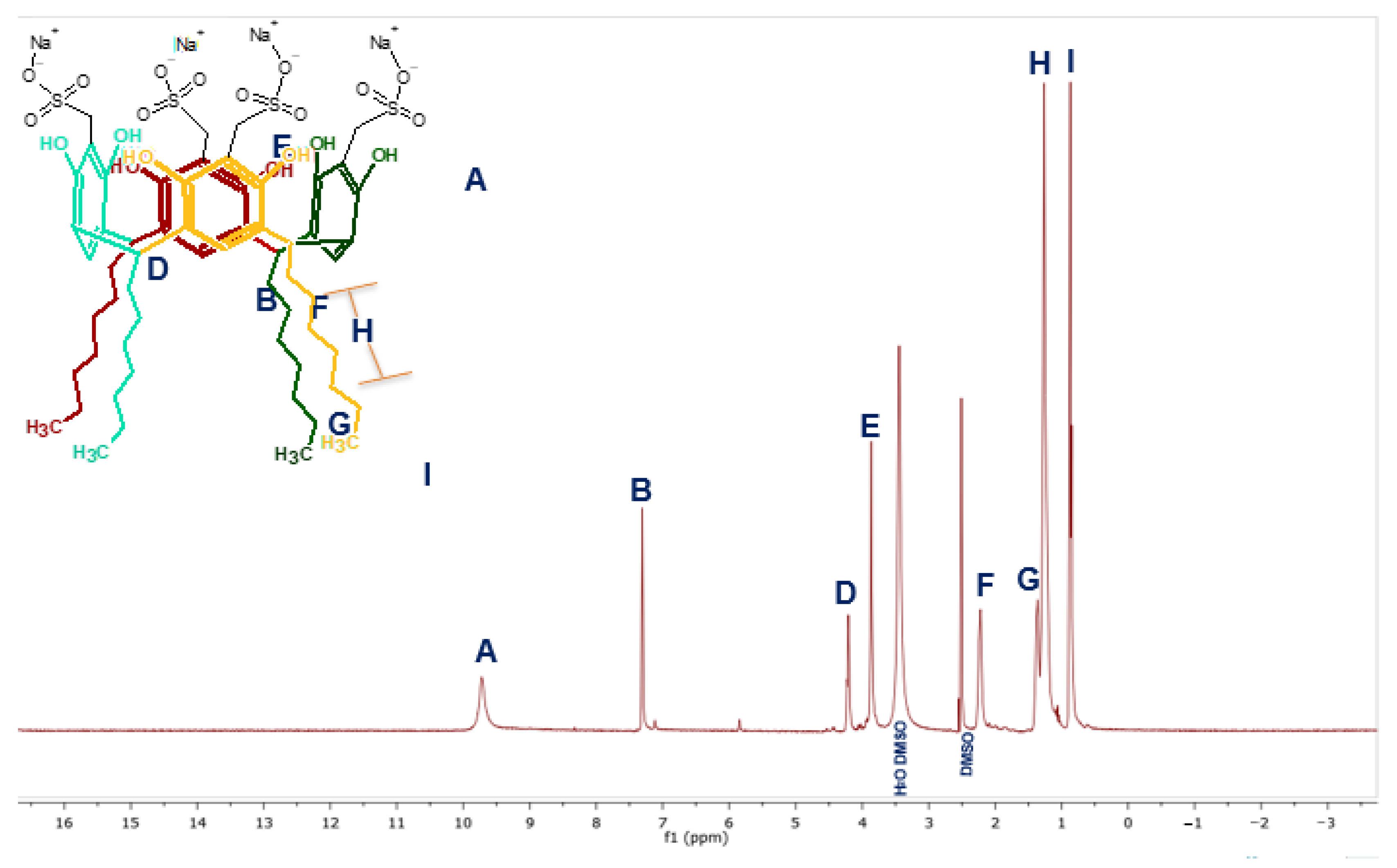
3.2. Sulfomethylation of Resorcinarenes
3.3. Obtention of Poly(butyl methacrylate) and Impregnation with Resorcinarenes
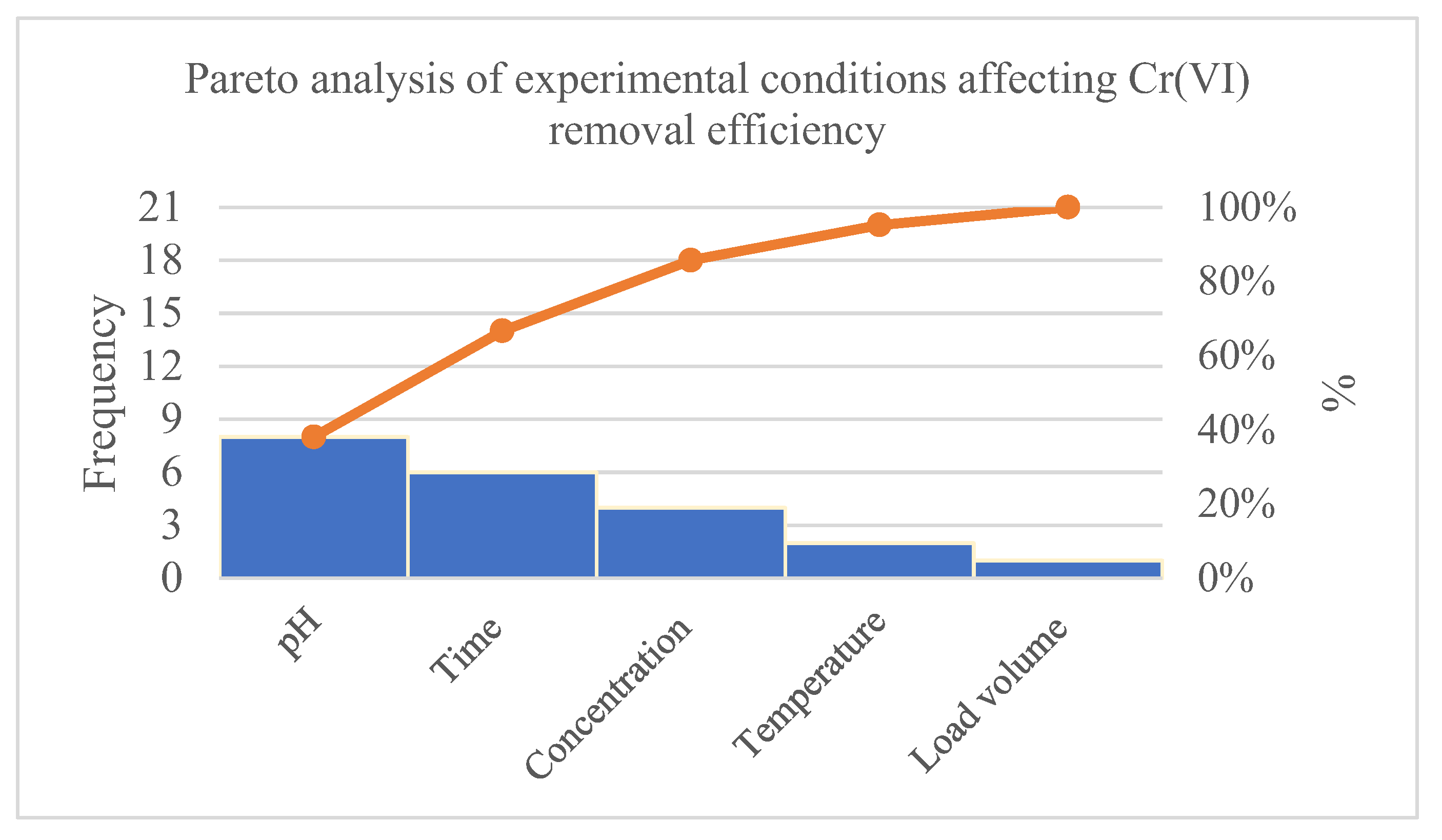
3.4. Determination of Chromium (VI) Removal Conditions
3.5. The Dichromate Anion (Cr2O72−) Removal Experiments in Aqueous Media
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerur, S.S.; Bandekar, S.; Hanagadakar, M.S.; Nandi, S.S.; Ratnamala, G.M.; Hegde, P.G. Removal of hexavalent Chromium-Industry treated water and Wastewater: A review. Mater. Today Proc. 2021, 42, 1112–1121. [Google Scholar] [CrossRef]
- Hanjra, M.A.; Blackwell, J.; Carr, G.; Zhang, F.; Jackson, T.M. Wastewater irrigation and environmental health: Implications for water governance and public policy. Int. J. Hyg. Environ. Health 2012, 215, 255–269. [Google Scholar] [CrossRef] [PubMed]
- US EPA ORD NCEA Integrated Risk Information System. Hexavalent Chromium in Support of Summary Information on the Integrated Risk Information System (IRIS). 1998. Available online: http://www.epa.gov/iris (accessed on 22 June 2025).
- Guidelines for Drinking-Water Quality Fourth Edition WHO Library Cataloguing-in-Publication Data Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; Available online: http://www.who.int (accessed on 22 June 2025).
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef] [PubMed]
- Benjjar, A.; Eljaddi, T.; Kamal, O.; Touaj, K.; Lebrun, L.; Hlaibi, M. The development of new supported liquid membranes (SLMs) with agents: Methyl cholate and resorcinarene as carriers for the removal of dichromate ions (Cr2O72−). J. Environ. Chem. Eng. 2014, 2, 503–509. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223–224, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.S.; Aparna, N.; Mathew, M. Hexavalent chromium removal using reduced graphene oxide-zinc oxide composite fabricated via simple pyrolysis method. Appl. Surf. Sci. Adv. 2024, 19, 100535. [Google Scholar] [CrossRef]
- Leyva, R.; Flores, J.V.; Díaz, P.E.; Berber, M.S. Adsorción de Cromo (VI) en Solución Acuosa sobre Fibra de Carbón Activado Adsorption of Chromium (VI) from Aqueous Solution onto Activated Carbon Fiber. Inf. Tecnol. 2008, 19, 27–36. [Google Scholar] [CrossRef]
- Dobrosz-Gómez, I.; Gómez, M.-Á.; Santa, C.; Dobrosz-Gómez, I.; Gómez, M.-Á.; Santa, C. Optimización del Proceso de Adsorción de Cr(VI) sobre Carbón Activado de Origen Bituminoso. Inf. Tecnol. 2018, 29, 43–56. [Google Scholar] [CrossRef][Green Version]
- Higuera Cobos, O.F. Estudio de la biosorción de cromo con hoja de café. Rev. Ing. Investig. 2009, 29, 59–64. [Google Scholar][Green Version]
- Medina, E.C. Estudio de la Adsorción de Cromo Hexavalente Utilizando Como Biomaterial la Ectodermis de Opuntia. Quivera 2008, 10, 16–31. [Google Scholar][Green Version]
- Mohan, D.; Pittman, C.U. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef] [PubMed]
- Ali, I. New Generation Adsorbents for Water Treatment. Chem Rev 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Iftekhar, S.; Pizzetti, F.; Zarepour, A.; Zare, E.N.; Ashrafizadeh, M.; Agarwal, T.; Padil, V.V.; Mohammadinejad, R.; Sillanpaa, M.; et al. Functionalization of polymers and nanomaterials for water treatment, food packaging, textile and biomedical applications: A review. Environ. Chem. Lett. 2021, 19, 583–611. [Google Scholar] [CrossRef]
- Severiche Sierra, C.A.; González García, H. Assesment of an Analytical Method for Determining Hexavalent Chromiun in Water Using Spectrophotometry. Ing. USBMed 2013, 4, 22–26. [Google Scholar] [CrossRef]
- Semenovitch, G.; Lipatov, Y.; Todosijchuk, T.; Chornaya, V. Adsorption of Poly(butyl methacrylate) and Its Mixtures with Polystyrene from Solutions: Adsorption Kinetics and Structure of Adsorption Layers. J. Colloid Interface Sci. 1996, 184, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Aguirre, A.; Maldonado, M.; Esteso, M.A. Removal of Toxic Metal Ions Using Poly(BuMA–co–EDMA) Modified with C-Tetra(nonyl)calix[4]resorcinarene. Toxics 2022, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, E.; Esteso, M.A.; Pérez-Redondo, A.; Vargas, E.; Maldonado, M. Synthesis and characterization of two sulfonated resorcinarenes: A new example of a linear array of sodium centers and macrocycles. Molecules 2015, 20, 9915–9928. [Google Scholar] [CrossRef] [PubMed]
- Urquijo, C.; Vela, M.; Sarmiento, R.; Maldonado, M. Molecular Interaction of Water-Soluble Resorcinarenes for Potential Choline Detectors. Processes 2025, 13, 553. [Google Scholar] [CrossRef]
- Lee, J.; Chul Kim, S. Synthesis and Thermal Properties of Polyurethane, Poly(butylmethacrylate), and Poly(methylmethacrylate) Multi-Component IPN’s. Polym. J. 1984, 16, 453–459. [Google Scholar] [CrossRef]
- Deus, L.C.; Silva, C.M.F.; Martins, M.F.; Aversa, T.M.; Lucas, E.F. Removing ammonium from water using porous resins: Influence of polymer structure, ion exchange capacity and porosity. DYNA 2021, 88, 237–246. [Google Scholar] [CrossRef]
- López-Solis, C.L.; Córdoba-Jiménez, D.G.; Cuervo-Ochoa, G.; Martín-Franco, J.; Gutiérrez-Valencia, T.M. Extracción selectiva de oro mediante membranas basadas en un nuevo material polimérico con sitios activos complejantes. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2020, 44, 814–827. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.; Jiang, K.; Fan, Z.; Wang, D.; Gu, P.; Zhou, S.; Li, Z. Post-synthetic sulfonation of resorcinarene-based porous organic polymer for superfast adsorption of diverse organic pollutants. Polymer 2024, 313, 127738. [Google Scholar] [CrossRef]
- Velásquez-Silva, B.A.; Castillo-Aguirre, A.; Rivera-Monroy, Z.J.; Maldonado, M. Aminomethylated calix[4]resorcinarenes as modifying agents for glycidyl methacrylate (GMA) rigid copolymers surface. Polymers 2019, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Zawierucha, I.; Nowik-Zajac, A.; Kozlowski, C.A. Removal of Pb(II) ions using polymer inclusion membranes containing calix[4]resorcinarene derivative as ion carrier. Polymers 2019, 11, 2111. [Google Scholar] [CrossRef] [PubMed]
- Rivas, B.L. Materiales poliméricos con capacidad para retener iones metálicos con impacto en el medio ambiente. Rev. Quim. 2004, 18, 23–30. [Google Scholar]
- Huang, J.; Fang, Y.; Dehaen, W. Macrocyclic arenes functionalized with BODIPY: Rising stars among chemosensors and smart materials. Chemosensors 2020, 8, 51. [Google Scholar] [CrossRef]
- Navarro, A.E.; Ramos, K.P.; Campos, K.; Maldonado, H.J. Elucidación del Efecto del pH en la Adsorción de Metales Pesados Mediante Biopolímeros Naturales: Cationes Divalentes y Superficies Activas. Rev. Iberoam. Polímeros 2006, 7, 113–126. [Google Scholar]
- Martínez-Vertel, J.J.; Villaquirán-Vargas, A.P.; Villar-García, Á.; Moreno-Díaz, D.F.; Rodríguez-Castelblanco, A.X. Polymer adsorption isotherms with NaCl and CaCl2 on kaolinite substrates. Rev. DYNA 2019, 86, 66–73. [Google Scholar] [CrossRef]
- Tero, T.R.; Nissinen, M. A perspective to resorcinarene crowns. Tetrahedron 2014, 70, 1111–1123. [Google Scholar] [CrossRef]
- Anandkumar, J.; Mandal, B. Removal of Cr(VI) from aqueous solution using Bael fruit (Aegle marmelos correa) shell as an adsorbent. J. Hazard. Mater. 2009, 168, 633–640. [Google Scholar] [CrossRef] [PubMed]
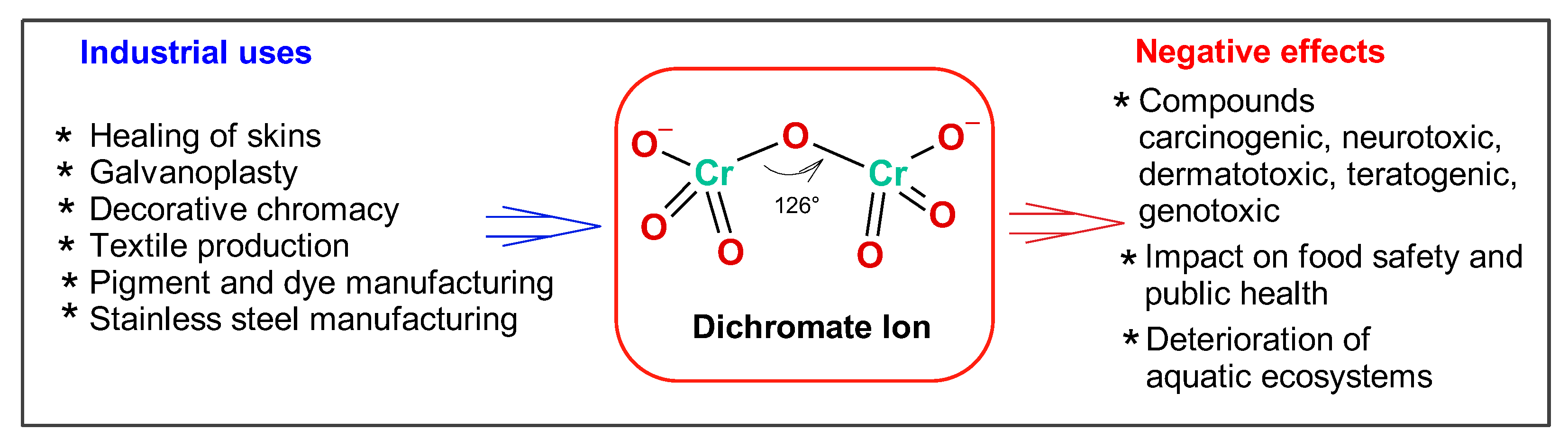

| Compound | Anion Type | Industrial Applications | Environmental and Health Impacts |
|---|---|---|---|
| Potassium dichromate (K2Cr2O7) | Dichromate (Cr2O72−) | Oxidizing agent in organic synthesis, electroplating, analytical chemistry | Highly toxic, carcinogenic, contaminates water and soil |
| Sodium dichromate (Na2Cr2O7) | Dichromate (Cr2O72−) | Pigments, leather tanning, corrosion inhibitors, metal finishing | Strong oxidizer, damages liver and kidneys, persistent in water |
| Ammonium dichromate ((NH4)2Cr2O7) | Dichromate (Cr2O72−) | Pyrotechnics, chemical demonstrations, oxidizing agent | Fire hazard, toxic by inhalation and ingestion, soil contaminant |
| Chromic acid (H2Cr2O7/CrO3 + H2O) | Acidic dichromate form | Metal cleaning, etching, glassware cleaning in labs | Corrosive, harmful to aquatic life, toxic vapors |
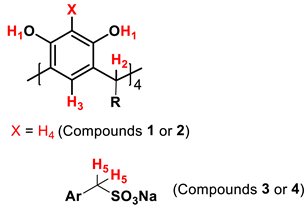 | |||||
| (1) | (2) | (3) | (4) | ||
| RI (ATR-ZnSe /cm−1) | (O–H) | 3205 | 3206 | 3334 | 3393 |
| (ArC-H), | 3050 | 3050 | 3020 | 3020 | |
| (Aliph. C-H) | 2923–2863 | 2920–2850 | 2923–2852 | 2919–2850 | |
| C=C | 1618–1446 | 1617–1452 | 1610–1472 | 1608–1469 | |
| C-C | 1170 | 1172 | 1142 | 1144 | |
| C-O | 1083 | 1088 | 1038 | 1039 | |
| O=S=O | --- | --- | 1308–1177 | 1307–1182 | |
| C-S | --- | --- | 776 | 770 | |
| 1H NMR (ppm) | H1 [8H, OH] | 8.86 | 9.56 | 9.71 | 9.72 |
| H3 [4H, m. OH] | 7.11 | 7.23 | 7,31 | 7.23 | |
| H4 [4H, o. OH] | 6,14 | 6.13 | --- | --- | |
| H2 [4H, CH] | 4.22 | 4.32 | 4.21 | 4.20 | |
| H5 [8H, CH2] | --- | --- | 3.86 | 3.84 | |
| R | 1.98, 1.22, 1.18, 0.84 | 2.23, 1.35, 1.29, 0.90 | 2.24, 1.24, 1.25, 0.87 | 2.18, 1.33, 1.25, 0.86 | |
| 13C NMR (ppm) | (1) C (1–12) | 152.2, 125.3, 123.4, 102.8, 34.6, 33.4, 31.8, 29.7, 29.3, 28.2, 22.5, 14.3 | |||
| (2) C (1–16) | 150.4, 124.9, 108.1, 103.0, 33.3, 31.9, 29.7, 29.4, 28.1, 22.7, 22.6, 14.3 | ||||
| (3) C (1–13) | 150.5, 125.2, 123.3, 109.5, 48.7, 34.6, 34.1, 31.9, 29.9, 28.9, 22.6, 19.9, 14.4 | ||||
| (4) C (1–17) | 150.5, 125.1, 109.6, 99.6, 48.4, 40.5, 40.3, 40.1, 39.9, 39.7, 39.5, 39.3, 31.9, 29.7, 29.3, 22.6, 14.4 | ||||
| PMBA | (5) | (6) | (7) | (8) | ||
|---|---|---|---|---|---|---|
| RI (ATR-ZnSe /cm−1) | (O–H) | --- | 3326 | 3320 | 3427 | 3320 |
| (ArC-H), | --- | 3020 | 3020 | 3020 | 3030 | |
| (Aliph. C-H) | 2956–2872 | 2926–2855 | 2919–2851 | 2956–2871 | 2956–2872 | |
| C=C | --- | 1618–1494 | 1608–1468 | 1618–1445 | 1633–1465 | |
| C=O | 1722 | 1725 | 1722 | 1721 | 1721 | |
| C-C | 1238 | 1242 | 1229 | 1238 | 1239 | |
| C-O | 1142 | 1153 | 1144 | 1143 | 1142 | |
| O=S=O | --- | --- | --- | 1300–1170 | 1380–1180 | |
| C-S | --- | --- | --- | 746 | 747 |
| Polymer | O-H cm−1 | C=O cm−1 | μmol/g |
|---|---|---|---|
| (5) | 3326 | 1725 | 1290 |
| (6) | 3320 | 1722 | 37 |
| (7) | 3427 | 1721 | 1114 |
| (8) | 3320 | 1721 | 882 |
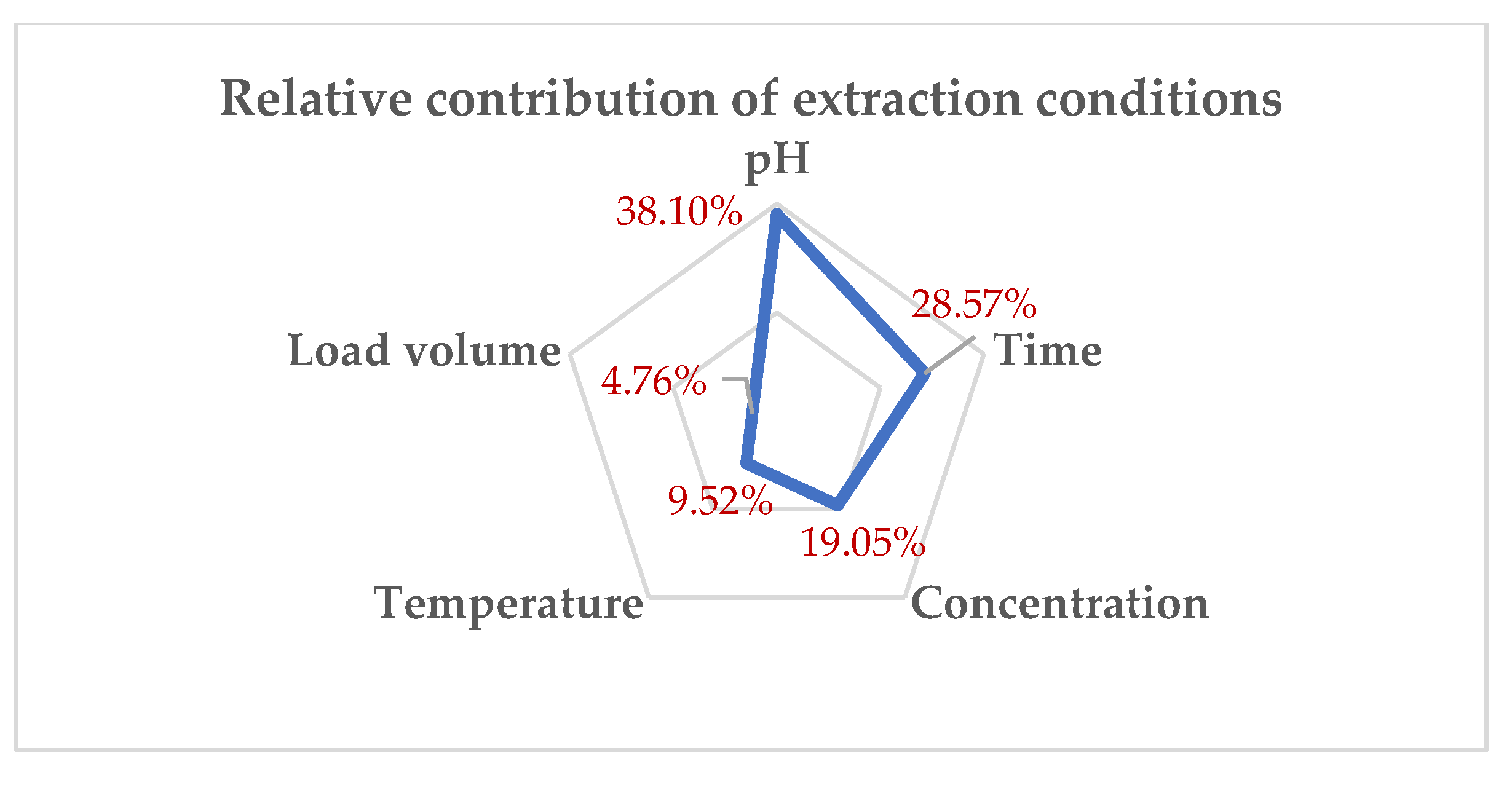
| pH | 8 | 38.10% | 38.10% |
|---|---|---|---|
| Time | 6 | 28.57% | 66.67% |
| Concentration | 4 | 19.05% | 85.72% |
| Temperature | 2 | 9.52% | 95.24% |
| Load volume | 1 | 4.76% | 100.00% |
| 21 | 100.00% |
| Matrix | ||||||||
|---|---|---|---|---|---|---|---|---|
| pH | Time (min) | Concentration [M] | Initial Absorbance | (5) ** | (6) * | (7) ** | (8) | (PBMA) |
| Absorbance, % Removal | ||||||||
| 2.0 | 60 | 3.36 × 10−4 | 0.981 | 0.961 | --- | 1.071 | 1.507 | 1.020 |
| 2.00% | --- | --- | --- | --- | ||||
| 3.0 | 60 | 3.36 × 10−4 | 0.954 | --- | 1.052 | 1.114 | 0.986 | |
| 6.00% | --- | --- | --- | 0.030 | ||||
| 120 | 3.36 × 10−4 | 0.999 | --- | 1.110 | 1.168 | 0.988 | ||
| 2.00% | --- | --- | --- | 0.030 | ||||
| 4.5 | 30 | 3.36 × 10−4 | 1.084 | 1.066 | --- | 1.071 | 1.097 | 1.066 |
| 1.66% | --- | 1.20% | --- | 1.66% | ||||
| 60 | 3.36 × 10−4 | 1.035 | --- | 1.088 | 1.083 | 1.034 | ||
| 4.52% | --- | --- | 0.09% | 4.61% | ||||
| 90 | 3.36 × 10−4 | 1.043 | --- | 1.107 | 1.127 | 1.054 | ||
| 3.78% | --- | --- | 2.77% | |||||
| 120 | 3.36 × 10−4 | 1.041 | --- | 1.115 | 1.149 | 1.045 | ||
| 3.97% | --- | --- | --- | 3.60% | ||||
| 150 | 3.36 × 10−4 | 1.042 | 1.128 | 1.124 | 1.055 | |||
| 3.87% | --- | --- | --- | 2.68% | ||||
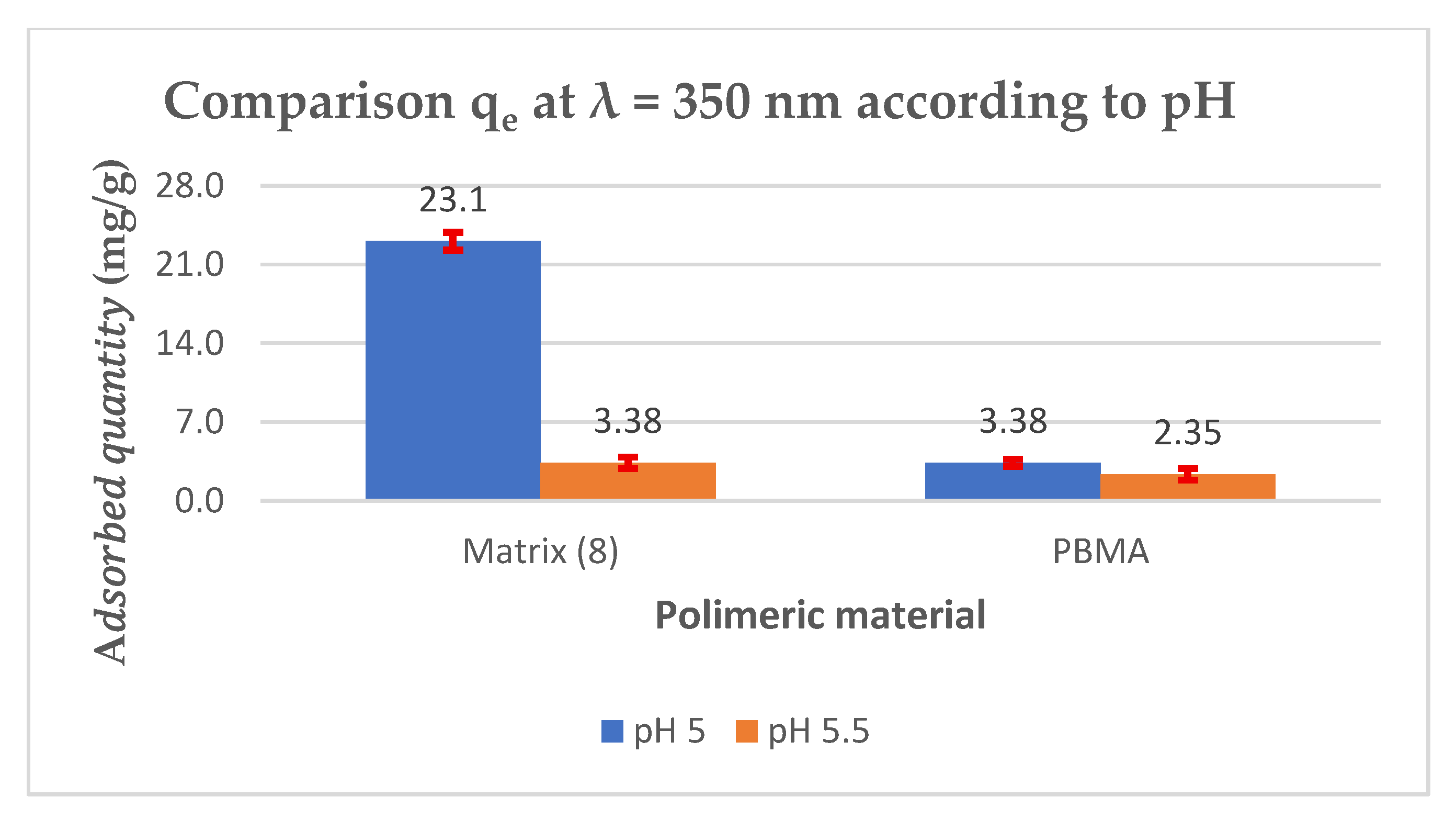
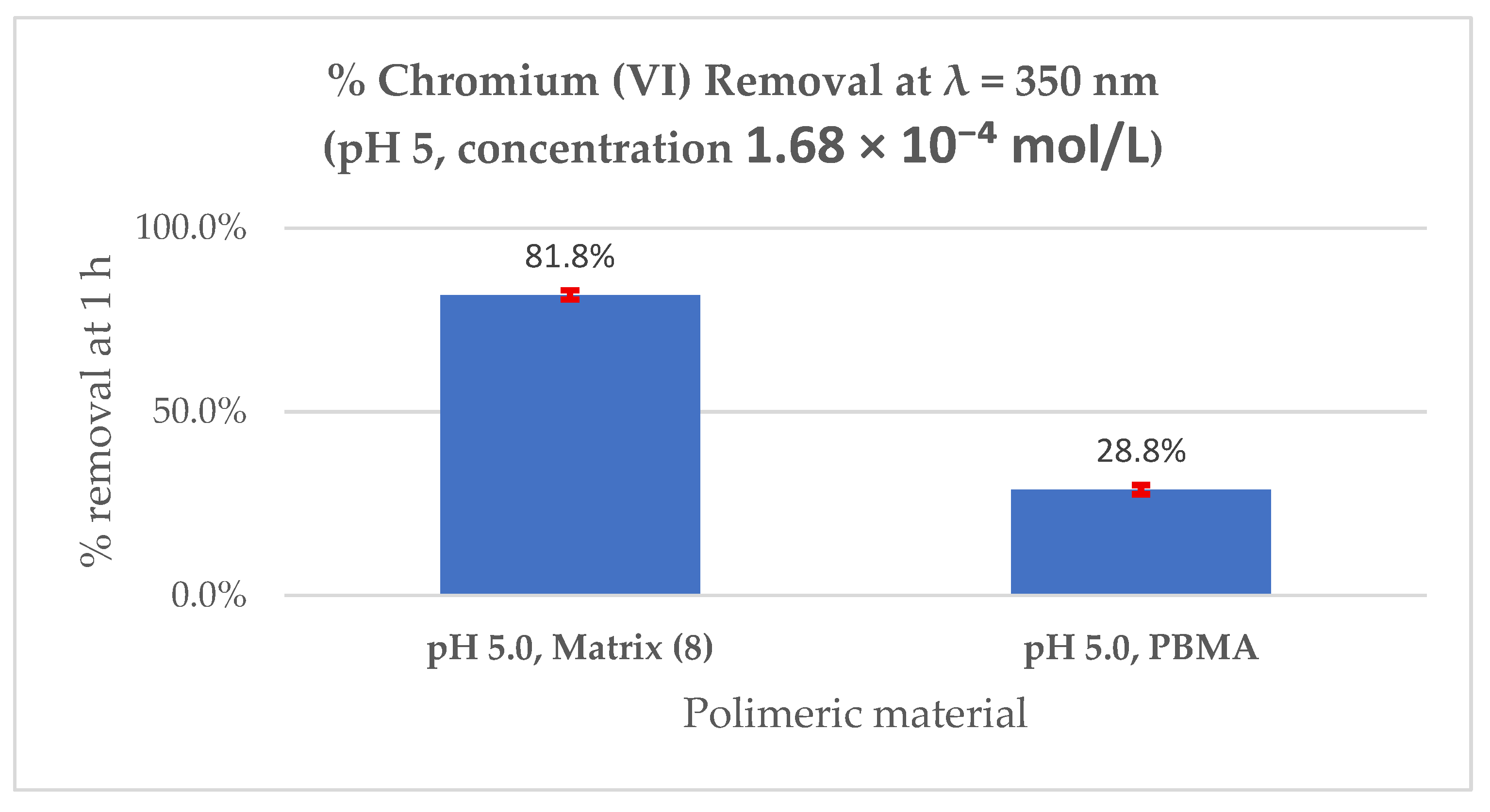
| 5.0 | 60 | 1.64 × 10−4 | 0.111 | --- | --- | --- | 0.021 | 0.079 |
| 81.1% | 28.8% | |||||||
| 5.5 | 60 | 0.113 | --- | --- | --- | 0.111 | 0.078 | |
| 1.8% | 30.9% | |||||||
| 5.0 | 120 | 3.36 × 10−4 | 0.173 | --- | --- | --- | 0.091 | 0.161 |
| 47.4% | 6.9% | |||||||
| 5.5 | 120 | 0.180 | --- | --- | --- | 0.133 | 0.163 | |
| 23.1% | 5.8% | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urquijo, C.; Maldonado, M. Surface Modification of Poly(butyl methacrylate) with Sulfomethylated Resorcinarenes for the Selective Extraction of Dichromate Ion in Aqueous Media. Analytica 2025, 6, 24. https://doi.org/10.3390/analytica6030024
Urquijo C, Maldonado M. Surface Modification of Poly(butyl methacrylate) with Sulfomethylated Resorcinarenes for the Selective Extraction of Dichromate Ion in Aqueous Media. Analytica. 2025; 6(3):24. https://doi.org/10.3390/analytica6030024
Chicago/Turabian StyleUrquijo, Cielo, and Mauricio Maldonado. 2025. "Surface Modification of Poly(butyl methacrylate) with Sulfomethylated Resorcinarenes for the Selective Extraction of Dichromate Ion in Aqueous Media" Analytica 6, no. 3: 24. https://doi.org/10.3390/analytica6030024
APA StyleUrquijo, C., & Maldonado, M. (2025). Surface Modification of Poly(butyl methacrylate) with Sulfomethylated Resorcinarenes for the Selective Extraction of Dichromate Ion in Aqueous Media. Analytica, 6(3), 24. https://doi.org/10.3390/analytica6030024





