Derivatizing Agent Selection for Hydrophilic Lysine- and Arginine-Containing Tetradecapeptide Analysis in Human Plasma by RP HPLC-MS/MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Peptide Therapeutic to Study
2.2. Mass Spectrum
2.3. Derivatizing Agent Selection
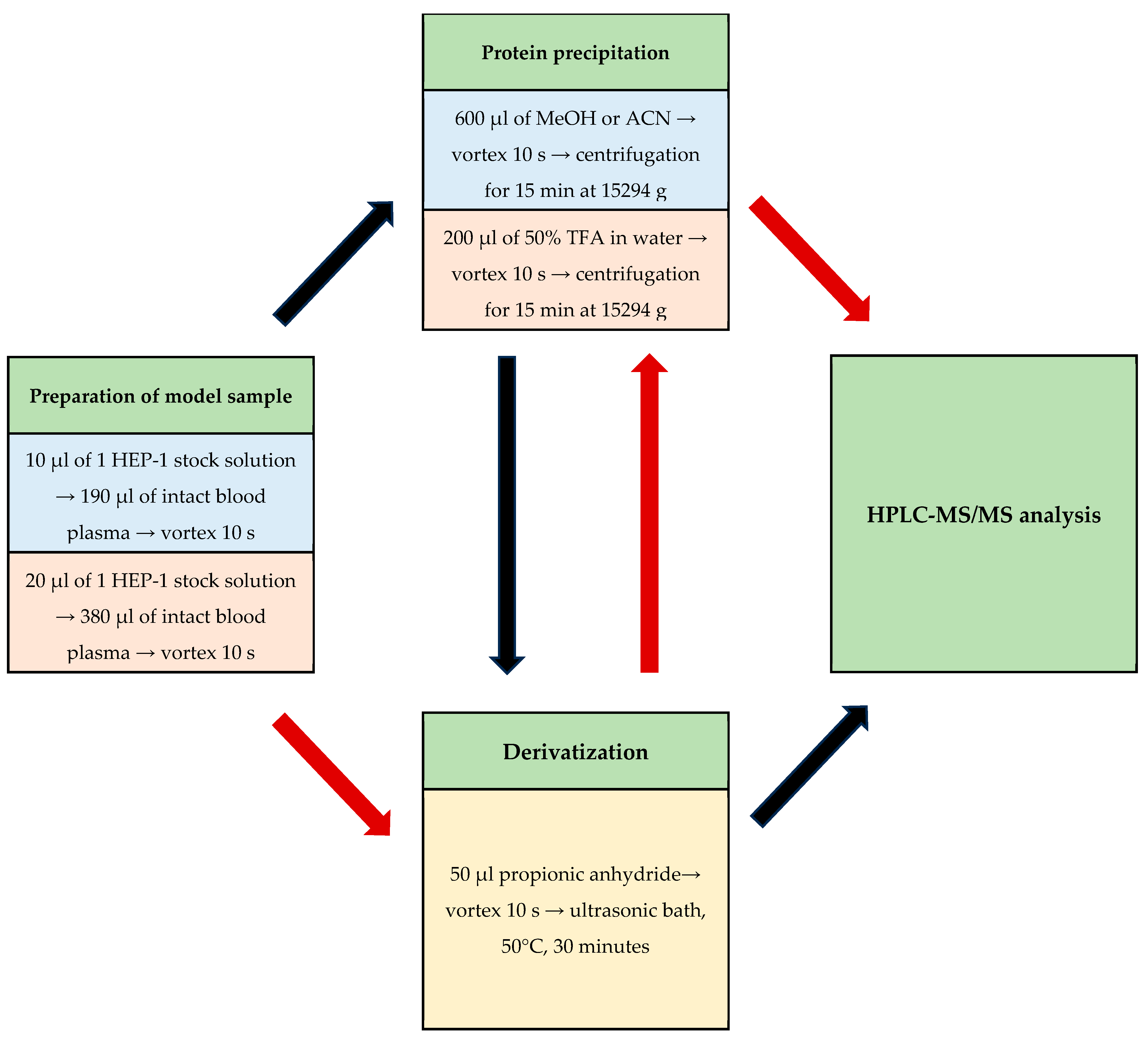
2.4. Sample Preparation Selection
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Apparatus and HPLC Conditions
| Time, min | Eluent B, % |
|---|---|
| 0.00–0.50 | 5 |
| 0.50–2.50 | 5 → 30 |
| 2.50–2.60 | 30 → 100 |
| 2.60–3.60 | 100 |
| 3.60–4.10 | 100 → 5 |
| 4.10–5.00 | 5 |
| Time, min | Eluent B, % |
|---|---|
| 0.00–0.50 | 5 |
| 0.50–4.70 | 5 → 55 |
| 4.70–4.80 | 55 → 100 |
| 4.80–5.80 | 100 |
| 5.80–6.50 | 100 → 5 |
| 6.50–7.00 | 5 |
4.3. Preparation of Solutions
4.3.1. Preparation of HEP-1 Stock Solution
4.3.2. Preparation of Derivatizing Agents Solutions
4.3.3. Preparation of Buffer Solutions
4.4. Preparation of Analytical Samples
4.4.1. Preparation of Dansyl, Tosyl, and PITC Derivative Samples of HEP-1
4.4.2. Preparation of Fmoc-Osu, Boc Anhydride, Cbz-Osu Derivative Samples of HEP-1
4.4.3. Preparation of Benzoic Anhydride Derivative Samples of HEP-1
4.4.4. Preparation of Propionic Anhydride Derivative Samples of HEP-1
4.4.5. Preparation of Propionic Anhydride Derivative Samples of HEP-1 in Blood Plasma
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ELISA | enzyme-linked immunosorbent assay |
| MS | mass spectrometry |
| HPLC-MS/MS | high-performance liquid chromatography with tandem mass spectrometry |
| MALDI | matrix-assisted laser desorption ionization source |
| CPP | cell-penetrating peptide |
| RP | reversed-phase |
| HILIC | hydrophilic interaction liquid chromatography |
| ICAT | isotope-coded affinity tag |
| ICPL | isotope-coded protein label |
| iTRAQ | isobaric tag for relative and absolute quantitation |
| TMT | tandem mass tag |
| HEP-1 | human ezrin peptide-1 |
| IFNs | interferons |
| HIV | Human Immunodeficiency Virus |
| PASC | Post-Acute Sequelae of Coronavirus disease |
| MRM | multiple reaction monitoring |
| CID | collision-induced dissociation |
| dansyl chloride | dimethylaminonapthalene-5-sulfonyl chloride |
| tosyl chloride | 4-Toluenesulfonyl chloride |
| PITC | phenylisothiocyanate |
| Fmoc-OSu | N-(9H-fluorene-2-ylmethoxy)succinimide |
| Boc anhydride | Di-tert-butyl dicarbonate |
| Cbz-OSu | N-(Benzyloxycarbonyloxy)succinimide |
| ESI | electrospray ionization |
| TFA | trifluoroacetic acid |
| ACN | acetonitrile |
| TEA | triethylamine |
Appendix A
| Dansyl Group Monoisotopic Mass, Da | 234.1 | ||||||
| Derivatized Groups | Charge | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | 2051.0 | 1026.0 | 684.3 | 513.5 | 411.0 | 342.7 | 293.9 |
| 2 | 2284.1 | 1142.5 | 762.0 | 571.8 | 457.6 | 381.5 | 327.2 |
| 3 | 2517.1 | 1259.1 | 839.7 | 630.0 | 504.2 | 420.4 | 360.5 |
| 4 | 2750.2 | 1375.6 | 917.4 | 688.3 | 550.8 | 459.2 | 393.7 |
| 5 | 2983.2 | 1492.1 | 995.1 | 746.6 | 597.5 | 498.0 | 427.0 |
| 6 | 3216.3 | 1608.6 | 1072.8 | 804.8 | 644.1 | 536.9 | 460.3 |
| 7 | 3449.3 | 1725.2 | 1150.4 | 863.1 | 690.7 | 575.7 | 493.6 |
| Tosyl Group Monoisotopic Mass, Da | 155.0 | ||||||
| Derivatized Groups | Charge | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | 1972.0 | 986.5 | 658.0 | 493.8 | 395.2 | 329.5 | 282.6 |
| 2 | 2126.0 | 1063.5 | 709.3 | 532.3 | 426.0 | 355.2 | 304.6 |
| 3 | 2280.0 | 1140.5 | 760.7 | 570.8 | 456.8 | 380.8 | 326.6 |
| 4 | 2434.0 | 1217.5 | 812.0 | 609.3 | 487.6 | 406.5 | 348.6 |
| 5 | 2588.0 | 1294.5 | 863.3 | 647.8 | 518.4 | 432.2 | 370.6 |
| 6 | 2742.0 | 1371.5 | 914.7 | 686.3 | 549.2 | 457.8 | 392.6 |
| 7 | 2896.0 | 1448.5 | 966.0 | 724.8 | 580.0 | 483.5 | 414.6 |
| PITC Group Monoisotopic Mass, Da | 135.0 | ||||||
| Derivatized Groups | Charge | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | 1953.0 | 977.0 | 651.7 | 489.0 | 391.4 | 326.3 | 279.9 |
| 2 | 2088.0 | 1044.5 | 696.7 | 522.8 | 418.4 | 348.8 | 299.1 |
| 3 | 2223.0 | 1112.0 | 741.7 | 556.5 | 445.4 | 371.3 | 318.4 |
| 4 | 2358.0 | 1179.5 | 786.7 | 590.3 | 472.4 | 393.8 | 337.7 |
| 5 | 2493.0 | 1247.0 | 831.7 | 624.0 | 499.4 | 416.3 | 357.0 |
| 6 | 2628.1 | 1314.5 | 876.7 | 657.8 | 526.4 | 438.8 | 376.3 |
| 7 | 2763.1 | 1382.0 | 921.7 | 691.5 | 553.4 | 461.4 | 395.6 |
| (Fluoren-9-ylmethoxy)carbonyl (Fmoc) Group Monoisotopic Mass, Da | 223.1 | ||||||
| Derivatized Groups | Charge | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | 2040.0 | 1020.5 | 680.7 | 510.8 | 408.8 | 340.8 | 292.3 |
| 2 | 2262.1 | 1131.6 | 754.7 | 566.3 | 453.2 | 377.9 | 324.0 |
| 3 | 2484.2 | 1242.6 | 828.7 | 621.8 | 497.6 | 414.9 | 355.7 |
| 4 | 2706.2 | 1353.6 | 902.8 | 677.3 | 542.1 | 451.9 | 387.5 |
| 5 | 2928.3 | 1464.7 | 976.8 | 732.8 | 586.5 | 488.9 | 419.2 |
| 6 | 3150.4 | 1575.7 | 1050.8 | 788.4 | 630.9 | 525.9 | 450.9 |
| 7 | 3372.4 | 1686.7 | 1124.8 | 843.9 | 675.3 | 562.9 | 482.6 |
| Trert-butoxycarbonyl (Boc) Group Monoisotopic Mass, Da | 101.1 | ||||||
| Derivatized Groups | Charge | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | 1918.0 | 959.5 | 640.0 | 480.3 | 384.4 | 320.5 | 274.9 |
| 2 | 2018.1 | 1009.5 | 673.4 | 505.3 | 404.4 | 337.2 | 289.2 |
| 3 | 2118.1 | 1059.6 | 706.7 | 530.3 | 424.4 | 353.9 | 303.5 |
| 4 | 2218.2 | 1109.6 | 740.1 | 555.3 | 444.4 | 370.5 | 317.7 |
| 5 | 2318.2 | 1159.6 | 773.4 | 580.3 | 464.5 | 387.2 | 332.0 |
| 6 | 2418.3 | 1209.6 | 806.8 | 605.3 | 484.5 | 403.9 | 346.3 |
| 7 | 2518.3 | 1259.7 | 840.1 | 630.3 | 504.5 | 420.6 | 360.6 |
| Benzyloxycarbonyl (Cbz) Group Monoisotopic Mass, Da | 135.0 | ||||||
| Derivatized Groups | Charge | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | 1952.0 | 976.5 | 651.3 | 488.8 | 391.2 | 326.2 | 279.7 |
| 2 | 2086.0 | 1043.5 | 696.0 | 522.3 | 418.0 | 348.5 | 298.9 |
| 3 | 2220.1 | 1110.5 | 740.7 | 555.8 | 444.8 | 370.9 | 318.0 |
| 4 | 2354.1 | 1177.6 | 785.4 | 589.3 | 471.6 | 393.2 | 337.2 |
| 5 | 2488.2 | 1244.6 | 830.1 | 622.8 | 498.4 | 415.5 | 356.3 |
| 6 | 2622.2 | 1311.6 | 874.7 | 656.3 | 525.2 | 437.9 | 375.5 |
| 7 | 2756.2 | 1378.6 | 919.4 | 689.8 | 552.1 | 460.2 | 394.6 |
| Benzoyl Group (From Benzoic Anhydride) Monoisotopic Mass, Da | 105.0 | ||||||
| Derivatized Groups | Charge | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | 1922.0 | 961.5 | 641.3 | 481.3 | 385.2 | 321.2 | 275.4 |
| 2 | 2026.0 | 1013.5 | 676.0 | 507.3 | 406.0 | 338.5 | 290.3 |
| 3 | 2130.1 | 1065.5 | 710.7 | 533.3 | 426.8 | 355.8 | 305.2 |
| 4 | 2234.1 | 1117.5 | 745.4 | 559.3 | 447.6 | 373.2 | 320.0 |
| 5 | 2338.1 | 1169.6 | 780.0 | 585.3 | 468.4 | 390.5 | 334.9 |
| 6 | 2442.1 | 1221.6 | 814.7 | 611.3 | 489.2 | 407.9 | 349.7 |
| 7 | 2546.2 | 1273.6 | 849.4 | 637.3 | 510.0 | 425.2 | 364.6 |
| Propionyl Group (from Propionic Anhydride) Monoisotopic Mass, Da | 57.0 | ||||||
| Derivatized Groups | Charge | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | 1874.0 | 937.5 | 625.3 | 469.3 | 375.6 | 313.2 | 268.6 |
| 2 | 1930.0 | 965.5 | 644.0 | 483.3 | 386.8 | 322.5 | 276.6 |
| 3 | 1986.0 | 993.5 | 662.7 | 497.3 | 398.0 | 331.9 | 284.6 |
| 4 | 2042.1 | 1021.5 | 681.4 | 511.3 | 409.2 | 341.2 | 292.6 |
| 5 | 2098.1 | 1049.6 | 700.0 | 525.3 | 420.4 | 350.5 | 300.6 |
| 6 | 2154.1 | 1077.6 | 718.7 | 539.3 | 431.6 | 359.9 | 308.6 |
| 7 | 2210.2 | 1105.6 | 737.4 | 553.3 | 442.8 | 369.2 | 316.6 |
References
- Al-Amrani, S.; Al-Jabri, Z.; Al-Zaabi, A.; Alshekaili, J.; Al-Khabori, M. Proteomics: Concepts and Applications in Human Medicine. World J. Biol. Chem. 2021, 12, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Porfiryeva, N.N.; Semina, I.I.; Moustafine, R.I.; Khutoryanskiy, V.V. Intranasal Administration as a Route to Deliver Drugs to the Brain (Review). Drug Dev. Regist. 2021, 10, 117–127. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, K.K.; Sharma, A.; Jain, R. Peptide-Based Drug Discovery: Current Status and Recent Advances. Drug Discov. Today 2023, 28, 103464. [Google Scholar] [CrossRef]
- Rauh, M. LC–MS/MS for Protein and Peptide Quantification in Clinical Chemistry. J. Chromatogr. B 2012, 883–884, 59–67. [Google Scholar] [CrossRef]
- Chen, G.; Pramanik, B.N. Application of LC/MS to Proteomics Studies: Current Status and Future Prospects. Drug Discov. Today 2009, 14, 465–471. [Google Scholar] [CrossRef]
- Campbell, J.L.; Blanc, J.Y.L. Peptide and Protein Drug Analysis by MS: Challenges and Opportunities for The Discovery Environment. Bioanalysis 2011, 3, 645–657. [Google Scholar] [CrossRef]
- Neagu, A.-N.; Jayathirtha, M.; Baxter, E.; Donnelly, M.; Petre, B.A.; Darie, C.C. Applications of Tandem Mass Spectrometry (MS/MS) in Protein Analysis for Biomedical Research. Molecules 2022, 27, 2411. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.-A.; Lee, H. Bottom-Up Proteomics: Advancements in Sample Preparation. Int. J. Mol. Sci. 2023, 24, 5350. [Google Scholar] [CrossRef]
- Zeng, K.; Geerlof-Vidavisky, I.; Gucinski, A.; Jiang, X.; Boyne, M.T. Liquid Chromatography-High Resolution Mass Spectrometry for Peptide Drug Quality Control. AAPS J. 2015, 17, 643–651. [Google Scholar] [CrossRef]
- Kang, L.; Weng, N.; Jian, W. LC–MS Bioanalysis of Intact Proteins and Peptides. Biomed. Chromatogr. 2020, 34, e4633. [Google Scholar] [CrossRef]
- Fisher, E.N.; Melnikov, E.S.; Shohin, I.E. Development and Validation of HPLC-MS/MS Method for Busereline Quantitation in Animal Blood Plasma. Drug Dev. Regist. 2019, 8, 79–84. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef]
- Böttger, R.; Hoffmann, R.; Knappe, D. Differential Stability of Therapeutic Peptides with Different Proteolytic Cleavage Sites in Blood, Plasma and Serum. PLoS ONE 2017, 12, e0178943. [Google Scholar] [CrossRef] [PubMed]
- John, H.; Walden, M.; Schäfer, S.; Genz, S.; Forssmann, W.-G. Analytical Procedures for Quantification of Peptides in Pharmaceutical Research by Liquid Chromatography–Mass Spectrometry. Anal. Bioanal. Chem. 2004, 378, 883–897. [Google Scholar] [CrossRef]
- Adermann, K.; John, H.; Ständker, L.; Forssmann, W.-G. Exploiting Natural Peptide Diversity: Novel Research Tools and Drug Leads. Curr. Opin. Biotechnol. 2004, 15, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Pellois, J.-P. Hydrophobicity Is a Key Determinant in the Activity of Arginine-Rich Cell Penetrating Peptides. Sci. Rep. 2022, 12, 15981. [Google Scholar] [CrossRef]
- Sunar, S.Z.; Acar, T.; Sahin, F. Chemically Peptide Synthesis and Role of Arginine and Lysine in the Antimicrobial and Antiviral Activity of Synthetic Peptides: A Comprehensive Review. Pept. Sci. 2024, 116, e24368. [Google Scholar] [CrossRef]
- Shatilov, A.A.; Shatilova, A.V.; Saprygina, L.V.; Babikhina, M.O.; Timotievich, E.D.; Kovchina, V.I.; Andreev, S.M.; Smirnov, V.V.; Kudlay, D.A.; Shilovsky, I.P.; et al. New synthetic antiviral peptides. Design, preparation, physicochemical analysis, and activity studies. Immunology 2024, 45, 162–170. [Google Scholar] [CrossRef]
- Dongré, A.R.; Jones, J.L.; Somogyi, Á.; Wysocki, V.H. Influence of Peptide Composition, Gas-Phase Basicity, and Chemical Modification on Fragmentation Efficiency: Evidence for the Mobile Proton Model. J. Am. Chem. Soc. 1996, 118, 8365–8374. [Google Scholar] [CrossRef]
- Yoshida, T. Peptide Separation by Hydrophilic-Interaction Chromatography: A Review. J. Biochem. Biophys. Methods 2004, 60, 265–280. [Google Scholar] [CrossRef]
- Yeung, D.; Spicer, V.; Krokhin, O.V. Peptide Retention Time Prediction for Hydrophilic Interaction Liquid Chromatography at Acidic pH in Formic-Acid Based Eluents. J. Chromatogr. A 2024, 1736, 465355. [Google Scholar] [CrossRef] [PubMed]
- Yao, X. Derivatization or Not: A Choice in Quantitative Proteomics. Anal. Chem. 2011, 83, 4427–4439. [Google Scholar] [CrossRef]
- Qi, B.-L.; Liu, P.; Wang, Q.-Y.; Cai, W.-J.; Yuan, B.-F.; Feng, Y.-Q. Derivatization for Liquid Chromatography-Mass Spectrometry. TrAC Trends Anal. Chem. 2014, 59, 121–132. [Google Scholar] [CrossRef]
- Srinivas, N.R. New Thinking in the Development of Novel Derivatization Reagents for Liquid Chromatography-Mass Spectrometric Detection. Biomed. Chromatogr. 2009, 23, 107–108. [Google Scholar] [CrossRef]
- Timms, M.; Hall, N.; Levina, V.; Vine, J.; Steel, R. A High-throughput LC-MS/MS Screen for GHRP in Equine and Human Urine, Featuring Peptide Derivatization for Improved Chromatography. Drug Test. Anal. 2014, 6, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Z.; Bastian, K.C.; Moore, R.D.; Stobaugh, J.F.; Borchardt, R.T. Quantitative Analysis of a Model Opioid Peptide and Its Cyclic Prodrugs in Rat Plasma Using High-Performance Liquid Chromatography with Fluorescence and Tandem Mass Spectrometric Detection. J. Chromatogr. B 2002, 780, 269–281. [Google Scholar] [CrossRef]
- Pan, G.; Wang, X.; Huang, Y.; Gao, X.; Wang, Y. Development and Validation of a LC–MS/MS Method for Determination of Bivalirudin in Human Plasma: Application to a Clinical Pharmacokinetic Study. J. Pharm. Biomed. Anal. 2010, 52, 105–109. [Google Scholar] [CrossRef]
- Hering, A.; Jieu, B.; Jones, A.; Muttenthaler, M. Approaches to Improve the Quantitation of Oxytocin in Human Serum by Mass Spectrometry. Front. Chem. 2022, 10, 889154. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Z.; Wang, X.; Qiu, C. Sample Preparation. J. Chromatogr. A 2008, 1184, 191–219. [Google Scholar] [CrossRef]
- Ataullakhanov, R.I.; Holms, R.D.; Katlinskiĭ, A.V.; Deriabin, P.G.; Narovlianskiĭ, A.N.; Mezentseva, M.V.; Ershov, F.I. Ani immunomodulator Hepon inhibits hepatitis C virus replication in human cell cultures in vitro. Antibiot. Khimioter. 2002, 47, 9–11. [Google Scholar]
- Holms, R.D. Long COVID (PASC) Is Maintained by a Self-Sustaining Pro-Inflammatory TLR4/RAGE-Loop of S100A8/A9 > TLR4/RAGE Signalling, Inducing Chronic Expression of IL-1b, IL-6 and TNFa: Anti-Inflammatory Ezrin Peptides as Potential Therapy. Immuno 2022, 2, 512–533. [Google Scholar] [CrossRef]
- Kuzmina, D.A.; Shabashova, N.V.; Novikova, V.P.; Orishak, E.A.; Boitsov, A.G.; Moroz, B.T. Microbiocenosis and innate immunity of oral mucosa in decompensated caries before and after treatment with “Hepon” immunomodulator. Pediatr. Dent. Dent. Prophyl. 2009, 8, 16–20. [Google Scholar]
- Peptide Mass Calculator—Peptide Protein Research (PPR Ltd.). Available online: https://www.peptidesynthetics.co.uk/tools/ (accessed on 8 April 2025).
- Nadler, W.M.; Waidelich, D.; Kerner, A.; Hanke, S.; Berg, R.; Trumpp, A.; Rösli, C. MALDI versus ESI: The Impact of the Ion Source on Peptide Identification. J. Proteome Res. 2017, 16, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.R. [12] Dansyl Chloride Procedure. In Methods in Enzymology; Enzyme Structure; Academic Press: Cambridge, MA, USA, 1967; Volume 11, pp. 139–151. [Google Scholar]
- Theodoropoulos, D.; Gazopoulos, J. Peptide Synthesis. I. The Use of p-Toluenesulfonyl Chloride for Carboxyl Activation. J. Org. Chem. 1962, 27, 2091–2093. [Google Scholar] [CrossRef]
- Jullian, M.; Hernandez, A.; Maurras, A.; Puget, K.; Amblard, M.; Martinez, J.; Subra, G. N-Terminus FITC Labeling of Peptides on Solid Support: The Truth behind the Spacer. Tetrahedron Lett. 2009, 50, 260–263. [Google Scholar] [CrossRef]
- Koller, M.; Eckert, H. Derivatization of Peptides for Their Determination by Chromatographic Methods. Anal. Chim. Acta 1997, 352, 31–59. [Google Scholar] [CrossRef]
- Chhanikar, P.T.; Gupta, K.R.; Umekar, M.J. Derivatizing Reagents for Detection of Organic Compounds By HPLC. Asian J. Appl. Chem. Res. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- El-Faham, A.; Albericio, F. Carpino’s Protecting Groups, beyond the Boc and the Fmoc. Pept. Sci. 2020, 112, e24164. [Google Scholar] [CrossRef]
- Gartenmann, K.; Kochhar, S. Short-Chain Peptide Analysis by High-Performance Liquid Chromatography Coupled to Electrospray Ionization Mass Spectrometer after Derivatization with 9-Fluorenylmethyl Chloroformate. J. Agric. Food Chem. 1999, 47, 5068–5071. [Google Scholar] [CrossRef]
- Sharma, K.K.; Ravi, R.; Maurya, I.K.; Kapadia, A.; Khan, S.I.; Kumar, V.; Tikoo, K.; Jain, R. Modified Histidine Containing Amphipathic Ultrashort Antifungal Peptide, His [2-p-(n-Butyl)Phenyl]-Trp-Arg-OMe Exhibits Potent Anticryptococcal Activity. Eur. J. Med. Chem. 2021, 223, 113635. [Google Scholar] [CrossRef]
- Nguyen, D.P.; Alai, M.M.G.; Virdee, S.; Chin, J.W. Genetically Directing Ɛ-N, N-Dimethyl-l-Lysine in Recombinant Histones. Chem. Biol. 2010, 17, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Sidoli, S.; Yuan, Z.-F.; Lin, S.; Karch, K.; Wang, X.; Bhanu, N.; Arnaudo, A.M.; Britton, L.-M.; Cao, X.-J.; Gonzales-Cope, M.; et al. Drawbacks in the Use of Unconventional Hydrophobic Anhydrides for Histone Derivatization in Bottom-up Proteomics PTM Analysis. Proteomics 2015, 15, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- van Faassen, M.; Bischoff, R.; Eijkelenkamp, K.; de Jong, W.H.A.; van der Ley, C.P.; Kema, I.P. In Matrix Derivatization Combined with LC-MS/MS Results in Ultrasensitive Quantification of Plasma Free Metanephrines and Catecholamines. Anal. Chem. 2020, 92, 9072–9078. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.A.; Mollah, S.; Ueberheide, B.M.; Busby, S.A.; Muratore, T.L.; Shabanowitz, J.; Hunt, D.F. Chemical Derivatization of Histones for Facilitated Analysis by Mass Spectrometry. Nat. Protoc. 2007, 2, 933–938. [Google Scholar] [CrossRef]
- Grundler, V.; Gademann, K. Direct Arginine Modification in Native Peptides and Application to Chemical Probe Development. ACS Med. Chem. Lett. 2014, 5, 1290–1295. [Google Scholar] [CrossRef]
- Aydoğmuş, Z.; Sarı, F.; Ulu, S.T. Spectrofluorimetric Determination of Aliskiren in Tablets and Spiked Human Plasma through Derivatization with Dansyl Chloride. J. Fluoresc. 2012, 22, 549–556. [Google Scholar] [CrossRef]
- Onisko, B.; Dynin, I.; Requena, J.R.; Silva, C.J.; Erickson, M.; Carter, J.M. Mass Spectrometric Detection of Attomole Amounts of the Prion Protein by nanoLC/MS/MS. J. Am. Soc. Mass. Spectrom. 2007, 18, 1070–1079. [Google Scholar] [CrossRef]
- Kole, P.L.; Venkatesh, G.; Kotecha, J.; Sheshala, R. Recent Advances in Sample Preparation Techniques for Effective Bioanalytical Methods. Biomed. Chromatogr. 2011, 25, 199–217. [Google Scholar] [CrossRef]
- Yuan, L. Sample Preparation for LC-MS Bioanalysis of Peptides. In Sample Preparation in LC-MS Bioanalysis; Li, W., Jian, W., Fu, Y., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 284–303. ISBN 978-1-119-27429-2. [Google Scholar]
- Tokareva, M.A.; Melnikov, E.S.; Belova, M.V.; Fisher, E.N.; Rodina, T.A.; Shohin, I.E. HPLC-MS/MS method development and validation for the determination of tetradecapeptide in human plasma. Drug Dev. Regist. 2024, 13, 171–180. [Google Scholar] [CrossRef]
- Stevens, W.; Van Es, A. Mixed Carboxylic Acid Anhydrides: II. Esterification of Alcohols with the Formic Acid/Acetic Anhydride Reaction Mixture. Recl. Trav. Chim. Pays-Bas 1964, 83, 1287–1293. [Google Scholar] [CrossRef]
- Shiina, I.; Kubota, M.; Oshiumi, H.; Hashizume, M. An Effective Use of Benzoic Anhydride and Its Derivatives for the Synthesis of Carboxylic Esters and Lactones: A Powerful and Convenient Mixed Anhydride Method Promoted by Basic Catalysts. J. Org. Chem. 2004, 69, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Otera, J. Transesterification. Chem. Rev. 1993, 93, 1449–1470. [Google Scholar] [CrossRef]
- Chen, G.; Liu, H.; Wang, X.; Li, Z. In Vitro Methylation by Methanol: Proteomic Screening and Prevalence Investigation. Anal. Chim. Acta 2010, 661, 67–75. [Google Scholar] [CrossRef] [PubMed]

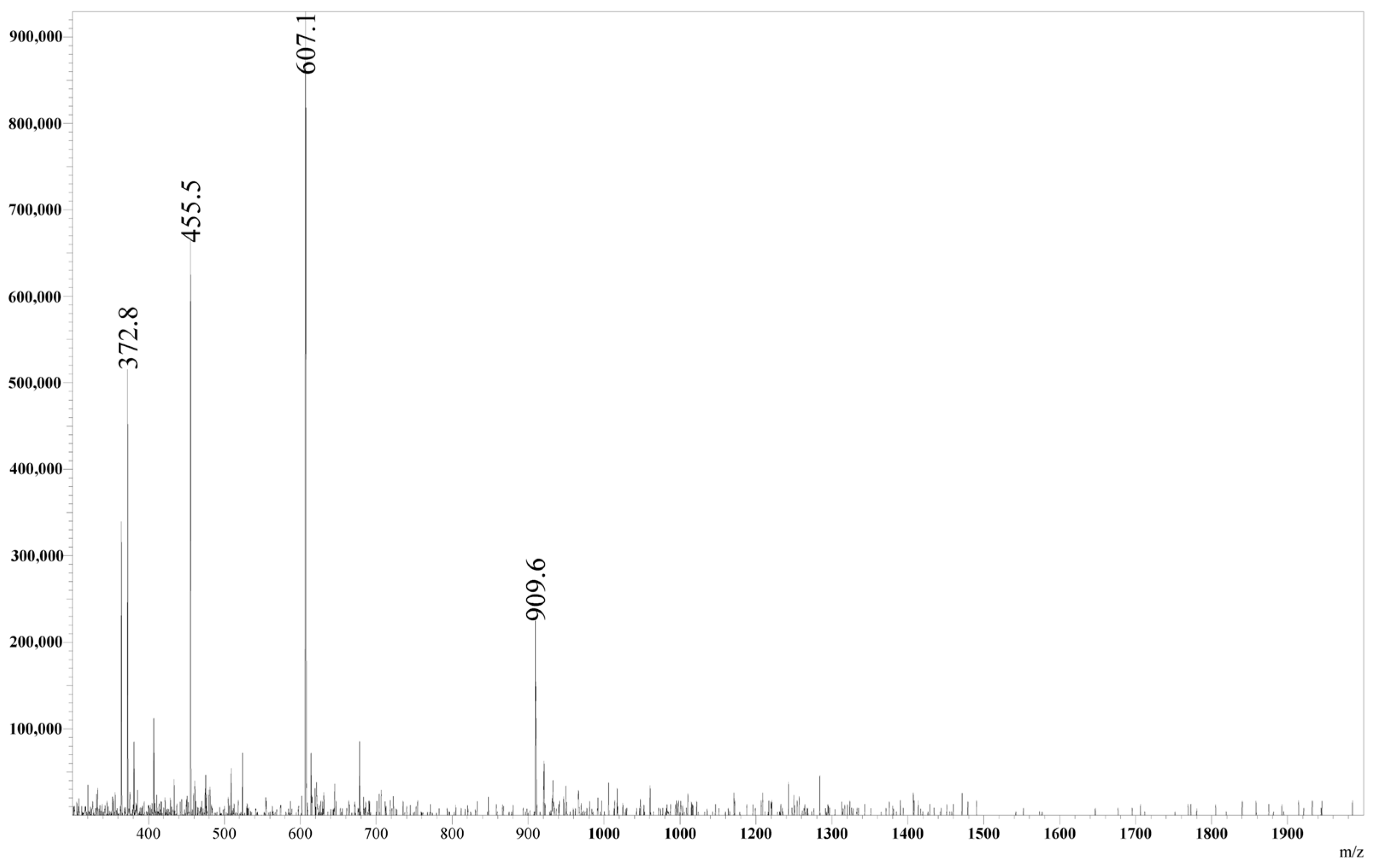
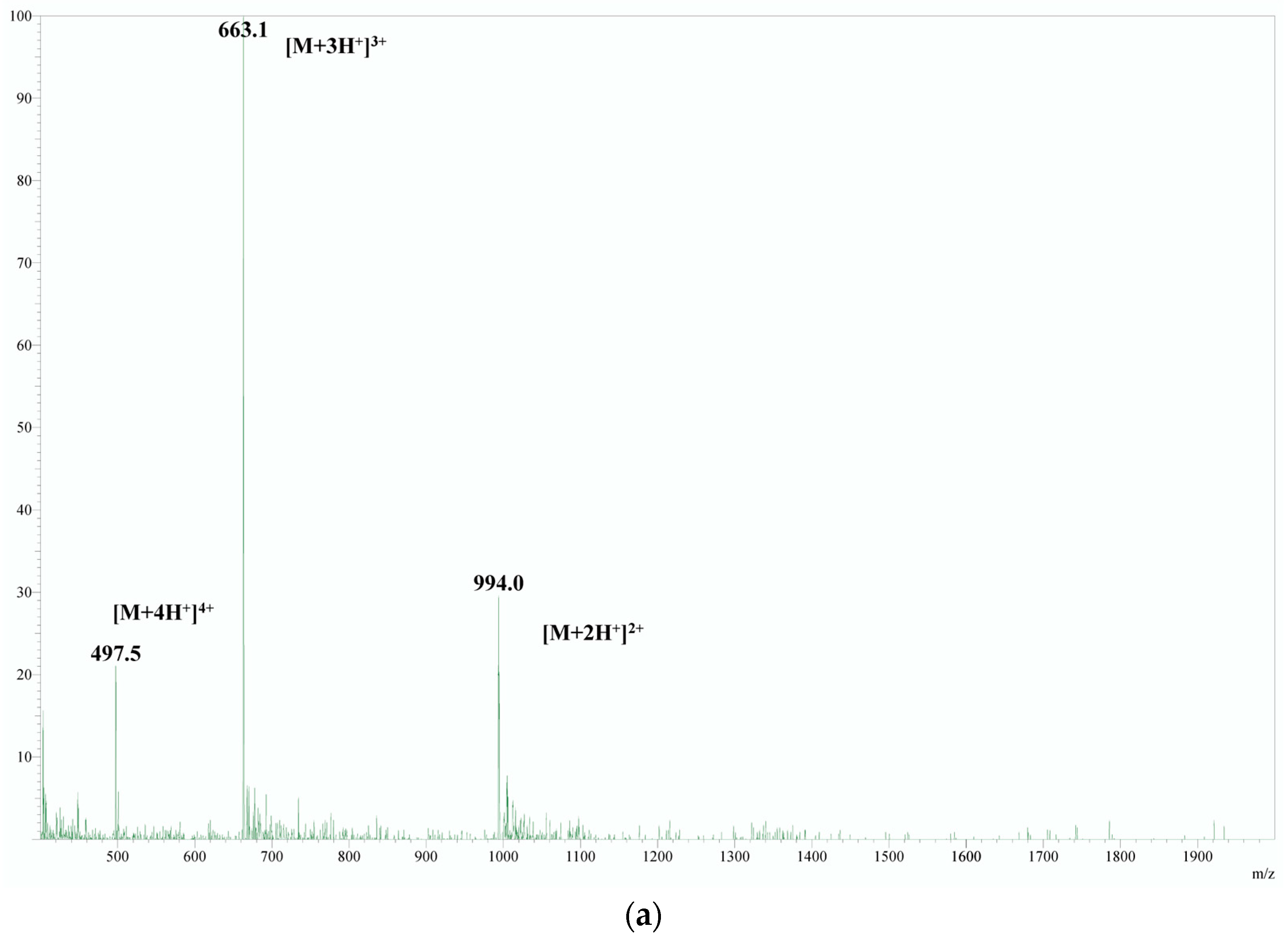
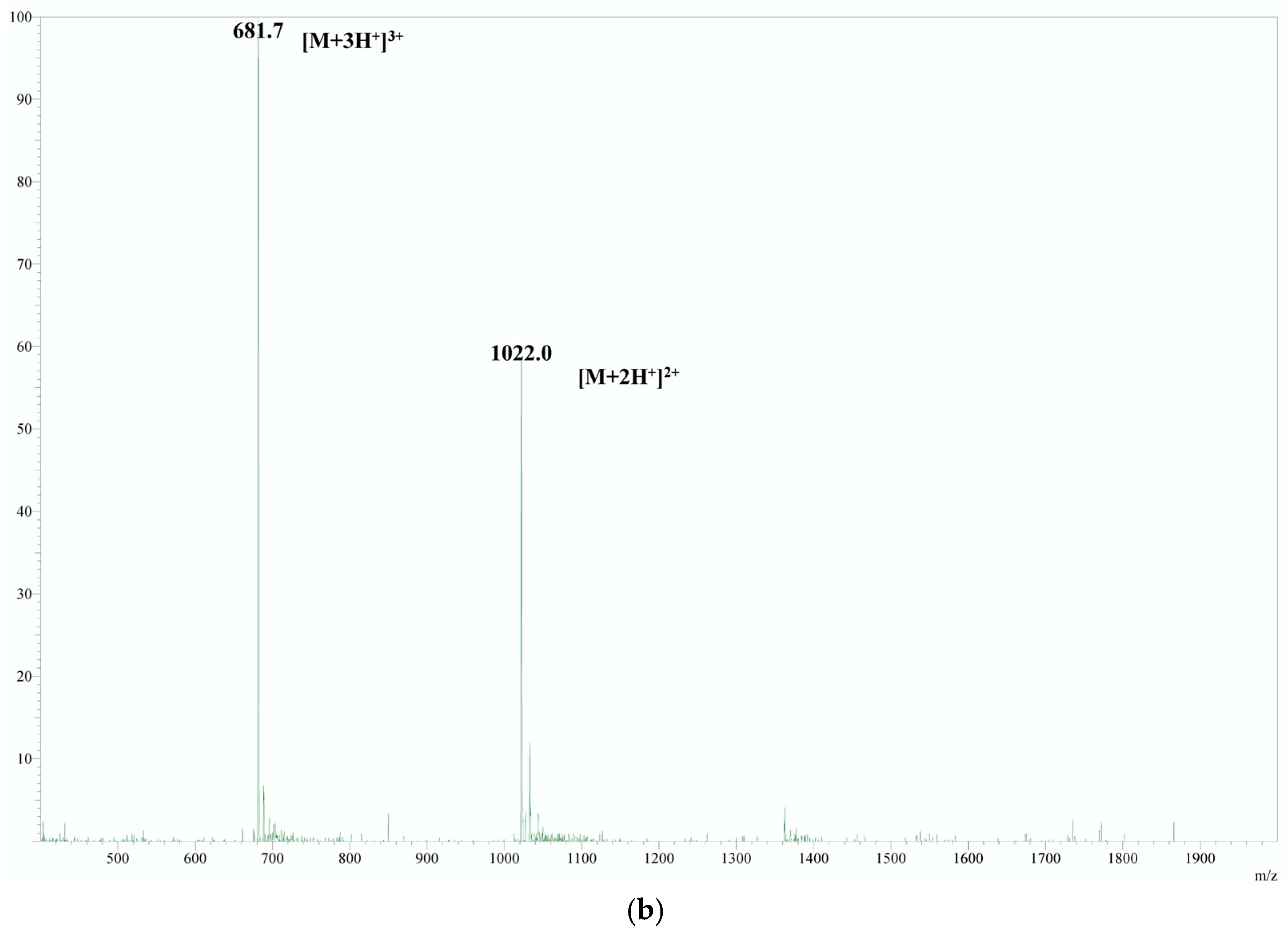
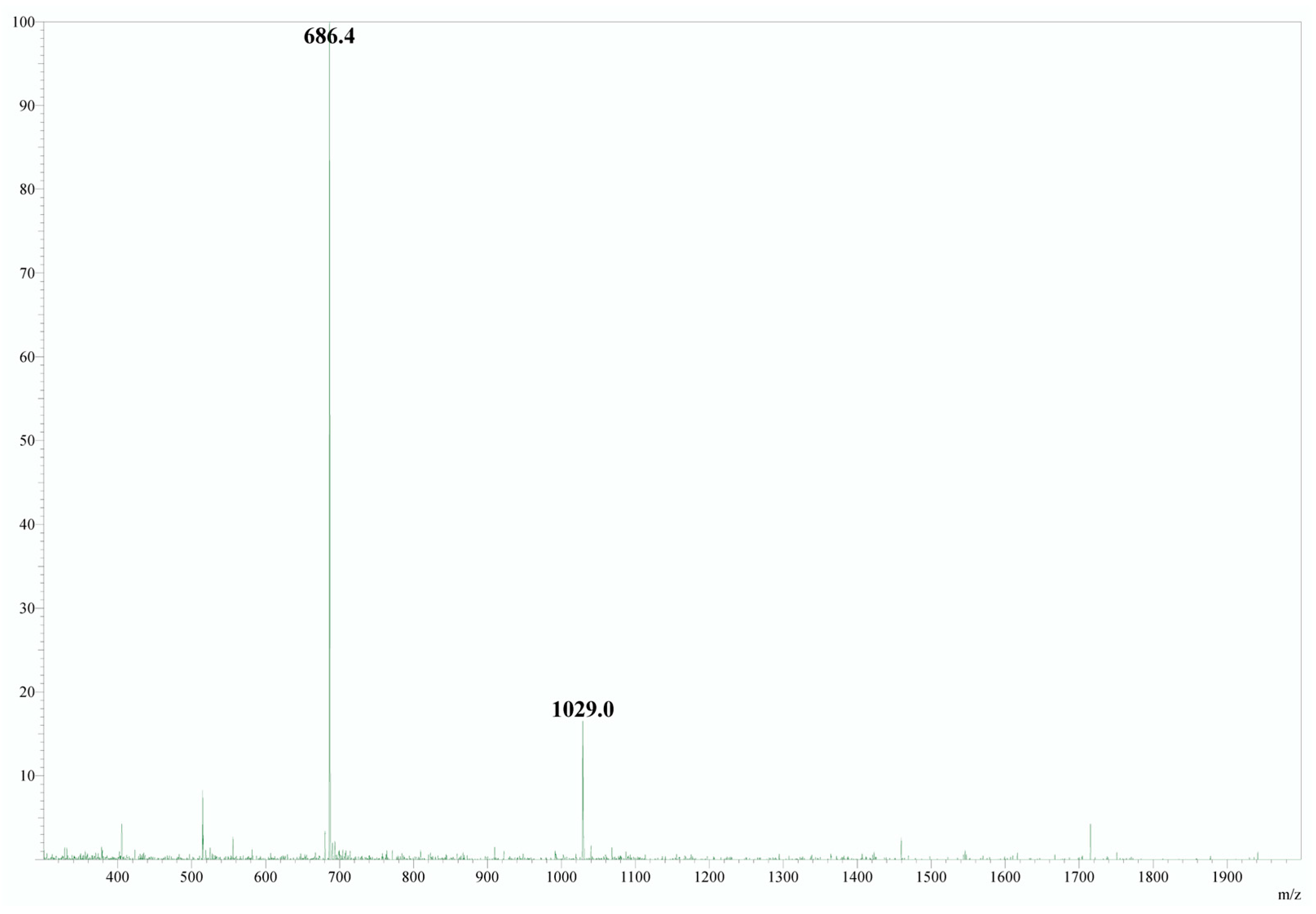
| n (H+) | m/z Calculated | m/z Obtained |
|---|---|---|
| 1 | 1818.0 | - |
| 2 | 909.5 | 909.6 |
| 3 | 606.7 | 607.1 |
| 4 | 455.3 | 455.5 |
| 5 | 364.4 | 364.6 |
| Derivatizing Agent | Reaction Condition | Derivative Product Mass Spectra | 4-Substituted RT | Informative Product Ion Scan | MRM Developed | Controlled Yield Reaction | Applied in Plasma | Achieved High Sensitivity |
|---|---|---|---|---|---|---|---|---|
| - | Native HEP-1 without derivatization | - | 0.55 | - | - | - | - | - |
| Dansyl chloride | 50 °C for 30 min in a carbonate buffer (pH 9) with a mixed solvent (water-acetonitrile, 1:1) | + | 4.63 | - | - | - | - | - |
| Tosyl chloride | + | 3.91 | + | + | - | - | - | |
| PITC | + | 3.95 | + | + | - | - | - | |
| Fmoc-OSu | Room temperature in the dark for 30 min in triethylamine at pH 9 | + | 5.13 | + | - | - | - | - |
| Boc anhydride | + | 3.85 | + | + | - | - | - | |
| Cbz-OSu | + | 4.08 | + | + | - | - | - | |
| Benzoic anhydride | Room temperature overnight | + | 3.91 | + | - | - | - | - |
| Propionic anhydride | 50 °C for 30 min in a propionic acid solution | + | 2.42 | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokareva, M.A.; Melnikov, E.S.; Fisher, E.N.; Rodina, T.A.; Shohin, I.E.; Belova, M.V. Derivatizing Agent Selection for Hydrophilic Lysine- and Arginine-Containing Tetradecapeptide Analysis in Human Plasma by RP HPLC-MS/MS. Analytica 2025, 6, 23. https://doi.org/10.3390/analytica6030023
Tokareva MA, Melnikov ES, Fisher EN, Rodina TA, Shohin IE, Belova MV. Derivatizing Agent Selection for Hydrophilic Lysine- and Arginine-Containing Tetradecapeptide Analysis in Human Plasma by RP HPLC-MS/MS. Analytica. 2025; 6(3):23. https://doi.org/10.3390/analytica6030023
Chicago/Turabian StyleTokareva, Margarita A., Evgeny S. Melnikov, Elizaveta N. Fisher, Tatiana A. Rodina, Igor E. Shohin, and Maria V. Belova. 2025. "Derivatizing Agent Selection for Hydrophilic Lysine- and Arginine-Containing Tetradecapeptide Analysis in Human Plasma by RP HPLC-MS/MS" Analytica 6, no. 3: 23. https://doi.org/10.3390/analytica6030023
APA StyleTokareva, M. A., Melnikov, E. S., Fisher, E. N., Rodina, T. A., Shohin, I. E., & Belova, M. V. (2025). Derivatizing Agent Selection for Hydrophilic Lysine- and Arginine-Containing Tetradecapeptide Analysis in Human Plasma by RP HPLC-MS/MS. Analytica, 6(3), 23. https://doi.org/10.3390/analytica6030023







