Abstract
The GPHF-Minilab™ is a portable toolkit for performing qualitative methods such as thin-layer chromatography (TLC) on common pharmaceuticals. It is particularly useful in resource-limited locations where it is more challenging to monitor for substandard and falsified (SF) medicines. However, the GPHF-Minilab™ TLC methods are only semi-quantitative at best and thus have issues monitoring product quality effectively. We have improved on the GPHF-Minilab™ TLC method for metronidazole, a common antibiotic, by making it fully quantitative. Sample solutions were spotted on TLC plates alongside three metronidazole standards at different concentrations. After development, plates were imaged in a lightbox with two different smartphone cameras. Images were processed through the open-source program ImageJ and resulting pixel data from the standard spots were used to create a calibration curve, enabling quantitation of the sample. The USP Metronidazole Tablet high-performance liquid chromatography (HPLC) assay was used as the reference method. We validated this TLC method using 250 and 500 mg metronidazole tablets from different manufacturers and assessed linearity, range, accuracy, precision, intermediate precision, specificity, and robustness. These improvements should enhance the GPHF-Minilab™ TLC methods for metronidazole product screening. Additionally, the procedure is extensible to other analytes, although further validation would be required for each Minilab method.
1. Introduction
Metronidazole is an antibiotic on the World Health Organization’s (WHO) Essential Medicines List [1] and is commonly used to treat several different bacterial and parasitic infections worldwide. It is available in the global market and can be distributed in many low- and middle-income countries (LMICs) [2]. LMICs can be particularly vulnerable to substandard and falsified (SF) medicines as they can lack the resources to maintain effective regulatory systems and rigorously assure pharmaceutical product quality through testing programs. SF medical products can lead to prolonged illness, antibiotic resistant bacterial strains, and even fatalities. In 2017, the WHO estimated that one in ten medicines in LMICs were SF [3]. Metronidazole is not immune to this: Tchounga et al. recently found 7.9% of ciprofloxacin and metronidazole tablets in three cities in Cameroon to be SF [4]. Therefore, it is critical that effective methods exist to monitor pharmaceutical product quality.
Traditional methods for assessing metronidazole tablet product quality can be complicated and require expensive equipment and/or large volumes of materials such as solvents. The assay method in the metronidazole tablet International Pharmacopoeia (Ph. Int.) monograph involves a seemingly simple approach using extraction and titration but uses perchloric titrants that require additional safety considerations [5]. The United States Pharmacopoeia (USP) assay method uses high performance liquid chromatography (HPLC), which requires relatively costly instruments [6]. Therefore, a simple, inexpensive, and rapid method for quantification and quality screening of metronidazole products would be beneficial in resource-limited settings. In these settings, products that undergo quality screening and are identified as high-risk could then be prioritized for additional analysis with more extensive and complex test methods.
Thin-layer chromatography (TLC) is a rapid and inexpensive, but generally not quantitative, analytical technique primarily used for qualitative compound identification and purity evaluations. Standard TLC has been used extensively to screen active pharmaceutical ingredient (API) identity and product purity. A quantitative variation in high performance TLC (HPTLC) does exist, with performance comparable to HPLC, but requiring a similar level of financial investment [7] due to the use of specialized equipment such as densitometers, automated spotters, and/or TLC plates using stationary phases with finer particle sizes to achieve the necessary resolution and precision. Several HPTLC methods for the analysis of metronidazole have been described in the literature [8,9,10,11,12]. HPTLC is predicated on densitometry, which uses the Beer–Lambert relationship between light absorbance and concentration of a substance. In HPTLC, compounds spotted onto plates may absorb visible or ultraviolet light and show up as dark spots when illuminated. Digital images of illuminated plates yield pixel values, which are related to light intensity and correlated to concentration, allowing for quantitation of the sample. There have been efforts to reduce the financial investment required to develop quantitative densitometric TLC methods using less expensive scanners or digital cameras [13,14,15,16,17], along with open-source software for the quantitation of digital images of TLC plates such as quanTLC [18] and qTLC [19] (version 2.0.1).
The Global Pharma Health Fund (GPHF), a charitable organization voluntarily supported by Merck KGaA (Darmstadt, Germany), has created the GPHF-Minilab™ (GPHF, Giessen, Germany), a portable collection of laboratory equipment and procedures for screening pharmaceutical product quality, intended for use in resource-constrained locations such as LMICs to combat substandard and falsified medicines [20]. The Minilab has seen widespread adoption, with over 1000 units in place in more than 100 countries [21]. The GPHF-Minilab™ uses TLC for several of its methods. Evaluations of the performance of the GPHF-Minilab™ show it to be somewhat effective at quality screening but not without issues [22,23,24]. Its methods are only roughly semi-quantitative, and require the analyst to make a subjective comparison of the intensity of a sample spot relative to an 80% standard spot, which can lead to problems with reproducibility [25]. Kovacs et al. described 42 technologies used to detect falsified and substandard medicines and graded each on a scale of 0 to 8 according to how useful the technology will be in LMICs (with higher scores being more useful), considering factors such as cost and ease of use in resource-constrained environments [26]. They scored the GPHF-Minilab™ TLC methods a 4 with a “Low” performance rating, noting that these methods are “only able to detect grossly substandard products (less than 80% of the active ingredient)”, an issue which has been noted multiple times in the literature [27,28].
In recent years, there have been attempts to improve and enhance GPHF-Minilab™ methods, such as by combining them with other methods to increase performance or translating them to HPTLC [29]. Such techniques have been used specifically to improve the metronidazole TLC method. Kaale et al. [30] and Zhang et al. [31] independently transferred the GPHF-Minilab™ method to HPTLC. Hauk et al. developed TLCyzer, an Android app to quantitate digital images of standard TLC plates, and evaluated it on several GPHF-Minilab™ methods, including metronidazole [32].
Here we report a validated method for quantitating metronidazole in tablets by expanding TLC plate quantification capabilities of the GPHF-Minilab™. Our objective is to improve the performance of the TLC method for pharmaceutical quality screening in limited-resource settings. Digital images of developed TLC plates housed in a 3D printed light box are collected using smartphone cameras and analyzed to determine the metronidazole concentration with free and open-source ImageJ software. The use of ImageJ as the analysis platform enables the use of any type of digital camera, and this validation includes data from two smartphone models.
2. Materials and Methods
2.1. TLC Method
The TLC method used is derived from the GPHF-Minilab™ metronidazole tablet method [20]. A general flowchart of the procedure is shown in Figure S1. Metronidazole tablets used in method development and validation were purchased from a local pharmacy and included USP 250 and 500 mg tablets from Rising (East Brunswick, NJ, USA), USP 250 mg metronidazole tablets from Unichem Laboratories (Mumbai, India), and USP 500 mg tablets from Cadila Healthcare (Ahmedabad, India). Both 250 mg and 500 mg tablets were used in this method validation. Results are not associated with brand to protect the manufacturer’s confidentiality. Metronidazole standards (pharmaceutical secondary standard certified reference material, Sigma Aldrich, St. Louis, MO, USA) and samples were prepared at concentrations relative to 5.0 mg/mL (denoted as 100%), with methanol (HPLC grade, VWR, West Chester, PA, USA) as the solvent. TLC standard solutions were prepared by creating a stock solution and diluting to lower concentrations. For the 75% (3.75 mg/mL), 100%, and 125% (6.25 mg/mL) standard solutions, the 125% solution served as the stock, and the 75% and 100% solutions were created by diluting that stock. The 50% (2.5 mg/mL), 100%, and 150% (7.5 mg/mL) standard solutions were created by diluting a 200% (10 mg/mL) stock solution. At the beginning of accuracy and precision evaluations, 10 tablet sample composites of either 250 mg or 500 mg tablets were prepared by grinding with a mortar and pestle until homogeneous. The composite samples were used to create fresh stock sample solutions each day and were stored away from light and in a desiccator between uses. Each day a sample was prepared, the required powder weight was measured out and used to prepare the sample solutions, with the average tablet weight used for 100% concentration solutions, and that weight adjusted for 75% or 125% concentrations accordingly. Solutions were filtered with 0.7 µm pore size, 25 mm diameter Gf/f Whatman syringe filters (Cytiva, Marlborough, MA, USA) before spotting. Silica gel 60 F254 5 × 10 cm aluminum TLC plates (Merck, Rahway, NJ, USA) were used. Solutions were spotted 1 cm apart on a line 2 cm from the bottom of the plate using 2 µL glass capillary tubes (manufacturer specified as To Contain, ±1% volume; Drummond Scientific Co., Broomall, PA, USA), allowing four spots per plate (three different standard concentrations and one sample). Plates were developed for 12 min (migration distance of approximately 3 cm, Rf = 0.34) in a solution of 45 mL ethyl acetate (HPLC grade, VWR, West Chester, PA, USA), 15 mL methanol, and 30 drops of 25% ammonium hydroxide (Sigma Aldrich, St. Louis, MO, USA) in an equilibrated glass chamber (27.0 × 26.5 × 7.0 cm L × W × H; VWR, West Chester, PA, USA) lined with filter paper. After developing, the solvent front was marked in pencil and the plates were allowed to air dry before being photographed.
2.2. Image Acquisition and Analysis
Our image collection and analysis approach is largely based on previous work from our group and that of our collaborators [33,34]. Plates were photographed in a custom 3D printed light box that held the plate, camera, and UV lamp in fixed positions (Figure 1); this box has been described previously [35]. The plates were illuminated with a 254 nm UV lamp (Analytik Jena, Jena, Germany). As the metronidazole absorbs at this wavelength, it shows up as a dark spot against a bright green background. Each plate was photographed using two smartphone cameras: a Pixel 4a 5G (Google, Mountain View, CA, USA), and an iPhone SE (2020) (Apple, Cupertino, CA, USA). Pictures were collected using the Adobe Lightroom app (version 6.3). Camera settings depended on the phone being used as each camera had different available camera options, particularly exposure time, so it was not possible to set the two cameras to the same exact settings. For the Pixel 4a, the exposure time was 1/30 s with ISO 150. For the iPhone SE, the exposure time was 1/100 s with ISO 100. Both phones were set to fluorescent white balance and 20% focus. These settings were chosen for each phone to give a good contrast between plate and spot, without oversaturating the image. Pictures were collected as RAW images (.dng) and exported as .jpeg files at 100% quality.
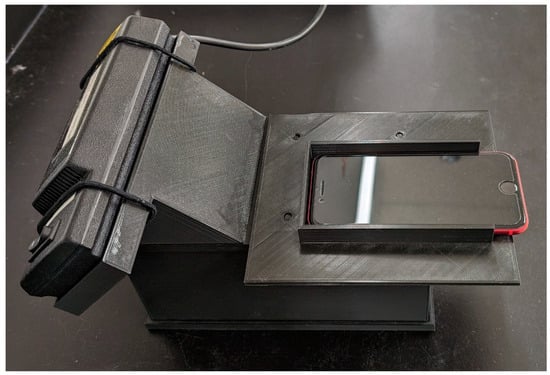
Figure 1.
Light box design housing the 254 nm UV lamp, with the smartphone situated on top for photo collection. The box has a removable base with slots to hold the TLC plates.
The plate images were analyzed using the FIJI (FIJI Is Just ImageJ; version 2.14.0) distribution of ImageJ [36]. The ImageJ image processing procedure (provided as an ImageJ macro in Supplemental Material) was based on one we have previously described in the literature [33], with one major modification: no adjustment for the background image was made, as the method we previously used was found to decrease the quality of results. Other methods of background correction were attempted, including manual baseline assignment and the “rolling ball” algorithm in ImageJ, but neither of these methods improved performance. Our ImageJ macro automatically determined the location of each spot in the plate image and determined the integrated density of that spot, which is the product of the mean gray pixel value and the area of the spot. The mean gray pixel value is proportional to the concentration of metronidazole in the spot. The integrated densities of standards spotted on each plate are used to create a calibration curve to determine the concentration of the unknown sample. This linear regression was performed using the Python programming language (version 3.10), and the package pyimagej [37] (version 1.41) was used to automate analysis of the thousands of images generated in this method validation.
2.3. HPLC Method
The USP Metronidazole Tablets assay method was used as the reference method [6] for accuracy determination. TLC sample solutions were diluted by a factor of 10 with mobile phase (20:80 v/v methanol:water) to target concentrations of 0.5 mg/mL at 100% and used directly for HPLC analysis. The metronidazole standards used in the HPLC assay were prepared separately at 0.5 mg/mL and not diluted from the TLC standard solutions. For HPLC analysis, an HPLC 1200 system with autosampler (G1329A), quaternary pump (G1311A), thermostat (G1330B), thermostatted column compartment (G1316A), diode-array detector (G1315B), and OpenLab CDS ChemStation software (Rev C.01.07 SR3; Agilent, Santa Clara, CA, USA) was used. The instrument was equipped with a Luna C8(2) 5 µm 150 × 4.6 mm 100 Å column (Phenomenex, Torrance, CA, USA) operating at 25 °C. The flow rate was 1.0 mL/min. The detector was an ultraviolet spectrophotometer set at a wavelength of 254 nm. The injection volume was 10 μL.
2.4. Validation
This method is intended to be a screening method, and as such it would fall under application IV as defined in USP <1850> [38]. Therefore, we assessed linearity, range, accuracy, precision, intermediate precision, and specificity. Detection and quantitation limits were not evaluated.
2.4.1. Linearity and Range
Due to the small size of the TLC plates and broad metronidazole spots, only four spots can reliably fit on the 5 × 10 cm plates used in this GPHF-Minilab™ method. Additionally, due to the differences between TLC plates, and to account for and varying lighting conditions over time and any potential differences in plate development, a standard calibration must be run on each plate with the sample. Therefore, linearity was evaluated across two ranges: 75–125% (normally used for sample analysis) and 50–150%, with each range comprising 3 concentrations and evaluated separately. Both ranges included standard solutions at 100% concentration. For each range, the three standards were spotted in increasing concentration from left to right across the plate, followed by a metronidazole tablet sample. Linearity was evaluated in this way to fully mimic the experimental setup as it would be during accuracy and precision evaluations. The order of spotting on the TLC plates was examined during robustness and spotting the standards in increasing concentration from left to right, followed by the sample spot, yielded the best results. The sample spot was not quantified during linearity evaluations; it was used purely as a placeholder. Each set of standards was used to spot three different TLC plates, and five images were collected in rapid succession from each plate to average out pixel noise (total of n = 15 images per set of standards). Linear regression was performed on the measured integrated density values determined from the image analysis process. The slope, y-intercept, and correlation coefficient (R2) were calculated.
2.4.2. Accuracy and Precision
The accuracy of this method was tested for both 250 mg and 500 mg metronidazole tablet samples, each at three concentrations: 75% (3.75 mg/mL), 100% (5.0 mg/mL), and 125% (6.25 mg/mL). Sample solutions were prepared from the composite sample. TLC plates were spotted from left to right with 75%, 100%, and 125% standards, followed by the sample. The three standards are used to create a calibration curve allowing for quantitation of the sample spot. For each concentration, three replicate plates were prepared with the same samples and five images were collected of each plate with each phone (total of n = 15 images per set of standards at each concentration). For the accuracy determination, TLC sample solutions were diluted by a factor of 10 with mobile phase to target concentrations of 0.5 mg/mL at 100% and used directly for HPLC analysis. The metronidazole standards used in the HPLC assay were prepared separately and not diluted from the TLC standard solutions. Percent recovery was calculated by dividing the TLC determined concentration by the concentration determined by HPLC.
2.4.3. Repeatability and Intermediate Precision
Repeatability and intermediate precision were determined by conducting the precision analysis on each of three days. Each day, fresh 75%, 100%, and 125% concentration samples were prepared for each of the 250 mg and 500 mg metronidazole tablet composites. Three plates were prepared at each concentration of each tablet, with each set of three plates spotted with the same set of solutions. Five images were taken of each plate by each smartphone (Google Pixel 4a 5G and iPhone SE (2020)). To assess precision, the within-days, or repeatability, variance (Sr), and intermediate precision variance (SIP), along with associated relative standard deviations (RSDr and RSDIP), were calculated as defined by González et al. [39]. Pooled Sr and SIP were calculated according to Equation (1), where k is the number of different standard deviations pooled and sk is the individual standard deviation value. Repeatability and intermediate precision were determined at each concentration level (75%, 100%, and 125%, each using all 45 images from the 3 plates run each day) and overall (pooling the three concentrations).
2.4.4. Specificity
As the proposed procedure is based on the established method for metronidazole TLC analysis in the GPHF-Minilab™, with no chemical modifications to the standard and sample solutions nor to the developing solvent and chromatography, the method specificity is primarily based on the reference method. Additional testing of a sample placebo containing common excipients was performed to further demonstrate method specificity. Since we do not have the manufacturing facilities to be able to prepare a true placebo tablet, we prepared a placebo mixture to imitate the tablet by mixing equal parts of the reagents in Table 1. The reagents were determined by comparing excipient lists from several generic metronidazole tablet manufacturers. A powder weight equivalent to one tablet was weighed out, and a placebo sample for TLC was prepared according to the solution preparation procedure for metronidazole tablet samples. This placebo sample was spotted onto a TLC plate along with a 100% metronidazole standard solution and 250 mg tablet sample, and the plate was developed according to the method.

Table 1.
Materials used to create the metronidazole tablet placebo mixture.
2.4.5. Robustness
Additional camera settings were tested during method development and validation. During the accuracy, precision, and intermediate precision portions of the validation, we collected images of all plates using a second set of camera settings. For the Pixel camera, ISO 200 and exposure 1/20 s were used, while ISO 200 and exposure 1/50 s were used for the iPhone camera. Accuracy, precision, repeatability, and intermediate precision were determined for the second set of camera settings and compared to the settings used for the rest of the validation.
The TLC plates that come with the GPHF-Minilab™ use a thin aluminum substrate, which can curl slightly at the top and bottom. This could potentially impact pictures of the plates and alter results. To assess the impact of this, we also obtained TLC plates with a glass substrate (silica gel 60 F254 5 × 10 cm glass TLC plates; Sigma Aldrich, St. Louis, MO, USA). The glass TLC plates have a different silica gel particle size and layer thickness (10–12 µm particle size at 200 µm layer thickness for the aluminum plate and 8.0–12.0 µm particle size at 250 µm layer thickness for the glass plates). We prepared a separate 250 mg metronidazole tablet sample at 100% concentration and ran three TLC plates spotted with the same standard and sample solutions and took five images of each plate with both the iPhone and Pixel, each with several different camera settings. Percent label claim results were compared between the plate types.
3. Results
3.1. Linearity and Range
The summarized linearity results for plates spotted from left to right with 75%, 100%, and 125% or 50%, 100%, and 150% metronidazole standards followed by a 250 mg metronidazole tablet sample are shown in Table 2. Linear regression results for each image collected during the linearity evaluation are shown in Table S1. All the plates show good linearity across both the 75–125% and 50–150% ranges. Both Apple and Android images yielded overall average R2 > 0.99 for the 75–125% plates and >0.97 for the 50–150% plates. The RSD for R2 was around 1% or lower. Results were consistent from Apple to Android images. Slopes and intercepts were consistent within different images of the same plates, and roughly consistent overall, although there is some difference plate-to-plate. The potential for high RSDs for slope and intercept requires running the standards and developing a linear regression on each plate used in the method. The 75–125% range was used for the accuracy determinations as it had a higher R2.

Table 2.
Linearity across two ranges as determined by both the Google Pixel 4a 5G and Apple iPhone SE (2020) smartphone cameras.
3.2. Accuracy and Precision
The TLC label claim and percent recovery at each of the three concentrations as determined by both cameras are shown in Table 3, along with overall label claim and percent recovery and associated RSDs. The HPLC assay results are shown in Table S2 for reference. The percent recoveries (average for all 15 images of the 3 plates for each concentration, combined results for 250 mg and 500 mg tablet samples) ranged from 94.4% to 100.8%, and the label claims ranged from 94.7% to 101.2%, respectively. The best percent recovery (closest to 100%) for the Pixel was at 75% (99.1%), although it came with a high RSD (9.6%). The best percent recovery for the iPhone was at 75% (100.8%), again with high RSD (7.9%), although the percent recovery at 100% is close (99.1%), with much lower RSD (2.2%). The percent recoveries for both phones at 125% was the furthest from 100%: 95.6% for the Pixel and 94.4% for the iPhone, although RSDs were again much lower than at 75% (3.7% and 2.7%, respectively). The RSDs at each concentration varied from 1.4% to 9.2% for label claim and 1.4% to 9.6% for percent recovery. The overall label claim was 97.4% (RSD 5.4%) for the Pixel and 99.7% (6.3% RSD) for the iPhone. The 75% sample concentration had the highest percent recovery and label claim RSDs, while the 100% concentration had the lowest RSDs. The high RSD appears to be due to plate-to-plate differences, as the percent recovery RSDs for the five images collected of each plate generally fall under 2% (Table S3). Additionally, the lower concentration solutions result in spots that are not as dark, and consequently, there is less contrast in the plate image leading to less reproducible image analysis. It is possible that at higher concentrations spots become saturated with sample and the measured integrated density responds slightly less linearly to concentration (supported by linearity results with the wider range). Thus, it appears that the best performance of this method is within the vicinity of 100% concentration (5 mg/mL). This is acceptable for the intended use case of this method in product screening, as results near 75% (3.75 mg/mL) or 125% (6.25 mg/mL) concentration would flag the product for further screening using methods such as HPLC. While there can be up to about a 2.5% difference between the Pixel and iPhone label claims and percent recoveries, the trends were generally similar across the different smartphone types.

Table 3.
Accuracy (label claim and percent recovery) and precision (RSD) for both 250 and 500 mg metronidazole tablets at 75%, 100%, and 125% concentrations and overall, from both cameras.
3.3. Repeatability and Intermediate Precision
The combined repeatability and intermediate precision results for 250 and 500 mg tablets are shown in Table 4. These results only include percent label claim, as the determination of weight of API in tablets is not directly comparable between the 250 and 500 mg tablets. The within-days, or repeatability, variance, Sr, and intermediate precision variance, SIP, and associated RSDr and RSDIP, were very close for the two cameras, differing 0.2% or less. The Sr at 75% was 6.3%, 3.2% at 100%, and 2.9–3.0% at 125% concentration, and the pooled values were 4.2% for both cameras. The SIP was 6.1–6.2% at 75%, 5.4% at 100%, and 4.1–4.4% at 125% concentration, and the pooled value was 5.6% for the Pixel and 5.5% for the iPhone. The RSDr and RSDIP values were similar to the Sr and SIP as the RSD is the respective S divided by the overall average label claim, which is close to 100%. The lowest Sr and SIP values were from the images at 125% concentration, with 100% and 75% successively higher, likely because the higher concentration resulted in darker spots and higher contrast, making image analysis more reproducible.

Table 4.
Repeatability (Sr) and intermediate precision (SIP) results with associated relative standard deviations (RSD) at 75%, 100%, and 125% concentrations and pooled across all 3 concentrations for both the Google Pixel 4a 5G and Apple iPhone SE (2020), including both 250 and 500 mg metronidazole tablets.
3.4. Specificity
A representative iPhone image of a TLC plate spotted with the placebo sample, as well as a metronidazole standard and a 250 mg metronidazole tablet sample, is shown in Figure 2. No spots are visible in the placebo sample lane, demonstrating that the TLC method is specific for metronidazole in the tablets used in this method.
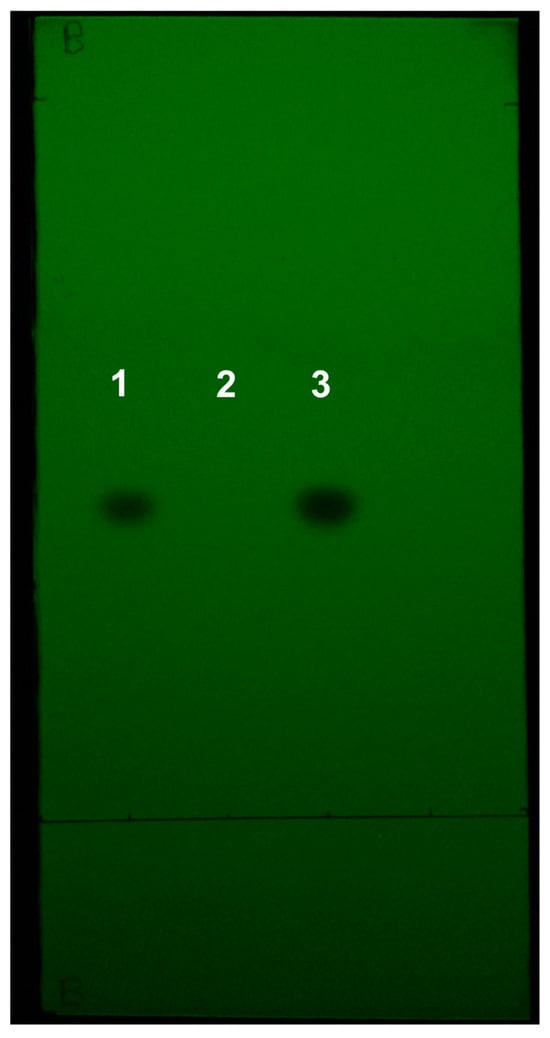
Figure 2.
Representative image of the metronidazole tablet placebo TLC plate. Spotted: (1) metronidazole standard solution, (2) placebo solution, (3) 250 mg metronidazole tablet sample.
3.5. Robustness
The accuracy and precision results from the modified camera settings are shown in Table S4, and the repeatability and intermediate precision results are shown in Table S5. Both sets of results are quite close to the results at the original camera settings, with the largest difference being the percent recovery determined by the Pixel at 100% was 2.2% higher with the robustness camera settings compared to the validation camera settings. These results indicate that different camera settings can be used to generate similar results for this method, provided there is enough exposure of the plate.
The glass and aluminum TLC plate label claim results (with error bars for plus and minus one standard deviation) for the Pixel and iPhone images are shown in Figure 3. The glass plates had overall label claims closer to 100% at all camera settings but the average RSD was higher. Tukey honestly significant difference (HSD) tests were performed between each aluminum/glass pair at the same camera settings. Tukey HSD p-values < 0.05 indicate that the means are statistically different at a 95% confidence level. All plates photographed with the Android phone had p-values < 0.05, while six of the nine pairs photographed with the iPhone had p-values > 0.05. We currently do not know if the glass plates are more accurate overall (closer to 100% label claim) or simply systematically lead to lower predictions than aluminum plates; further testing is needed. If the glass plates are more accurate overall, then the tradeoff is with precision and price, as the glass plates cost more than the aluminum ones. For the purposes of product screening, the level of difference should still be adequate to flag product outside the normal acceptance range of 90–110% of the label claim.
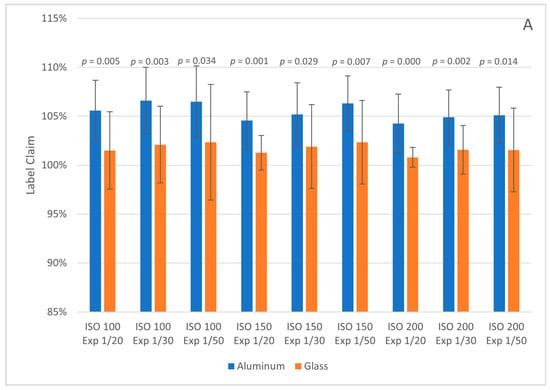
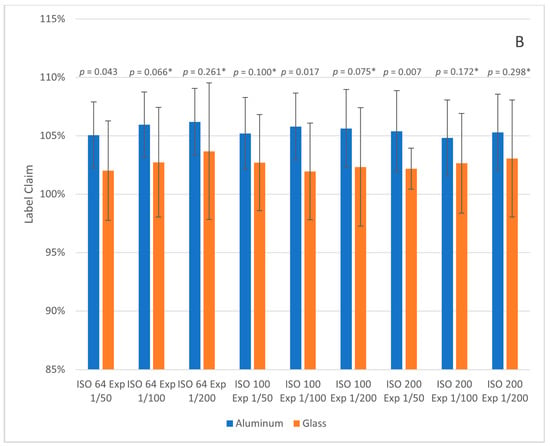
Figure 3.
Label claim results for 250 mg metronidazole tablet samples on aluminum and glass TLC plates at different camera settings (ISO and Exposure, denoted as Exp). Images were collected with the Google Pixel 4a 5G (A) and Apple iPhone SE 2020 (B). Error bars represent plus and minus one standard deviation. Tukey HSD p-values are given above each pair; values denoted with * indicate pairs with p-value > 0.05.
The ordering of standard spots on the TLC plates was also investigated. Metronidazole standards at 75/100/125% or 50/100/150% concentrations were spotted in different orders from left to right on the TLC plate. Each ordering was spotted onto three plates, and each plate was imaged five times. The linear regression results are given in Table S6. In general (with some exceptions), the 75/100/125% standards had higher average R2 than the 50/100/150% standards, and the ordering of 75/100/125% from left to right had the highest average R2 and lowest R2 RSD. This is the ordering that was used for the validation work.
4. Discussion
Our validation results demonstrate that we have successfully taken a semi-quantitative method and made it fully quantitative (within 75–125% of the 5 mg/mL target concentration, with accuracy and precision of approximately 5 to 6%). While our results do not reach the level of accuracy and precision of HPLC analysis, our method is not intended to replace HPLC applications, just as the original GPHF-Minilab™ method was not meant to replace HPLC methods [40]. Rather, this method is meant to serve as an enhanced screening procedure. Products can be flagged for further testing using HPLC. Our validation results demonstrate that this method should improve the GPHF-Minilab™ method performance at the 80–100% concentration range, which is where the technique has struggled, as noted previously.
While not calculated the same way, the accuracy, repeatability, and intermediate precision results presented are comparable to those generated by TLCyzer analysis on the GPHF-Minilab™ metronidazole TLC method by Hauk et al. [32]. The similarity suggests that the errors and variations are inherent to the materials and method rather than the image analysis approach. Our results show that different cameras and camera settings generate similar results. The largest variation in results seems to be plate to plate; this could be due to differences in analyst spotting performance and/or differences between the plates and their silica gel coatings. Manual TLC methods such as the GPHF-Minilab™ methods could be made more repeatable from incorporating automated spotters [41,42], but additional equipment requirements would make the method less useful in LMICs. While the TLCyzer app is open source, as of the time of preparation of this manuscript, the app is no longer available on the Google Play Store [43]. Therefore, this approach provides a useful alternative and could potentially be improved by ideas implemented by Hauk et al., including a polynomial fitting for background estimation and correction, and keeping only the top 15% of pixels in each spot during the linear regression.
5. Conclusions
Our objective is to improve the performance of the GPHF-Minilab™ metronidazole TLC method for applications in quality screening. Our results show that with minimal additional investment, the GPHF-Minilab™ metronidazole TLC method can be made quantitative, making it a viable option for resource limited settings. No major alterations of the GPHF TLC procedure itself were made, suggesting that this approach should be easily extendable to the other GPHF-Minilab™ methods for different active pharmaceutical ingredients, although each method would require its own validation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/analytica5040036/s1, ImageJ image processing macro, Figure S1: General flowchart of the TLC quantitation procedure, Table S1: Linear regression results for each linearity image, Table S2: Accuracy HPLC results for 250 and 500 mg metronidazole tablets, Table S3: TLC percent recovery and RSD of 250 and 500 mg metronidazole tablets, Table S4: TLC accuracy and precision for 250 and 500 mg metronidazole tablets, Table S5: TLC repeatability and intermediate precision results at different camera settings than used in validation work, Table S6: TLC linear regression results of metronidazole standards in varying orders.
Author Contributions
Conceptualization, C.L.H., M.E.S., E.B. and D.J.; Data curation, C.L.H., S.B. and M.E.S.; Formal analysis, C.L.H. and S.B.; Funding acquisition, D.J.; Investigation, C.L.H. and S.B.; Methodology, C.L.H., M.E.S., E.B. and D.J.; Project administration, D.J.; Resources, C.L.H. and D.J.; Software, C.L.H. and M.E.S.; Supervision, D.J.; Validation, C.L.H. and S.B.; Visualization, C.L.H.; Writing—original draft, C.L.H.; Writing—review and editing, C.L.H., S.B., M.E.S., E.B. and D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by USAID Contract No. AID-OAA-C-15-00001, Global Health Supply Chain Quality Assurance Program (GHSC-QA).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization (WHO). Metronidazole. Available online: https://list.essentialmeds.org/medicines/313 (accessed on 4 June 2024).
- Tebano, G.; Li, G.; Beovic, B.; Bielicki, J.; Brink, A.; Enani, M.A.; Godman, B.; Hinrichsen, S.L.; Kibuule, D.; Gabriel, L.-H.; et al. Essential and Forgotten Antibiotics: An Inventory in Low- and Middle-Income Countries. Int. J. Antimicrob. Agents 2019, 54, 273–282. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151342-5. [Google Scholar]
- Tchounga, C.A.W.; Sacré, P.-Y.; Hamuli, P.C.; Mballa, R.N.; Bleye, C.D.; Ziemons, E.; Hubert, P.; Djang’eing’a, R.M. Prevalence of Poor Quality Ciprofloxacin and Metronidazole Tablets in Three Cities in Cameroon. Am. J. Trop. Med. Hyg. 2023, 108, 403. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Metronidazole Tablets (Metronidazoli Compressi). In The International Pharmacopoeia, 11th ed.; World Health Organization: Geneva, Switzerland, 2022; Volume 6. [Google Scholar]
- United States Pharmacopoeia (USP). Metronidazole Tablets. In USP-NF; USP: Rockville, MD, USA, 2023. [Google Scholar] [CrossRef]
- Kaale, E.; Manyanga, V.; Makori, N.; Jenkins, D.; Michael Hope, S.; Layloff, T. High-performance thin layer chromatography to assess pharmaceutical product quality. Trop. Med. Int. Health 2014, 19, 747–751. [Google Scholar] [CrossRef]
- Pyka-Pająk, A. TLC–Densitometric Analysis of Selected 5-Nitroimidazoles. Processes 2023, 11, 170. [Google Scholar] [CrossRef]
- Meshram, D.; Bagade, S.; Tajne, M. TLC-Densitometric Analysis of Clotrimazole and Metronidazole in Combined Dosage Forms. J. Planar Chromatogr.–Mod. TLC 2008, 21, 277–282. [Google Scholar] [CrossRef]
- Meshram, D.B.; Bagade, S.B.; Tajne, M.R. Simultaneous Determination of Metronidazole and Miconazole Nitrate in Gel by HPTLC. Pak. J. Pharm. Sci. 2009, 22, 323–328. [Google Scholar]
- Elkady, E.F.; Mahrouse, M.A. Reversed-Phase Ion-Pair HPLC and TLC-Densitometric Methods for the Simultaneous Determination of Ciprofloxacin Hydrochloride and Metronidazole in Tablets. Chromatographia 2011, 73, 297–305. [Google Scholar] [CrossRef]
- Ali, N.W.; Gamal, M.; Abdelkawy, M. Chromatographic Methods for Simultaneous Determination of Diiodohydroxyquinoline and Metronidazole in Their Binary Mixture. Pak. J. Pharm. Sci. 2013, 26, 865–871. [Google Scholar] [PubMed]
- Johnson, M.E. Rapid, Simple Quantitation in Thin-Layer Chromatography Using a Flatbed Scanner. J. Chem. Educ. 2000, 77, 368. [Google Scholar] [CrossRef]
- Hess, A.V.I. Digitally Enhanced Thin-Layer Chromatography: An Inexpensive, New Technique for Qualitative and Quantitative Analysis. J. Chem. Educ. 2007, 84, 842. [Google Scholar] [CrossRef]
- Soponar, F.; Moţ, A.C.; Sârbu, C. Quantitative Determination of Some Food Dyes Using Digital Processing of Images Obtained by Thin-Layer Chromatography. J. Chromatogr. A 2008, 1188, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.G.; Mohammed Fayez, Y.; Kelani, K.M.; Mahmoud, A.M. TLC-Smartphone in Antibiotics Determination and Low-Quality Pharmaceuticals Detection. RSC Adv. 2021, 11, 19196–19202. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-T.; Zhao, J.; Li, S.-P. Application of Smartphone in Detection of Thin-Layer Chromatography: Case of Salvia Miltiorrhiza. J. Chromatogr. A 2021, 1637, 461826. [Google Scholar] [CrossRef]
- Fichou, D.; Morlock, G.E. quanTLC, an Online Open-Source Solution for Videodensitometric Quantification. J. Chromatogr. A 2018, 1560, 78–81. [Google Scholar] [CrossRef]
- Mac Fhionnlaoich, N.; Ibsen, S.; Serrano, L.A.; Taylor, A.; Qi, R.; Guldin, S. A Toolkit to Quantify Target Compounds in Thin-Layer-Chromatography Experiments. J. Chem. Educ. 2018, 95, 2191–2196. [Google Scholar] [CrossRef]
- Jähnke, R.W.; Dwornik, K. A Concise Quality Control Guide on Essential Drugs and Other Medicines; Manual issued by the Global Pharma Health Fund (GPHF) for its GPHF-Minilab™, GPHF: Giessen, Germany, 2008. [Google Scholar]
- Global Use of the GPHF-MinilabTM. Available online: https://www.gphf.org/en/minilab/einsatzgebiete.htm (accessed on 13 May 2024).
- Petersen, A.; Held, N.; Heide, L. Surveillance for Falsified and Substandard Medicines in Africa and Asia by Local Organizations Using the Low-Cost GPHF Minilab. PLoS ONE 2017, 12, e0184165. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ba-Thein, W. Diagnostic Accuracy of Global Pharma Health Fund MinilabTM in Assessing Pharmacopoeial Quality of Antimicrobials. Am. J. Trop. Med. Hyg. 2018, 98, 344–348. [Google Scholar] [CrossRef]
- Sherma, J.; Rabel, F. Advances in the Thin Layer Chromatographic Analysis of Counterfeit Pharmaceutical Products: 2008–2019. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 367–379. [Google Scholar] [CrossRef]
- Risha, P.; Msuya, Z.; Ndomondo-Sigonda, M.; Layloff, T. Proficiency Testing as a Tool to Assess the Performance of Visual TLC Quantitation Estimates. J. AOAC Int. 2006, 89, 1300–1304. [Google Scholar] [CrossRef]
- Kovacs, S.; Hawes, S.E.; Maley, S.N.; Mosites, E.; Wong, L.; Stergachis, A. Technologies for Detecting Falsified and Substandard Drugs in Low and Middle-Income Countries. PLoS ONE 2014, 9, e90601. [Google Scholar] [CrossRef]
- Vickers, S.; Bernier, M.; Zambrzycki, S.; Fernandez, F.M.; Newton, P.N.; Caillet, C. Field Detection Devices for Screening the Quality of Medicines: A Systematic Review. BMJ Glob. Health 2018, 3, e000725. [Google Scholar] [CrossRef] [PubMed]
- Zambrzycki, S.C.; Caillet, C.; Vickers, S.; Bouza, M.; Donndelinger, D.V.; Geben, L.C.; Bernier, M.C.; Newton, P.N.; Fernández, F.M. Laboratory Evaluation of Twelve Portable Devices for Medicine Quality Screening. PLoS Negl. Trop. Dis. 2021, 15, e0009360. [Google Scholar] [CrossRef] [PubMed]
- Opuni, K.F.-M.; Nettey, H.; Larbi, M.A.; Amartey, S.N.A.; Nti, G.; Dzidonu, A.; Owusu-Danso, P.; Owusu, N.A.; Nyarko, A.K. Usefulness of Combined Screening Methods for Rapid Detection of Falsified and/or Substandard Medicines in the Absence of a Confirmatory Method. Malar. J. 2019, 18, 403. [Google Scholar] [CrossRef] [PubMed]
- Kaale, E.; Risha, P.; Reich, E.; Layloff, T.P. An Interlaboratory Investigation on the Use of High-Performance Thin Layer Chromatography to Perform Assays of Lamivudine-Zidovudine, Metronidazole, Nevirapine, and Quinine Composite Samples. J. AOAC Int. 2010, 93, 1836–1843. [Google Scholar] [CrossRef]
- Zhang, D.; Armour, E.; Sherma, J. Development of Quantitative HPTLC–Densitometry Methods Following a Model Approach for Transfer of TLC Screening Methods for Pharmaceutical Products of Cefixime, Cefuroxime Axetil, cephalexin⋅H2O, Ciprofloxacin HCl, Levofloxacin, and Metronidazole. Acta Chromatogr. 2017, 29, 484–486. [Google Scholar] [CrossRef]
- Hauk, C.; Boss, M.; Gabel, J.; Schäfermann, S.; Lensch, H.P.A.; Heide, L. An Open-Source Smartphone App for the Quantitative Evaluation of Thin-Layer Chromatographic Analyses in Medicine Quality Screening. Sci. Rep. 2022, 12, 13433. [Google Scholar] [CrossRef]
- Sowers, M.E.; Ambrose, R.; Bethea, E.; Harmon, C.; Jenkins, D. Quantitative Thin Layer Chromatography for the Determination of Medroxyprogesterone Acetate Using a Smartphone and Open-Source Image Analysis. J. Chromatogr. A 2022, 1669, 462942. [Google Scholar] [CrossRef]
- Yu, H.; Le, H.M.; Kaale, E.; Long, K.D.; Layloff, T.; Lumetta, S.S.; Cunningham, B.T. Characterization of Drug Authenticity Using Thin-Layer Chromatography Imaging with a Mobile Phone. J. Pharm. Biomed. Anal. 2016, 125, 85–93. [Google Scholar] [CrossRef]
- Sowers, M.E.; Bethea, E.; Xiao, W.; Phase, N.; Harmon, C.; Jenkins, D. Quantification of Isoniazid in Tablets for Tuberculosis Treatment by Thin Layer Chromatography with Smartphone Image Capture and ImageJ Analysis. Microchem. J. 2024, 202, 110796. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Rueden, C.T.; Hiner, M.C.; Evans, E.L.; Pinkert, M.A.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Eliceiri, K.W. PyImageJ: A Library for Integrating ImageJ and Python. Nat. Methods 2022, 19, 1326–1327. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopoeia (USP). ⟨1850⟩ Evaluation of Screening Technologies for Assessing Medicine Quality. In USP-NF; USP: Rockville, MD, USA, 2023. [Google Scholar] [CrossRef]
- González, A.G.; Herrador, M.Á.; Asuero, A.G. Intra-Laboratory Assessment of Method Accuracy (Trueness and Precision) by Using Validation Standards. Talanta 2010, 82, 1995–1998. [Google Scholar] [CrossRef] [PubMed]
- Jähnke, R. Letter to the Editor on Previously Published GPHF-Minilab Assessment. Am. J. Trop. Med. Hyg. 2018, 98, 1880. [Google Scholar] [CrossRef] [PubMed]
- Boulgakov, A.A.; Moor, S.R.; Jo, H.H.; Metola, P.; Joyce, L.A.; Marcotte, E.M.; Welch, C.J.; Anslyn, E.V. Next-Generation TLC: A Quantitative Platform for Parallel Spotting and Imaging. J. Org. Chem. 2020, 85, 9447–9453. [Google Scholar] [CrossRef]
- Woortman, D.V.; Haack, M.; Mehlmer, N.; Brück, T.B. Additive Analytics: Easy Transformation of Low-Cost Fused Deposition Modeling Three-Dimensional Printers for HPTLC Sample Application. ACS Omega 2020, 5, 11147–11150. [Google Scholar] [CrossRef]
- TLCyzer. Available online: https://tlcyzer.github.io/ (accessed on 4 June 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).