Abstract
Contamination of trace levels of volatile organic compounds (VOCs) in enclosed spaces is not usually a significant cause for concern; however, it can be relevant in the case of canine scent detection training as a canine’s superior sense of smell makes them highly likely to detect low levels of contamination, contributing to inefficient training. Thus, herein, we address the need for a simple, low-cost, robust, vapochromic sensor to determine the cross-contamination of VOCs within closed containers, such as canine training aid kits. This study focuses on the development of a vapor sensor, which produces a rapid colorimetric change when a target chemical vapor is present. A pH indicator is used as the colorimetric dye and its incorporation into a sol–gel matrix on a paper substrate is confirmed via SEM characterization. The sensor’s stability and performance is tested against exposure to various levels of sunlight and temperature. The design allows the sensor to present a clear and unambiguous visible response to the release of the volatile target within a closed container. It can be readily incorporated into existing training kits and functions as a straightforward reminder of when training aids need to be changed or a new containment system should be considered.
1. Introduction
Volatile organic compounds (VOCs) can often be found at harmful levels in some workplace environments and can be observed as contamination in many products of interest [1,2,3]. Cross-contamination of VOCs can easily occur when chemicals are stored closely together in enclosed spaces, which can be an issue for first responders [4] and food and beverage industries [5], as well as detection canines [6]. The presence of unwanted chemicals on stored canine odor detection training aids can lead to a decrease in detection proficiency [3]. Humans often contribute to inefficient canine detection training as they remain unaware of cross-contamination between their training materials, though their canine’s superior sense of smell is very likely to detect it, even at trace levels. An example of this was observed in 1997, where researchers observed that canines trained on multiple explosives were better able to find explosive training aids that were cross-contaminated with associated highly volatile chemicals instead of the quality control aids, which were not cross-contaminated [7]. To our knowledge, there are no methods in place at present that would indicate to canine handlers that their training materials may be contaminated. Recent research has looked at potential cross-contamination of explosive training aids using gas chromatography–mass spectrometry (GC–MS) and found that it may occur during manufacturing, transportation, or storage of the training aids [8]. To address this issue, this research developed and optimized the development of a paper-based sensor with a sol–gel coating infused with a colorimetric dye to detect potential vapor cross-contamination in closed containers.
The development of chemical vapor sensors—which can produce a fluorescent, electric, or colorimetric response based on chemical stimuli originating from their surrounding environment—has previously been of particular interest in the detection of toxic vapors [9]. However, many of such sensors can be costly and/or involve further complicated steps for final analysis [1,10,11,12]. Colorimetric sensors have a unique advantage of inducing a response that can be easily visible to the human eye [13]. In most cases, they are also cost-effective and versatile. The proposed sensor utilizes a paper substrate because it is robust, widely available, of low-cost, and easy to use and dispose. Its vapochromic capabilities are derived from chemoresponsive compounds, such as pH indicator dyes. The pH indicator dyes are compounds that change color due to the acidity or basicity of their surroundings [11,12]. Similarly, vapochromism is a change in color when a substance is exposed to different vapors [14]. The ensuing color changes following solvent or vapor exposure are to be conveniently observed by the naked eye. Previous work on vapor sensors using similar colorimetric compounds has noted a lack of response when exposed to the target vapor [15,16] and difficulty in immobilizing the indicator to the substrate. To address this shortcoming, in the research herein, the colorimetric indicator was incorporated into a polymer matrix created via sol–gel synthesis which increases its vapochromic response [15]. Sol–gel synthesis has the additional advantage of being physically robust with high thermal, solvent, and chemical stability [17]. Sol–gel synthesis involves the formation of a three-dimensional cross-linked structure starting from a colloid solution known as the “sol”, which is subsequently gelled (“gel”) through a cascade of catalytic reactions at room temperature [18]. The resulting polymer matrix is then treated to remove any leftover solvents. The colorimetric indicator used should remain contained within the sol–gel layer due to its the porous nature.
In previous work during 2015, researchers used a color-changing reagent to produce a colorimetric sol–gel-based sensor for the detection of 3,4-Methylenedioxymethamphetamine (MDMA) [19]. Another study from 2020 successfully developed a colorimetric paper-based sol–gel sensor to monitor freshness of fish via detection of the total volatile basic nitrogen levels through plastic film by incorporating bromocresol green into their synthesized material [20]. While there are often difficulties with many sensing materials in detecting chemical agents in the vapor phase, sol–gel synthesis has proven to be an effective adsorbent material for vaporous analytes [21,22]. In general, sol–gel synthesis involves simple, low-cost procedures that allow for the design of an inexpensive product that can be easily distributed for widespread use. In theory, use of the sol–gel increases sensitivity as the vapor in the enclosed container will be absorbed into the three-dimensional sponge-like structure formed from the sol–gel synthesis and interact with the colorimetric agent present there, thus inducing a visible color change.
Multiple techniques have been implemented in recent years to develop vapor sensors to detect common environmental hazards such as ammonia, benzene, or other vapors, and have been noted to be highly sensitive and/or efficient in the detection of their respective vapor analytes. For example, Khachornsakkul et al. developed a paper-based colorimetric sensor using methyl orange to detect trace levels of ammonia with a limit of detection at 2.0 ppbv [23]. Although not reusable, it was found to be superior to multiple other electrochemical and colorimetric sensors developed for ammonia. Additionally, Lee et al. synthesized two novel solvatochromic dyes and affixed them to cotton fabrics to produce textile-based sensors that would detect multiple relevant solvents in the vapor phase [24]. Most notably, this sensor was found to be reusable for at least ten cycles with similar performance levels as the initial trial. Additionally, graphene-based [25], electrochemical [26], and semiconductor gas sensors [27,28] are highly sensitive devices that have recently become more popular in the detection of vapor analytes. Nevertheless, for the purposes of our goal, a rapid colorimetric sensor with minimal complexity might better suit canine handlers so that it can be easily introduced into current canine training aid kits and can reduce potential end-user error.
These visible color responses are crucial for correct interpretation of any analyses implementing colorimetric sensors and, unfortunately, they can be affected by a variety of environmental factors, such as interfering VOCs, pH, environment humidity, and temperature. Other colorimetric sensors have previously been noted to have a decrease in sensitivity at low target concentrations with the presence of moisture [29]. Choodum et al. studied the stability of their MDMA sol–gel colorimetric sensor for nearly three months and found that the sensor was stable at freezing temperatures with very minute deviations in the color intensity of their product [19]. Furthermore, they found that the sensor color darkened, and its responses started to vary slightly after being exposed to light for three hours. Similarly, Choudhary and Philip (2021) found that their paper-based colorimetric phosphate sensor was stable for 90 days at different temperatures ranging from 25 °C to 60 °C [30]. To confidently implement the proposed sensor in real-life applications, it was essential to study common factors that could affect the colorimetric response.
This research developed and optimized the formulation for a paper-based vapochromic sensor with a sol–gel coating infused with a colorimetric dye to detect cross-contamination of vapors. The sensor produces a colorimetric response when it comes into contact with the chosen target chemical agent in the vapor phase inside a closed container, such as a training aid kit. In testing the sensor application, this study evaluated the overall effects on the vapochromic sensor when exposed to some environmental factors—such as temperature and light. The sensor was designed in a manner to present a clear and unambiguous visible response to the release of the volatile target, indicative of possible cross-contamination of adjacent canine training aids.
2. Materials and Methods
2.1. Reagents
The following reagents were acquired from Sigma Aldrich (Burlington, Massachusetts, USA): trifluoroacetic acid (TFA), tetraethyl orthosilicate (TEOS), methanol (MeOH), sodium hydroxide (NaOH), ammonium hydroxide (NH4OH), bromocresol (BCG), bromophenol blue (BB), and phenol red (PR). All reagents and materials were used as purchased, without prior modifications or purification. All sensor development procedures were performed at room temperature and at an average humidity of 58.4% RH, unless stated otherwise.
2.2. Sensor Formula Optimization
2.2.1. General Sensor Preparation
The sol–gel synthetic procedure followed three basic steps: hydrolysis, condensation, and gelation (solidification). In this formulation, 100 μL of an inorganic precursor—TEOS—was mixed with 480 μL of either MeOH or DI water in a 2 mL microtube. The TEOS molecules underwent hydrolysis with the addition of 34 μL TFA (0.1 M in methanol) acting as an acid catalyst. This solution was homogenized by vortexing for 3 min, following the addition of each compound, and then left to react overnight (~19 h). Thereafter, condensation of the TEOS polymer was induced with the addition of a base catalyst, ammonium hydroxide (NH4OH; 0.25 M in DI water). The mixture was vortexed for 1 min and gelation followed shortly after the addition of the base. The molar ratio between TEOS:water:TFA (0.1 M in methanol):NH4OH (0.25 M in DI water) was maintained at 1:56:1.8:5.8–17.5. Prior to complete gelation, 5 L drops of the sol–gel mixture were deposited onto the chosen substrate, Whatman No. 1 filter paper (Sigma Aldrich, St. Louis, MO, USA). The paper was left to air dry for 30 min and was then placed in an oven at 70 °C for at least 30 min to remove any remaining solvent. Some parameters for this formulation—such as gelation time, indicator used, and indicator concentration—were optimized. Thereafter, the colorimetric indicator was included into the initial TEOS/solvent mixture for the sol–gel. To prepare the indicator solution, 0.01 N NaOH was added in a dropwise fashion to 10 mg of the pH indicator dye until it was dissolved in a 25 mL volumetric flask. This solution was then diluted to the mark with water. Methanol was initially used as a solvent/porogenic agent for the sol–gel material; however, it was replaced with DI water due to the dye solubility.
2.2.2. Gelation vs. Base Catalyst
The amount of the base catalyst in the formulation was tested to allow time for deposition on the paper substrate prior to gelation time. This amount was varied in increments of 50 (50–150) to establish a reasonable gelation time to allow for the deposition of the solution onto the substrate before solidifying. Changing the amount of base catalyst to 50 , 100 , and 150 varied the molar ratio of TEOS/NH4OH (0.25 M in DI water) to 1:5.8, 1:11.7, and 1:17.5, respectively. In theory, an increase in the base catalyst would allow for a shorter gelation time.
2.2.3. Choosing Compatible Analyte and Colorimetric Indicator
To determine colorimetric indicator and analyte compatibility, a screening of 17 indicator dyes was completed (Table S1). The indicators were tested first against vinegar (containing 5% acetic acid) and then against diluted NH4OH (0.25 M). These targets were chosen as they are volatile, inexpensive, and low-hazard chemicals that are commonly found in household products.
Once the more responsive colorimetric dyes were identified, they were tested against a series of diluted solutions of their target vapor to determine which was most sensitive. Five milliliters of a stock solution of the target chemical agent was prepared in DI water and diluted to 100, 50, 40, 30, 20, and 10 ppm in 40 mL headspace vials. To allow each dilution to reach equilibrium, the solutions were left in the sealed containers for at least 24 h prior to testing the sensor. The sensors were suspended in the headspace for each solution, and the time it took each sensor to change color was noted.
2.2.4. Loading of Dye in Sensor
The optimum loading amount of the colorimetric indicator in the sol–gel sensor was evaluated to achieve the most sensitive response without diminishing colorimetric response. Four solutions were prepared for bromocresol green at 0.02% w/v, 0.04% w/v, 0.08% w/v, and 0.12% w/v in NaOH 0.01 N and DI water. The vapochromic sensors were prepared at these corresponding concentrations and placed in the headspace of 100, 50, and 10 ppm NH4OH dilutions.
2.3. Estimating Limit of Detection in Terms of pH
A pH meter (PC60, Apera Instruments, Columbus, Ohio, USA) was utilized to measure the pH of liquid solutions of NH4OH as well as their corresponding headspace. The NH4OH was diluted from a stock solution of 8760 ppm to 500, 100, 50, 20, 10, and 4 ppm. It must be noted that, by definition, pH is only measured in an aqueous phase; thus, what was measured here is the moisture within the headspace that condenses on the electrode of the pH meter. A small amount of each solution with enough volume to cover the bottom surface was pipetted into the Apera Instruments pH meter container. The PC60 meter configuration has an O-ring that allows the user to seal the pH electrode with the container. The headspaces of the solutions were left to equilibrate for 10 min before measurement collection. Afterwards, the Shapiro–Wilkes test was conducted to determine if the data obtained were normally distributed. Thereafter, IBM SPSS Statistics software (Version 28.0.1.0 (142)) was used to perform an independent samples t-test to determine if pH results between headspace and liquid samples as well as between consecutive concentrations, were significantly different from each other (i.e., stock vs. 50, 50 vs. 40, 40 vs. 30, 30 vs. 20 (ppm)).
While it is uncommon to measure headspace pH, previously, researchers have used pH test paper to measure and monitor the acidity of an indoor museum air [31]. Using chromatography, they were then able to characterize acids in that environment. With this method, the museum was able to develop protocols to add ventilation for exhibition cases and, thus, allow them to take better care of their exhibits. Herein, pH is similarly detected by the paper-based sensor; thus, the limit of detection was determined by pH.
2.4. Sensor Characterization
Sensor surface morphologies were examined at 200× magnification using a JSM-IT500HR scanning electron microscope (SEM; Jeol, Tokyo, Japan) at a voltage of 5 kV. Prior to SEM imaging, the samples were prepared by coating small square pieces of the sensor with gold to make them conductive. Two samples were examined, including plain filter paper and the bromocresol green sol–gel-coated sensor.
2.5. Sensor Stability
The stability of the vapochromic sensor over time was examined in three commonly used canine training aid cases (or kits). Three sensors were placed inside cases from three manufacturers, namely, Pelican, Nanuk, and Ray Allen Manufacturing (PEL, NAN, and RAM, respectively) for 10 weeks. The cases were empty except for the sensors and the foam lining fitted to the case by their original manufacturers. The sensors were removed weekly for observation, placed inside a photo box assembled in-house, and had their image recorded using a Dino-Lite digital microscope (AM3111 model, Dunwell Tech., Inc., Los Angeles, CA, USA) for image documentation. The cases were kept closed and were stored at room temperature. The red, green, and blue (RGB) color data for the sensor were extracted from the recorded images with ImageJ (ImageJ 1.53e; Java 1.8.0_172), an open-source software. Additionally, ImageJ was used to calculate a brightness value (0.299R + 0.587G + 0.114B) based on the RGB pixels, and this value was used to normalize the data and remove light interference originating from the Dino-Lite microscope.
2.6. Exposure to Sunlight
The effect of sunlight on the vapochromic sensor was examined over time at three different levels. Two sensors were separately placed inside either a clear glass vial, an amber glass vial, or a clear glass vial wrapped in aluminum foil (CLR, AMB, and ALM, respectively) for 10 weeks. The vials were kept closed, stored at room temperature, and exposed directly to sunlight. The CLR vial had the highest exposure to sunlight, followed by the AMB vial, which had a colored coating that reduces sunlight exposure. Finally, the ALM vial had the least exposure to sunlight. All vials were precleaned and empty except for the sensors. The sensors were removed weekly for observation, placed inside a photo box assembled in-house, and had their image recorded using the Dino-Lite digital microscope for image documentation. The red, green, and blue (RGB) color data for the sensor were extracted from the recorded images with ImageJ. Similar to the previously mentioned stability experiment, the RGB results were normalized by the provided brightness value. Experimental schematics for Section 2.5 and Section 2.6 can be found in the Supplemental Information Figures S1 and S2.
2.7. Effect of Temperature on Performance
The sensor’s performance was examined under different temperatures (25 °C, 50 °C, and 70 °C). Training aid kits are often stored in locations such as garages or vehicle trunks. Previous research on vehicle cabin temperatures has shown that the hottest temperatures ever reached inside a vehicle were near 70 °C [30] and, as such, this value was chosen as the highest temperature value in this study. The experimental setup included three mason jars (A, B, and C) inside a Nanuk hard plastic case (Figures S3 and S4). Jar A contained 5.0 mL of 1000 ppm NH4OH solution in water. This jar was enclosed in a heat-sealed 8 MIL plastic bag alongside two vapochromic sensors. Jar B contained a blank training aid which was composed of a 2″ × 2″ piece of clean gauze inside a 2 MIL plastic bag. Finally, Jar C contained a common, volatile, commercially available explosive training aid, which contained 2-ethyl-1-hexanol (2E1H). The cases were placed inside an oven at the corresponding temperatures and monitored daily for up to three days to check for any change in the sensor color and to monitor for cross-contamination. For this experiment, cross-contamination was determined to have occurred once 2E1H was detected in the headspace of Jar B (the blank training aid).
The headspace of the blank training aid was analyzed daily using SPME–GC–MS to detect any presence of 2E1H. A gray (DVB/CAR/PDMS) SPME fiber was exposed to the Jar B headspace for 10 min for sample collection. An HP-5MS UI column (30 m × 0.25 mm I.D. × 0.25 um, Agilent Technologies) was used with a 1 mL/min flow rate using helium as carrier gas with a splitless injection. The oven temperature parameters had 105 °C as the initial temperature, increased by 40 °C/min until 280 °C. Standard MS parameters were used with the MS source, MS Quad, and instrument transfer line at 230 °C, 150 °C, and 280 °C, respectively. The electron ionization (EI) had an energy of 70 eV and the scan range was m/z 33–300. Blank testing of the jars and the blank training aid did not show prior presence of 2E1H. Two test kits and one control kit were placed inside the oven for analysis at a time. The control kit contained the same materials as the test kits, except that it did not have any NH4OH or 2E1H. The control kit was used as a negative control to evaluate the sensor performance in the absence of any targets.
3. Results
3.1. Optimization
3.1.1. Gelation Time vs. Base Catalyst
Table 1 displays the results for the gelation time based on the amount of base catalyst added to induce polymerization. Based on the results, the amount of 100 was chosen as it provided the fastest gelation time. Further increase and decrease in base catalyst was found to extend the gelation time unnecessarily.

Table 1.
Gelation time change with different addition of base catalyst, 0.25 M NH4OH in water (n = 6).
3.1.2. Choosing Compatible Analyte and Colorimetric Indicator
Three pH indicator dyes, out of all colorimetric indicators screened, were observed to respond to the vapor of either vinegar or ammonium hydroxide in under 3 min (Table 2). All other responses for the colorimetric indicators tested were longer than 3 min and, thus, eliminated. However, both bromocresol green and phenol red sensor were much faster to respond to the vapor from neat ammonium hydroxide (immediately (<1 s)) than bromophenol blue was to the vapor from neat vinegar (1–3 min). Bromocresol green also responded to vinegar but at a much slower rate than 0.25 M NH4OH (~7 min vs. <1 s). As such, further sensor optimization preceded bromocresol green and phenol red as potential colorimetric indicators and NH4OH as the target chemical agent.

Table 2.
Three pH indicator dyes that changed color in the presence of either vinegar (5% acetic acid) or ammonium hydroxide (0.25 M).
Table 3 compares the performance of bromocresol green and phenol red. As the concentration of NH4OH decreased, the response time of the sensor decreased as well. In Table 4, it can be observed that PR stopped reacting at 100 ppm NH4OH or less while BCG stopped reacting near 10 ppm; however, it should be noted that the criteria for “No reaction” or “N/A” is that there was no color change after 10 min. Based on these observations, further optimization focused on BCG sensors over PR sensors as they reacted to lower concentrations of NH4OH.

Table 3.
Comparison between time of reaction of BCG and PR sensors to NH4OH dilutions (n = 6). Concentration reported as concentration of NH4OH in solution.

Table 4.
Final sol–gel sensor formulation.
3.1.3. Loading of Dye in Sensor
The results for the four BCG sensor concentrations can be seen in Figure 1. The resulting sensor with 0.02% w/v of BCG was too lightly colored to clearly discern the color change response. The general trend observed was that the reaction time across each NH4OH dilution decreased with BCG concentration. From this experiment, it was determined that 0.04% w/v was the optimal concentration for bromocresol green in this sensor configuration.
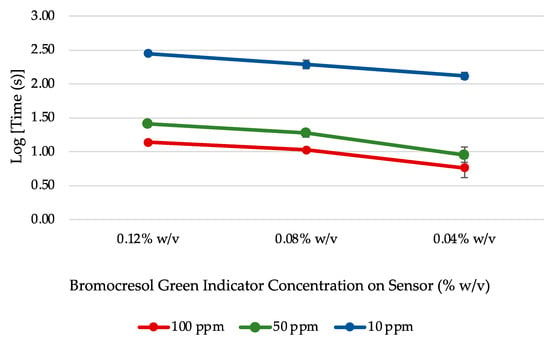
Figure 1.
Average reaction time for BCG sensors prepared at different dye concentrations exposed to various NH4OH dilutions (n = 3).
3.1.4. Optimized Vapochromic Sensor Formulation
The final optimized formulation for the sensor is seen in Table 4. A sulfonephthalein dye, bromocresol green, was chosen as the colorimetric indicator to detect the presence of a base environment inside a closed container. These dyes are known for their intense colors and widespread use in analytical chemistry [32]. It was hypothesized in the early 1900s that their color-changing mechanism was mainly due to the formation of a resonance stabilized quinone–phenolate ion complex [33]. As bromocresol green dye is deprotonated to form this complex, it can transition from an intense yellow color at low pH to green and then, finally, blue in conditions of higher pH. Figure 2 shows the initial sensor color in green (left) next to a blue sensor (right) exposed to the headspace of an ammonium hydroxide solution. After use, the sensor was found to be able to be restored to its initial green coloring after heating in the oven at 50 °C, indicating its potential for reuse.

Figure 2.
Bromocresol green sensor color change from green (acid) to blue (basic).
3.2. Estimating Sensor Limit of Detection
The developed sensor was not exclusively selective to the vapor of ammonia or ammonium hydroxide. Instead, it will detect a change to a high-pH environment. For this reason, pH was used to define its limit of detection. All pH measurements in both solution and headspace were seen to have pH values above the bromocresol green’s stated pH range of 3.8–5.4 [34] (Figure 3 and Table 5). Initial statistical testing indicated that the acquired data were normally distributed. As such, an independent sample t-test was implemented for the comparison of means between two sample conditions using the IBM SPSS Statistics software (version 28.0.1.0). For each concentration, the means between the pH of headspace and solutions were found be significantly different, as noted by the p-values, which were all <0.001 (Table 5). Moreover, the p-values were also less than 0.05 when comparing pH values between subsequent NH4OH dilutions, indicating that they are also significantly different. This highlights the differences in pH that the sensor encounters both in the headspace and in solution as ammonium hydroxide is diluted. Based on these results and when the sensor stopped reacting to NH4OH dilutions (Table 3), the sensor’s limit of detection was estimated to be at a range between pH 7.53 ± 0.19–7.89 ± 0.06 in the headspace of the NH4OH dilutions.
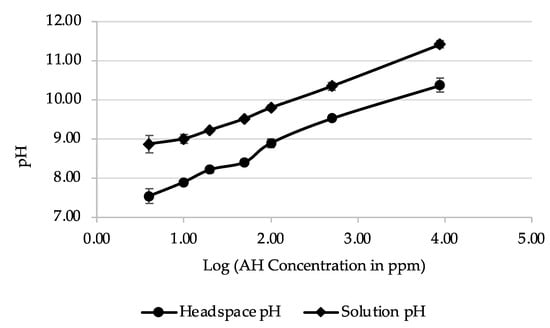
Figure 3.
Comparison of pH measurements for NH4OH dilutions in headspace and in solution (n = 4).

Table 5.
Measurements of pH for NH4OH liquid solutions and their corresponding headspace (n = 4) as well as results from independent sample t-test from SPSS.
3.3. Sensor Characterization
SEM imaging of the filter paper before and after preparation of the vapochromic sensor indicated successful coating of the paper substrate. When comparing Figure 4A,B, a coating is seen on the surface of the filter paper at 200× magnification; however, even with the additional layer, the fibrous and porous nature of the filter paper (i.e., cellulose) is still distinguishable in this image. This is a good indication of the large amount of surface area that can be utilized by the sensor to interact with the target vapor.
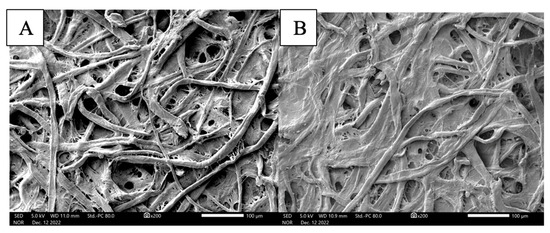
Figure 4.
SEM imaging of (A) plain filter paper and (B) bromocresol green sol–gel-coated sensor.
3.4. Sensor Stability
Some widely available hard plastic cases (Ray Allen Manufacturing, Nanuk, and Pelican brands) used for training aid kits were used to test the stability of the sensor over 10 weeks in these containers. As time passed, the sensor color was visually observed to be altered by its surrounding environment in different degrees (Figure 5). Figure 6 shows the relationship between time and the normalized RGB data for a sensor in each case tested. As color changed, the RGB values shifted as well. From the graphs shown in Figure 6, it can be seen that the sensors in the Ray Allen Manufacturing case had the most drastic separation between the RGB values. The increasing separation between the red, blue, and green lines across time corresponded to a change in the overall sensor color. The larger the separation, the more significant the change of color. Visually, the RAM case sensors were seen to begin transitioning from green to blue between 19 and 26 days, indicating a basic environment (Figure 5). The PEL and NAN case sensors did not show as wide a separation for the RGB lines as the RAM case. However, when looking at Figure 5 and Figure 6, it is noted that at approximately 26 days the Pelican case sensors began acquiring a blue ring of color as did the Nanuk case sensors after 30 days. This is suspected to be due to minor contamination from the ambient environment. In general, regardless of the outer blue ring color, they remained dominantly green and, thus, were still functional for detection of NH4OH. One possible explanation for the significant color change in the RAM case is the foam lining contained within the kit. As opposed to the Pelican and Nanuk cases, Ray Allen Manufacturing uses an adhesive to affix the foam to the plastic case. This adhesive may have released VOCs that generated a basic environment within the case, causing the change in color.
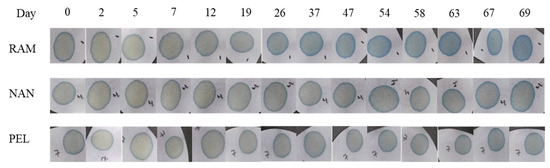
Figure 5.
Progression of sensor color throughout the 10-week period for the stability experiment.
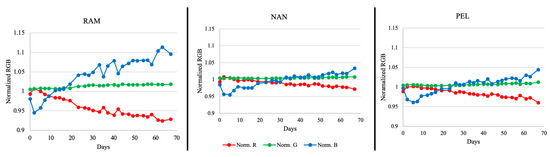
Figure 6.
Individual sensor RGB value trends across 10 weeks for each of the cases examined over time (normalized data; n = 3).
3.5. Sunlight Exposure
The progression of the sensor colors over a 10-week period is shown in Figure 7 and the graphs in Figure 8 show the degree of separation between the RGB values over time. In this experiment, the sensors in the clear vial (most light) showed the most separation between RGB values, followed by the sensors in the amber vial (less light), indicating that exposure to light caused a substantial change in color. Visually, it was observed that the CLR vial sensors transitioned from their initial green color to a faded gray color occurring at approximately 4–7 days (Figure 7). Meanwhile, the color transition was harder to visually discern for the AMB vial sensors but, according to the data in Figure 8, they appeared to have taken approximately 32 to 37 days to reach the same faded color. On the other hand, the sensors contained within aluminum (no light) retained most of their initial light green color, with only a slight fading, and had RGB values that remained close together. Taking the sensor out of the ALM wrapped vial for image recording could account for some sunlight exposure leading to small color changes.
Figure 7.
Progression of sensor color throughout the 10-week period for the sunlight experiment.
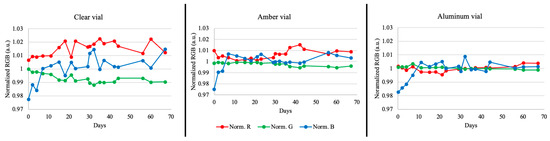
Figure 8.
Individual sensor RGB values across 10 weeks for each of the sunlight exposure variables (normalized data; n = 2).
3.6. Effects of Temperature on Sensor Application
The sensor’s performance was tested at three different temperatures at which canine training aid kits could be frequently stored (Table 6). This experiment was evaluated through a present/not present paradigm that would reflect future end-user practice. Kits can be stored at either indoor or outdoor locations and the chosen temperatures reflect either possibility. In this experiment, no cross-contamination of 2E1H was detected by instrumental headspace analysis after three days at 25 °C, the average time a canine handler may leave the training aids stored between uses. Likewise, there was no change in color in the sensor for that time at that temperature. On the other hand, at 50 °C and 70 °C, cross-contamination of 2E1H was detected in the headspace of the blank jar within 48 h and 24 h, respectively; however, the sensor changed from green to blue within the first 24 h for both environments. Further analysis of the training aid kit itself showed that, at 50 °C, 2E1H was detected via SPME–GC–MS inside the kit after 24 h even if it was not detected inside Jar B; thus, the color change was indeed indicative of contamination within the kit.

Table 6.
Results for sensor performance indicating the time that contamination was detected and the sensor changes color.
4. Discussion
Bromocresol green was successfully embedded into a sol–gel matrix and then deposited onto a filter paper base to build a vapochromic sensor. The deposition was confirmed by both the visible green color of the sensor and the surface image of the sensor obtained via scanning electron microscopy. Some aspects of the sol–gel formulation were optimized, including the amount of base catalyst and indicator dye added. In addition to other factors, such as catalyst concentration and solution temperature, gelation time for the sol–gel solution has previously been observed to be dependent on the time of hydrolysis for the tetraethyl orthosilicate (TEOS) alkyl chains [35]. However, in the case of the two-catalyst process implemented here, it was the amount of base catalyst that had a more immediate and visible role in the polycondensation of the sol–gel material. Contrary to the initial hypothesis, gelation time did not decrease with the increase in the base catalyst. Based on the results in Table 1, both an excess and deficiency of the base catalyst caused a longer gelation time in this formulation. Similarly, an excess of pH indicator dye loaded into the sensor (0.12% w/v and 0.08% w/v) caused a decrease in reaction time, whereas an insufficient amount (0.02% w/v) did not produce a sensor with an indistinguishable color change.
During this sensor development, ammonium hydroxide (NH4OH) was identified as a potential target chemical that would generate a basic vapor in its surrounding environment and trigger a colorimetric response in the sensor. In the headspace of the target chemical, the sensor will change color from green to blue (Figure 2). Depending on the NH4OH concentration, this reaction can take place between 2 s to 132 s on average. Once exposed to NH4OH, the paper sensor can be returned to its original state by placing it in the oven at 50 °C to evaporate any lingering chemicals. It cannot be assumed that other ammonium-containing compounds will also generate a sensor response. Initial testing in the headspace of ammonium nitrate (NH4NO3 or AN) did not trigger a positive response since AN does not create a basic environment; it exists in an equilibrium with ammonia and nitric acid [36]. This is indicative of a nonbasic environment in the headspace and, thus, would not allow the sensor to react and change color. Other compounds with similar alkaline properties to ammonium hydroxide may also work as a target chemical for the sensor’s application. The reported working range for the bromocresol green pH indicator is pH 3.8–5.4 [34]; however, the BCG sensor was found to cease color change between 10 ppm and 4 ppm dilutions of the target chemical, corresponding to a limit of detection at a pH range between pH 7.53 ± 0.19 and 7.89 ± 0.06 in the headspace of the NH4OH dilutions. Low standard deviations throughout the analyses performed on the sensors indicated good reproducibility for the developed sensor. The decrease in sensitivity in the vapor phase compared to aqueous BCG solutions might be due to a limited contact between the indicator molecules and the air moisture as opposed to the direct contact that occurs when they are submerged or mixed into a solution.
Bromocresol green was chosen among 17 potential indicators for the development of this vapochromic sensor due to its superior performance against the analytes tested. However, all possible dye/target combinations were not tested; thus, it is possible that higher sensitivity or faster response times could be obtained by dyes or target chemicals that were not tested herein. For example, in 2021, another vapor sensor was designed specifically for the detection of ammonia gas and was able to obtain an LOD of 2.0 ppbv with a reaction time of 3 min [23]; however, unlike the current developed cross-contamination sensor, it was found to not be reusable. Moreover, Maity and Ghosh (2018) also created a colorimetric ammonia gas sensor with an LOD near 10 ppm and gave a color change for ammonia within 10 s when concentrations were over 20 ppm [37]. Again, this color change was irreversible. More recently, in 2023, Xiaowei et al. developed a fluorescent ammonia gas sensor to monitor chicken freshness in real time but could only monitor the fluorescent signal every 24 h [38]. In general, there have been many studies that have worked to build sensors capable of detecting ammonia for their own different purposes that may or may not also be applicable to our described application; however, the sensor described in this paper was designed solely as a prototype tool for the canine handler community which required a low-cost, robust, and reusable sensor.
This developed sensor was designed as a general alert system for canine handlers to become aware of when cross-contamination may be occurring within their training materials. In its application, the target chemical is included inside the closed vessel and stored with the same containment parameters as the other items within the container. The vapochromic sensor is also included in the container alongside the target chemical. In theory, the containment of the stored materials may fail in extreme circumstances involving high temperatures or rough handling. This would induce a color change in the sensor as a response to the vapor of the target chemical, and thereby alert the end-user that their materials have leaked due to a potential containment failure. It is only intended to give a color response when the chosen method of containment (i.e., mason jars, plastic bag, etc.) fails uniformly due to a systematic event. The sensor was not designed for specific detection of the training aid odors themselves. Thus, this vapochromic sensor will not alert if the containment for one of the training aids is defective, but the rest are functional. Overall, it was observed that the cases manufactured by Ray Allen were not compatible with this sensor and its intended application, while the Nanuk and Pelican brands (the latter of which is more widely used) were. Additionally, the sensor must be stored in the dark to prevent any degradation of the product’s effectiveness. The sensors were also found to be functional in high-temperature environments (relative to ambient car cabin temperatures).
Future directions of this study should expand the performance testing of the sensor and involve testing the sensor in other commonly used containers such as odor-proof bags, training aid delivery devices (TADDs), aluminum bags, metal containers, plastic bags, etc. There is also room for improvement in the end-user means of determining the sensor response. The final color response may be interpreted differently by each individual due to bias, colorblindness, or other reasons. As such, future studies should also look to create a computer/phone interface that would allow for a more unbiased determination of the color response to help overcome these difficulties and give definitive notification of cross-contamination.
5. Conclusions
A vapochromic sensor that targets an extraneous vapor in the environment—generated by a chosen chemical reactant—was successfully developed. This sensor is intended to be implemented in the detection of the target vapor within a canine training aid kit (closed containment). Of the three colorimetric indicators tested that changed color in the presence of either vinegar or ammonium hydroxide, bromocresol green was observed to be the most responsive indicator for the chemical reactants tested; it was best paired with ammonium hydroxide as the target reactant. The sensor’s limit of detection for the target vapor was found to be within a range of pH 7.53 ± 0.19 to 7.89 ± 0.06, which is a higher pH than reported for the bromocresol green indicator in solution. It is recommended that the sensor be stored in the dark and away from any environment that may generate compounds with high pH, such as some cleaning products or adhesives. This sensor and target chemical can be incorporated into canine training aid kits to detect possible cross-contamination and containment failure for the training aids. Additionally, it could be potentially implemented in determining cross-contamination in other settings such as food storage areas, industrial or laboratory storage units, and law enforcement evidence lockers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/analytica5030019/s1, Table S1: Colorimetric indicator dyes tested; Figure S1: Experiment schematic for testing sensor response to three different commercially available hard case containers over time; Figure S2: Experiment schematic for sunlight exposure testing. Vial A is a clear, transparent vial. Vial B is an amber vial. Vial C is a clear vial wrapped in aluminum foil; Figure S3: Experiment schematic for temperature testing detailing what the training aid kits contained; Figure S4: Experiment set-up for Section 2.7. Effect of Temperature on Sensor Performance.
Author Contributions
Conceptualization, L.E.D. and A.K.; methodology, A.K. and J.C.-C.; formal analysis, J.C.-C., J.C. and A.S.; data curation, J.C.-C., J.C. and A.S.; investigation, J.C.-C., J.C. and A.S.; resources, L.E.D. and K.G.F.; writing—original draft preparation, J.C.-C.; writing—review and editing, L.E.D., A.K., K.G.F. and J.C.-C.; visualization, J.C.-C.; supervision, A.K. and L.E.D.; project administration, K.G.F. and L.E.D.; funding acquisition, L.E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was, in part, financially supported by a FIU University Graduate School Dissertation Year Fellowship.
Data Availability Statement
Data available on request from the authors.
Acknowledgments
The authors would like to thank Ping Jiang from the Trace Evidence Analysis Facility at Florida International University for assistance in the SEM analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Feng, L.; Musto, C.J.; Kemling, J.W.; Lim, S.H.; Zhong, W.; Suslick, K.S. Colorimetric Sensor Array for Determination and Identification of Toxic Industrial Chemicals. Anal. Chem. 2010, 82, 9433–9440. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Wu, K.K. Determination of Ethylene Oxide by Solid-Phase Microextraction Device with on-Fiber Derivatization. J. Chromatogr. A 2003, 991, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Lazarowski, L.; Singletary, M.; Barrow, J.; Van Arsdale, K.; Angle, T.; Waggoner, P.; Giles, K. A Review of the Types of Training Aids Used for Canine Detection Training. Front. Vet. Sci. 2020, 7, 313. [Google Scholar] [CrossRef] [PubMed]
- Larner, J.; Durrant, A.; Hughes, P.; Mahalingam, D.; Rivers, S.; Matar, H.; Thomas, E.; Barrett, M.; Pinhal, A.; Amer, N.; et al. Efficacy of Different Hair and Skin Decontamination Strategies with Identification of Associated Hazards to First Responders. Prehospital Emerg. Care 2020, 24, 355–368. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef] [PubMed]
- Goss, K.U. The Physical Chemistry of Odors—Consequences for the Work with Detection Dogs. Forensic Sci. Int. 2019, 296, 110–114. [Google Scholar] [CrossRef]
- Hallowell, S.F.; Fischer, D.S.; Brasher, J.D.; Malone, R.L.; Gresham, G.L.; Rae, C. Effectiveness of Quality-Control Aids in Verifying K-9-Team Explosive Detection Performance. Chem. Biol. Based Technol. Contraband Detect. 1997, 2937, 227–234. [Google Scholar] [CrossRef]
- Davis, K.; Goodpaster, J.V. Analysis of the Cross-Contamination of Explosive Canine Training Aids during Manufacturing and Storage. Forensic Chem. 2024, 38, 100571. [Google Scholar] [CrossRef]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent Progress on the Development of Chemosensors for Gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef]
- Park, D.H.; Heo, J.M.; Jeong, W.; Yoo, Y.H.; Park, B.J.; Kim, J.M. Smartphone-Based VOC Sensor Using Colorimetric Polydiacetylenes. ACS Appl. Mater. Interfaces 2018, 10, 5014–5021. [Google Scholar] [CrossRef]
- Suslick, K.S. An Optoelectronic Nose: “Seeing” Smells by Means of Colorimetric Sensor Arrays. MRS Bull. 2004, 29, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S.; Rakow, N.A.; Sen, A. Colorimetric Sensor Arrays for Molecular Recognition. Tetrahedron 2004, 60, 11133–11138. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, J.; Hu, G.; Zhang, E.; Yu, H.H. Colorimetric Sensors for Chemical and Biological Sensing Applications. Sensors 2023, 23, 2749. [Google Scholar] [CrossRef]
- Wenger, O.S. Vapochromism in Organometallic and Coordination Complexes: Chemical Sensors for Volatile Organic Compounds. Chem. Rev. 2013, 113, 3686–3733. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.C.; Cheema, J.A.; Yildiz, U.H.; Palaniappan, A.; Liedberg, B. Vapor Phase Solvatochromic Responses of Polydiacetylene Embedded Matrix Polymers. J. Mater. Chem. C Mater. 2017, 5, 1803–1809. [Google Scholar] [CrossRef]
- Pumtang, S.; Siripornnoppakhun, W.; Sukwattanasinitt, M.; Ajavakom, A. Solvent Colorimetric Paper-Based Polydiacetylene Sensors from Diacetylene Lipids. J. Colloid. Interface Sci. 2011, 364, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Kehagia, M.; Kabir, A.; Furton, K.G. Matrix Molecularly Imprinted Mesoporous Sol-Gel Sorbent for Efficient Solid-Phase Extraction of Chloramphenicol from Milk. Anal. Chim. Acta 2016, 914, 62–74. [Google Scholar] [CrossRef]
- Prasad, S.; Kumar, V.; Kirubanandam, S.; Barhoum, A. Engineered Nanomaterials: Nanofabrication and Surface Functionalization; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Choodum, A.; Kanatharana, P.; Wongniramaikul, W.; NicDaeid, N. A Sol-Gel Colorimetric Sensor for Methamphetamine Detection. Sens. Actuators B Chem. 2015, 215, 553–560. [Google Scholar] [CrossRef]
- Liu, X.; Chen, K.; Wang, J.; Wang, Y.; Tang, Y.; Gao, X.; Zhu, L.; Li, X.; Li, J. An On-Package Colorimetric Sensing Label Based on a Sol-Gel Matrix for Fish Freshness Monitoring. Food Chem. 2020, 307, 125580. [Google Scholar] [CrossRef]
- Bunkoed, O.; Davis, F.; Kanatharana, P.; Thavarungkul, P.; Higson, S.P.J. Sol-Gel Based Sensor for Selective Formaldehyde Determination. Anal. Chim. Acta 2010, 659, 251–257. [Google Scholar] [CrossRef]
- Makote, R.; Collinson, M.M. Organically Modified Silicate Films for Stable PH Sensors. Anal. Chim. Acta 1999, 394, 195–200. [Google Scholar] [CrossRef]
- Khachornsakkul, K.; Hung, K.H.; Chang, J.J.; Dungchai, W.; Chen, C.H. A Rapid and Highly Sensitive Paper-Based Colorimetric Device for the on-Site Screening of Ammonia Gas. Analyst 2021, 146, 2919–2927. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Kim, T. Synthesis of Vapochromic Dyes Having Sensing Properties for Vapor Phase of Organic Solvents Used in Semiconductor Manufacturing Processes and Their Application to Textile-Based Sensors. Sensors 2022, 22, 4487. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhao, K.; Wu, J.; Deng, G.; Tang, C.; Zhang, C.; Xu, H.; Wang, Q.; Chen, K.; Chiang, K.S. A Vapochromic Dye/Graphene Coated Long-Period Fiber Grating for Benzene Vapor Sensing. Mater. Chem. Front. 2022, 6, 2438–2446. [Google Scholar] [CrossRef]
- Singh, P.; Mandal, D.; Chandra, A.; Singh, T. Zinc Stannate Oxide Perovskite Nanomaterial Based Electrochemical Detection of Ammonia. Sens. Actuators A Phys. 2024, 366, 114955. [Google Scholar] [CrossRef]
- Humayun, M.; Bououdina, M.; Usman, M.; Khan, A.; Luo, W.; Wang, C. Designing State-of-the-Art Gas Sensors: From Fundamentals to Applications. Chem. Rec. 2024, 24, e202300350. [Google Scholar] [CrossRef] [PubMed]
- Uma, S.; Shobana, M.K. Metal Oxide Semiconductor Gas Sensors in Clinical Diagnosis and Environmental Monitoring. Sens. Actuators A Phys. 2023, 349, 114044. [Google Scholar] [CrossRef]
- Piriya, V.S.A.; Joseph, P.; Daniel, S.C.G.K.; Lakshmanan, S.; Kinoshita, T.; Muthusamy, S. Colorimetric Sensors for Rapid Detection of Various Analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar] [CrossRef]
- Choudhary, V.; Philip, L. Stable Paper-Based Colorimetric Sensor for Selective Detection of Phosphate Ion in Aqueous Phase. Microchem. J. 2021, 171, 106809. [Google Scholar] [CrossRef]
- Sano, C. Air Pollutants Trapped in a PH Test Paper for Indoor Air Henshoku Shiken-Shi. Sci. Conserv. 1999, 38, 15–22. (In Japanese) [Google Scholar]
- Magnaghi, L.R.; Zanoni, C.; Alberti, G.; Biesuz, R. The Colorful World of Sulfonephthaleins: Current Applications in Analytical Chemistry for “Old but Gold” Molecules. Anal. Chim. Acta 2023, 1281, 341807. [Google Scholar] [CrossRef] [PubMed]
- Lubs, H.A.; Acree, S.F. On the Sulfonphthalein Series of Indicators and the Quinone-Phenolate Theory. J. Am. Chem. Soc. 1916, 38, 2772–2784. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Firoozbakht, F. Determination of the Acidity Constants of Neutral Red and Bromocresol Green by Solution Scanometric Method and Comparison with Spectrophotometric Results. Beni Suef Univ. J. Basic Appl. Sci. 2016, 5, 13–20. [Google Scholar] [CrossRef][Green Version]
- Boonstra, A.H.; Bernards, T.N.M. The Dependence of the Gelation Time on the Hydrolysis Time in a Two-Step SiO2 Sol-Gel Process. J. Non Cryst. Solids 1988, 105, 207–213. [Google Scholar] [CrossRef]
- Stelson, A.W.; Friedlander, S.K.; Seinfeld, J.H. A Note on the Equilibrium Relationship between Ammonia and Nitric Acid and Particulate Ammonium Nitrate. Atmos. Environ. 1979, 13, 369–371. [Google Scholar] [CrossRef]
- Maity, A.; Ghosh, B. Fast Response Paper Based Visual Color Change Gas Sensor for Efficient Ammonia Detection at Room Temperature. Sci. Rep. 2018, 8, 16851. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, W.; Sun, W.; Li, Z.; Zhang, N.; Shi, J.; Zhang, Y.; Zhang, X.; Shen, T.; Zou, X. A Paper-Based Ratiometric Fluorescent Sensor for NH3 Detection in Gaseous Phase: Real-Time Monitoring of Chilled Chicken Freshness. Food Chem. X 2024, 21, 101054. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).