Abstract
Fingerprints are essential for human identification and are valuable tools in criminal investigations. The pursuit of new materials for digital printing is expanding, with increasing interest in natural compounds such as bixin, sourced from annatto seeds. Despite its traditional use as a natural dye with medicinal properties, the potential of bixin in papilloscopy remains largely untapped. In this study, we meticulously extracted bixin from annatto seeds and meticulously developed composites incorporating zinc carbonate (bixin/ZnCO3) and kaolinite (bixin/kaolinite). UV-visible spectroscopy was used for characterization, and the extracted bixin showed absorption peaks at 429, 453, and 481 nm, which were very similar to standard peaks at 429, 457, and 487 nm. The two samples also had the same retention times (7.07 min) according to further liquid chromatography analysis. Sweat pores were easier to detect thanks to the effectiveness of the bixin/ZnCO3 and bixin/kaolinite composites in creating high contrast sebaceous and natural latent fingerprints. These results highlight the composites’ potential as novel and fascinating instruments for papilloscopy applications, which might also improve forensic investigations.
1. Introduction
Papilloscopy is the science of studying human identification through fingerprints, which are a valuable source of evidence at crime scenes because they differ not only between fingers but also between individuals. Fingerprints have been used to identify people for more than a century [1]. In the field of forensic sciences, fingerprints are rooted in Locard’s exchange principle, which states that every contact between two objects results in a transfer of traces between them. Thus, the contact of a fingerprint facilitates the transfer of materials from the papillary ridges of the fingers to the surface during this interaction [2]. In criminal investigations, fingerprints take the form of physical evidence as the residue left by the fingermark can preserve exogenous compounds such as drugs, explosives, and chemicals. Additionally, the physical properties of fingerprints are utilized in identifying the perpetrator of a crime, given that the details of the ridges generate a unique pattern [3].
Frequently, fingerprints found are latent, meaning they are invisible to the naked eye, necessitating the use of specific methods for their visualization [4]. Various methods for the revelation of latent fingerprints (LFP) are employed, including optical (ultraviolet laser), chemical (fuming with cyanoacrylate), and physical (spraying and powder deposition) [5]. The technique for revealing latent fingerprints using either conventional or unconventional powders is contingent upon the physicochemical properties of their components and the particle size that constitutes them.
The use of powder to develop fingerprints is a reliable and well-known procedure. However, currently available powders still have some limitations, including toxicity, low contrast on the surface, and poor adherence to latent fingerprints [6]. This prompts the exploration of the use of natural sources, such as flowers, seeds, plants, algae, roots, etc. Vadivel et al. (2021) reviewed several studies using commonly available products (i.e., cosmetics, food powders, and plant materials) and categorized the scenarios in which these materials had positive and negative results. Turmeric powder, custard powder, gram flour, and durian powder are some successful examples with high efficiency according to the authors [7].
In one of these reviewed papers, Garg, Kumari, and Kaur (2011) focused on saffron powder to reveal latent sebaceous fingerprints on nine different surfaces (plain paper, bond paper, thermal paper, aluminum foil, transparency sheet, wood [bright solar mica], plastic sheet, painted steel, top, and the writing surface of a CD). Remarkably, saffron powder showed superior results on contrasting surfaces compared to other examined materials. An interesting aspect to highlight is that latent fingerprints, after being revealed with saffron powder on the compact disc’s writing surface, did not damage the data contained therein, making them usable for further analysis. This last point proves to be particularly relevant in forensic investigations [8].
Fuller’s earth (Multani Mitti) powder, a particular clay from India, is known for its availability, low toxicity, low-priced material, usages for adsorbing different compounds, and more recently, good performance in helping to identify latent fingerprints on surfaces like glass, steel, wood and plastic [9]. Marine biomasses, such as Lessonia searlesiana and Spirulina sp., have also been reported in the literature as yielding satisfactory results in developing natural and sebaceous latent fingerprints on a glass surface which could be a feasible bio-renewable source due to its easy obtainability [10].
Another successful study in the application of non-toxic natural compounds was conducted by Ferreira, Okuma, and Costa in 2023. In this research, powders of non-toxic natural products, such as red beet, hibiscus, Spirulina algae, indigo carmine, and tartrazine, were employed in the development of latent fingerprints deposited on varied materials with non-porous surfaces, including rough Formica, varnished wood, raw metal, galvanized metal, and glass. The powders of Spirulina algae, indigo carmine, and tartrazine demonstrated superior development of latent fingerprints due to their finer texture [11]. Hence, it is crucial to assess and investigate novel avenues for enhancing fingerprints, aiming to identify materials with minimal or no toxicity, superior surface contrast, and enhanced adhesion to latent fingerprints.
With that in mind, carotenoid-based composites could be a viable alternative for papilloscopy applications, as they typically exhibit high contrast and low toxicity. Carotenoids comprise more than 700 compounds, are lipophilic, and generally exhibit yellow, orange, or red colors [12]. Compounds resulting from the cleavage of carotenoids are commonly called apocarotenoids, representing carotenoids in which the carbon skeleton has been shortened by removing fragments from one end or both ends. Also, apocarotenoids are attracting attention due to their potential contributions to positive health promotion actions [13].
One example of these apocarotenoids is bixin, a liposoluble compound extracted from the seeds of annatto (Bixa orellana L.), a shrub native to tropical countries in the Americas. However, its cultivation has now expanded to the Caribbean, Africa, and Asia. The pigment extracted from its seeds is commercially referred to as annatto, typically used as a spice and dye, providing a yellow-orange hue to various food products [14,15]. Besides that, it is approved as a colorant and additive by the Food and Drug Administration (FDA) and can be extracted using one or a suitable combination of food-grade solvents, such as acetone, ethylene dichloride, hexane, isopropyl alcohol, methyl alcohol, methylene chloride, and trichloroethylene [16,17]. Additionally, it exhibits various beneficial health properties, including antioxidant effects [18], antimicrobial activity [19], potential anticancer properties [20], anti-inflammatory properties [21], and nephroprotective activity [22].
Bixin (C25H30O4) constitutes 80% of the annatto seed extract and is composed of an open chain of 25 carbons with a methyl ester and a carboxylic group at the end [17]. To analyze annatto, specifically bixin, various detection techniques are used, including ultraviolet (UV), and mass spectrometry (MS), in conjunction with liquid chromatography (LC) [23]. The identification of carotenoids is commonly performed using UV-Vis detectors to easily identify functional groups [24].
Furthermore, according to Scotter (2009), the isomerization of bixin for commercial purposes is achieved by heating a suspension of the cis isomer in oil at 130 °C under vacuum, and as noted by Curi-Borda et al. (2021) [25], dimerization and thermal degradation of solid bixin occur above 70 °C. This thermal stability makes the molecule suitable for applications in the field of papilloscopy. In addition to this characteristic, it is noteworthy that bixin does not exhibit toxicity, genotoxic, teratogenic, or mutagenic effects, as evidenced by previous studies [26]. This arouses interest in the development of composites based on natural products.
As described above, a potential alternative would be the use of bixin-based composites for application in papilloscopy, adopting an innovative approach, considering the lack of literature records regarding the use of bixin in this context. The choice to use bixin extracted from annatto seeds is due to the high cost associated with the standard Sigma-Aldrich bixin. This decision is supported by the fact that these seeds can be obtained at a more affordable cost, and in addition to the low cost of extracting bixin, it can be a low-cost alternative to the commercially available powders.
In the realm of developing new materials for fingerprint development, the combination of organic and inorganic compounds offers an effective affinity for substances commonly found in fingerprints, as highlighted by Da Rosa et al. (2023). ZnCO3, for example, is a natural crystal with high chemical stability in the air and is notable for its wide applications in optical, physical, and chemical fields [27]. It is known for having small particles, good adhesion, and the ability to reveal clear fingerprints on dark-colored items [1]. ZnCO3 has been widely used for fingerprint development as it allows the development of latent fingerprints on a wide variety of wet and non-porous surfaces [28,29,30]. Rohatgi et al. (2015) developed a formulation containing a ZnCO3 suspension, crystal violet dye, and a commercial liquid detergent, and the results obtained were satisfactory [31]. The formulation was able to reveal clear, sharp, and detailed fingerprints on non-porous items and can be applied on both wet and dry surfaces. Similarly, formulations containing ZnCO3, commercial liquid detergent, water, and eosin Y produced positive results under destructive conditions, where the successful development of latent fingerprints was observed on smooth surfaces exposed to high temperatures of 200 °C and water [32]. Thus, even under destructive crime scene conditions, zinc carbonate is a suitable reagent for developing latent marks.
Kaolinite is a natural, environmentally friendly, low-cost and one of the most abundant minerals in terms of soils and sediments [33,34]. Its structure and composition are based on Al, Si, O, and (OH)−, structured in a 1:1 clay layer, comprising one layer of Al octahedra and another layer of Si tetrahedra [35]. In addition to that, it is a non-explored inorganic material which can be promising to reduce the concentration of ZnCO3-based formulations. It is a mineral clay used as an adhesive polymer due to its intrinsic properties, such as chemical and thermal stability, easy processing, abundant resources, and low cost. However, it lacks intercalated charge-balancing cations due to little or no isomorphic substitution in tetrahedral and octahedral sheets. Thus, adjacent layers are bound by hydrogen bonds, making the direct intercalation of kaolinite with inorganic and/or organic molecules much more challenging than other clays, such as expansive clays. Additionally, it is also challenging to homogenize kaolinite surfaces, leading to low dispersion in polymeric matrices [36]. Despite kaolinite presenting interesting properties for application in papilloscopy, there are no reports in the literature on its use in this regard.
The objectives of the experiments conducted for this study were to extract and characterize bixin using Ultraviolet-visible spectroscopy (UV-Vis) and High-performance Liquid Chromatography (HPLC) and to create two different composites based on bixin, using kaolinite and ZnCO3 as additives. After that, tests were conducted on glass surfaces using the bixin/kaolinite and bixin/ZnCO3 composites as latent fingerprint developers.
2. Materials and Methods
Bixin (9-cis-6,6′-Diapo-ψ,ψ-carotenedioic acid 6-methyl ester—purity ≥ 90.0% (HPLC)) and kaolinite® were purchased from Sigma-Aldrich (St. Louis, MI, USA). Bixa orellana seeds were purchased at a local market in Pelotas, Rio Grande do Sul—Brazil. Methanol was purchased from J.K.Baker SOLUSORB® (San Pedro Xalostoc, Mexico City, Mexico—HPLC ≥ 99.96%). ZnCO3 and formic acid were of analytical grade from commercial sources.
2.1. Extraction of Bixin
The extraction methodology was adapted from the literature [37]. Bixin was extracted from whole annatto seeds using a reflux system at 60 °C and a seed-to-solvent ratio (methanol) of 1:15 (w/v), under magnetic stirring for 30 min. The extracted product was filtered, followed by solvent evaporation under vacuum, and dried in an oven at 50 °C. Figure 1 shows the raw annatto seed and the extracted bixin.

Figure 1.
Picture of annatto seeds (left) and extracted dried bixin (right).
2.2. Preparation of Bixin Composites
After preliminary optimization, two composites were prepared, bixin/ZnCO3 and bixin/kaolinite, using a 70:30 (w/w) ratio. To obtain the formulations, powders were macerated using a glass mortar and pistil, and a homogeneous powder was obtained.
2.3. Characterization
2.3.1. Ultraviolet-Visible Spectroscopy
Ultraviolet-visible spectroscopy (UV-Vis) of standard and extracted bixin was performed in an LGS53 instrument (Monza, Italy—BEL Engineering), with diluted samples in methanol at a concentration of 100 ppm (w/v).
2.3.2. High-performance Liquid Chromatography (HPLC) Analyses
The chromatographic analyses were performed using a Thermo Scientific Ultimate 3000 model (Waltham, MA, USA) with a standard auto-injector and UV-Vis detector. The separation of analytes was carried out on a C18 column (Ascentis, Bellevue, WA, USA) with a particle size of 5 μM, a pore size of 90 Å, and 15 cm × 4.6 mm in size. The method employed was adapted from the literature [38], where the isocratic mode (A:B 5:95) was used with a mobile phase consisting of water–formic acid (98:2, v/v) (solvent A) and methanol–formic acid (98:2, v/v) (solvent B). The analysis was performed at a temperature of 29 °C, with an injection volume of 20 µL and a flow rate of 0.9 mL/min. The detection wavelength was 459 nm. Solutions were prepared for spectrophotometric measurements in 10 mL volumetric flasks with methanol solvent at a concentration of 10 ppm (w/v), filtered with a PTPF filter with a pore size of 0.45 µm, and introduced into the HPLC. The extracted bixin peak was compared with the reference peak of a bixin standard.
2.4. Fingermark Deposition
Fingerprints revelation was performed using a powder technique, in which specific brushes (132LBW and CFB100, acquired from Sirchie®, Youngsville, LA, USA) were used. For preliminary tests, glass slides were used to deposit fingerprints from four random donors. The method by Pacheco et al. (2021), was used for the deposition of natural and sebaceous fingerprints. To simulate a natural fingerprint deposition, participants washed their hands using neutral soap and water, subsequently engaging in their routine activities for 30 min. For a sebaceous deposition, the donor rubbed their thumb on facial areas, including the forehead and nose, followed by fingertip rubbing to enhance the print with oily components, due to the lack of sebaceous glands on hands [39]. During the fingerprint placement, the pressure applied was subjectively firm (exerting medium pressure), contact time was between 3 and 5 s, and fingerprints were revealed using commercial and composite powders after 24 h. For comparison, the same fingerprint was deposited on two glass surfaces, with the left half of the fingerprint revealed using the prepared composites and the right half using the White® fingerprint powder acquired from Sirchie (Youngsville, LA, USA). Subsequently, the fingerprints revealed were photographed with a Canon EOS Rebel T6 18MP digital camera.
3. Results
In Figure 2, the Ultraviolet-visible spectroscopy (UV-Vis) absorbance spectrum is shown for standard and extracted bixin, both dissolved in methanol. The absorption of extracted bixin reached peaks at 429, 453, and 481 nm. Meanwhile, the standard bixin had maximum absorption peaks at 429, 457, and 487 nm. Given that UV-Vis analysis is not confirmatory, we sought to reinforce the information obtained through this technique by performing High-performance Liquid Chromatography (HPLC).
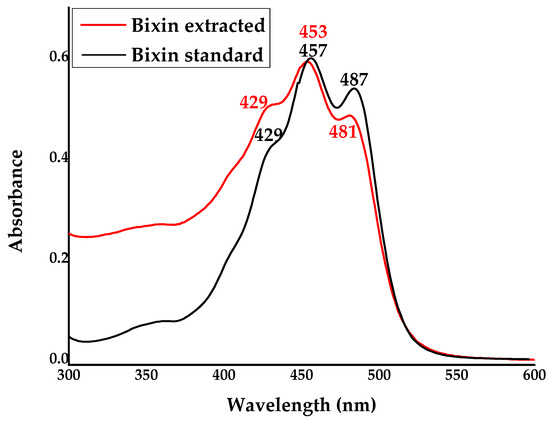
Figure 2.
UV–vis absorption spectra of standard and extracted bixin in MeOH at a concentration of 100 ppm.
The HPLC chromatograms of standard bixin (a) and extracted bixin (b) are represented in Figure 3 in which peaks at retention times of 7.07 (1) and 8.04 (2) minutes, were observed in both samples.
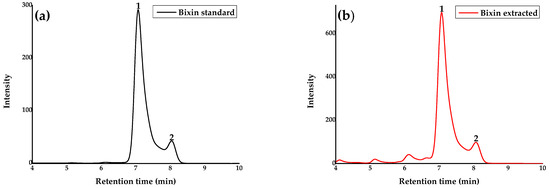
Figure 3.
Chromatograms of standard bixin (a) and extracted bixin (b) at 10 ppm. Peak 1 is from cis-bixin and peak 2 is from its corresponding isomer. Conditions: column C18 15 cm × 4.6 mm, 5 μM, mobile phase of water–formic acid (98:2, v/v) (solvent A), and methanol–formic acid (98:2, v/v) (solvent B) isocratic elution at 0.9 mL/min 29 °C, were monitored at λ = 459 nm.
The bixin/kaolinite and bixin/ZnCO3 composites were then applied to develop fingerprints by the physical spray method on glass substrates. Figure 4 shows the results obtained for natural and sebaceous fingerprints from a donor. In Supplementary Figures S1 and S2, it is possible to observe all fingerprints obtained for the natural and sebaceous fingerprints from the four donors.

Figure 4.
Natural (N) and sebaceous (S) latent fingerprints deposited onto glass surfaces developed using bixin/kaolinite and bixin/ZnCO3.
Figure 5 demonstrates the same fingerprint (natural and sebaceous) developed on the left with the composites and on the right with the commercial white powder. In Supplementary Figures S3 and S4, it is possible to observe the development obtained for the same natural and sebaceous fingerprints from the four donors, with the composites and the commercial powder.
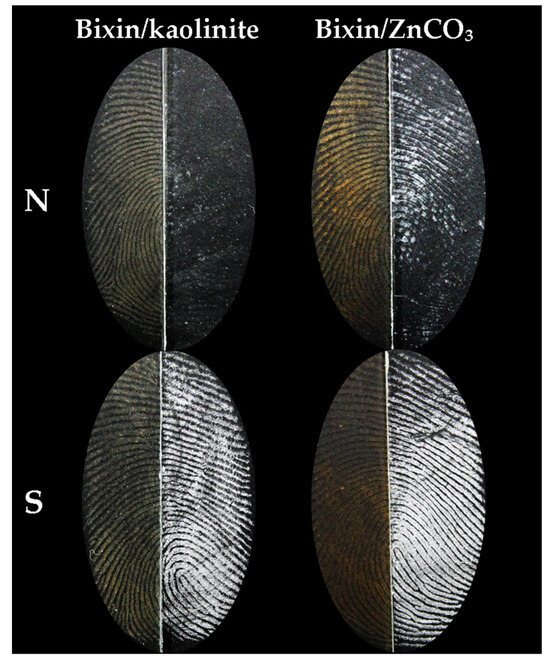
Figure 5.
Fingerprint (Natural (N) and sebaceous (S)) sectioned and revealed the left half with composites and the right half with White® commercial powder purchased from Sirchie.
Finally, a detailed evaluation of the natural fingerprint developed with the bixin/kaolinite composite is shown in Figure 6, showing the predominance of a left loop fingerprint pattern and some characteristic regions of pores, ridge ending, eye, lake, dot, bifurcation, and double bifurcation.
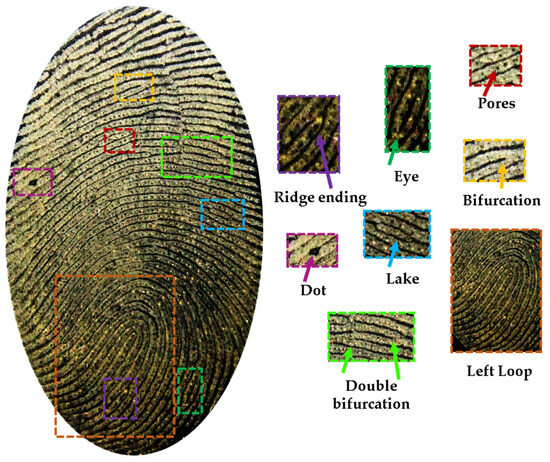
Figure 6.
In detail, the fingermark developed with composite bixin/kaolinite and its minutiae identification.
4. Discussion
Spectrophotometric analysis in the visible spectrum provided crucial information about the compounds extracted from annatto seeds. In Ultraviolet-visible spectroscopy (UV-Vis), carotenoids typically exhibit three peaks of maximum absorption. According to Saini et al. (2022) [40], the UV/Visible wavelength spectrum for carotenoids generally exhibits three peaks of maximum absorption (nm), where the first is only an inflection point, the second is located at approximately 452 nm, and the third is close to 476 nm. It is reported that the apocarotenoid bixin displays absorption bands above 400 nm due to an electronic dipole transition of the molecule [41]. The energy required to induce the transition is relatively low, corresponding to an absorption in the visible region, attributed to the peaks above 400 nm obtained in the spectrum. The higher intensity of bands above 400 nm is characteristic of bixin [41,42]. Thus, the literature data align with the experimentally obtained results, where the extracted bixin showed peaks with maximum absorption at 429, 453, and 481 nm, similarly to the standard bixin, which exhibited peaks at 429, 457, and 487 nm, indicating a successful extraction. Please refer to Figure 2.
The chromatogram in Figure 3a represents the standard bixin, which has a purity level ≥ 90.0%, and it was possible to observe two peaks. Several reports in the literature indicate that the use of a High-performance Liquid Chromatography (HPLC) system in isocratic mode may result in the lack of separation of cis and trans structures of bixin [43]. In light of this, it is possible to consider the hypothesis that peak 1 represents cis-bixin, as described in the packaging provided by the Sigma standard, while peak 2 would be its corresponding isomer. The chromatogram of the extracted bixin (Figure 3b) corroborated the results obtained in the standard sample, presenting the same chromatographic profile, and thereby confirming the effectiveness of the bixin extraction process from annatto seeds.
To assess the influence that the donor can have on the development of latent fingerprints, Supplementary Figures S1 and S2 display images of natural and sebaceous fingerprints from four different donors, revealed using bixin/kaolinite and bixin/ZnCO3 composites. The donor’s impact may vary depending on factors such as sweat rate, quantity and composition of secretions, age, health status, mental stress, biological sex, and ethnicity, among others [44]. Moreover, residues from fingerprints constitute a complex mixture of different water-soluble and lipid-soluble compounds. Because of that, natural and sebaceous fingerprints were developed to assess the interaction of the composites with aqueous or lipidic residues from fingerprints [39].
As a result, it was possible to observe that both composites were able to provide images of natural and sebaceous fingerprint marks for all donors (Supplementary Figures S1 and S2). The contrast difference between the composites is noticeable, with the bixin/ZnCO3 composite standing out. Both powders used to formulate the composites effectively enhanced the adhesion of bixin, a key characteristic to be improved. This is particularly significant as bixin already exhibits intense coloration, i.e., high contrast, making it well-suited for application in the field of papilloscopy.
In general, when comparing natural and sebaceous fingerprints, a more significant enhancement in the development of natural fingerprints is observed with the use of composites. However, the results do not differ substantially for sebaceous fingerprints. For the bixin/kaolinite composite (Supplementary Figure S1), no significant differences were identified in natural fingerprints among the four donors. Regarding sebaceous fingerprints, clearer visualization was noted in donors 1, 3, and 4, with donor 3 exhibiting slightly lower contrast, but not compromising the visibility of characteristic points. Similar patterns were observed in the results of sebaceous fingerprints developed with the bixin/ZnCO3 composite (Supplementary Figure S2), highlighting a better development in the fingerprints of donors 1, 3, and 4. On the other hand, natural fingerprints showed a more noticeable improvement in donors 1, 2, and 3, with some fewer sharp areas in the case of donor 4. In this way, the donor’s influence in the fingerprint development process became evident, considering that the same deposition method was uniformly applied to all. Despite individual variations, consistent and satisfactory results were observed, enabling the identification of all analyzed fingerprints.
Furthermore, Supplementary Figures S3 and S4 demonstrate that the prepared composites were able to develop natural and sebaceous fingerprints similarly or even better than what is currently found in terms of image quality. As evidenced in Supplementary Figure S3, a notable improvement in the development of natural fingerprints was observed when using the bixin/kaolinite composite for donors 2 and 3. Donors 1 and 4, on the other hand, exhibited a development comparable to that obtained with the commercial powder. Regarding sebaceous fingerprints, a similar development pattern was observed for all four donors. Similar results were observed in natural fingerprints revealed by the bixin/ZnCO3 composite (Supplementary Figure S4), indicating a significant improvement for donors 2 and 3. In contrast, donors 1 and 4 showed a development comparable to that obtained with the commercial powder. Concerning sebaceous fingerprints, it was noted that the bixin/ZnCO3 composite provided a more pronounced contrast for donors 1 and 4 while showing a lower contrast for donors 2 and 3, compared to the employed commercial powder.
Finally, a thorough assessment of a natural fingerprint revealed using the bixin/kaolinite composite was conducted (Figure 6). In this regard, biometric fingerprint recognition involves three levels of evaluation. The first level focuses on the macro details of the fingerprint, encompassing the four fundamental types: left loop, right loop, arch, and whorl, of which the first one can be seen in Figure 6. The second level involves minutiae, where ridge ending, eye, lake, dot, bifurcation, and double bifurcation are also evidenced in the figure. Lastly, the third level incorporates all dimensional attributes of ridges, including width, shape, contour, sweat pores, scars, folds, and other permanent details. In the fingerprint presented here, the pores positioned in unique ways with varying shapes, quantities, and dimensions from person to person can be particularly crucial at this level and play a critical role in automatic fingerprint recognition methods [45].
5. Conclusions
This study proposed the extraction of bixin from annatto seeds, employing techniques such as Ultraviolet-visible spectroscopy (UV-Vis) and High-performance Liquid Chromatography (HPLC) for characterization. With the successful extraction, a simple methodology was developed to obtain bixin, a natural compound yet unexplored in the field of papilloscopy. The choice of bixin was motivated by its remarkable contrast, strategically combined with kaolinite and zinc carbonate, providing essential adherence, and making it a highly effective revealing powder.
Bixin/kaolinite and bixin/ZnCO3 were particularly notable for their exceptional sharpness and adherence to dermal ridges, which greatly enhanced the fingerprints’ ability to be identified. The advancement made by the compounds is remarkable because it makes it possible to see pores in fingerprints from a variety of donors, going beyond different nuances and basic fingerprint types.
The materials developed in this study emerge as promising alternatives, noted for their simplicity, sensitivity, and effectiveness in revealing crucial details, thus opening new perspectives and opportunities in papilloscopy. This progress promotes an innovative and efficient approach to fingerprint identification and analysis, while aligning with the principles of green chemistry, supporting the exploration of eco-friendly alternatives to toxic fingerprint developers. Additionally, this development demonstrates the promise and advancement of research, particularly in forensic fingerprint analysis, as the evaluated compounds show potential to enhance fingerprint revelation techniques.
Lastly, it is important to highlight that before the commercial use of this revealing powder formulation, further work is required to analyze the thermal and over-time-stability, ensuring its shelf life, and techno-economic assessment, to evaluate the commercial viability of these materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/analytica5010007/s1, Figure S1: Natural (N) and sebaceous (S) latent fingerprints deposited onto glass surfaces developed using bixin/kaolinite. Figure S2: Natural (N) and sebaceous (S) latent fingerprints deposited onto glass surfaces developed using bixin/ZnCO3. Figure S3: Digital mark (natural (N) and sebaceous (S)) sectioned and revealed left half with composite bixin/kaolinite and right half with White® commercial powder purchased from Sirchie. Figure S4: Digital mark (natural (N) and sebaceous (S)) sectioned and revealed left half with composite bixin/ZnCO3 and right half with White® commercial powder purchased from Sirchie.
Author Contributions
D.T.B.: Conceptualization, investigation, and writing—original draft. A.F.L.: Investigation, data curation, and writing—review and editing. R.L.C.: Investigation, data curation, and writing—review and editing. C.J.-A.: Investigation, data curation, and writing—review and editing. E.G.B.: Investigation and writing—review and editing. J.P.d.S.: Methodology and investigation. G.Q.S.: Methodology and investigation. K.d.C.M.: Validation and funding acquisition. C.M.P.d.P.: Resources, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this research by FAPERGS (Research Support Foundation of the Rio Grande do Sul State 22/2551-0000840-2), Coordination for Improvement of Higher-Level Personnel (CAPES), and the Forensic National Institute of Science and Technology Grant number (465450/2014-8).
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding author.
Acknowledgments
The authors are thankful to FAPERGS, CAPES, Forensic National Institute of Science and Technology, and the Brazilian Federal Police for their assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barros, H.L.; Tavares, L.; Stefani, V. Dye-doped starch microparticles as a novel fluorescent agent for the visualization of latent fingermarks on porous and non-porous substrates. Forensic Chem. 2020, 20, 100264. [Google Scholar] [CrossRef]
- Khare, V.; Singla, A. A review on the advancements in chemical examination of composition of latent fingerprint residues. Egypt. J. Forensic Sci. 2022, 12, 6. [Google Scholar] [CrossRef]
- Robson, R.; Ginige, T.; Mansour, S.; Khan, I.; Assi, S. Analysis of fingermark constituents: A systematic review of quantitative studies. Chem. Pap. 2022, 76, 4645–4667. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Chu, J.; Xi, P.; Wang, C.; Liu, R.; Wang, X.; Cheng, B. Dual-mode luminescent multilayer core-shell UCNPs@SiO2@TEuTbB nanospheres for high-level anti-counterfeiting and recognition of latent fingerprints. Appl. Surf. Sci. 2022, 581, 152395. [Google Scholar] [CrossRef]
- Ansari, A.A.; Aldajani, K.M.; Alhazaa, A.N.; Albrithen, H.A. Recent progress of fluorescent materials for fingermarks detection in forensic science and anti-counterfeiting. Coord. Chem. Rev. 2022, 462, 214523. [Google Scholar] [CrossRef]
- Barros, H.L.; Stefani, V. Micro-structured fluorescent powders for detecting latent fingerprints on different types of surfaces. J. Photochem. Photobiol. A Chem. 2019, 368, 137–146. [Google Scholar] [CrossRef]
- Vadivel, R.; Nirmala, M.; Anbukumaran, K. Commonly available, everyday materials as non-conventional powders for the visualization of latent fingerprints. Forensic Chem. 2021, 24, 100339. [Google Scholar] [CrossRef]
- Garg, R.K.; Kumari, H.; Kaur, R. A new technique for visualization of latent fingerprints on various surfaces using powder from turmeric: A rhizomatous herbaceous plant (Curcuma longa). Egypt. J. Forensic Sci. 2011, 1, 53–57. [Google Scholar] [CrossRef]
- Thakur, P.; Garg, R.K. New developing reagent for latent fingermark visualization: Fuller’s earth (Multani Mitti). Egypt. J. Forensic Sci. 2016, 6, 449–458. [Google Scholar] [CrossRef]
- Passos, L.F.; Berneira, L.M.; Poletti, T.; Mariotti, K.d.C.; Carreño, N.L.V.; Hartwig, C.A.; Pereira, C.M.P. Evaluation and characterization of algal biomass applied to the development of fingermarks on glass surfaces. Aust. J. Forensic Sci. 2021, 53, 337–346. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Okuma, A.A.; Costa, L.M. Evaluation of alternative powders for Forensic Papilloscopy. Rev. Bras. Crim. 2023, 12, 129–136. [Google Scholar] [CrossRef]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. J. Chem. 2016, 2016, 3164312. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef]
- Enayati, A.; Rezaei, A.; Falsafi, S.R.; Rostamabadi, H.; Malekjani, N.; Akhavan-Mahdavi, S.; Kharazmi, M.S.; Jafari, S.M. Bixin-loaded colloidal nanodelivery systems, techniques and applications. Food Chem. 2023, 412, 135479. [Google Scholar] [CrossRef]
- Shadisvaaran, S.; Chin, K.-Y.; Mohd-Said, S.; Leong, X.-F. Therapeutic potential of bixin on inflammation: A mini review. Front. Nutr. 2023, 10, 1209248. [Google Scholar] [CrossRef]
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=73.30&SearchTerm=annatto (accessed on 1 February 2024).
- Ashraf, A.; Ijaz, M.U.; Muzammil, S.; Nazir, M.M.; Zafar, S.; Zihad, S.N.K.; Uddin, S.J.; Hasnain, S.; Nayak, A.K. The role of bixin as antioxidant, anti-inflammatory, anticancer, and skin protecting natural product extracted from Bixa orellana L. Fitoterapia 2023, 169, 105612. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.R.; A Maioli, M.; Medeiros, H.C.; Guelfi, M.; Pereira, F.T.; E Mingatto, F. Protective effect of bixin on carbon tetrachloride-induced hepatotoxicity in rats. Biol. Res. 2014, 47, 49. [Google Scholar] [CrossRef] [PubMed]
- Handayani, I.; Haryanti, P.; Sulistyo, S. Color and antibacterial activity of annatto extracts at various pH of distilled. Food Res. 2021, 5, 247–253. [Google Scholar] [CrossRef]
- Dos Santos, G.C.; Mendonça, L.M.; Antonucci, G.A.; Dos Santos, A.C.; Antunes, L.M.G.; Bianchi, M.D.L.P. Protective effect of bixin on cisplatin-induced genotoxicity in PC12 cells. Food Chem. Toxicol. 2012, 50, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.D.G.; Gasparin, A.T.; Jesus, C.H.A.; Sotomaior, B.B.; Ventura, A.C.S.S.B.; Redivo, D.D.B.; Cabrini, D.D.A.; Dias, J.D.F.G.; Miguel, M.D.; Miguel, O.G.; et al. Antinociceptive and Anti-Inflammatory Effects of Bixin, a Carotenoid Extracted from the Seeds of Bixa orellana. Planta Medica 2019, 85, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Y.; Wei, S.; Chen, L.; Xue, L.; Tian, H.; Tao, S. Bixin Protects Against Kidney Interstitial Fibrosis Through Promoting STAT6 Degradation. Front. Cell Dev. Biol. 2020, 8, 576988. [Google Scholar] [CrossRef] [PubMed]
- Noppe, H.; Abuin Martinez, S.; Verheyden, K.; Van Loco, J.; Companyó Beltran, R.; De Brabander, H.F. Determination of bixin and norbixin in meat using liquid chromatography and photodiode array detection. Food Addit. Contam. 2009, 26, 17–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zia-Ul-Haq, M.; Dewanjee, S.; Riaz, M.; Author, A. Carotenoids: Structure and Function in the Human Body; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Curi-Borda, C.K.; Tannira, V.; Gentile, N.; Alvarado, J.-A.; Bergenståhl, B. Colloids and Surfaces A: Physicochemical and Engineering Aspects Model for measuring light stability of photolabile substances in powder beds using spray dried bixin microcapsules. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127131. [Google Scholar] [CrossRef]
- De Oliveira Júnior, R.G.; Bonnet, A.; Braconnier, E.; Groult, H.; Prunier, G.; Beaugeard, L.; Grougnet, R.; da Silva Almeida, J.R.G.; Ferraz, C.A.A.; Picot, L. Bixin, an apocarotenoid isolated from Bixa orellana L., sensitizes human melanoma cells to dacarbazine-induced apoptosis through ROS-mediated cytotoxicity. Food Chem. Toxicol. 2019, 125, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Bai, J.; Li, Z.; Meng, Y.; Liu, K.; Li, L. Crystal growth, structure and thermal properties of anhydrous zinc carbonate (ZnCO3). J. Alloy. Compd. 2022, 898, 162916. [Google Scholar] [CrossRef]
- Kapoor, S.; Gurvinder, S.S.; Sanjiv, K. Visualization of Latent Fingermarks using Rhodamine B: A New Method. Int. J. Forensic Sci. Pathol. 2015, 3, 199–201. [Google Scholar] [CrossRef]
- Pattarith, K.; Benchawattananon, R. The Novel Photoluminescence Powder Synthesized from Zinc Carbonate Nanoparticles Associated with Fluorescein Dye For Its Latent Fingerprint Detection. New Innov. Chem. Biochem. 2021, 36, 14–25. [Google Scholar] [CrossRef]
- Bumbrah, G.S. Small particle reagent (SPR) method for detection of latent fingermarks: A review. Egypt. J. Forensic Sci. 2016, 6, 328–332. [Google Scholar] [CrossRef]
- Rohatgi, R.; Sodhi, G.; Kapoor, A. Small particle reagent based on crystal violet dye for developing latent fingerprints on non-porous wet surfaces. Egypt. J. Forensic Sci. 2015, 5, 162–165. [Google Scholar] [CrossRef]
- Dhall, J.K.; Kapoor, A. Development of latent prints exposed to destructive crime scene conditions using wet powder suspensions. Egypt. J. Forensic Sci. 2016, 6, 396–404. [Google Scholar] [CrossRef]
- Miranda-Trevino, J.C.; Coles, C.A. Kaolinite properties, structure and influence of metal retention on pH. Appl. Clay Sci. 2003, 23, 133–139. [Google Scholar] [CrossRef]
- Yang, H.; Tong, D.; Dong, Y.; Ren, L.; Fang, K.; Zhou, C.; Yu, W. Kaolinite: A natural and stable catalyst for depolymerization of cellulose to reducing sugars in water. Appl. Clay Sci. 2020, 188, 105512. [Google Scholar] [CrossRef]
- Bauer, A.; Velde, B.; Berger, G. Kaolinite transformation in high molar KOH solutions. Appl. Geochem. 1998, 13, 619–629. [Google Scholar] [CrossRef]
- Su, L.; Zeng, X.; He, H.; Tao, Q.; Komarneni, S. Preparation of functionalized kaolinite/epoxy resin nanocomposites with enhanced thermal properties. Appl. Clay Sci. 2017, 148, 103–108. [Google Scholar] [CrossRef]
- Miguel, K.B.; Cardoso, V.L.; Reis, M.H.M. Concentration of Pigments and Health-Promoting Bioactive Compounds from Annatto Seeds by Ultrafiltration. Waste Biomass-Valorization 2024, 15, 127–137. [Google Scholar] [CrossRef]
- Chisté, R.C.; Yamashita, F.; Gozzo, F.C.; Mercadante, A.Z. Simultaneous extraction and analysis by high performance liquid chromatography coupled to diode array and mass spectrometric detectors of bixin and phenolic compounds from annatto seeds. J. Chromatogr. A 2011, 1218, 57–63. [Google Scholar] [CrossRef]
- Pacheco, B.S.; Da Silva, C.C.; Da Rosa, B.N.; Mariotti, K.C.; Nicolodi, C.; Poletti, T.; Segatto, N.V.; Collares, T.; Seixas, F.K.; Paniz, O.; et al. Monofunctional curcumin analogues: Evaluation of green and safe developers of latent fingerprints. Chem. Pap. 2021, 75, 3119–3129. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Calogero, G.; Bartolotta, A.; Di Marco, G.; Di Carlo, A.; Bonaccorso, F. Vegetable-based dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3244–3294. [Google Scholar] [CrossRef]
- Perotti, G.; Silva, F.; De Couto, R.; Lima, F.; Petrilli, H.; Leroux, F.; Ferreira, A.; Constantino, V. Intercalation of Apocarotenoids from Annatto (Bixa orellana L.) into Layered Double Hydroxides. J. Braz. Chem. Soc. 2020, 31, 2211–2223. [Google Scholar] [CrossRef]
- Scotter, M. The chemistry and analysis of annatto food colouring: A review. Food Addit. Contam. Part A 2009, 26, 1123–1145. [Google Scholar] [CrossRef]
- De Alcaraz-Fossoul, J.; Patris, C.M.; Muntaner, A.B.; Feixat, C.B.; Badia, M.G. Determination of latent fingerprint degradation patterns—A real fieldwork study. Int. J. Leg. Med. 2013, 127, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, M.D.A.; Marana, A.N. Reconhecimento Automático de Impressões Digitais Baseado em Características Biométricas de Terceiro Nível. São Paulo, Brazil, 2015. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).