Abstract
The removal of contaminants of emerging concern (CECs) occurring in wastewater effluents, such as pharmaceutically active substances (PhACs) and personal care products, pose a big research challenge since they can be a major source of pollution for water bodies and a danger to public health. The objective of this work was to perform a comprehensive monitoring of a broad set of PhACs (>130) in a wastewater treatment plant (WWTP) close to Thessaloniki (Greece), as well as to evaluate the potential of heterogeneous catalytic ozonation for the removal of CECs from wastewater through a continuous flow system. The high-resolution mass spectrometry analysis revealed the highest average concentrations for irbesartan (1817 ng/L). Antihypertensives along with antibiotics, psychiatrics, and β-blockers were found to aggravate the effluents. Removal efficiency after conventional treatment was >30%. The results from catalytic ozonation unit operation indicate that the introduction of a proper solid material that acts as catalyst can enhance the removal of CECs. A preliminary risk assessment using the risk quotient (RQ) revealed that irbesartan and telmisartan entail high acute risk. The overall results underline the urgent need to incessantly monitor PhACs and expand the toxicological studies to establish the sublethal and chronic effects on aquatic organisms.
1. Introduction
Nowadays, the vast population growth, the increase of medicine consumption (up to 7% within the last 5 years), the uncontrollable consumption of over-the-counter (OTC) drugs [1], as well as the extensive use of medicinal products for human and veterinary purpose has rendered the pharmaceutical active compounds (PhACs) as well-recognized contaminants of emerging concern (CECs) [2]. Their worldwide occurrence in waterways has reached dangerous levels for the environment and human health [3]. Wastewater treatment plants (WWTPs) comprise a key point source of PhACs in the environment, considering that 80% of the world’s wastewater is discharged untreated into the environment [4]. Therefore, PhACs find their way to the aquatic bodies either alone or in mixtures, posing a global threat to the aquatic environment and detrimentally to human health.
Wastewater as a source of data is of primordial importance owing to the unique advantage of the statistical significance, given that the obtained results capture the mean drug consumption of the whole community linked to a given WWTP [5]. Moreover, the usefulness to conduct monitoring campaigns at a regular basis is also highlighted by the scarcity or inappropriateness of ecotoxicity endpoints. According to recent studies, an array of the most environmentally relevant PhACs, some of them belonging to the 3rd Watch List (WL), are often reported at concentrations exceeding the established ecotoxicity endpoints. Such examples are the very talked-about diclofenac, and other NSAIDs such as ibuprofen, naproxen, ketoprofen, the analgesic paracetamol, broad-spectrum antibiotics (sufamethoxazole + trimethoprim), clarithromycin, etc.
Besides the advances in the wastewater treatment technology and the upgrade of conventional WWTPs, their efficiency in removing CECs remains insufficient. In essence, unit’s size, operating conditions, location, as well as climate/meteorological conditions, and finally the inherent physico-chemical properties of the compounds play a pivotal role in eliminating CECs. In addition, the potential interaction between the CECs and the solid particles can heavily affect the elimination rate. That is, contaminants with low sorption coefficients are more susceptible in remaining in the aqueous phase and have elevated mobility, so they transfer into receiving water bodies. Consequently, WWTPs not equipped with highly advanced treatment facilities cannot hinder the non-degraded CECs from passing the barriers and they finally discharge in the environment as parent compounds, metabolites, or transformation products. Although no legal limits have been established to regulate the levels of PhACs in effluents and water bodies, owing to EU-wide systematic monitoring the updated WL of the EU [6] includes eight pharmaceuticals so far, implying the importance to propagate significant improvement of sewage systems by enhancing the remediation technologies that are of primary importance to tackle with the problem. Owing to the remarkable evolution of analytical tools, such as the employment of high-resolution mass spectrometry analyzers (Orbitrap, ToF) the gap in the knowledge on the environmental occurrence of such compounds has been notably filled during the last years.
In the light of the above, there is an urgent need to implement innovative stage for purification which counterbalance the conventional treatment. Advanced treatment processes can achieve higher and more consistent CECs removal. Ozonation and Advanced Oxidation Processes (AOPs) are effective redox technologies that prevail over conventional treatments, due to their high degradation rates and non-selectivity. However, high ozonation efficiency may also present undesirable side effects, such as specific by-product formation, which can be as harmful as the original contaminants treated [7]. To further reduce parent compounds and oxidation by-products, the ozonation process can be optimized through the presence of an appropriate catalyst (catalytic ozonation), which is based on the degradation of organic compounds via the decomposition of O3 into hydroxyl radicals (•OH), which are a powerful and non-selective oxidant [8].
The objective of this study is to perform a comprehensive monitoring for a wide set of PhACs in the second largest WWTP of Thessaloniki (Northern Greece). To this end, influent and effluent concentration levels, respectively removal efficiencies were investigated, while a preliminary risk assessment was carried out to capture the potential impact of the WWTP in the non-target organisms. Specific objectives of this work were (a) the assessment of the pharmaceuticals discharge of the WWTP effluents, including PhACs from the European Union WL, (b) the evaluation of the removal efficiency toward plenty of PhACs, by applying both conventional secondary treatment and ozone disinfection, (c) the comparison of the ozonation over the catalytic ozonation employed at large-scale experiments, (d) the appraisal of the impact of the WWTP discharge on non-target organisms, by performing a risk-quotient-based risk assessment for acute and chronic toxicity study deriving from the most relevant PhACs. To reach the specific objectives of the study, monitoring of PhACs concentrations in influent wastewater and effluent wastewater after secondary treatment (before and after ozone disinfection) was performed, employing the powerful advanced analytical technique of liquid chromatography–high resolution and mass accuracy mass spectrometry for quantitative analysis, including a robust quality control procedure to ensure the reliability of the reported results.
2. Materials and Methods
2.1. Description of the “Aineias” WWTP and the Pre-Industrial Unit
The municipal WWTP “Aineias” (40.480324, 22.831869) is located in Aggelochori (Central Macedonia, Greece), approximately 35 km away from the metropolitan area of the city of Thessaloniki. The plant treats the wastewater from the touristic zone of Thessaloniki (Figure S1). Started-up in 1997 and designed to serve a population equivalent (PE) of 87,000 inhabitants, it is a conventional treatment plant which nowadays receives about 8500 m3 of influent per day. However, the sludge processing units (thickening and anaerobic digestion) was put into operation in October 2014. The plant consists of a combination of mechanical pretreatment processes, secondary biological treatment and final ozone disinfection. Preliminary treatment includes coarse screening, grit, grease, and sand removal. There are two primary and secondary sedimentation tanks; one of the primary sedimentation tanks is used as equilibration/ homogenization tank of domestic septic wastewater. The effluent from primary treatment is further treated by aerobic biological processes (“carousel”-type tank with surface aerators) after which the effluent is disinfected by ozonation and discharged into the sea (Thermaikos Gulf). The primary and the secondary sludge are thickened by gravity thickeners. After that, all sludge is treated in anaerobic digesters, where the sludge is stabilized, the sludge volume is reduced, and biogas is produced. Finally, the sludge is dewatered in belt filters and eventually it can be used as soil amendment product. The variation of BOD5, COD, SS, T-N, NH4-N, NO3-N, and T-P at the entrance and treated outflow of WWTP for the years within the current study took place, are provided in Supplementary Material (Figure S2 and Figure S3).
To investigate the application of heterogeneous catalytic ozonation for CECs removal from wastewater, a pre-industrial level unit was designed, constructed, and operated at AINEIA’s WWTP. The unit is comprised of four distinct operating sections, namely (a) post-filtration, (b) ozone dilution, (c) catalytic ozonation, and (d) biological stabilization, as presented in Figure 1. Details about the operating sections, as well as a detailed flowchart and a photo of the pre-industrial unit are provided in Supplementary Materials (Section S1, Tables S1 and S2 and Figures S4 and S5).
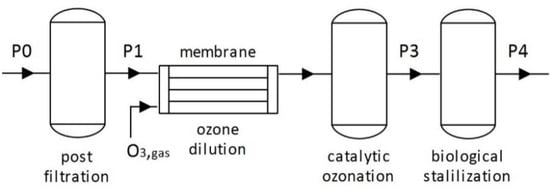
Figure 1.
Flowchart of the pre-industrial level unit.
2.2. Sampling
Composite (24 h) influent (IWW) and effluent (EWW) wastewater samples were collected during eight sampling campaigns, from 2018 to 2021. The samples were collected into 1-L amber glass bottles (pre-rinsed with deionized water) and then transferred to the laboratory in portable freezers. The influent concentrations of CECs were determined by analyzing the samples from the inlet of WWTP, while the effluent from the ones collected after the conventional disinfection (by ozonation). The measured concentrations were also used to estimate the removal efficiency of the WWTP after the secondary treatment, as well as to perform a risk assessment aiming to assess the potential impacts of the output in the receiving bodies and non-target organisms. On the other hand, for the efficacy evaluation of the catalytic ozonation, two different monitoring campaigns (February and May 2021) were carried out by collecting samples from the different treatment stages of the pre-industrial pilot unit (Table S3) applying zeolite and PET as catalysts.
2.3. Chemicals, Reagents, and Materials
Methanol (MeOH), water, and formic acid (FA) used in the present study were of LC-MS grade and were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The Oasis hydrophilic-lipophilic balance polymer (HLB, 200 mg/6 mL) cartridges used for extraction were obtained from Waters Corporation (Milford, MA, USA).
All reference standards of PhACs were purchased at high purity (>98%) from Promochem (Wesel, Germany), Fluka and Sigma-Aldrich (Steinheim, Germany) in a high purity grade (>98%) and were used to prepare initially a stock mixture (10 mg/L) in methanol, while working standards were prepared in methanol/water 90/10 (v/v) and their stability was checked monthly. The target analytes selection was based on the authors’ previous experience, environmental relevance, inclusion in WFD list(s), potential environmental hazards, national market as well as the necessity to fill the gaps in unavailable data. Detailed information on the main properties of the target compounds can be found in Table S4.
2.4. Analytical Procedure
2.4.1. Sample Pretreatment
Upon arrival, the samples were pretreated in the laboratory, and if not feasible, they were stored in the dark, in the freezer (−20 °C) for maximum one week. In brief, a protocol recently published by the authors was used [9], after slight modifications. Briefly, 100 mL influent and 250 mL effluent samples were filtered to eliminate the interfering particulate matter. Meanwhile, Oasis HLB cartridges were conditioned with 5 mL MeOH, and 5 mL water. The samples were loaded onto the cartridges at a flow rate of 2–3 mL min–1, and once the whole volume was percolated, the cartridges were vacuum-dried for 30 min. The elution was achieved by adding 7 mL of methanol; the extract was evaporated to 400 μL under a gentle stream of nitrogen at 30 °C, and 100 μL of water was added to obtain a final volume 500 μL. The final extracts were stored at <−20 °C until analysis. Prior to injection to the UHPLC-Orbitrap MS/MS system, the samples were filtered through PTFE 0.22 μm filters.
2.4.2. LC-Orbitrap MS/MS Analysis
An Orbitrap Q ExactiveTM Focus (Thermo Fisher Scientific, Bremen, Germany) equipped with an Ion Max heated electrospray ionization (HESI-II), coupled with a Dionex Ultimate 3000 system was employed for the multi-residue analysis, operating in switching ionization mode. The separation of target analytes was achieved on a Thermo Hypersil GOLD aQ column (50 × 2.1 mm, 1.9 μm within a 15-min gradient elution program [9], using water (A) and MeOH (B) as mobile phase, both acidified with 0.1% formic acid. The flow rate was stable at 200 μL, injection volume was 5 μL, and the internal autosampler temperature was set at 10 °C. Identification and confirmation of the target analytes were achieved within a single-run, by setting (a) a full-scan (FS) MS experiment (scan range 100–1000 m/z, resolution 35,000), and (b) a data-dependent MS2 experiment (confirmation mode, scan range 50–750 m/z, resolution 15,000), respectively. Stepped collision energy of 15, 30, and 50 eV was applied for fragmentation. Detailed information about LC conditions, HESI-MS parameters, and LC-HRMS data are available in the Supplementary Material (Tables S5 and S6). Xcalibur 4.1 was used for the instrument control, and Trace Finder 4.1 EFS quantification purposes.
2.4.3. Quality Assurance/Quality Control (QA/QC) and Data Treatment
The identification, confirmation, and quantification of the positive findings was based on the guidelines provided by DG SANTE 12682/2019 and ISO 17025:2017 [10,11]. The criteria for the positive identification are summarized in SM. Briefly, the criteria fulfilled to consider a positive identification were: (a) Allowed retention time (tR) drift < 0.2 min, for the peak of the adduct ion in the extracted ion chromatogram and that of the (matrix) standard; (b) mass error (Δ) for the adduct ion in the full-scan spectrum < 5 ppm; (c) presence of the isotopes’ cluster in the full-scan spectrum with a relative isotope abundance (RIA) score > 70%; (d) at least one fragment in the MS2 spectrum (Δ < 5 ppm; (e) S/N for the quantification peak ≥10. Since background noise is difficult to be traced in HRMS instruments, signals <104 were considered as noise and not further considered as positive findings, as recommended elsewhere [12]. The quantification of the target analytes was conducted by means of a matrix-matched calibration curve, prepared in the initial composition of the mobile phase. A blank sample was analyzed for every batch so that any occurring concentrations of the selected CECs be subtracted from the standards and real samples. With the view to check the instrumental drift, a five-point calibration curve was injected for every twenty samples. Moreover, two QC samples, one spiked at the lowest method calibration limit (LMCL) and upper method calibration limit (UMCL) were extracted and analyzed along with every batch. All the method performance characteristics are available in Supplementary Material (Table S7). Finally, descriptive statistics, including mean, median, minimum, and maximum values were calculated with the Microsoft® Excel. For data analysis, concentration values for not detected compounds were set MDL/2, while in cases where the concertation was between MDL and MQL the value was set at MQL/2. Box-plots and heatmaps were constructed by using Prism 9.0.0 (GraphPad Software, San Diego, CA, USA, www.graphpad.com, (accessed on 24 February 2022)).
3. Results
3.1. Overview of the Occurrence of PhACs in the WWTP
In total, 141 PhACs, belonging to 38 different therapeutic classes were included in this monitoring of which 41 PhACs and some personal care products (UV-filters, an antiseptic, an insect repellent) belonging to 27 different classes were detected at least once at levels over the method detection limit. The highest mean effluent concentrations were observed for antihypertensives (413.8 ng/L), an insect repellent (186.8 ng/L), an antifungal (88.2 ng/L), antiepileptics (53.2 ng/L), antihistamines (30.2 ng/L), and caffeine (37.4 ng/L). Mean concentrations for the rest of compounds ranged below 20 ng/L. Table 1 summarizes the results categorized by therapeutical classes, while Table S8 presents the occurrence values (ng/L) for PhACs detected in the effluents of the studied WWTP.

Table 1.
Occurrence values (ng/L) for the therapeutic classes of PhACs detected in the effluents of the studied WWTP. Concentrations (ng/L) are calculated as the minimum, maximum, and average from all sampling campaigns.
3.1.1. Caffeine (Stimulant)
Caffeine, one of the widely consumed compounds globally, is a typical stimulant that is extensively detected in the inlets of WWTPs at high levels, thus being a marker to track anthropogenic pollution [13]. This is unsurprising, considering that caffeine is one of the main ingredients of coffee, tea, beverages, chocolate as well as several OTC drugs (e.g., to accelerate the action of analgesics or dietary supplements etc.). Herein, the mean concentration of caffeine during the whole sampling campaign was calculated to be 16,745 ng/L, while the maximum observed concentration (May 2021) was found as high as 34,385 ng/L. Albeit elevated enough, this concentration is much lower than the highest observed influent concentration in Europe (150,413 ng/L), which was reported in a study carried out in UK wastewaters [14].
3.1.2. Non-Steroidal Anti-Inflammatory Drugs and Analgesics
The median municipal wastewater concentrations of analgesics in Europe have been estimated at 1707 ng/L [15]. In comparison with most of the other pharmaceutical classes, analgesics occur at highly elevated concentrations in municipal wastewater worldwide, reaching even at concentrations as high as 1,407,000 ng/L for aspirin [15]. The extremely high concentrations of analgesic in influents are not that surprising, since they are administered to alleviate the pain and inflammation that are the symptoms for a plethora of diseases. On top of that, several analgesics are available over-the-counter, while high daily dosages are allowed, reaching even a defined daily dose (DDD) of 3 g/day for acetaminophen [16]. Accordingly, acetaminophen has been quantified as high as 482,687 ng/L in Wales [17], while ibuprofen and ketoprofen have reached 603,000 ng/L and 8560 ng/L (Seville, Spain) [18].
The varying concentrations of analgesics in influents from different areas can be attributed to the population served by WWTPs, monitoring diligence, proximity of the WWTP to hospitals and pharmaceutical companies as well as different societal drug consumption patterns [19].
The pioneer of the analgesic drugs was the OTC paracetamol, with average influent and effluent concentration equal to 11,394 ng/L and 8 ng/L, respectively, whereas the maximum concentration in the influents reached even at 31,413 ng/L in February 2021. Such an incidence may be attributed to the COVID19 outburst, for which paracetamol was the drug of choice to treat fever and common flu symptoms. Interestingly, tramadol, a synthetic opioid belonging to the controlled substances administered to cope with severe pains such as operation, cancer, fracture, arthralgia, neuralgia [20], showed a mean influent concentration of 196 ng/L, reaching at 411 ng/L in February 2021. The effluent concentrations were much lower (approximately 20 ng/L). The investigation of tramadol seems to attract scientific attention as a biomarker and probably is widely used across countries [20].
As regards the NSAIDs, diclofenac was by far the predominant drug in the influents, demonstrating an average concentration of 680 ng/L, while a maximum concentration of 3409 ng/L was measured in February 2021. The elevated influent levels of diclofenac are due to its usage to alleviate pain either administered through pills, creams, or injections. This recalcitrant NSAID is the mostly detected PhAC in the aquatic environment and adversely affect aquatic organisms, causing morphological and anti-ovulatory effects among others. Remarkably, diclofenac is one of the first introduced compounds in the WL that gained a lot of attention, it is one of the most documented compounds in particular in WWTPs and is usually suspected to exert toxicity to non-target organisms. However, effluent concentrations were almost negligible, a fact that partially justifies its withdrawal from the latest WL. The other relevant NSAIDs emerged herein were naproxen, ketoprofen, and tolfenamic acid, exhibiting influent concentrations 41 ng/L, 147 ng/L, and 63 ng/L, respectively. Naproxen and ketoprofen were decreased 2–5 times in the effluents, while tolfenamic acid seems to be more recalcitrant, exhibiting a similar effluent concentration. Tolfenamic acid is a drug administered to treat acute migraine attacks, and disorders like dysmenorrhea, rheumatoid, andosteoarthritis, and it is less frequently detected in comparison with the other NSAIDs investigated in this study. In Greece, it has been reported in hospital influents at 48,586 ng/L [2].
3.1.3. Antihypertensive Drugs (β-Blockers and Sartans)
Nowadays, more and more antihypertensive drugs, such as β-blockers, and angiotensin II receptor antagonists (sartans), are being prescribed, especially in aging societies [21]. Substantial amounts of β-blockers (and their metabolites) occur in wastewater and end up in freshwaters exerting ecotoxicity to aquatic organisms that seem to be sensitive to these PhACs, used to treat hypertension and patients after heart attacks to prevent recurrences. The most commonly reported β-blockers worldwide are atenolol, propranolol, and metoprolol. In this study, atenolol showed the highest concentration in the influents (196 ng/L), followed by metoprolol (93 ng/L). Despite the fact that atenolol was frequently detected at elevated concentrations, it is detected at relatively higher concentrations. More specifically, according to a recent review [15], the global municipal wastewater influent levels of β-blockers ranged from <1 ng/L to approximately 3 μg/L (atenolol in India) [22], while slightly lower maximum concentrations are reported in Europe (33,106 ng/L) [17,22,23,24]. Regarding metoprolol, the influent concentrations ranged from 2 to 79,500 ng/L [15]. The effluent concentrations were <10 ng/L for all the β-blockers explored in this study.
Sartans, such as valsartan, irbesartan, losartan, telmisartan, etc., demonstrate related chemical structures thus their elimination, persistence, and toxicity are expected to be similar [21]. In addition, antihypertensives tend to be among the most relevant groups within monitorings. For instance, in a recent study conducted in Italian wastewaters, they were the predominant group, with valsartan on the top followed by irbesartan and losartan. Herein, both influent and effluent concentrations in this study followed the same trend: valsartan > irbesartan > telmisartan > losartan. More specifically, valsartan was measured approximately four times higher than irbesartan in the influents, while in the effluents their average concentration was almost equal. Losartan and telmisartan showed the same occurrence levels in the influents (182 to 205 ng/L), but losartan was almost eliminated in the effluents. As for valsartan highest concentration in Europe, this has been reported in Portugal (8400 ng/L) [22]. Valsartan exhibited also the highest concentrations among the total investigated compounds in raw wastewater in autumn [25]. Within the same study valsartan showed again very high concentrations in spring, while irbesartan was also highly detected. Other non-sartan antihypertensives studied herein, namely ramipril and enalapril were detected at substantially lower concentrations in the influents (mean concentrations 4 and 20 ng/L, respectively), while they were almost completely eliminated in the effluents. Limited toxicity and environmental data are available for this group of antihypertensives, therefore much more effort should be put to their monitoring with the view to exploit the data for the assessment of their potential ecotoxicity.
3.1.4. Psychiatric Drugs
Psychoactive drugs, encompassing antidepressants, antipsychotics, anticonvulsants, sedatives-hypnotics, and others are receiving widespread attention as emerging contaminants since they occur in wastewaters at high levels, sometimes even higher than antibiotics and analgesics. The average concentrations of the studied psychiatric pharmaceuticals in influent varied from <MQL (doxepine) to 1806 ng/L (carbamazepine). Regarding the sub-classes of the psychoactive drugs, the average concentration levels were antiepileptics/anticonvulsants > antidepressants > antipsychotics. Among the antiepileptics, the omnipresent carbamazepine showed the highest mean and maximum concentration in the influents (1806 ng/L and 10,112 ng/L, respectively), followed by gabapentin (682 ng/L and 3774 ng/L), pregabalin (88 ng/L and 372 ng/L), and lamotrigine (43 ng/L and 67 ng/L). The same pattern was observed for the effluent concentrations for this class. According to a large number of studies, the antiepileptic carbamazepine is very often measured at a detection frequency close to 100% in the influents [26,27,28], which is partially justified due to its DDD that reaches 1 g. As regards the antidepressants’ influent concentrations, the antidepressant venlafaxine, a neuroendocrine disruptor routinely detected in the aquatic environment and included in the 3rd WL, was found at double average concentration (60 ng/L) compared to amitriptyline that followed with 27 ng/L. Citalopram was also detected but at lower levels (13 ng/L mean concentration). In terms of WBE, the elevated occurrence of antidepressants in influents may reflect the elevated consumption of drugs belonging to this class to tackle with several psychological disorders due to the COVID-19 pandemic on a global scale in last year, owing to the depression in the general population changes in lifestyle, reduction of physical activity, self-isolation, stress, uncertainty, and unemployment with the effects of the COVID-19 pandemic. This claim is in good accordance with the fact that the maximum concentrations of antidepressants were noted during February 2021, the period of the second lockdown in Greece. On the other hand, the concentrations of all antidepressants in the effluents were close to zero, highlighting that these compounds are largely removed whereas their monitoring in the influents remain of primary importance to draw conclusions on the psychological problems of a given community. Last, the antipsychotic drug amisulpride was found at a mean concentration as high as 138 ng/L in the effluents, while it was eliminated in the effluents. The overall median concentrations of various psychoactives reported in wastewaters in Europe was 129 ng/L [15].
3.1.5. Antibiotics
Antibiotics are a particular group of medicine, accounting for about 11% of the world’s total applied medicines [29], that has gained a lot of attention during the past decades because of the development of the antibiotic resistance genes (ARGs). Their widespread occurrence is partially explained by their consumption by both humans and livestock. Data from scientific literature and national/ local surveillance systems from 71 countries over the past decade reveals that the usage of antibiotics is on an incessant worldwide (30%) [30]. In addition, their supply without prescription in many developing countries has contributed to their unproper usage. On top of that, they are not fully metabolized in humans (rate up to 75%), resulting as parent compounds in the WWTPs. In an interesting study carried out in Italy, antibiotics were the second most predominant class (after carbamazepine itself) with amoxicillin, ciprofloxacin, and clarithromycin showing concentration ranges: nd–1629 ng/L, nd–130 ng/L, 111–1034 ng/L, respectively [25]. High levels of antibiotics in wastewater evidence widespread bacterial infections. Moreover, seasonal patterns are highly associated with antibiotics; in particular, the highest concentrations in winter when antibiotics are administered to treat the increased incidences of flu that are common in colder seasons. In addition, their photodegradation may be poor during the cold period due to less sunlight.
Among the investigated antibiotics, the fluoroquinolone ciprofloxacin was measured by far at the highest mean concentration (205 ng/L) in the influents. In a recent study of Rodriguez-Mozaz et al. (2020) [30], it was found up to 1435.5 ng/L in Portugal, while the authors considered this antibiotic as a marker of antibiotic pollution, because its RQ exceeded the threshold of 0.1. Sulfamethoxazole, a common broad-spectrum sulfonamide exhibited a mean concentration of 40 ng/L in the influents, while it was almost eliminated in the effluents. The simultaneous prescription of sulfamethoxazole + trimethoprim at 5:1 for a variety of infections is not clearly reflected in the influents studied herein, although there is a similar ratio (3.3) given to trimethoprim’s influent concentration equal to 12 ng/L. On the contrary, in a study reporting on the concentrations of 53 antibiotics from seven European countries, sulfamethoxazole was detected in the final effluents, with maximal values of 583.6 ng/L in Norway and 220.9 ng/L in Cyprus, respectively [30]. Our results are in good accordance with the relevant literature according to which ciprofloxacin, sulfamethoxazole and trimethoprim, erythromycin, and tetracycline are some of the most frequently detected antibiotics in the influents at a global scale. The quinolone antibiotics were detected at concentrations norfloxacin = moxifloxacin > levofloxacin, with average concentrations 35 and 25 ng/L, respectively. In the effluents, the concentrations followed the same pattern, with norfloxacin reaching 11 ng/L, while moxifloxacin and levofloxacin were close to zero. The macrolide clarithromycin was calculated at 21 ng/L in the influents, while the glycoside antibiotic lincomycin, administered for both human and veterinary use, was found at 15 ng/L. Metronidazole, a nitroimidazole antibiotic used for a wide spectrum of skin diseases as well as for vaginal infections, was found to be 41 ng/L in the influent. Data on its occurrence in real wastewaters are scarce but it seems to be efficiently removed in effluents [29]. Among the other studied antibiotics, sulfadiazine and rifaximin were detected at negligible levels in the influents (<6 ng/L).
3.1.6. Other PhACs
Apart from the therapeutic classes discussed above, interesting findings were recorded for some individual PhACs or PhACs-related products. First, DEET (N, N-diethyl-m-toluamide), one of the most frequently detected contaminants in wastewaters used primarily as an insect repellent, demonstrated a high average concentration in the influents (563 ng/L), with a peak concentration in August 2020, probably attributed to the extended use of anti-repellent products. In addition, DEET very high concentration is associated with the fact that it is the active ingredient for many different formulations such as spray, liquid, and lotion in concentrations as high as 100% or incorporated into consumer products like clothes. It is remarkable that there are 524 DEET-containing registered products [31]. Although DEET is ubiquitously present in the environment, it is susceptible in degradation, and it is successfully eliminated during treatment.
Metformin is a widely used first-line medication around the world for the treatment of diabetes and other metabolic diseases, thus it has been found in various WBE methods.
Herein, metformin was detected at 350 ng/L in the influents, while it showed a maximum concentration at 1153 ng/L in May 2020. Higher concentrations, reaching the high μg/L scale have been reported elsewhere (1.7 μg/L to 239.0 μg/L, with an average value of 68.3 μg/L) [32]. Metformin is found to be ubiquitous [33,34,35] proving its good functionality to predict diabetes [32].
Cytostatic/antineoplastic drugs are synthetic and natural chemicals used in the cancer therapy and chemotherapy. Despite the fact that cancer diseases comprise one of the biggest problems of today’s world, significantly less attention has been paid to cytostatic agents [36]. Different studies have evidenced that cytostatics are highly stable in WWTPs due to poor degradation [37]. Among the antineoplastics included in the present study, cyclophosphamide was detected at higher levels in the effluents (42 ng/L). Similar results have been reported for cyclophosphamide that was the only cytostatic found in April 2018 in Italian wastewaters [25]. Cytarabine was found at 20 ng/L in the influents, much lower than the concentrations reported elsewhere [38,39]. In addition, the diuretic furosemide was found up to 368 ng/L in the influents (mean concentration 146 ng/L). Furosemide has been widely detected within the last years, while in a recent research concentrations up to 1.6 μg/L have been reported [40].
The lowest average concentration PhAC (<MQL-10 ng/L in the influents) levels were observed for the antivirals abacavir and acyclovir, the a1 receptor antagonist alfuzosin, the anesthetic prilocaine, the H2 receptor antagonist ranitidine, and the diuretic diltiazem. In addition, regarding the personal care products, the antiseptic-antibacterial triclosan (<5 ng/L in the influents) which has been banned since it is an endocrine disruptor, especially in the thyroid, and that its effects include antibiotic resistance [41]. Moreover, the UV filters BP1, BP2, BP3 were detected < MQL in both influents and effluents.
3.2. Removal of PhACs
Technically, the removal is a rough comparison between the average influent and effluent concentrations that can provide valuable information on the efficiency of the operating system against the CECs. Τhe removal efficiency (%) expresses the decrease in the concentration of a compound during treatment, which is technically the concentration difference between the influent and the effluent, measured according to the Equation (1).
The obtained results were plotted as Box–Whisker graphs for a better visualization of the removals. Where Cinfluent and Ceffluent are the concentrations of CECs quantified at the influent and the effluent, respectively.
Given the medium to high polarity of most pharmaceuticals, as well as the fact that some less polar ones can be absorbed onto the solid particles and remain in the sludge, the applied approach is reasonable. The calculation of the removal efficiency during the monitoring campaigns is critical, since it is statistically significant and vary from time to time. Ofrydopoulou et al. [9] grouped the investigated PhACs according to their logKow to explore if there is any correlation between the removal and polarity. Although sometimes such a correlation can be evidenced, this would be a generalization. For these reasons, herein, the removals are illustrated for the most relevant therapeutic groups, based on their concentration in the effluents (Figure 2). The lines in each box from the Box and Whisker graphs demonstrate the lower quartile (25%) and upper quartile (75%) of the determined values of each PhAC. The whiskers or lines extending from each box represent the extent of the data up to 1.5 times the interquartile range (IQR).
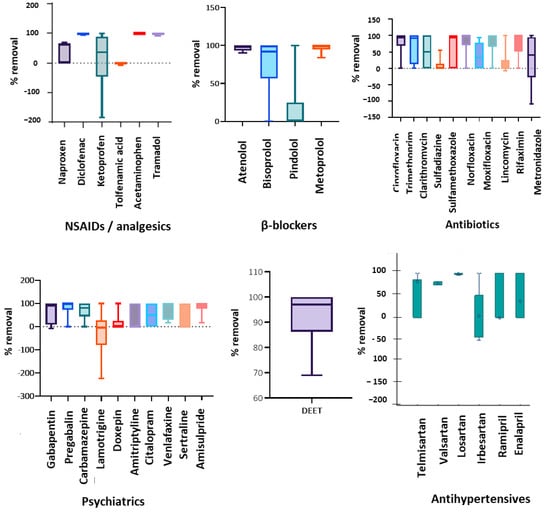
Figure 2.
Removal efficiency for the main therapeutic classes investigated in this study.
According to the results, the vast majority of the detectable PhACs are eliminated either partially or totally after the ozonation treatment. Antibiotics demonstrated various removal values, from low up to 100%, except for metronidazole that was the only antibiotic that showed negative removal as well (February 2021). Diclofenac, acetaminophen, and tramadol were among the NSAIDs/analgesics that were constantly removed close to 100%, while naproxen, and ketoprofen demonstrated discrepancies in their removal, with ketoprofen to show negative removals as well. Very good removals were also observed for the PhACs from the group of β-blockers, especially atenolol and metoprolol, followed by bisoprolol. Lower removals were observed for pindolol. Concerning the large group of psychiatrics investigated herein, most of them were efficiently removed, while lamotrigine was the most resilient to the ozonation treatment, showing strong negative removals. The ubiquitous antihypertensives, showed satisfactory removals. However, more effort should be put to enhance the elimination of these compounds since they reach extremely high concentrations in the influents, and they are not polar enough so that their removal be favored. Telmisartan was the most recalcitrant among the sartans and the other antihypertensives. In addition, the insect repellent DEET showed satisfactory removal, up to 100%. Finally, among the “notorious” PhACs occurring both at high detection frequency and high levels, caffeine and metformin demonstrated 100% removals in all sampling campaigns.
Notably, the negative removal efficiency observed for some PhACs, since the effluent concentrations are higher than in influents, is in accordance to previous literature [28,42]. Around 30% of pharmaceuticals were partially removed (50–75%), a fact that can be attributed to the employment of ozone disinfection apart from the conventional primary and secondary treatment.
3.3. Catalytic Ozonation
Τhe potential of heterogeneous catalytic ozonation for CECs removal from wastewater was evaluated at the pre-industrial level unit applying zeolite and PET as catalysts. Occurrence values for CECs detected in the effluents of the studied WWTP before and after conventional disinfection (by ozonation) and after the different treatment stages of the pre-industrial level unit are presented in Tables S9 and S10 for zeolite and PET application respectively. During the monitoring period that zeolite was used, degradation rates obtained by the application of conventional disinfection ranged between 47% and complete removal, depending on the specific contaminant, with an average value of about 90%. Experimental results from the treatment of secondary effluent in the pilot unit revealed that part of CECs was removed through filtration of residual suspended solids (about 45%), i.e., at the first treatment stage of the unit (post filtration column), while degradation carried out at catalytic ozonation column led to complete removal of all CECs studied except ciprofloxacin.
During the monitoring campaign that PET was applied, removal of CECs by conventional disinfection ranged between 31% and complete degradation, with an average value of 83%. Treatment of the secondary effluent of the same period in the pilot unit led to a removal rate of about 34% of examined CECs in the first treatment stage of the unit, while degradation carried out at catalytic ozonation column led to an average of 80%. The biological stabilization of the effluent after catalytic ozonation (final treatment stage of the unit) led to complete degradation for the majority of examined contaminants and an average removal rate of about 90%.
Nevertheless, comparison of CECs’ residual concentrations after the application of conventional (single) and catalytic ozonation indicated the enhancement of contaminants removal through ozonation by the introduction of a proper solid material, acting as catalyst. Specifically, application of zeolite led to smaller or equal residual concentrations for the 95% of examined contaminants, while for the case of PET use the respective percentage was 82%.
3.4. Environmental Risk Assessment
Several compounds detected in the effluents of the investigated WWTP are continually released into the aquatic environment entailing ecotoxicity. Exploiting the dataset of the PhACs measured in the effluents, an environmental risk assessment based on the risk quotient (RQ) approach was employed, presuming that those final effluents were discharged in freshwater systems.
The (RQ), suggested by EMEA [43] was employed to assess the environmental impact of the detected target PhACs to the three levels of the aquatic life (algae, invertebrates, fish). To this end, the ratio between the measured environmental concentration (MEC) using the worst-case scenario (maximum measured concentration for each PhAC) and the predicted non-effect concentration (PNEC) were calculated for all the three taxa. PNEC is the concentration below which a chemical will likely have no adverse effect in an ecosystem, and it is expressed as either the lowest values of LC50/EC50 found from available measured data reported in literature [2,44,45] or predicted from the ECOSAR software, and it was divided by an assessment factor (AF) of (a) 1000 for acute toxicity, (b) 100, 50, and 10 when one, two, or three non-observed effective concentrations (NOEC) from the three aquatic organisms are available, respectively, for chronic toxicity. The following equations (Equations (2)–(4)) were used for calculations:
The considered levels of concern were:
(i) RQ > 1, high ecological risk, (ii) 0.1 < RQ ≤ 1, moderate risk, (iii) 0.01 < RQ ≤ 0.1, low risk. Given that it is still not feasible to include all PhACs in the environmental risk assessment, the RQ approach was applied for the individual PhACs detected at concentrations exceeding the quantification limits and quantified at levels >10 ng/L, as suggested elsewhere [46]. Hence, the RQ was determined for 25 out of the 41 quantified PhACs. Among them, the compounds exceeding the lower threshold limit of 0.1 for at least one species, are summarized in Figure 3.
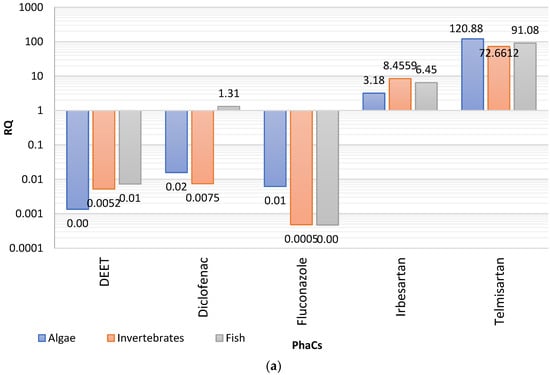
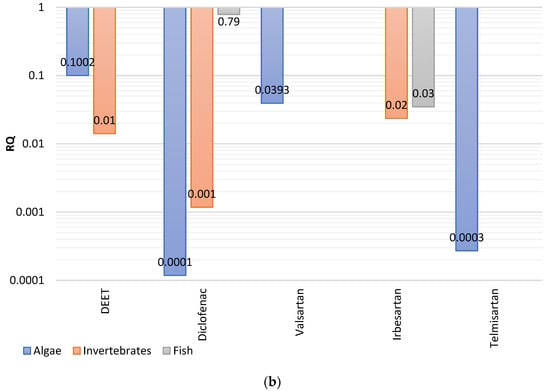
Figure 3.
Risk quotients (RQ) calculated for the (a) acute, and (b) chronic toxicity deriving from the most concentrated compounds detected in effluents.
According to the results, low to high acute toxicity relevance was observed for telmisartan > irbesartan > diclofenac > DEET, fluconazole. The highest environmental risk was found for the antihypertensive telmisartan, which demonstrate RQ 121, 73, and 91, for algae, invertebrates, and fish, respectively. The extremely high RQ values could be attributed not only to its high maximum concentration (133 ng/L) and low PNEC, but also to its high logP (8.2) that involves high potential bioaccumulation. Likewise, the other common antihypertensive, irbesartan, exhibits also high risk for all the three levels of aquatic organisms, despite the lower values, compared to those of telmisartan. More specifically, although concentrations of irbesartan were much higher than for telmisartan, it showed RQs from 3 to 8.5, mainly attributed to the highest PNECs, and probably to its lower lipophilicity (logP = 5.3). As regards the “notorious” NSAID diclofenac, it demonstrated high risk for fish and moderate risk for algae and invertebrates, a result that involves higher toxicity for the organisms at the upper levels of trophic chain. The average risk of diclofenac could be expected given its concentration, PNEC, and medium polarity (logP = 4.5) that involves slightly elevated bioaccumulation. The calculated RQs for acute toxicity of diclofenac are in good accordance with recent studies [25,46] as well as with its predicted accumulation potential, inferred from the logKow values (>4.5). Moderate to low hazard was calculated for the insect repellent DEET (algae < invertebrates < fish), and the antifungal fluconazole (algae > invertebrates ≈ fish).
As regards to the chronic toxicity, the lowest hazards referred to the algae, while slightly higher RQs were measured for invertebrates and fish. More specifically, diclofenac, telmisartan showed negligible risks for the algae, while low risks were estimated for valsartan. DEET was on the borderline of very low and low risk for algae. Similar patterns were observed for invertebrates, with potential chronic toxicity following the order diclofenac < DEET < irbesartan. Regarding the high trophic level organisms (fish), only low to moderate hazard were observed, for diclofenac > irbesartan.
To sum up, the potential risks estimated based on the maximum concentration values (worst-case scenario) of the selected PhACs in the effluent samples from Aineias WWTP entail environmental risks for certain groups, thus further studies for potential bioaccumulation should take place. In addition, the extremely high RQs demonstrated by the antihypertensives reflect the high consumption rate of these drugs by a large part of the population, their insufficient elimination and necessity to advance the treatments methods to tackle the issue, as well as the need to expand the exotoxicity tests so that more data are available to perform risk assessment.
Taking the occasion to pay more attention to environmental risks, it should be considered that PhACs occur in the aquatic environment as a mixture of the same class or mixtures of various therapeutic groups. Such mixture may present synergistic/antagonistic actions and they may exacerbate the status of the receiving aquatic bodies. In a nutshell, monitoring surveys based on reliable and sensitive analytical methods are mandatory and broaden the risk assessment studies.
4. Conclusions
The present wide scope monitoring is the first study performed in the WWTP of Aineias that delineates the comparison between a catalytic ozonation performed on a pre-industrial unit and the conventional ozonation operated in the plant. This work primarily enabled the assessment of the effluent wastewater quality status from one of the two main WWTPs discharging in Thermaikos Gulf. Contrary to conventional methods to acquire data related to major diseases (market investigation, nationwide census, etc.), wastewater-based epidemiology (WBE) studies demonstrate an array of advantages, such as the low-cost opportunity to obtain accurate data, high frequency of data acquisition, absence of bias owing to self-reporting, etc. To this end, owing to this monitoring it was feasible to document the prevalence of certain worrisome classes of drugs, such as antibiotics, antidepressants, antihypertensives, and others, with the view to capture the consumption pattern at the given community. According to the obtained results on both the occurrence, removal, and potential ecotoxicity, it could be recommended that antihypertensives can be considered as markers of pharmaceutical pollution. Moreover, compounds posing high pharmaceutical loads were some antiepileptics-antidepressants, antibiotics, NSAIDs, and β-blockers. It is remarkable that all pharmaceuticals included in the 3rd WL were investigated herein, examining the compliance with EU legislation. The pharmaceutical pollution originated from WWTP can exacerbate in the near future because of a plethora of factors such as rapid population growth, climate change, industrial level agriculture, intensification of industrial activities, etc. Overall, such campaigns comprise prospects of WBE in drug utilization research and they are more than necessary to mirror society and reflect pharmaceutical use. In addition, the continuous implementation of WBE campaigns could be a promising early warning tool for pharmaceutical misuse as well as a means to evaluate community-wide pharmaceutical use in spatio-temporal resolutions.
Otherwise, advances in scientific instrumentation, especially chromatography and mass spectrometry-based techniques have significantly increased the spectrum of detectable chemicals in environmental aqueous matrices such as wastewater. The obtained data involves that the removal efficiency can be enhanced with the application of an advanced catalytic ozonation technology. The treatment of secondary effluent in the pilot unit revealed that a significant part of micropollutants (more than 40%) was removed through filtration of residual suspended solids, while degradation carried out at catalytic ozonation column led to complete removal of them, when applying zeolite as catalyst, and an average of 80% removal by using PET. In the latter case, the biological stabilization of the effluent after catalytic ozonation led to complete degradation for the majority of examined contaminants and an average removal rate of about 90%.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/analytica3020014/s1.
Author Contributions
C.N.: investigation, data curation, formal analysis, software, visualization, original draft preparation; E.K.: investigation, data curation, visualization, original draft preparation; S.P.: investigation, data curation; M.S.: investigation, data curation; P.-A.P.: project administration, writing—review and editing; P.D.: writing—review and editing; D.A.L.: writing—review and editing; M.M.: conceptualization, methodology, writing—review and editing; A.Z.: supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship, and Innovation, under the call RESEACH-CREATE-INNOVATE (project code: T1EDK-02397).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AF | Assessment Factor |
| AOPs | Advanced Oxidation Processes |
| ARGs | Antibiotic Resistance Genes |
| CECs | Contaminants of Emerging Concern |
| DDD | Defined Daily Dose |
| EWW | Effluent |
| FS | Full-Scan |
| IWW | Influent |
| LMCL | Lowest Method Calibration Limit |
| MDL | Method Detection Limit |
| MEC | Measured Environmental Concentration |
| MQL | Method Quantification Limit |
| NOEC | Non-Observed Effective Concentrations |
| OTC | Over-The-Counter |
| PhACs | Pharmaceutically Active Substances |
| PNEC | Predicted Non-Effect Concentration |
| QA | Quality Assurance |
| QC | Quality Control |
| RQ | Risk Quotient |
| tR | Retention Time |
| UMCL | Upper Method Calibration Limit |
| WBE | Wastewater-based epidemiology |
| WFD | Water Framework Directive |
| WL | Watch List |
| WWTPs | Wastewater Treatment Plants |
References
- Quintiles IMS. Outlook for Global Medicines through 2021; Quintiles IMS Inst.: Durham, NC, USA, 2016; pp. 1–54. Available online: http://static.correofarmaceutico.com/docs/2016/12/12/qiihi_outlook_for_global_medicines_through_2021.pdf (accessed on 24 February 2022).
- Kosma, C.I.; Kapsi, M.G.; Konstas, P.-S.G.; Trantopoulos, E.P.; Boti, V.I.; Konstantinou, I.K.; Albanis, T.A. Assessment of multiclass pharmaceutical active compounds (PhACs) in hospital WWTP influent and effluent samples by UHPLC-Orbitrap MS: Temporal variation, removals and environmental risk assessment. Environ. Res. 2020, 191, 110152. [Google Scholar] [CrossRef] [PubMed]
- O’Flynn, D.; Lawler, J.; Yusuf, A.; Parle-McDermott, A.; Harold, D.; Cloughlin, T.M.; Holland, L.; Regan, F.; White, B. A review of pharmaceutical occurrence and pathways in the aquatic environment in the context of a changing climate and the COVID-19 pandemic. Anal. Methods 2021, 13, 575–594. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Water Quality and Wastewater. 2021. Available online: https://www.unwater.org/water-facts/quality-and-wastewater/ (accessed on 24 February 2022).
- Bodík, M.; Mackuľak, T.; Feher, M.; Staňová, A.V.; Grabicová, K.; Varjúová, D.; Bodík, I. Searching for the correlations between the use of different groups of pharmaceuticals from wastewaters. Ecotoxicol. Environ. Saf. 2021, 228, 112973. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Decision (EU) 2020/1161. Off. J. Eur. Union 2020, L257, 32–35. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2020.257.01.0032.01.ENG&toc=OJ:L:2020:257:TOC (accessed on 24 February 2022).
- Lashuk, B.; Yargeau, V. A review of ecotoxicity reduction in contaminated waters by heterogeneous photocatalytic ozonation. Sci. Total Environ. 2021, 787, 147645. [Google Scholar] [CrossRef]
- Nawrocki, J. Catalytic ozonation in water: Controversies and questions. Discussion paper. Appl. Catal. B Environ. 2013, 142–143, 465–471. [Google Scholar] [CrossRef]
- Ofrydopoulou, A.; Nannou, C.; Evgenidou, E.; Lambropoulou, D. Sample preparation optimization by central composite design for multi class determination of 172 emerging contaminants in wastewaters and tap water using liquid chromatography high-resolution mass spectrometry. J. Chromatogr. A 2021, 1652, 462369. [Google Scholar] [CrossRef]
- EN ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. 3rd Version; International Organization for Standardization: Geneva, Switzerland, 2018.
- European Commission. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed, DG-SANTE/12682/2019. 2019. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 24 February 2022).
- Nannou, C.I.; Boti, V.I.; Albanis, T.A. A modified QuEChERS approach for the analysis of pharmaceuticals in sediments by LC-Orbitrap HRMS. Anal. Bioanal. Chem. 2019, 411, 1383–1396. [Google Scholar] [CrossRef]
- Buerge, I.J.; Poiger, T.; Müller, M.D.; Buser, H.-R. Caffeine, an Anthropogenic Marker for Wastewater Contamination of Surface Waters. Environ. Sci. Technol. 2003, 37, 691–700. [Google Scholar] [CrossRef]
- Baker, D.R.; Kasprzyk-Hordern, B. Spatial and temporal occurrence of pharmaceuticals and illicit drugs in the aqueous environment and during wastewater treatment: New developments. Sci. Total Environ. 2013, 454–455, 442–456. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Xue, J.; Zhao, Y.; Taylor, A.A.; Zenobio, J.E.; Sun, Y.; Han, Z.; Salawu, O.A.; Zhu, Y. Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments. J. Hazard. Mater. 2021, 424, 127284. [Google Scholar] [CrossRef] [PubMed]
- WHO. Defined Daily Dose (DDD). Available online: https://www.who.int/tools/atc-ddd-toolkit/about-ddd, (accessed on 24 February 2022).
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Aparicio, I.; Callejón, M.; Alonso, E. Occurrence of pharmaceutically active compounds during 1-year period in wastewaters from four wastewater treatment plants in Seville (Spain). J. Hazard. Mater. 2009, 164, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Gupta, A.K.; Ghosal, P.S.; Varma, M. A review on hospital wastewater treatment: A special emphasis on occurrence and removal of pharmaceutically active compounds, resistant microorganisms, and SARS-CoV-2. J. Environ. Chem. Eng. 2020, 9, 104812. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Zhou, Z.; Wang, Z.; Xu, Z.; Zheng, Q.; Li, X.; He, J.; Li, X.; Cheng, H.; Thai, P.K. Analysing wastewater to estimate fentanyl and tramadol use in major Chinese cities. Sci. Total Environ. 2021, 795, 148838. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Asner, R.; Schüssler, W.; Kopf, W.; Weiß, K.; Sengl, M.; Letzel, M. Behavior of sartans (antihypertensive drugs) in wastewater treatment plants, their occurrence and risk for the aquatic environment. Environ. Sci. Pollut. Res. 2014, 21, 10830–10839. [Google Scholar] [CrossRef] [PubMed]
- Masoner, J.R.; Kolpin, D.W.; Cozzarelli, I.M.; Smalling, K.L.; Bolyard, S.C.; Field, J.A.; Furlong, E.T.; Gray, J.L.; Lozinski, D.; Reinhart, D.; et al. Landfill leachate contributes per-/poly-fluoroalkyl substances (PFAS) and pharmaceuticals to municipal wastewater. Environ. Sci. Water Res. Technol. 2020, 6, 1300–1311. [Google Scholar] [CrossRef]
- Mohapatra, S.; Huang, C.-H.; Mukherji, S.; Padhye, L.P. Occurrence and fate of pharmaceuticals in WWTPs in India and comparison with a similar study in the United States. Chemosphere 2016, 159, 526–535. [Google Scholar] [CrossRef]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef]
- Feo, M.L.; Bagnati, R.; Passoni, A.; Riva, F.; Manta, D.S.; Sprovieri, M.; Traina, A.; Zuccato, E.; Castiglioni, S. Pharmaceuticals and other contaminants in waters and sediments from Augusta Bay (southern Italy). Sci. Total Environ. 2020, 739, 139827. [Google Scholar] [CrossRef]
- Paíga, P.; Correia, M.; Fernandes, M.J.; Silva, A.; de Carvalho, M.M.M.; Vieira, J.; Jorge, S.; Silva, J.G.; Freire, C.; Delerue-Matos, C. Assessment of 83 pharmaceuticals in WWTP influent and effluent samples by UHPLC-MS/MS: Hourly variation. Sci. Total Environ. 2019, 648, 582–600. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Nannou, C.I.; Boti, V.I.; Albanis, T.A. Psychiatrics and selected metabolites in hospital and urban wastewaters: Occurrence, removal, mass loading, seasonal influence and risk assessment. Sci. Total Environ. 2019, 659, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Ofrydopoulou, A.; Nannou, C.; Evgenidou, E.; Christodoulou, A.; Lambropoulou, D. Assessment of a wide array of organic micropollutants of emerging concern in wastewater treatment plants in Greece: Occurrence, removals, mass loading and potential risks. Sci. Total Environ. 2021, 802, 149860. [Google Scholar] [CrossRef]
- Malakootian, M.; Kannan, K.; Gharaghani, M.A.; Dehdarirad, A.; Nasiri, A.; Shahamat, Y.D.; Mahdizadeh, H. Removal of metronidazole from wastewater by Fe/charcoal micro electrolysis fluidized bed reactor. J. Environ. Chem. Eng. 2019, 7, 103457. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Giustina, S.V.D.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- dos Santos, M.M.; Hoppe-Jones, C.; Snyder, S.A. DEET occurrence in wastewaters: Seasonal, spatial and diurnal variability-mismatches between consumption data and environmental detection. Environ. Int. 2019, 132, 105038. [Google Scholar] [CrossRef]
- Xiao, Y.; Shao, X.-T.; Tan, D.-Q.; Yan, J.-H.; Pei, W.; Wang, Z.; Yang, M.; Wang, D.-G. Assessing the trend of diabetes mellitus by analyzing metformin as a biomarker in wastewater. Sci. Total Environ. 2019, 688, 281–287. [Google Scholar] [CrossRef]
- Yan, J.-H.; Xiao, Y.; Tan, D.-Q.; Shao, X.-T.; Wang, Z.; Wang, D.-G. Wastewater analysis reveals spatial pattern in consumption of anti-diabetes drug metformin in China. Chemosphere 2019, 222, 688–695. [Google Scholar] [CrossRef]
- Scheurer, M.; Sacher, F.; Brauch, H.-J. Occurrence of the antidiabetic drug metformin in sewage and surface waters in Germany. J. Environ. Monit. 2009, 11, 1608–1613. [Google Scholar] [CrossRef]
- Van Nuijs, A.L.N.; Tarcomnicu, I.; Simons, W.; Bervoets, L.; Blust, R.; Jorens, P.G.; Neels, H.; Covaci, A. Optimization and validation of a hydrophilic interaction liquid chromatography–Tandem mass spectrometry method for the determination of 13 top-prescribed pharmaceuticals in influent wastewater. Anal. Bioanal. Chem. 2010, 398, 2211–2222. [Google Scholar] [CrossRef]
- Jureczko, M.; Kalka, J. Cytostatic pharmaceuticals as water contaminants. Eur. J. Pharmacol. 2020, 866, 172816. [Google Scholar] [CrossRef] [PubMed]
- Balcerzak, W.; Rezka, P. Occurrence of anti-cancer drugs in the aquatic environment and efficiency of their removal—The selected issues. Czas. Tech. 2014, 20, 11–18. [Google Scholar]
- Zhang, J.; Chang, V.W.; Giannis, A.; Wang, J.-Y. Removal of cytostatic drugs from aquatic environment: A review. Sci. Total Environ. 2013, 445–446, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and Ecotoxicological Risk Assessment of 14 Cytostatic Drugs in Wastewater. Water Air Soil Pollut. 2014, 225, 1896. [Google Scholar] [CrossRef]
- Ikonen, J.; Nuutinen, I.; Niittynen, M.; Hokajärvi, A.-M.; Pitkänen, T.; Antikainen, E.; Miettinen, I. Presence and Reduction of Anthropogenic Substances with UV Light and Oxidizing Disinfectants in Wastewater—A Case Study at Kuopio, Finland. Water 2021, 13, 360. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Decision (EU) 2016/110 of 27 January 2016. Euratom 2016, 2001, 20–30. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016D0110&from=EN (accessed on 24 February 2022).
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef] [Green Version]
- EMEA. Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use. 2006. Available online: http://www.emea.eu.int (accessed on 23 October 2018).
- Papageorgiou, M.; Zioris, I.; Danis, T.; Bikiaris, D.; Lambropoulou, D. Comprehensive investigation of a wide range of pharmaceuticals and personal care products in urban and hospital wastewaters in Greece. Sci. Total Environ. 2019, 694, 133565. [Google Scholar] [CrossRef]
- Kapelewska, J.; Kotowska, U.; Karpińska, J.; Kowalczuk, D.; Arciszewska, A.; Świrydo, A. Occurrence, removal, mass loading and environmental risk assessment of emerging organic contaminants in leachates, groundwaters and wastewaters. Microchem. J. 2018, 137, 292–301. [Google Scholar] [CrossRef]
- Styszko, K.; Proctor, K.; Castrignanò, E.; Kasprzyk-Hordern, B. Occurrence of pharmaceutical residues, personal care products, lifestyle chemicals, illicit drugs and metabolites in wastewater and receiving surface waters of Krakow agglomeration in South Poland. Sci. Total Environ. 2021, 768, 144360. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).