Variations in Female Pelvic Anatomy via MRI: A Retrospective Study at Single Academic Institution
Abstract
1. Introduction
2. Materials and Methods
3. Results
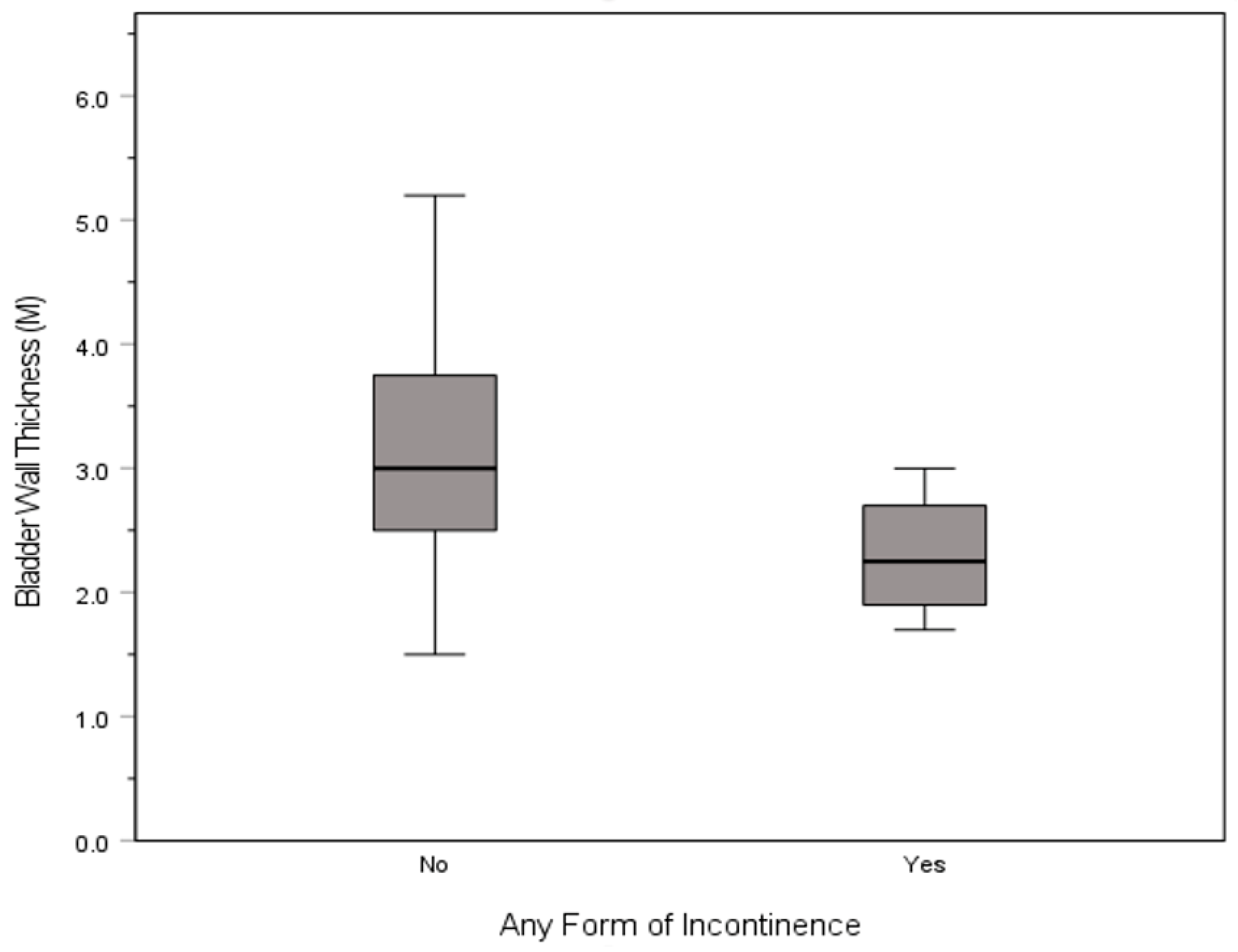
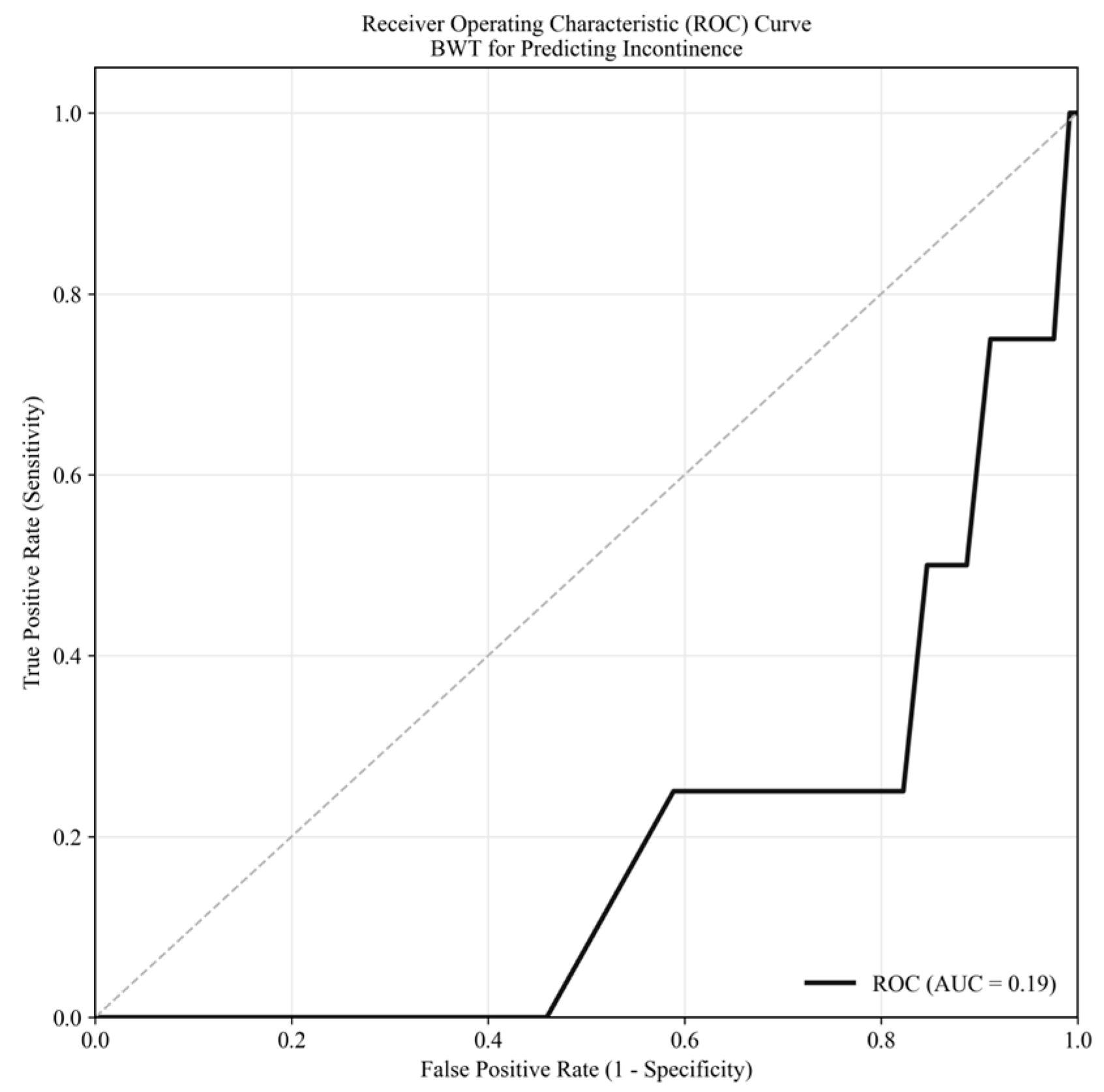
3.1. Incontinence
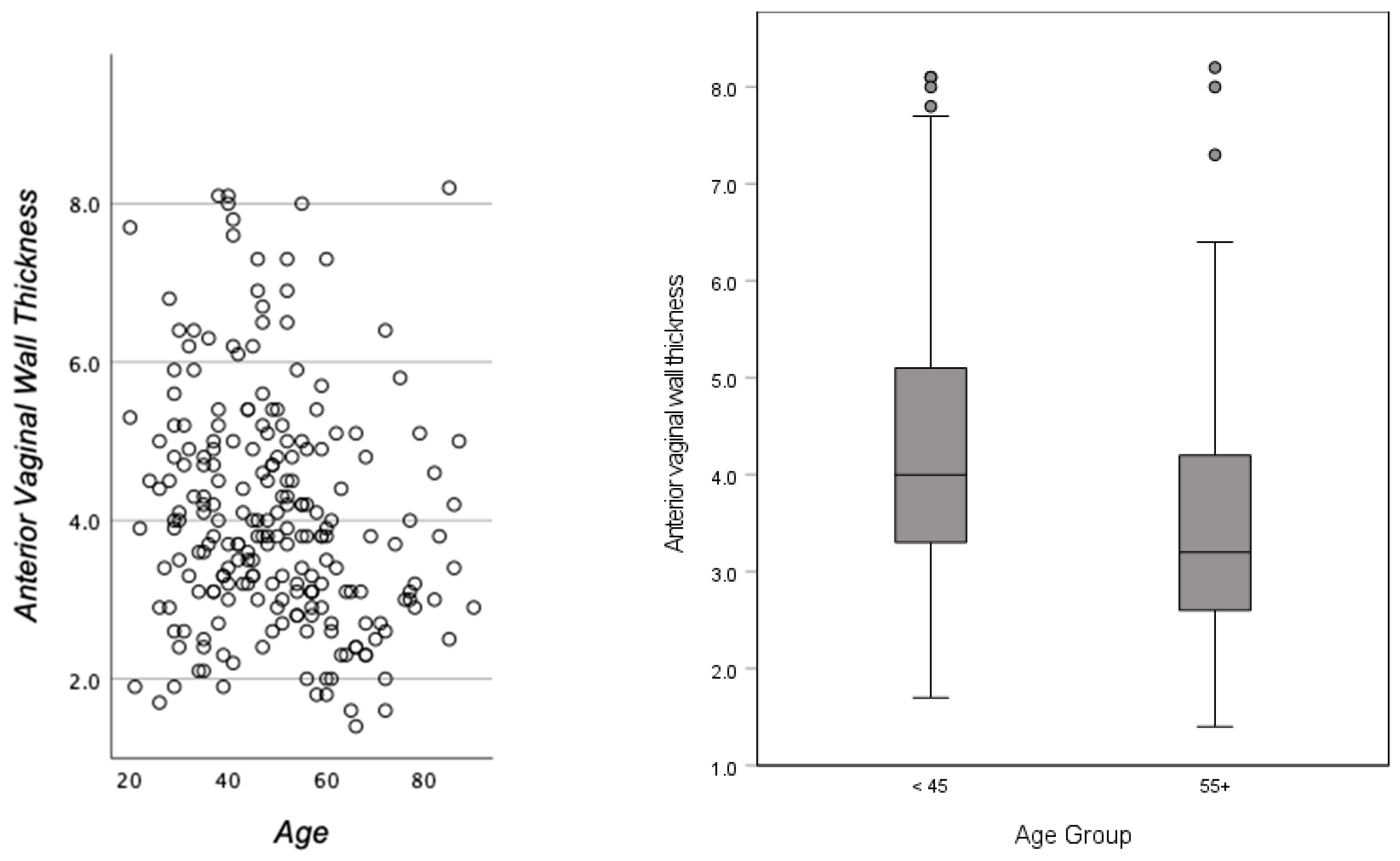
3.2. Age and Race
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AVWT | ANTERIOR VAGINAL WALL THICKNESS |
| BWT | Bladder Wall Thickness |
| VWBU | Vaginal Epithelium to Bladder Urothelium |
| UL | Urethral Length |
| MRI | Magnetic Resonance Imaging |
| PFD | Pelvic Floor Disorder |
| UI | Urinary Incontinence |
| SUI | Stress Urinary Incontinence |
| OAB | Overactive Bladder |
| POP | Pelvic Organ Prolapse |
| AI | Anal Incontinence |
| ESUR | European Society of Urogenital Radiology |
| ESGAR | European Society of Gastrointestinal and Abdominal Radiology |
| NICHD | National Institute of Child Health and Human Development |
| IRB | Institutional Review Board |
| BMI | Body Mass Index |
| SD | Standard Deviation |
| CI | Confidence Interval |
| MM | Millimeter |
References
- Hsu, Y.; Chen, L.; Delancey, J.O.L.; Ashton-Miller, J.A. Vaginal Thickness, Cross-Sectional Area, and Perimeter in Women With and Those Without Prolapse. Obstet. Gynecol. 2005, 105, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Panayi, D.C.; Khullar, V.; Fernando, R.; Digesu, G.A.; Tekkis, P. Vaginal wall thickness is related to the degree of vaginal prolapse. Ultrasound Obstet. Gynecol. 2009, 34, 58–59. [Google Scholar] [CrossRef]
- Bray, R.; Derpapas, A.; Fernando, R.; Khullar, V.; Panayi, D.C. Does the vaginal wall become thinner as prolapse grade increases? Int. Urogynecol. J. 2017, 28, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Kawasaki, A.; Hundley, A.F.; Dieter, A.A.; Myers, E.R.; Sung, V.W. Predicting the number of women who will undergo incontinence and prolapse surgery, 2010 to 2050. Am. J. Obstet. Gynecol. 2011, 205, 230.e1–230.e5. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.; Smith, V.; Bergstrom, J.; Colling, J.; Clark, A. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet. Gynecol. 1997, 89, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Ghoniem, G.M.; Shoukry, M.S.; Yang, A.; Mostwin, J. Imaging for urogynecology, including new modalities. Int. Urogynecol. J. 1992, 3, 212–221. [Google Scholar] [CrossRef]
- Rachaneni, S.; McCooty, S.; Middleton, L.J.; Parker, V.L.; Daniels, J.P.; Coomarasamy, A.; Verghese, T.S.; Balogun, M.; Goranitis, I.; Barton, P.; et al. Bladder ultrasound for diagnosing detrusor overactivity: Test accuracy study and economic evaluation. Health Technol. Assess. 2016, 20, 1–150. [Google Scholar] [CrossRef] [PubMed]
- DeLancey, J.O.L. What’s new in the functional anatomy of pelvic organ prolapse? Curr. Opin. Obstet. Gynecol. 2016, 28, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.L.; Huynh, Q.H.; Nguyen, P.N. Assessing the Clinical Characteristics and the Role of Imaging Modalities in Uterine Sarcoma: A Single-Center Retrospective Study From Vietnam. J. Clin. Ultrasound 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Spradling, K.; Khoyilar, C.; Abedi, G.; Okhunov, Z.; Wikenheiser, J.; Yoon, R.; Huang, J.; Youssef, R.F.; Ghoniem, G.; Landman, J. Redefining the Autonomic Nerve Distribution of the Bladder Using 3-Dimensional Image Reconstruction. J. Urol. 2015, 194, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Zenebe, C.B.; Chanie, W.F.; Aregawi, A.B.; Andargie, T.M.; Mihret, M.S. The effect of women’s body mass index on pelvic organ prolapse: A systematic review and meta analysis. Reprod. Health 2021, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, R.F.; Alt, C.D.; Maccioni, F.; Meissnitzer, M.; Masselli, G.; Manganaro, L.; Vinci, V.; Weishaupt, D. Magnetic resonance imaging of pelvic floor dysfunction—Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur. Radiol. 2017, 27, 2067–2085. [Google Scholar] [CrossRef] [PubMed]
- Talbott, J.M.V.; Yi, J.; Butterfield, R.J.; Wasson, M. Five Year Prevalence of Pelvic Organ Prolapse After Vaginal Hysterectomy with Prophylactic Apical Support. J. Gynecol. Surg. 2021, 37, 127–131. [Google Scholar] [CrossRef]
- Kupec, T.; Pecks, U.; Gräf, C.M.; Stickeler, E.; Meinhold-Heerlein, I.; Najjari, L. Size Does Not Make the Difference: 3D/4D Transperineal Sonographic Measurements of the Female Urethra in the Assessment of Urinary Incontinence Subtypes. Biomed Res. Int. 2016, 2016, 1810352. [Google Scholar] [CrossRef] [PubMed]
- Skorupska, K.; Wawrysiuk, S.; Bogusiewicz, M.; Miotła, P.; Winkler, I.; Kwiatkowska, A.; Rechberger, T. Impact of Hysterectomy on Quality of Life, Urinary Incontinence, Sexual Functions and Urethral Length. J. Clin. Med. 2021, 10, 3608. [Google Scholar] [CrossRef] [PubMed]
- Fugett, J.; Phillips, L.; Tobin, E.; Whitbrook, E.; Bennett, H.; Shrout, J.; Coad, J.E. Selective bladder denervation for overactive bladder (OAB) syndrome: From concept to healing outcomes using the ovine model. Neurourol. Urodyn. 2018, 37, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Mothes, A.R.; Mothes, H.K.; Kather, A.; Altendorf-Hofmann, A.; Radosa, M.P.; Radosa, J.C.; Runnebaum, I.B. Inverse correlation between urethral length and continence before and after native tissue pelvic floor reconstruction. Sci. Rep. 2021, 11, 22011. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.; Summers, A.; Hussain, H.K.; Guire, K.E.; Delancey, J.O.L. Levator plate angle in women with pelvic organ prolapse compared to women with normal support using dynamic MR imaging. Am. J. Obstet. Gynecol. 2006, 194, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Lara, L.A.; Da Silva, A.R.; Rosa-e-Silva, J.C.; Chaud, F.; Silva-de-Sá, M.F.; Meireles E Silva, A.R.; De Sá Rosa-e-Silva, A.C.J. Menopause Leading to Increased Vaginal Wall Thickness in Women with Genital Prolapse: Impact on Sexual Response. J. Sex. Med. 2009, 6, 3097–3110. [Google Scholar] [CrossRef] [PubMed]
- Chess-Williams, R.; Sellers, D.J. Pathophysiological Mechanisms Involved in Overactive Bladder/Detrusor Overactivity. Curr. Bladder Dysfunct. Rep. 2023, 18, 79–88. [Google Scholar] [CrossRef]
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Anterior vaginal wall thickness | ||||||||
| 2. Vaginal wall to bladder urothelium | 0.542 | |||||||
| 3. Urethral length | 0.143 | 0.078 | ||||||
| 4. Urethral width | 0.079 | 0.064 | 0.045 | |||||
| 5. Bladder wall thickness | 0.085 | 0.233 | 0.073 | 0.034 | ||||
| 6. Urethral length to sphincter | 0.246 | 0.207 | 0.214 | −0.290 | 0.602 | |||
| 7. Ureter mounds | 0.067 | 0.005 | −0.145 | 0.039 | −0.116 | −0.118 | ||
| 8. Inter-ureteral mounds to neck | 0.138 | 0.017 | −0.085 | 0.248 | −0.046 | −0.322 | 0.358 |
| Anterior Vaginal Wall Thickness | Vaginal Wall to Bladder Urothelium | Urethral Length | Urethral Width | Bladder Wall Thickness | Urethral Length to Sphincter | Inter-Ureteral Mounds | Inter-Ureteral to Neck | ||
|---|---|---|---|---|---|---|---|---|---|
| Category | |||||||||
| Overall Cohort | M (SD) | 4.1 (1.5) | 9.7 (3.5) | 33.2 (5.9) | 9.6 (2.8) | 3.1 (0.8) | 26.1 (3.6) | 31.9 (7.5) | 14.8 (5.9) |
| N | 221 | 231 | 174 | 114 | 204 | 12 | 114 | 112 | |
| IQR | 1.9 | 4.8 | 6.7 | 4.0 | 1.2 | 4.2 | 10.7 | 9.2 | |
| Hysterectomy | M (SD) | 4.0 (1.2) | 9.4 (3.2) | 34.3 (5.6) | 9.5 (2.8) | 3.0 (0.9) | 26.4 (-) | 30.8 (7.2) | 14.9 (5.9) |
| N | 44 | 47 | 35 | 24 | 41 | 1 | 30 | 30 | |
| IQR | 1.5 | 3.8 | 8.3 | 3.7 | 1.3 | 0.0 | 9.8 | 9.6 | |
| Prolapse | M (SD) | 4.6 (1.1) | 9.7 (3.1) | 35.4 (5.2) | 8.1 (4.3) | 3.1 (0.8) | 23.8 (1.1) | 31.0 (6.4) | 14.9 (3.6) |
| N | 9 | 10 | 9 | 5 | 8 | 2 | 4 | 4 | |
| IQR | 0.9 | 4.4 | 7.7 | 2.9 | 1.1 | 0.8 | 4.0 | 3.9 | |
| Incontinence, any | M (SD) | 5.1 (1.6) | 11.3 (3.7) | 33.4 (6.2) | 9.6 (3.3) | 2.3 (0.5) | 31.0 (4.9) | 14.8 (4.3) | |
| N | 9 | 11 | 11 | 7 | 4 | 0 | 5 | 5 | |
| IQR | 0.9 | 5.5 | 7.1 | 3.8 | 0.5 | 3.0 | 1.2 | ||
| Fibroid | M (SD) | 4.2 (1.5) | 10.0 (3.4) | 33.2 (6.5) | 8.9 (3.5) | 3.0 (0.8) | 27.1 (4.3) | 32.8 (8.0) | 13.9 (5.9) |
| N | 73 | 78 | 60 | 31 | 67 | 7 | 35 | 31 | |
| IQR | 1.7 | 5.0 | 8.0 | 5.6 | 1.0 | 7.1 | 11.0 | 7.7 |
| Anterior Vaginal Wall Thickness | Vaginal Wall to Bladder Urothelium | Urethral Length | Urethral Width | Bladder Wall Thickness | Urethral Length to Sphincter | Inter-Ureteral Mounds | Inter-Ureteral Mounds to Neck | ||
|---|---|---|---|---|---|---|---|---|---|
| Category | Statistic | ||||||||
| Overall Cohort | M (SD) | 4.1 (1.5) | 9.7 (3.5) | 33.2 (5.9) | 9.6 (2.8) | 3.1 (0.8) | 26.1 (3.6) | 31.9 (7.5) | 14.8 (5.9) |
| N | 221 | 231 | 174 | 114 | 204 | 12 | 114 | 112 | |
| IQR | 1.9 | 4.8 | 6.7 | 4.0 | 1.2 | 4.2 | 10.7 | 9.2 | |
| Age Group | |||||||||
| <45 | M (SD) | 4.3 (1.5) | 10.1 (3.5) | 33.3 (6.0) | 10.0 (2.8) | 3.0 (0.8) | 23.7 (1.5) | 31.7 (8.6) | 13.8 (5.6) |
| N | 95 | 103 | 79 | 50 | 90 | 5 | 44 | 42 | |
| IQR | 1.8 | 5.0 | 6.2 | 4.1 | 1.2 | 1.6 | 11.9 | 7.6 | |
| >65 | M (SD) | 3.6 (1.4) | 9.0 (3.3) | 32.3 (6.3) | 9.2 (2.9) | 3.2 (0.9) | 27.5 (3.9) | 32.2 (7.2) | 15.7 (6.1) |
| N | 78 | 80 | 60 | 38 | 71 | 4 | 49 | 50 | |
| IQR | 1.6 | 4.5 | 7.0 | 3.7 | 1.2 | 2.4 | 10.3 | 9.2 | |
| BMI Group | |||||||||
| <18.5 | M (SD) | 3.8 (1.0) | 8.5 (3.0) | 32.0 (3.7) | 9.4 (3.0) | 3.0 (0.8) | 27.0 (5.7) | 33.9 (9.3) | 13.0 (4.6) |
| N | 11 | 10 | 8 | 6 | 10 | 2 | 8 | 8 | |
| IQR | 0.4 | 5.1 | 4.6 | 4.4 | 1.4 | 4.0 | 16.8 | 4.3 | |
| 18.5–24.9 | M (SD) | 3.9 (1.4) | 9.4 (3.6) | 32.6 (6.3) | 9.4 (2.8) | 3.0 (0.8) | 26.6 (4.4) | 33.6 (7.2) | 14.1 (5.7) |
| N | 90 | 93 | 72 | 49 | 84 | 6 | 49 | 46 | |
| IQR | 1.6 | 5.3 | 7.2 | 3.7 | 1.1 | 5.9 | 10.7 | 8.3 | |
| 25.0–29.9 | M (SD) | 4.2 (1.5) | 9.8 (3.1) | 34.7 (5.4) | 9.8 (3.2) | 3.1 (0.9) | 24.7 (1.7) | 30.3 (7.4) | 15.1 (6.2) |
| N | 71 | 72 | 53 | 32 | 63 | 3 | 32 | 32 | |
| IQR | 2.0 | 3.8 | 7.7 | 4.2 | 1.2 | 1.7 | 11.0 | 9.8 | |
| 30+ | M (SD) | 4.3 (1.6) | 10.4 (3.8) | 33.1 (6.1) | 9.7 (2.4) | 3.2 (0.9) | 25.7 (-) | 29.9 (7.2) | 16.5 (6.3) |
| N | 47 | 54 | 39 | 26 | 45 | 1 | 25 | 26 | |
| IQR | 1.8 | 5.2 | 5.2 | 3.2 | 1.4 | 0.0 | 9.9 | 10.5 | |
| Race | |||||||||
| Asian | M (SD) | 4.1 (1.2) | 10.2 (3.6) | 34.0 (6.8) | 10.1 (2.4) | 3.3 (0.8) | 25.2 (3.5) | 32.0 (6.5) | 13.1 (6.2) |
| N | 45 | 46 | 38 | 21 | 40 | 4 | 23 | 22 | |
| IQR | 1.9 | 4.5 | 6.9 | 4.3 | 1.2 | 2.4 | 8.0 | 9.3 | |
| Black | M (SD) | 5.0 (1.6) | 11.3 (2.9) | 37.1 (2.4) | 7.2 (0.7) | 2.9 (0.6) | 32.2 (10.2) | 16.2 (4.7) | |
| N | 10 | 10 | 8 | 4 | 8 | 0 | 2 | 3 | |
| IQR | 1.6 | 3.9 | 2.5 | 0.8 | 0.7 | 7.2 | 4.2 | ||
| Hispanic | M (SD) | 4.4 (1.8) | 10.9 (3.8) | 33.2 (4.8) | 8.9 (3.3) | 2.8 (0.7) | 30.3 (7.3) | 14.5 (5.8) | |
| N | 32 | 39 | 27 | 14 | 30 | 0 | 16 | 17 | |
| IQR | 2.4 | 5.7 | 5.7 | 4.9 | 1.1 | 8.2 | 9.1 | ||
| White | M (SD) | 3.9 (1.4) | 9.1 (3.2) | 32.8 (6.2) | 9.6 (2.8) | 3.1 (0.9) | 24.7 (1.9) | 32.3 (8.4) | 15.7 (6.1) |
| N | 109 | 112 | 85 | 61 | 105 | 6 | 60 | 58 | |
| IQR | 1.8 | 3.7 | 6.8 | 3.5 | 1.3 | 2.8 | 13.8 | 9.1 | |
| Other | M (SD) | 3.8 (1.3) | 8.8 (3.6) | 33.2 (5.3) | 9.9 (2.3) | 3.5 (0.8) | 32.0 (1.5) | 30.1 (4.2) | 14.1 (6.0) |
| N | 15 | 15 | 9 | 7 | 13 | 2 | 6 | 6 | |
| IQR | 1.0 | 5.2 | 7.6 | 2.8 | 1.2 | 1.1 | 6.1 | 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoniem, G.; Phan, W.; Javaid, N.; Chowdhury, M.L.; Farhan, B.; Hammad, M.A.M.; Ahmed, A.; Csuka, D.; Saba, D.; Helmy, M.; et al. Variations in Female Pelvic Anatomy via MRI: A Retrospective Study at Single Academic Institution. Uro 2025, 5, 18. https://doi.org/10.3390/uro5030018
Ghoniem G, Phan W, Javaid N, Chowdhury ML, Farhan B, Hammad MAM, Ahmed A, Csuka D, Saba D, Helmy M, et al. Variations in Female Pelvic Anatomy via MRI: A Retrospective Study at Single Academic Institution. Uro. 2025; 5(3):18. https://doi.org/10.3390/uro5030018
Chicago/Turabian StyleGhoniem, Gamal, William Phan, Naila Javaid, Mashrin Lira Chowdhury, Bilal Farhan, Muhammed A. Moukhtar Hammad, Ahmed Ahmed, David Csuka, Dina Saba, Mohammad Helmy, and et al. 2025. "Variations in Female Pelvic Anatomy via MRI: A Retrospective Study at Single Academic Institution" Uro 5, no. 3: 18. https://doi.org/10.3390/uro5030018
APA StyleGhoniem, G., Phan, W., Javaid, N., Chowdhury, M. L., Farhan, B., Hammad, M. A. M., Ahmed, A., Csuka, D., Saba, D., Helmy, M., & Lee, S. (2025). Variations in Female Pelvic Anatomy via MRI: A Retrospective Study at Single Academic Institution. Uro, 5(3), 18. https://doi.org/10.3390/uro5030018






