1. Introduction
With newer concepts and newer techniques for the repair of an inguinal hernia, various prostheses have been developed. These prostheses are usually made from synthetic materials that add strength and stability to the defect producing the hernia. Most products were non-absorbable while adding reinforcement to the tissue around the defect. But, as with any surgical procedure, complications began to develop. In many cases, these prosthetic complications required further surgical intervention when a lump or mass formed in the repair area. Infection and erosion of the surgical site were followed by fistulization outward to the skin, requiring surgical removal of the mesh and later repair of a recurrence. Various prosthetics and shapes, such as flat, oblong, and two-sided mesh prostheses, were developed to avoid such concerns [
1,
2].
Herniorrhaphy of either the inguinal or abdominal wall defect is frequently required. Mesh reinforcement of the repair is readily utilized by many surgeons. Abdominal wall hernia repair, especially after previous surgery, is also a fairly frequent operation. Berger and Bientzle reported on 344 patients with endoscopic repair of incisional or parastomal hernias utilizing polyvinylidene fluoride (PVDF; DynaMesh-IPOM) in a two-layer surgical technique with a 2% postoperative reoccurrence rate and a low infection rate [
3]. However, Hussain et al. reported on the early occurrence of small bowel obstruction after laparoscopic ventral hernia repair with a mesh [
4].
Laparoscopic reduction in inguinal hernias with a mesh repair has the advantage of small non-inguinal incisions, while complications, including hematomas, seromas, and wound infection with fistulas, may still occur [
5]. This approach has also been reported to be the cause of a small bowel enteric fistula or a colocutaneous fistula [
6,
7]. Lauwers et al. reported on the use of the Stoppa preperitoneal repair for bilateral inguinal hernias, which resulted in the mesh eroding the colon after two years and the urinary bladder in another two years [
8]. In 2007, Goswami et al. reported on mesh erosion into the cecum following laparoscopic repair (TAPP) of an inguinal hernia [
9]. They then recommended a total extraperitoneal approach (TEP) rather than the TAPP procedure. Two other case reports also point out the occurrence of mesh migration of a synthetic prosthesis into the urinary bladder: Kurukahvecioglu reported on polytetrafluoroethylene (PTFE) migration into the bladder after laparoscopic inguinal herniorrhaphy and fixation with tackers, and Chowbey presented mesh migration into the urinary bladder after TEP (total extraperitoneal inguinal hernia repair). In both cases, infection was not the primary symptom [
10,
11].
Porous or nonporous and a plug or ring about the mesh gave further support for the repair. But new concerns then arose as the ring support of the mesh prosthesis could then cause pressure on the surrounding tissues and lead to infection, necrosis, recurrence, and migration of the prosthetic outward to the skin or inward into the abdomen, bladder, and colon with fistulization [
2]. Our patient developed both bladder and colon fistulas with hematuria, blood per rectum, and a large abdominal mass after inguinal hernia repair utilizing a mesh prosthesis, demonstrating the potential for long-term complications. This presentation attempts to point out some of the long-term untoward results of prosthetic inguinal hernia repair and also the possible reduction in such complications.
2. Case Presentation
This 51-year-old male patient was first seen in the gastrointestinal endosuite with a history of bowel and urinary complaints. Because of intermittent rectal bleeding associated with bowel movement, mild lower abdominal discomfort, and pneumaturia, he was seen by both the urologic and gastroenterologic consultants and scheduled for an endoscopy. A colonoscopy (during which I was called) revealed a hemorrhagic mesh-like material protruding into the colon. Cystoscopy demonstrated a hemorrhagic dome of the bladder with a mesh-like material eroding the bladder. He had no sign of pyelonephritis, renal failure, or temperature elevation.
The patient’s history was significant due to a left inguinal hernia repair ten years prior, a mesh implantation elsewhere more than 15 years ago (retrospectively, a small Kugel patch) (we do not have those records), and simultaneous bilateral scrotal vasectomy. Following surgery, he did well for ten years until he developed a urinary tract infection with pneumaturia and blood in his stools. He was otherwise in good physical health except for being 12–15 pounds overweight with no other medical conditions. He had no diabetes, no high blood pressure concerns, no allergies, and no renal disease.
Physical examination revealed a mildly obese, afebrile individual, in no distress, with a well-healed left lower quadrant abdominal scar, a heart rate of 72, normal blood pressure, and normal respiratory rate. When awake, no mass was palpable transabdominally, nor per rectal examination. The scrotum presented neither abnormal lesions nor signs of inflammation. Some resistance was noted in the left lower quadrant of the abdomen on physical examination, but no abdominal nor rebound tenderness was elicited and there was no inguinal or abdominal wall reddening nor induration.
Laboratory studies included a urinalysis, which showed numerous red and white blood cells, while the urine culture demonstrated E. coli (without signs of pyelonephritis or renal failure). Diagnostic testing also included a normal serum white cell count and a 13.9 gm hemoglobin. Computerized tomography demonstrated a large intra-abdominal mass involving the left lower abdominal cavity and the sigmoid colon. There were no cystoscopy photos taken, and unfortunately, the colonoscopy photos are very blurred. But they are available upon request.
Following endoscopy, the findings and possible treatment options, including exploratory laparotomy and resection of the mass, were discussed with the patient. Our plan was to reduce or remove the inflammatory process, close the bladder fistula, drain the bladder—either suprapubically or transurethral, remove the mesh, resect the inflamed colon, and perform a primary colocolostomy while avoiding a temporary diverting colostomy—if possible.
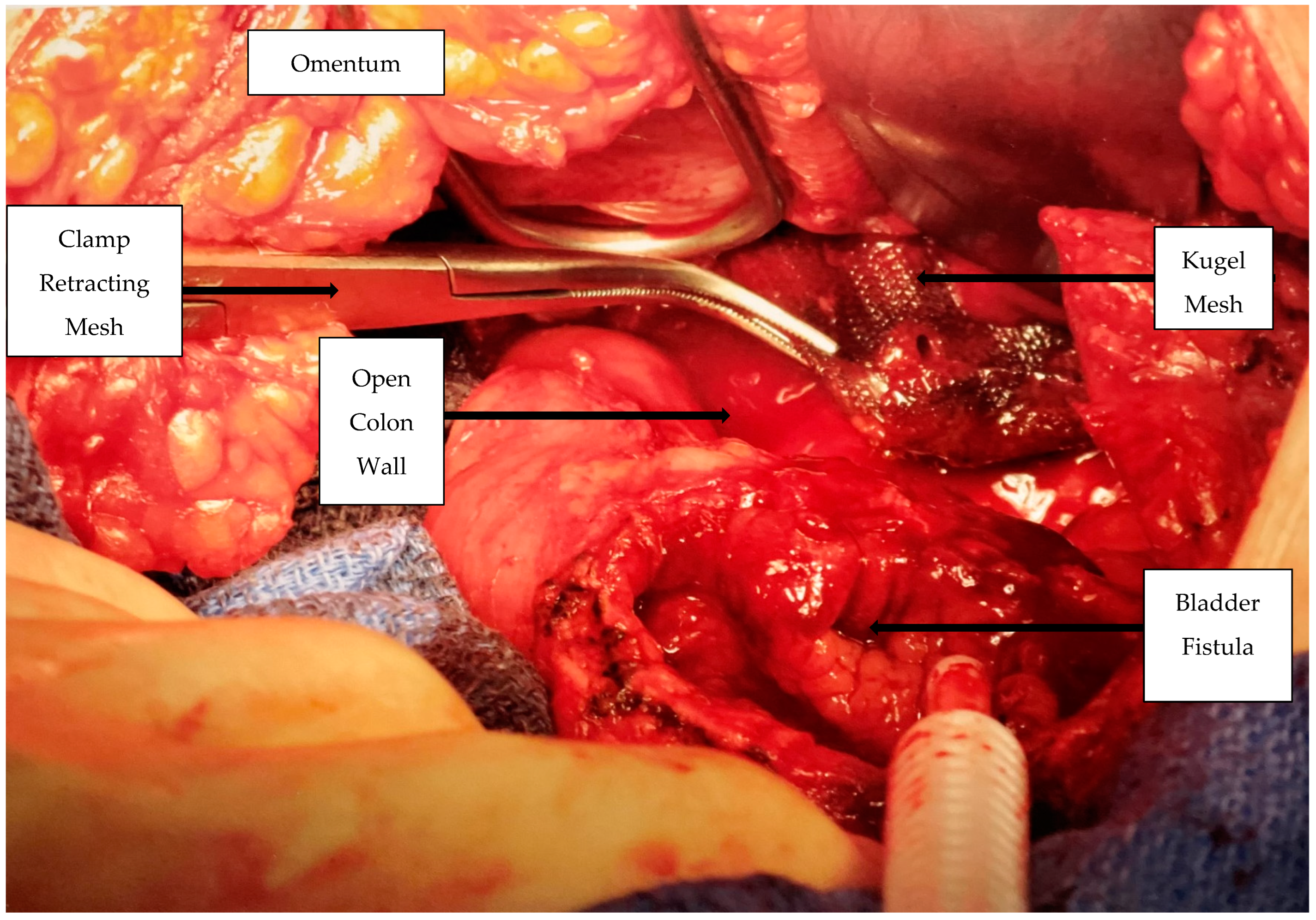
Surgical Procedure: After obtaining appropriate informed consent, the patient received a general endotracheal anesthetic. Following a lower abdominal vertical incision through a moderate amount of adipose tissue, a moderate-sized “mass” involving the sigmoid colon and the urinary bladder was found attached to the anterior abdominal wall. The inflammatory mass, which was freed from the left lower quadrant of the abdominal wall, contained the omentum, the loop of the colon, and the hernia mesh and was also attached to and involved the dome of the bladder (
Figure 1).
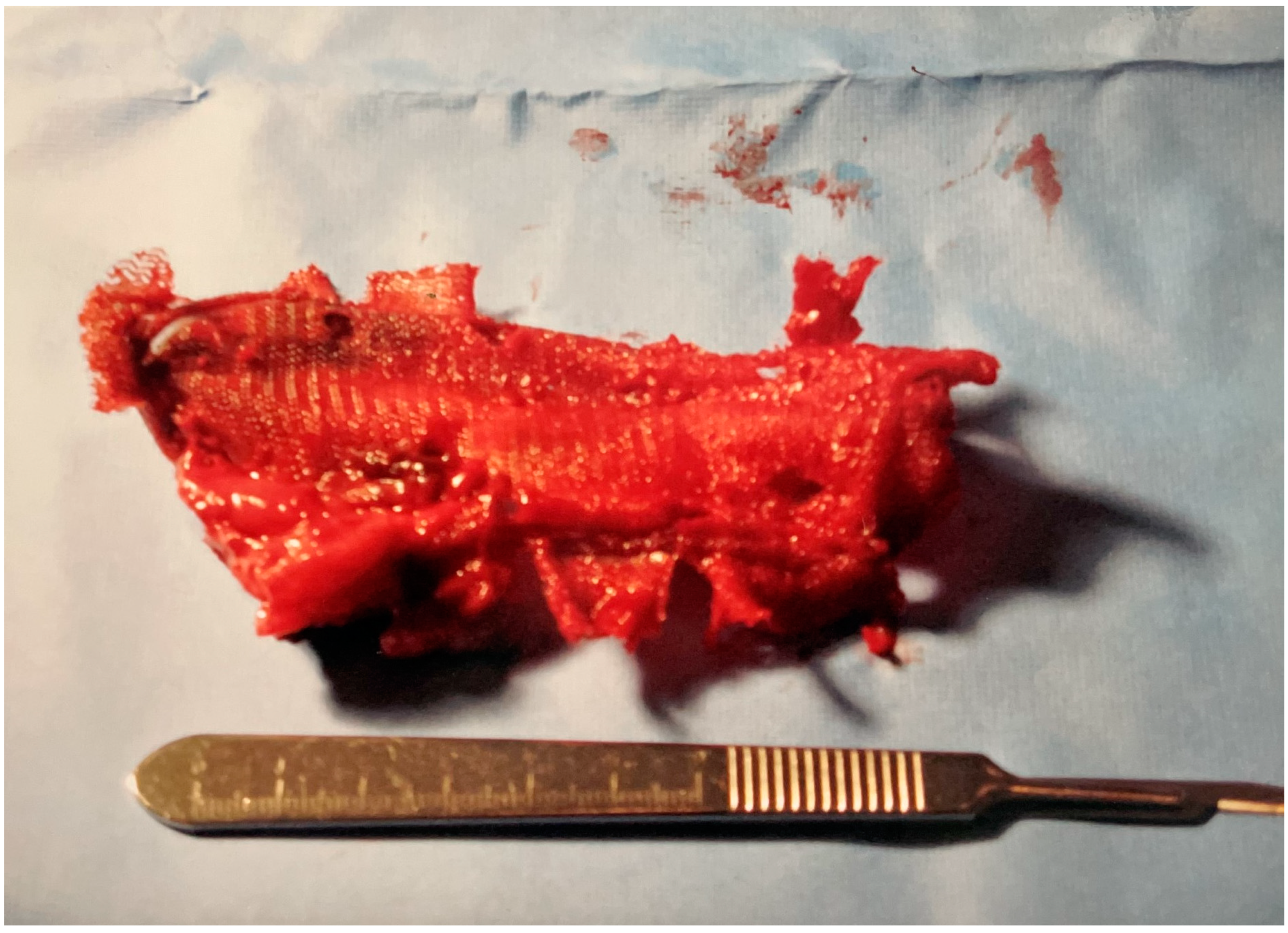
After removing the mesh (
Figure 2), the colon and bladder were separated. The bladder wall, which was thickened and firm about the site of perforation, was then closed with #1 absorbable suture. A Foley catheter was left in the bladder for long-term urinary drainage and healing. Following this, the thickened and inflamed area of the sigmoid colon, which contained the fistula and the majority of the mesh prosthesis, was resected with a clamp-clamp-tie technique. A side-to-side anastomosis of the proximal and distal colon was then performed with a GI endo stapler. The abdomen was closed with running #1 absorbable suture and staples on the skin after two hours of surgery. A nasogastric tube remained in place for 48 h while the patient received pre- and post-operative intravenous antibiotics (mefoxin (cefoxitin) 1.0 g Q 6 h for 24 h) and intravenous self-regulated morphine via a patient-controlled infusion pump for 48 h.
3. Outcome
Postoperatively, the patient received intravenous fluids and was NPO (nothing per mouth) for a few days until the bowel function returned. The Foley catheter remained in place for 3 1/2 weeks with a good urine output. The abdominal incision healed well with only slight erythema. The area of the previous inguinal hernia repair site and the scrotum showed no erythema, nor induration, and no bulge either preoperatively or postoperatively. The patient was discharged to outpatient follow-up care after placement on oral intake. At 6- and 12-month follow-up, the patient was doing well with no additional urinary, colon, or hernia concerns, while the abdominal wall and inguinal areas appeared normal.
4. Discussion
Inguinal hernia bulges, whether asymptomatic or symptomatic, are relatively common. They may be small and the size of a walnut or larger and reach to the ankle in males. Various methods, both surgical and non-surgical, have been devised to reduce the bulge and the symptoms. The direct primary inguinal surgical suture approach for hernia repair has been a standard for years. But, due to the incidence of recurrence, additional innovations have been added, including the addition of mesh prosthesis to the repair. Recurrence of the hernia was thus reduced, but, on occasion, other new concerns were reported following the use of a mesh in the open inguinal approach. Multiple materials, in multiple shapes and sizes, have been utilized in various positions and techniques to reduce and prevent the recurrence of the inguinal hernia. Unfortunately, no perfect complication-free material or method for hernia repair has been devised as yet.
Both the direct and indirect hernias repaired with mesh began to develop unexpected complications in addition to recurrence [
12]. Plugomas (mesh bulges) were seen when the prosthesis, such as the Prolene Hernia System (PHS), was utilized. In order to understand the difference between a significant post-surgery mass and a plugoma, Aganovic et al. then performed a C-T study on 564 patients to define the characteristic of the smooth or round proline mass versus a hernia defect adjacent to the inferior epigastric artery [
13].
Mesh infections, local external mesh erosion, and small or large bowel volvulus all occurred [
14,
15]. The more severe complications, including the occasional colocutaneous fistula, stimulated more concern regarding surgical technique [
16]. Authors emphasized in a letter to the editor that they reported no such occurrences in 1500 plugs over 12 years and that reducing mesh hernia repair complications required good “workmanship”, which included careful tissue dissection, adequate prosthesis fixation, avoiding tears in the peritoneum, closure of all peritoneal holes—despite the size—and flattening of the mesh plug [
16]. Lo et al. also advised not to place the plug deep in the defect and to secure the mesh [
1].
Others have advocated for the avoidance of strong tension, adequate implant space, avoidance of sharp edges, and other mesh modifications and techniques depending on the shape and construction of the mesh prosthesis. One such modification was seen in the Kugel patch, as used in this patient, which had a circular ring about the mesh to aid in positioning the patch and maintenance of the mesh diameter over the inguinal floor defect. However, that ring could create a constant pressure effect on the surrounding tissue and thus create erosion and migration into nearby structures over various time periods. This was not the case with other fistulas, as no ring was present in other reported cases.
Supporting information (Table S1: Some urinary and colon fistula patient history and Table S2: Endoscopy and surgery review of the above patient summaries) can be downloaded—please see
Supplementary Materials for download information.
Our patient, as also reported by Hagiwara et al. [
17], developed both urologic and colonic symptoms with pneumaturia, a bacterial-positive
E. coli urine culture, erosion of the bladder, and blood per rectum with bowel movements. A colonoscopy revealed the foreign material (mesh) protruding into the eroded and hemorrhagic area of the colon. Interestingly, our patient continued to have bowel movements despite the large colon erosion, as did another patient we had seen who sloughed the transverse colon after pancreas surgery. Of interest, Li and Cheng also reported a urinary bladder fistula following prosthetic hernia repair without mesh migration and healing without removal of the mesh [
2].
Abdominal pain and discomfort were seen with open or laparoscopic repair after a unilateral or bilateral mesh or Kugel repair [
12,
18,
19,
20]. A review of other randomly selected reports demonstrated all patients were male, with ages varying between 34 and 63 years of age and 3 to 10 years between the primary inguinal hernia repair and the fistula diagnosis. The original hernia was repaired in all patients with an inguinal incision. Symptomatology included mild lower abdominal discomfort, wound drainage, and a colocutaneous fistula. The diagnostic studies included a C-T scan and colonoscopy in four patients and a fistulogram in one patient. The urinalysis was abnormal in all patients with bladder involvement. The colonic and bladder surgical repair of the fistula was completed with open laparotomy. Narang et al. reported on mesh migration after both laparoscopic inguinal hernia repair and open inguinal hernia repair. The surgical corrective action included partial cystectomy (bladder only), colectomy, or ileocecal resection with partial cystectomy [
18].
These four patients had the mesh seen on a colonoscopy, with a colon polyp in two. All patients had an open laparotomy, with a colectomy in three patients (sigmoidectomy in two patients) and colon repair in one patient. Urinary patients had prolonged decompression postoperative bladder catheters. The suspected cause of the fistula included an incomplete peritoneal closure, localized irritation, and a second procedure in the same setting. Preoperative antibiotics were used in the four endoscopy patients and for two days postoperatively.
The use of mesh implantation reduced the incidence of hernia recurrence in both the inguinal and the abdominal wall hernia repairs. Newer surgical techniques, including endoscopic repair and small 1 to 2 cm incisions, reduced discomfort and tissue damage for the patient. However, when the mesh was inserted for surgical repair, as in this patient, for prevention of future recurrence, other potential adverse effects may develop, of which our patient is an example.
Because of the desire for complication reduction and prevention, surgeons have continued to search for other techniques to repair the abdominal or inguinal hernia. Others have fostered and promulgated newer trends. The robotic argument is that anatomic structures are better defined with enhanced tissue visualization and a reduction in surgical trauma. Hence, minimally invasive technology has continued, but with an increased cost, to expand with the hopes that surgical complications will be reduced along with the incidence of recurrence and mesh complications.
5. Conclusions
A patient with pneumaturia and passage of blood associated with bowel movements ten years after an open inguinal hernia repair is presented. Colonoscopy and cystoscopy demonstrated the synthetic hernia mesh eroding into the colon and the bladder dome. Via a laparoscopy, we removed the left inguinal hernia mesh, which had eroded into both the bladder and the colon. Following resection, there was complete healing of the abdominal incision and long-term normal urinary and colonic functions. The patient’s history defines our need to be selective in the performance of newer procedures and aware of risks associated with newer medical devices. Avoidance of surgical tension and prosthetic contact with the viscera should minimize the incidence of major hernia surgical complications.