Relationship between Androgen Deprivation Therapy and Abdominal Adipose Tissue
Abstract
1. Introduction
2. Methods
3. Results and Evidence
3.1. Effects of Androgen Deprivation Therapy on Abdominal Adipose Tissue
3.2. Role of Abdominal Adipose Tissue on Survival of Prostate Cancer Patients Treated with Androgen Deprivation Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vermeulen, A.; Goemaere, S.; Kaufman, J.M. Testosterone, body composition and aging. J. Endocrinol. Investig. 1999, 22, 110–116. [Google Scholar]
- Katznelson, L.; Finkelstein, J.S.; Schoenfeld, D.A.; Rosenthal, D.I.; Anderson, E.J.; Klibanski, A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J. Clin. Endocrinol. Metab. 1996, 81, 4358–4365. [Google Scholar] [PubMed]
- Bhasin, S.; Storer, T.W.; Berman, N.; Yarasheski, K.E.; Clevenger, B.; Phillips, J.; Lee, W.P.; Bunnell, T.J.; Casaburi, R. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J. Clin. Endocrinol. Metab. 1997, 82, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Stevens, R.E.; Hodges, C.V. Studies on prostatic cancer: The effect of castration on advanced carcinoma of the prostate gland. Arch. Surg. 1941, 43, 209–223. [Google Scholar] [CrossRef]
- James, N.D.; Spears, M.R.; Clarke, N.W.; Dearnaley, D.P.; De Bono, J.S.; Gale, J.; Hetherington, J.; Hoskin, P.J.; Jones, R.J.; Laing, R.; et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in the “Docetaxel Era”: Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur. Urol. 2015, 67, 1028–1038. [Google Scholar] [CrossRef]
- Rhee, H.; Gunter, J.H.; Heathcote, P.; Ho, K.; Stricker, P.; Corcoran, N.M.; Nelson, C.C. Adverse effects of androgen deprivation therapy in prostate cancer and their management. BJU Int. 2015, 115 (Suppl. S5), 3–13. [Google Scholar] [CrossRef]
- Van Londen, G.J.; Levy, M.E.; Perera, S.; Nelson, J.B.; Greenspan, S.L. Body composition changes during androgen deprivation therapy for prostate cancer: A 2-year prospective study. Crit. Rev. Oncol. Hematol. 2008, 68, 172–177. [Google Scholar] [CrossRef]
- Smith, J.C.; Bennett, S.; Evans, L.M.; Kynaston, H.G.; Parmar, M.; Mason, M.D.; Cockcroft, J.R.; Scanlon, M.F.; Davies, J.S. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J. Clin. Endocrinol. Metab. 2001, 86, 4261–4267. [Google Scholar] [CrossRef]
- Faris, J.E.; Smith, M.R. Metabolic sequelae associated with androgen deprivation therapy for prostate cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 240–246. [Google Scholar] [CrossRef]
- Freedland, S.J.; Aronson, W.J.; Kane, C.J.; Presti, J.C., Jr.; Amling, C.L.; Elashoff, D.; Terris, M.K. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: A report by the Shared Equal Access Regional Cancer Hospital database study group. J. Clin. Oncol. 2004, 22, 446–453. [Google Scholar] [CrossRef]
- WHO. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization Technical Report Series 894; WHO: Geneva, Switzerland, 2000; 253p. [Google Scholar]
- Park, Y.H.; Lee, J.K.; Kim, K.M.; Kook, H.R.; Lee, H.; Kim, K.B.; Lee, S.; Byun, S.S.; Lee, S.E. Visceral obesity in predicting oncologic outcomes of localized renal cell carcinoma. J. Urol. 2014, 192, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, R.; Sabatino, L.; Greco, F. Neck fat volume as a potential indicator of difficult intubation: A pilot study. Saudi J. Anaesth. 2018, 12, 67–71. [Google Scholar] [PubMed]
- Greco, F.; Cirimele, V.; Mallio, C.A.; Beomonte Zobel, B.; Grasso, R.F. Increased visceral adipose tissue in male patients with clear cell renal cell carcinoma. Clin. Cancer Investig. J. 2018, 7, 132–136. [Google Scholar] [CrossRef]
- Greco, F.; Mallio, C.A.; Cirimele, V.; Grasso, R.F.; Beomonte Zobel, B. Subcutaneous adipose tissue as a biomarker of pancreatic cancer: A pilot study in male patients. Clin. Cancer Investig. J. 2019, 8, 10–19. [Google Scholar] [CrossRef]
- Mallio, C.A.; Greco, F.; Pacella, G.; Schena, E.; Beomonte Zobel, B. Gender-based differences of abdominal adipose tissue distribution in non-small cell lung cancer patients. Shanghai Chest 2018, 2, 20. [Google Scholar] [CrossRef]
- Greco, F.; Quarta, L.G.; Carnevale, A.; Giganti, M.; Grasso, R.F.; Beomonte Zobel, B.; Mallio, C.A. Subcutaneous Adipose Tissue Reduction in Patients with Clear Cell Renal Cell Carcinoma and Peritumoral Collateral Vessels: A Retrospective Observational Study. Appl. Sci. 2021, 11, 6076. [Google Scholar] [CrossRef]
- Antoun, S.; Bayar, A.; Ileana, E.; Laplanche, A.; Fizazi, K.; di Palma, M.; Escudier, B.; Albiges, L.; Massard, C.; Loriot, Y. High subcutaneous adipose tissue predicts the prognosis in metastatic castration-resistant prostate cancer patients in post chemotherapy setting. Eur. J. Cancer 2015, 51, 2570–2577. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Reyes, D.K.; Rowe, S.P.; Pienta, K.J. CT-based assessment of body composition following neoadjuvant chemohormonal therapy in patients with castration-naïve oligometastatic prostate cancer. Prostate 2021, 81, 127–134. [Google Scholar] [CrossRef]
- Salji, M.; Hendry, J.; Patel, A.; Ahmad, I.; Nixon, C.; Leung, H.Y. Peri-prostatic Fat Volume Measurement as a Predictive Tool for Castration Resistance in Advanced Prostate Cancer. Eur. Urol. Focus 2018, 4, 858–866. [Google Scholar] [CrossRef]
- Sasaki, T.; Sugino, Y.; Kato, M.; Nishikawa, K.; Kanda, H. Pre-treatment ratio of periprostatic to subcutaneous fat thickness on MRI is an independent survival predictor in hormone-naïve men with advanced prostate cancer. Int. J. Clin. Oncol. 2020, 25, 370–376. [Google Scholar] [CrossRef]
- Cai, T.; Cocci, A.; Di Maida, F.; Chiodini, S.; Ciarleglio, F.; Luciani, L.G.; Pedrotti, G.; Palmieri, A.; Malossini, G.; Rizzo, M.; et al. Visceral adiposity is associated with worse urinary and sexual function recovery after radical prostatectomy: Results from a longitudinal cohort study. Arch. Ital. Urol. Androl. 2021, 93, 285–290. [Google Scholar] [CrossRef] [PubMed]
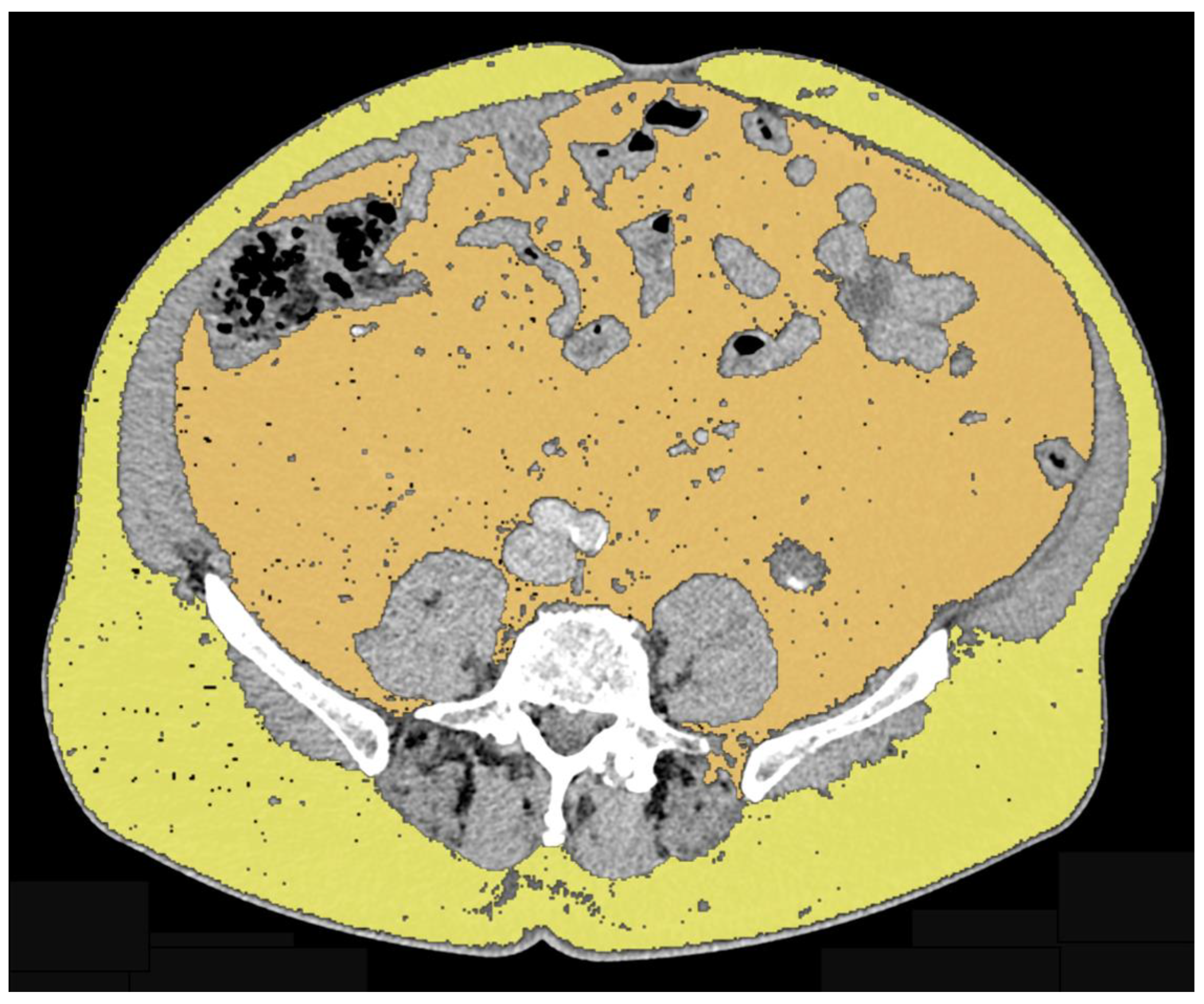
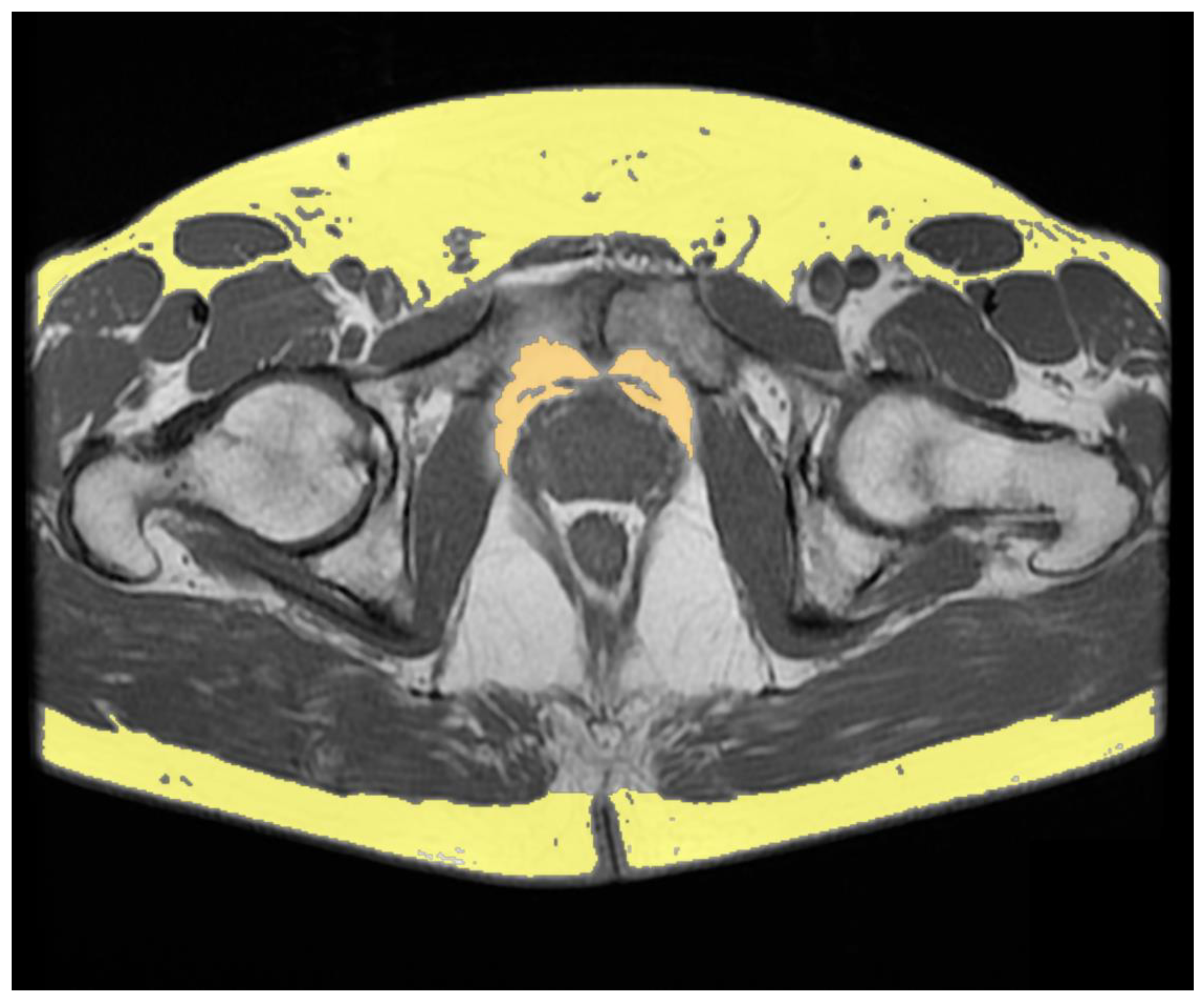
| Authors | Therapy | Adipose Tissue Compartments | Quantification Method | Number of Patients | Results |
|---|---|---|---|---|---|
| Antoun et al. (2015) [18] | Abiraterone acetateEnzalutamide | VAT SAT | Area at L3 level on axial plane using CT-based approach | 46 | Decrease in SAT (p < 0.001) Increase in VAT (p = 0.01) |
| Salji et al. (2018) [20] | Luteinizing hormone-releasing hormone analogues Androgen receptor blocker alone Estrogen patches Luteinizing hormone-releasing hormone antagonist | PPFV | Volume was calculated by consecutive areas using MRI-based approach on T2-weighted axial images | 61 | Higher PPFV in castration-resistant PCa patients compared to patients who showed a prolonged response to ADT (p < 0.0001). |
| Sasaki et al. (2020) [21] | Luteinizing hormone-releasing hormone agonist or antagonist Androgen blockade | Periprostatic adipose tissue thickness SAT thickness VAT SAT | Periprostatic fat thickness was measured on T2-weighted axial images at the femoral head and greater trochanter of the femur levels SAT thickness was measured on T2-weighted axial images at the maximum diameter of the bladder level VAT and SAT areas area were measured on axial plane using CT-based approach at the level of the umbilical position | 85 | Periprostatic adipose tissue/SAT thickness ratio ≥ 1 and overall survival Periprostatic adipose tissue/SAT thickness ratio < 1 and overall survival Univariate hazard ratio and multivariate hazard ratio showed p = 0.043 and p = 0.002, respectively VAT/SAT thickness ratio ≥ 1 and <1: no significant difference |
| Sheikhbahaei et al. (2021) [19] | Docetaxel Abiraterone Prednisone | TAT VAT SAT | Area at L3 level on axial plane using CT-based approach | 22 | Increased SAT areas on first and second follow-up CT exams (p = 0.002 and p < 0.001, respectively) Increased TAT area on second follow-up compared to baseline (p < 0.001) No significant change in VAT areas |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, F.; Tafuri, A.; Panunzio, A.; Beomonte Zobel, B.; Mallio, C.A. Relationship between Androgen Deprivation Therapy and Abdominal Adipose Tissue. Uro 2022, 2, 270-276. https://doi.org/10.3390/uro2040030
Greco F, Tafuri A, Panunzio A, Beomonte Zobel B, Mallio CA. Relationship between Androgen Deprivation Therapy and Abdominal Adipose Tissue. Uro. 2022; 2(4):270-276. https://doi.org/10.3390/uro2040030
Chicago/Turabian StyleGreco, Federico, Alessandro Tafuri, Andrea Panunzio, Bruno Beomonte Zobel, and Carlo Augusto Mallio. 2022. "Relationship between Androgen Deprivation Therapy and Abdominal Adipose Tissue" Uro 2, no. 4: 270-276. https://doi.org/10.3390/uro2040030
APA StyleGreco, F., Tafuri, A., Panunzio, A., Beomonte Zobel, B., & Mallio, C. A. (2022). Relationship between Androgen Deprivation Therapy and Abdominal Adipose Tissue. Uro, 2(4), 270-276. https://doi.org/10.3390/uro2040030










