1. Introduction
Clinical renal transplantation is so successful that the number of patients far exceeds the number of available organs by a ratio of 4:1 according to the United Network for Organ Sharing recent report [
1]. This organ shortage has forced transplant centers to use the expanded criteria donors with diabetes, hypertension, and advanced age to age-matched recipients. A large number of these kidneys with reduced creatinine clearance, which have been discarded as single grafts in the past, can be transplanted nowadays as dual kidneys to a single recipient to maximize the nephron mass. The first report on dual adult kidney transplants was presented at the 16th annual meeting of the American Society of Transplant Surgeons in Chicago in 1996 as a way to solve the donor–recipient discrepancy. However, despite over two decades of experience, due to technical difficulties, the dual transplant procedure is still underperformed in a few US centers, with only 1.6% of all kidneys transplanted as of 2017 [
2].
The majority of dual kidneys have been transplanted sequentially, one allograft after the other, in each iliac fossa [
3,
4], or through one long iliac transplant incision [
5,
6,
7]. They all required extensive surgical dissection, lengthy operative time, and exposed the elderly recipients to more risks attendant to their cardiovascular status. To avoid these drawbacks, a novel two-step technique of transplanting both kidneys with multiple vessels, en-bloc, into one iliac fossa, is described. A proposal for the identification of such kidneys, their procurement, and their placement is also presented. The study did not involve any identifiable patients and did not have to go through the institutional review board.
2. Material and Methods
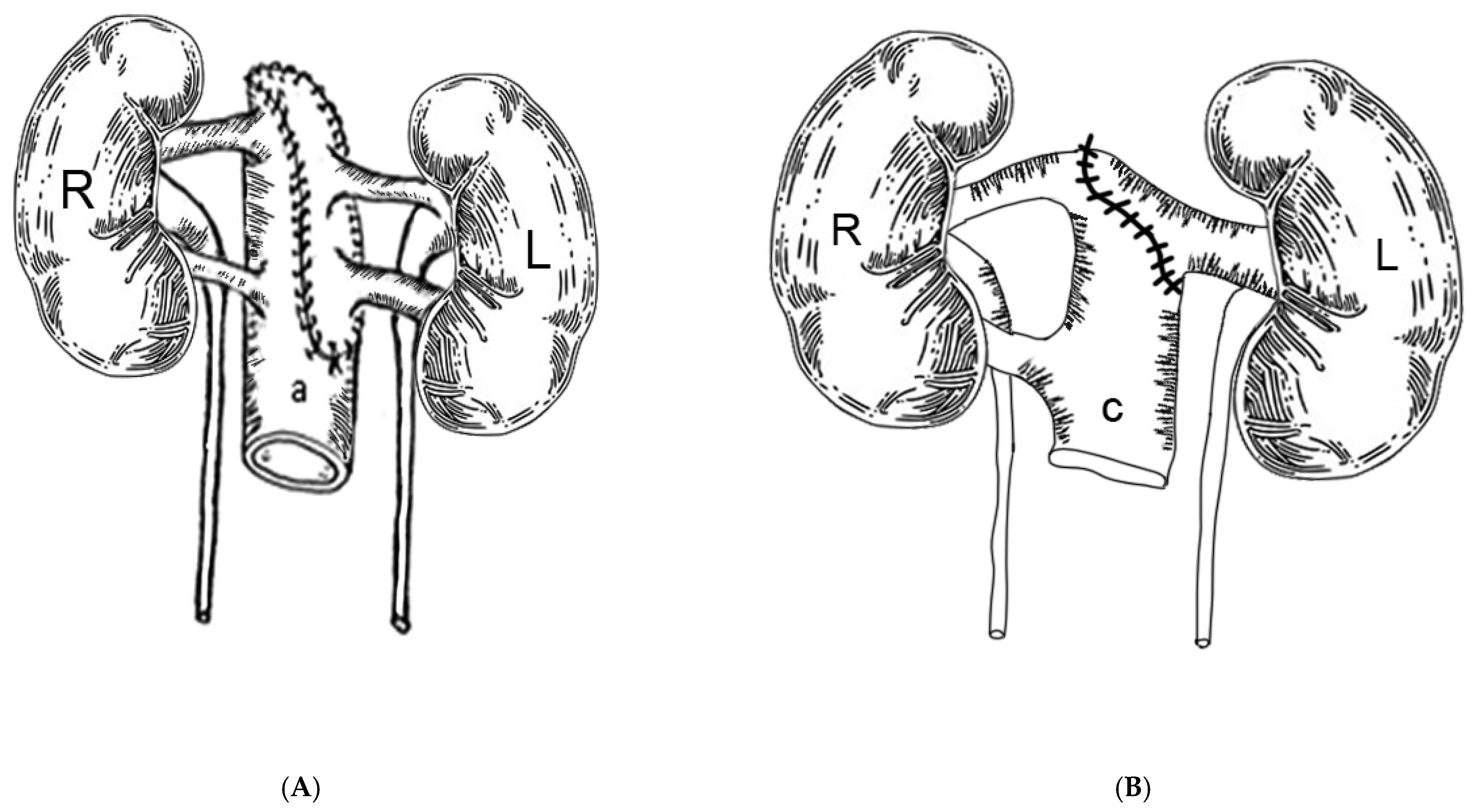
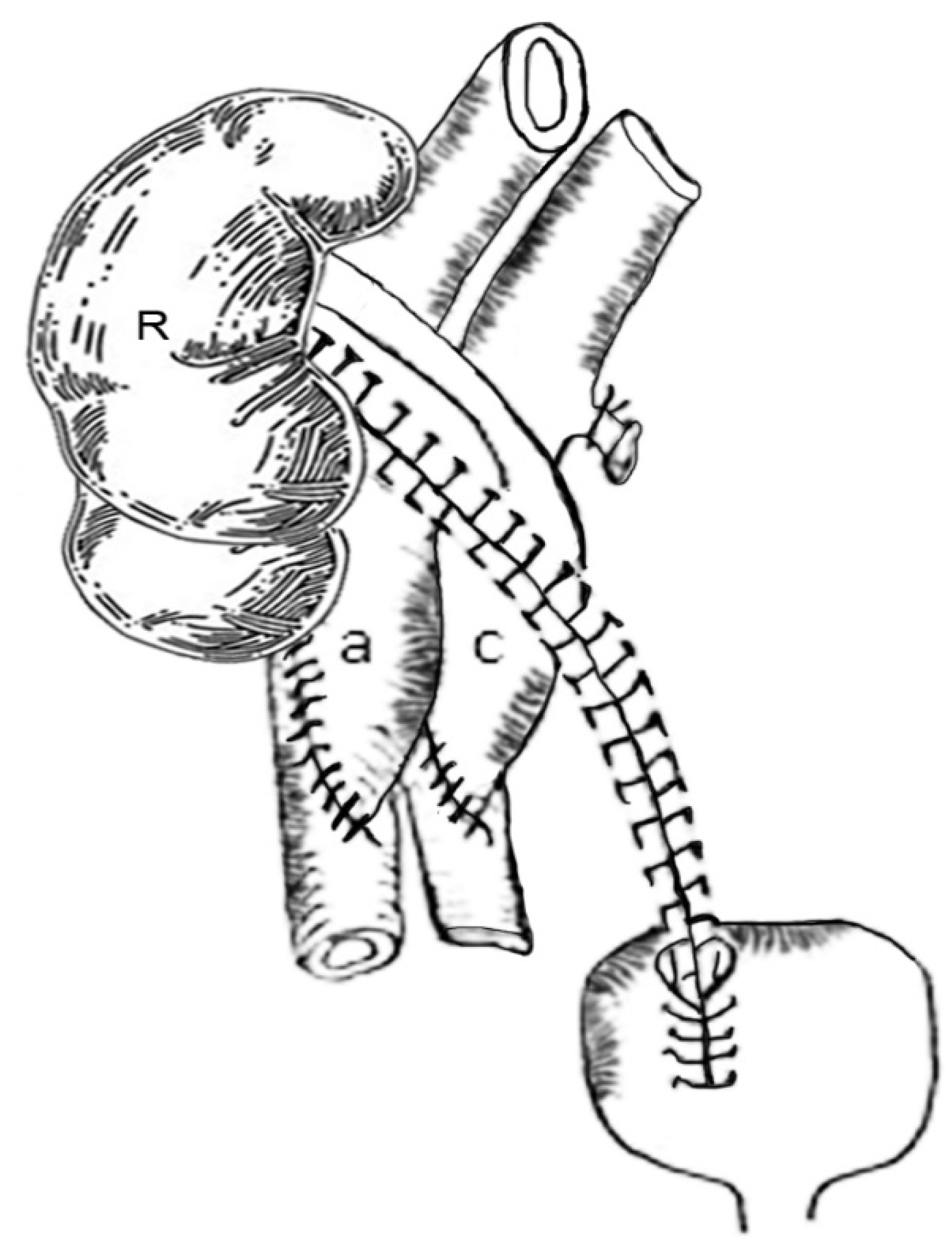
The technique is based first on the back table en-bloc reconstruction of the kidneys, as shown in the sketches. In most instances, both kidneys had been separated at the donor site, with the right kidney bearing 2 arteries on a short segment of arteriosclerosis free distal aorta and 3–5 cm of distal vena cava and a single vein, and the left kidney with a patch bearing two arteries and a long renal vein. The kidneys were dissected in a 4 °C ice saline bath, and the vessels were identified. The arterial patch bearing the left renal arteries was used to cap the proximal aortic cuff using continuous 5-0 polypropylene suture. Non-renal vessels were suture ligated. Thus, the distal aorta served as common arterial inflow to both kidneys (
Figure 1A). Next, the left renal vein was beveled and anastomosed to the proximal vena cava opening, making the distal vena cava the common venous outflow for both kidneys (
Figure 1B). A 12 F red rubber catheter was used as a temporary stent to avoid coaxial rotation of the renal vein during the reconstruction. When the vena cava segment was not available, the right renal vein was implanted end to side to the left renal vein, which then served as the common venous outflow for both kidneys. Non-renal venous collaterals were ligated. The ex vivo reconstruction took approximately one hour to perform while the elderly recipient was prepared by the anesthesia team.
Then, the reconstructed en-bloc kidneys were ready for transplantation. The right lower quadrant of the recipient was used preferentially, because the iliac vessels are more superficial, and more room is available than at the contralateral side. A regular right lower quadrant Gibson incision extending from the pubis to 2 cm above the anterior superior iliac spine was made, giving access to the right iliac retroperitoneal space. The external iliac vessels were dissected off their beds, and the lymphatic vessels were ligated carefully. Then, the en-bloc kidneys were inverted to place the venous outflow, i.e., the donor vena cava, in medial position, which is concordant with the iliac vein at the pelvic level. This step was crucial to prevent the scissoring of the aorta and the venous collaterals leading to renal vein thrombosis. When the left lower quadrant was used, because of a pre-existing right iliac kidney transplant or the presence of severe right iliac artery arteriosclerosis, no anatomic switch was necessary. The aorta and cava anastomoses were performed in an end-to-side fashion to the external iliac artery and vein, respectively, using #5-0 polypropylene sutures. At the time of venotomy, the iliac vein valve, if present, should be excised to facilitate the venous anastomosis. Afterwards, the distal and proximal venous and arterial vascular clamps, in this order, were removed. Both kidneys were re-perfused simultaneously. Once hemostasis was completed, the medial kidney (i.e., right donor kidney) was flipped over laterally and was stacked in front of the opposite kidney (i.e., left donor kidney). It usually wedged easily between the lateral kidney and the iliac wing without venous and arterial kinking. There was no need to “pex” the anterior kidney, since it will be maintained in place by the peritoneum falling back after closure of the incision. A 2 cm incision was made in the detrusor, and the underlying mucosa was freed extensively to accommodate loosely both ureters in their intravesical segment. The ureters were cut to length, spatulated posteriorly, anastomosed side to side at the ends with 5-0 polydioxanone sutures, and stented with a single 4.7 F × 22 cm long double pigtail catheter. Then, they were anastomosed to a distal small opening of the bladder mucosa in the extra-vesical fashion using an absorbable fine suture (
Figure 2). Then, the closure was tested by over-distending the bladder with irrigating antibiotic solution stained with indigo-carmine administered at 40 cm of water pressure through a Y connector attached to the Foley catheter. A blue-tinged leak should be closed by additional sutures. The detrusor incision was closed over the distal ureters using interrupted suture of 3-0 polydioxanone, thus burying both the ureterovesical anastomosis and the distal ureters. This extra-mucosal segment is responsible for maintaining the anti-reflux quality of the anastomosis; the abdominal incision was approximated without tension using #0 monofilament nonabsorbable suture for the muscular layer, #3-0 polypropylene for the fascia, and fine absorbable subcuticular sutures for the skin. Steri strips were applied. A Jackson Pratt drain was left in for 3 days. Despite the seemingly complicated procedure and the long vascular anastomoses, the entire procedure was completed within 3 h. This procedure was performed in four patients.
In three other patients, the kidneys have been recovered en-bloc and were shipped to our center en-bloc, with the cephalad aorta and vena cava stapled. The vessels were rapidly dissected, and the transplant procedure was carried out as described above.
All patients were treated initially with quadruple drug therapy with tacrolimus, mycophenolate mofetil, four days of steroids, and 5 days of anti-thymocyte globulin. They were subsequently maintained on tacrolimus and mycophenolate. Clinical rejection episodes were treated with 3–4 daily doses of 250 mg of methylprednisolone given intravenously. The Foley catheter was removed in 5–6 days, and the patient was instructed to void every 2 h. A bedside sonogram was obtained to ascertain complete bladder emptying prior to discharge. The ureteral stent was removed 6 weeks later by flexible cystoscopy during a clinic visit.
4. Discussion
Most of the dual kidneys from deceased donors can be transplanted, one after the other, in each iliac fossa through two separate iliac incisions, a midline incision [
3,
4], or a single long right Gibson incision [
5,
6,
7]. In the latter instance, the proximal kidney, most likely the right, was transplanted first and positioned behind the colon and outside of the true pelvis of the patient with the lengthened right renal vein connected to the vena cava or the common iliac vein. The arterial inflow came from the aorta or the common iliac artery. After the first kidney had been re-vascularized, the second kidney was transplanted distally and received its blood supply from the external iliac vessels. The two ureters were re-implanted in the bladder through one or two separate incisions. All these procedures required long and extensive surgical dissection and exposed the elderly recipients to more morbidity and complications. This touted “mono-lateral” or “ipsilateral” approach further exposed the long ureter of the proximal kidney to ischemic complications attendant to its length, leading to stenosis requiring secondary uretero-ureterostomy or pyelo-ureterostomy [
8]. It also increased considerably the operative time. Ekser reported that the operative time was significantly longer for dual kidneys (260 ± 36 min) than for single kidney transplants (157 ± 25 min) with
p < 0.001 [
6]. Gaber took 297 ± 32 min to perform dual transplantation, while single kidney transplantation took significantly less time with 219 ± 26 min with
p < 0.001 [
7]. Stratta recorded 1.4 h of cold ischemia time difference between the two transplants [
9]. On the contrary, Salehipour reported 180 min (range 165–210 min) for the en-bloc procedure [
10], while Tran et al. reported an operative time of 206 ± 57 min [
11]. Our operative time averaged 180 min. This technique greatly expedites the transplant procedure by requiring only two anastomoses to perform instead of four as required by the sequential procedure, when the kidneys have one single renal artery and one single renal vein. It can also accommodate multiple renal arteries and multiple veins as in our illustrated cases, since they all have a common aortic inflow and a single cava outflow, as shown in
Figure 1A,B. Technically, it is rather much simpler and less demanding to connect the donor aorta in an end-to-side fashion to the external iliac artery than to anastomose the long aortic patch encompassing two arteries, as described in the patch technique [
12,
13]. Indeed, it is accepted procedure now, twenty years after its introduction, to use the whole vena cava for venous outflow to simplify the venous anastomoses [
10,
11,
12,
13,
14]. It simplifies the organ recovery procedure by keeping the kidneys en-bloc and preserving both distal aorta and vena cava. It requires a donor aorta relatively free of arteriosclerosis. In fact, endarterectomy of the aorta has not been required in all our donors over 65 years old. Venous thrombosis affecting the medial kidney, a feared complication of the splayed out en-bloc dual adult transplantation with venous kinking, had not been encountered since the medial kidney had been positioned in front of the lateral kidney thus allowing the veins to course smoothly without kinking even after post-operative shifting.
The use of a single long double J ureteral stent made the ureteral re-implantation much easier and the stent removal faster, since it requires only one single cystoscopic pass [
15]. The systematic use of the stent combined with a short exposure to corticosteroid medications may have allowed the ureteral implantation site to heal without complications.
Long-term complications previously described [
7,
8], such as distal ureterovesical tunnel stenosis, or more extensive ureteral stenoses caused by ureteral ischemia, has not been observed in this series. In case of eventual thrombosis, absent in our series, the kidney or kidneys can be removed by the intra-capsular technique without difficulties [
16].
The recovery of the en-bloc kidneys is also simple, and the recovery surgeon does not need extra training. As usual, cannulation for aortic retrograde cold perfusion can be carried out routinely through one iliac artery with the ipsilateral iliac vein vented. After blanching of all intra-abdominal organs, the supra renal aorta and vena cava can be stapled twice and transected above the staple lines. The en-bloc kidneys can be lifted off the vertebral bodies after severing the posterior spinal collaterals, going from cephalad to caudad. No dissection of the renal pedicles is required. The ureters are cut at their junctions with the bladder. Then, the distal aorta and vena cava are transected proximal to the iliac bifurcation. The en-bloc kidneys are packed en-bloc in the usual manner and sent to the receiving transplant center [
17].
If the technique of en-bloc dual transplantation is straightforward, the selection of the kidneys for dual transplantation is still the subject of discussion by different Organ Procurement Organizations and the United Network for Organ Sharing, which allocated the kidneys according to the results of the HLA matching, the biopsy results, and other donor variables known as the Kidney Donor Profile Index [
18], which are all factors contributing to increasing the cold ischemia time and the organ discard from 10% in 1998 to 18.7% in 2014. Particularly for kidneys from donors >66 years old, the discard rate approached 60%. Of the 6643 kidneys recovered during the period 2010–2015, all kidneys with KDPI between 88% and 92% were discarded [
9]. Since the inception of our dual transplant program, in agreement with the board of directors of the Center for Organ Recovery and Education in Pittsburgh, Pennsylvania, we have elected to require from the kidney donor over age 65 a calculated creatinine clearance of 50–65 mL/min/1.73 m
2 (equivalent to the function of a living donor kidney) determined by the Cockcroft–Gault equation as criteria for the acceptance of dual kidneys [
19]. Even with these requirements, kidneys were considered for dual transplants only after they had been turned down by all other transplant centers in our OPO region, hence the small numbers of kidneys available for dual transplantation and the long cold ischemia time. We suggest that this calculated pre-recovery renal function, or the estimated GFR provided currently by the blood test, be adopted by all OPOs and transplant centers performing the dual transplant procedure as the standard for selection and allocation of dual kidneys from donors over the age of 66. This is in keeping with data using donor-estimated glomerular function rate as the criterion for the allocation of the extended criteria donor kidneys, showing that both the Dual Kidney Transplant group (with 81 dual kidneys) with an estimated GFR of 30–60 mL/min and the Single Kidney Transplant group (with 70 kidneys) with an estimated GFR of >60 mL/min had similar patient and graft survival at 2 years [
20].
This will efficiently expedite the dual kidney placement and reduce the cold ischemic time. Other data such as biopsy results, KDRI, and KDPI currently used for single organ allocation should not be applicable to dual adult kidneys identification and allocation. They only increase the cold ischemia time, which is particularly detrimental to the extended criteria kidneys donor by increasing the rate of delayed graft function [
8], and decrease the organ acceptance rate.
Finally, a list of transplant centers performing the dual transplant procedure should be drafted readily by UNOS and OPOs to simplify and expedite the placement of these kidneys and improve graft utilization and function. In our series, the long-term follow-up data revealed a mean serum creatinine level of 1.8 ± 0.16 mg/dL, which compared very favorably with 2.3 mg ± 0.16 mg/dL for other single kidneys from standard criteria donors performed during the same period.
 .
.
 .
.





