Multiple Adverse Drug Reactions to Calcineurin Inhibitors in a Renal Transplant Patient
Abstract
1. Introduction
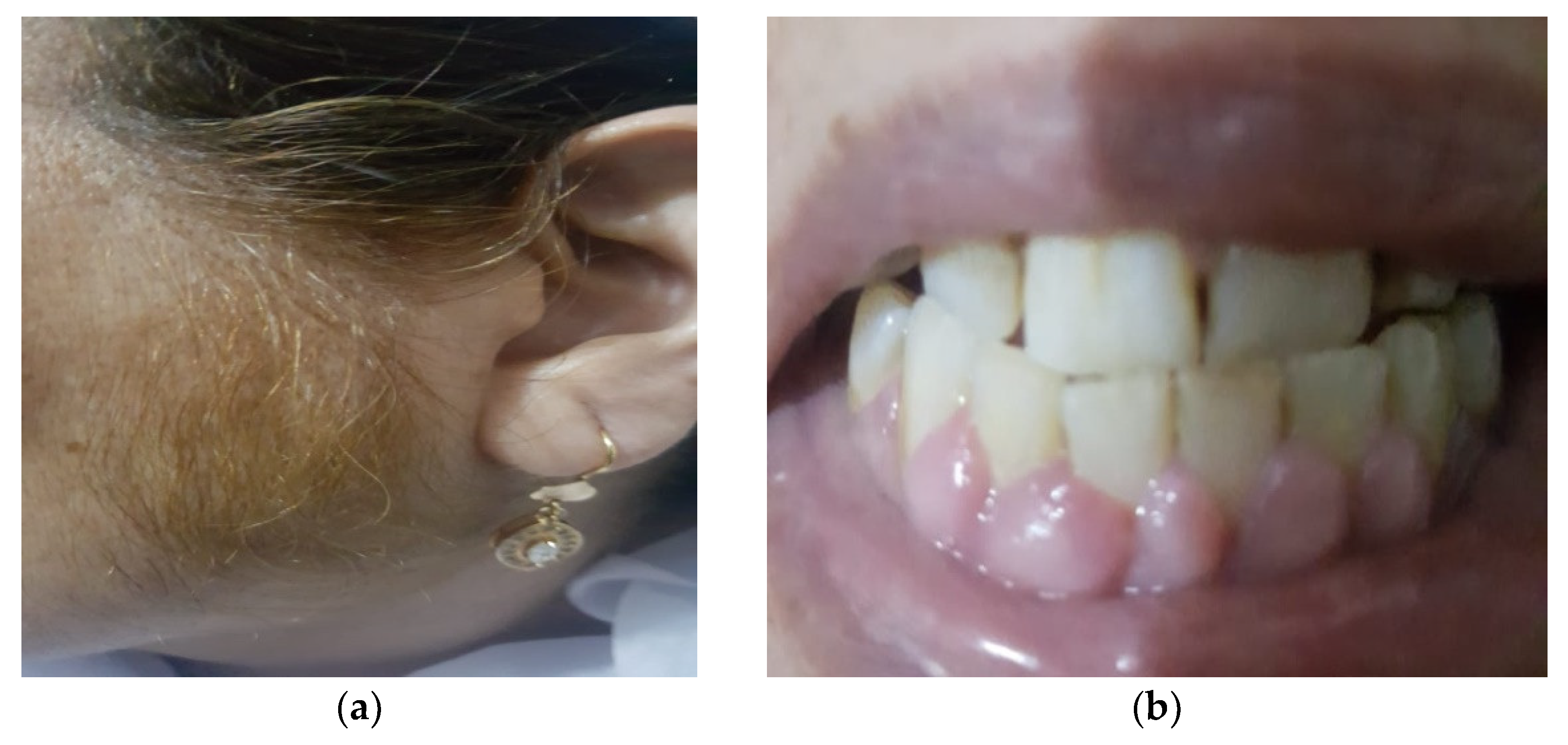
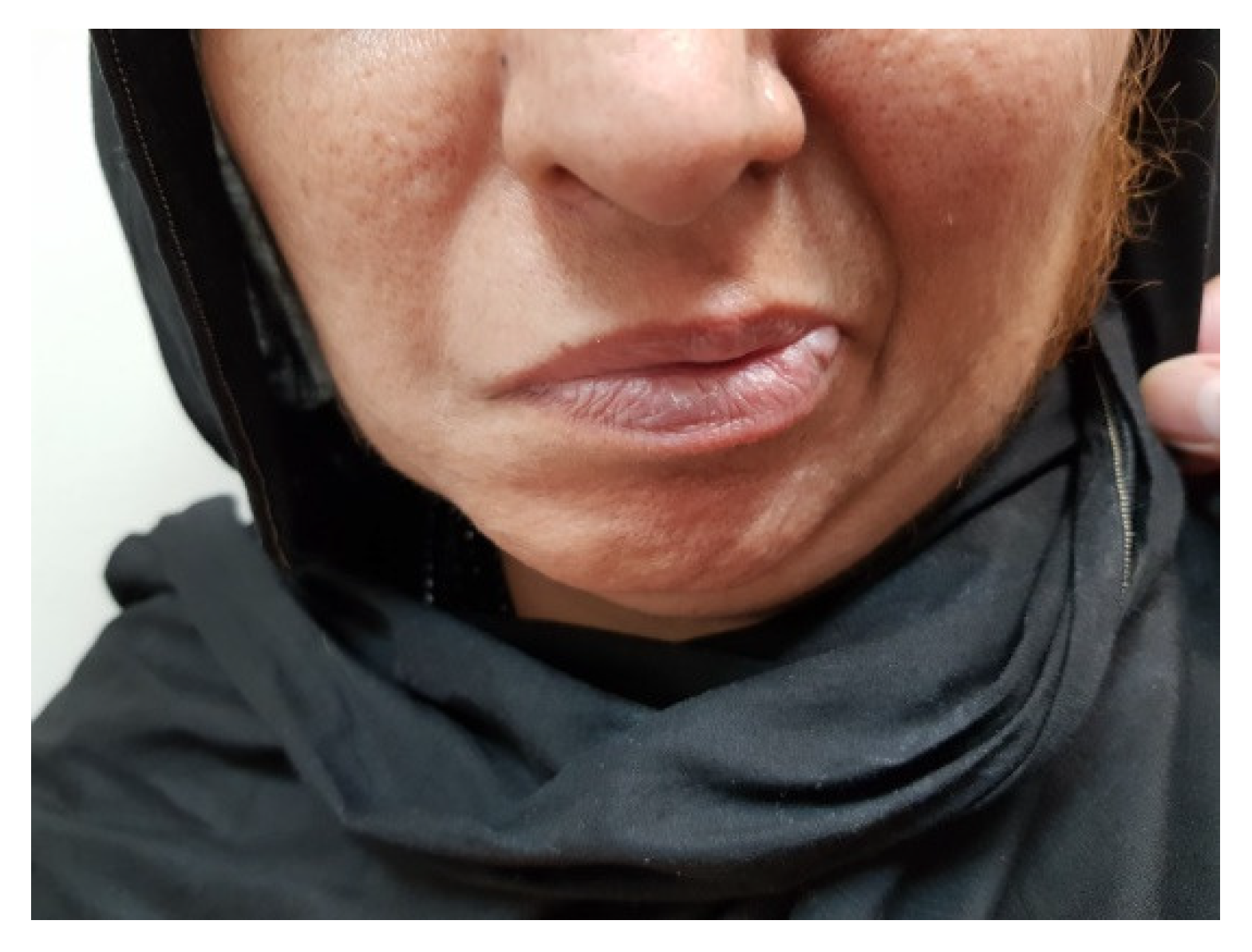
2. Case Presentation
3. Discussion
3.1. Gingival Hyperplasia (GH)
3.2. Hypertrichosis
3.3. Neurotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Liu, J.; You, R.; Guo, M.; Zeng, L.; Zhou, P.; Zhu, L.; Xu, G.; Li, J.; Liu, D. Tacrolimus versus cyclosporine as primary immunosuppressant after renal transplantation: A Meta-Analysis and economics evaluation. Am. J. Ther. 2016, 23, e810–e824. [Google Scholar] [CrossRef]
- Krejci, K.; Tichy, T.; Bachleda, P.; Zadrazil, J. Calcineurin inhibitor-induced renal allograft nephrotoxicity. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2010, 154, 297–306. [Google Scholar] [CrossRef]
- Ding, H.; Li, Z.; Zhang, J. A case report of cyclosporine-induced myopathy with subacute muscular atrophy as initial presentation. Medicine 2019, 98, e15206. [Google Scholar] [CrossRef]
- Barbarino, J.M.; Staatz, C.; Venkataramanan, R.; Klein, T.E.; Altman, R.B. PharmGKB summary: Cyclosporine and tacrolimus pathways. Pharm. Genom. 2013, 23, 563–585. [Google Scholar] [CrossRef]
- Thomson, A.W.; Bonham, C.A.; Zeevi, A. Mode of Action of Tacrolimus (FK506): Molecular and Cellular Mechanisms. Ther. Drug Monit. 1995, 17, 584–591. [Google Scholar] [CrossRef]
- Kino, T.; Hatanaka, H.; Miyata, S.; Inamura, N.; Nishiyama, M.; Yajima, T.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. J. Antibiot. 1987, 40, 1256–1265. [Google Scholar] [CrossRef]
- Kapturczak, M.; Meier-Kriesche, H.; Kaplan, B. (Eds.) Pharmacology of calcineurin antagonists. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Seymen, P.; Yildiz, M.; Türkmen, M.; Titiz, M.; Seymen, H. (Eds.) Effects of cyclosporine-tacrolimus switching in posttransplantation hyperlipidemia on high-density lipoprotein 2/3, lipoprotein a1/b, and other lipid parameters. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Rateitschak-Pluss, E.M.; Hefti, A.; Lörtscher, R.; Thiel, G. Initial observation that cyclosporin-A induces gingival enlargement in man. J. Clin. Periodontol. 1983, 10, 237–246. [Google Scholar] [CrossRef]
- Wysocki, G.P.; Gretzinger, H.A.; Laupacis, A.; Ulan, R.A.; Stiller, C.R. Fibrous hyperplasia of the gingiva: A side effect of cyclosporin A therapy. Oral Surg. Oral Med. Oral Pathol. 1983, 55, 274–278. [Google Scholar] [CrossRef]
- Kumar, S.; Guliani, A.; Vinay, K. Cyclosporine-Induced Gingival Hypertrophy. JAMA Dermatol. 2019, 155, 487. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Busto, U.; Sellers, E.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Nanda, T.; Singh, B.; Sharma, P.; Arora, K.S. Cyclosporine A and amlodipine induced gingival overgrowth in a kidney transplant recipient: Case presentation with literature review. BMJ Case Rep. 2019, 12, e229587. [Google Scholar] [CrossRef]
- Madi, M.; Shetty, S.; Babu, S.; Achalli, S. Amlodipine Induced Gingival Hyperplasia—A Case Report and Review. West Indian Med. J. 2015, 64, 279–282. [Google Scholar] [CrossRef][Green Version]
- Ponnaiyan, D.; Jegadeesan, V. Cyclosporine A: Novel concepts in its role in drug-induced gingival overgrowth. Dent. Res. J. 2015, 12, 499–506. [Google Scholar] [CrossRef]
- Gaphor, S.M.; Abdulkareem, S.A.; Abdullah, M.J. Cyclosporine induced gingival hyperplasia in kidney transplant: A case report and review of the literature. Eur. Sci. J. 2014, 10, 857–881. [Google Scholar]
- Ellis, J.; Seymour, R.; Thomason, J.; Monkman, S.; Idle, J. Gingival sequestration of amlodipine and amlodipine-induced gingival overgrowth. Lancet 1993, 341, 1102–1103. [Google Scholar] [CrossRef]
- James, J.A.; Marley, J.J.; Jamal, S.; Campbell, B.A.; Short, C.D.; Johnson, R.W.G.; Hull, P.S.; Spratt, H.; Irwin, C.R.; Boomer, S.; et al. The calcium channel blocker used with cyclosporin has an effect on gingival overgrowth. J. Clin. Periodontol. 2000, 27, 109–115. [Google Scholar] [CrossRef]
- Mehta, D.S.; Triveni, M.G.; Rudrakshi, C. Amlodipine-induced gingival overgrowth. J. Indian Soc. Periodontol. 2009, 13, 160–163. [Google Scholar] [CrossRef]
- Bencini, P.; Montagnino, G.; Sala, F.; De Vecchi, A.; Crosti, C.; Tarantino, A. Cutaneous Lesions in 67 Cyclosporin-Treated Renal Transplant Recipients. Dermatology 1986, 172, 24–30. [Google Scholar] [CrossRef]
- Tan, T.C.; Robinson, P.J. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant. Rev. 2006, 20, 49–60. [Google Scholar] [CrossRef]
- Wu, G.; Weng, F.L.; Balaraman, V. Tacrolimus-induced encephalopathy and polyneuropathy in a renal transplant recipient. BMJ Case Rep. 2013, 2013, bcr2013201099. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bonham, A.; Fukui, M. Immunosuppressive-associated leukoencephalopathy in organ transplant recipients. Transplantation 2000, 69, 467–472. [Google Scholar] [CrossRef]
- Gijtenbeek, J.M.M.; Bent, M.V.D.; Vecht, C.J. Cyclosporine neurotoxicity: A review. J. Neurol. 1999, 246, 339–346. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, R.; Hassan, Z.; Haseeb, A.; Masood, A.; Ali, I. Multiple Adverse Drug Reactions to Calcineurin Inhibitors in a Renal Transplant Patient. Uro 2021, 1, 180-186. https://doi.org/10.3390/uro1030018
Ahmed R, Hassan Z, Haseeb A, Masood A, Ali I. Multiple Adverse Drug Reactions to Calcineurin Inhibitors in a Renal Transplant Patient. Uro. 2021; 1(3):180-186. https://doi.org/10.3390/uro1030018
Chicago/Turabian StyleAhmed, Raheel, Zair Hassan, Abdul Haseeb, Aysha Masood, and Iftikhar Ali. 2021. "Multiple Adverse Drug Reactions to Calcineurin Inhibitors in a Renal Transplant Patient" Uro 1, no. 3: 180-186. https://doi.org/10.3390/uro1030018
APA StyleAhmed, R., Hassan, Z., Haseeb, A., Masood, A., & Ali, I. (2021). Multiple Adverse Drug Reactions to Calcineurin Inhibitors in a Renal Transplant Patient. Uro, 1(3), 180-186. https://doi.org/10.3390/uro1030018





