Hepatotoxicity of Herbal and Dietary Supplements a Review of Diagnosis, Histologic Features, and Common Culprits: Bodybuilding and Weight Loss Supplements
Abstract
1. Introduction
2. DILI Classification
3. Herbal and Dietary Supplements (HDS) Classification
4. Mechanisms of Liver Injury Induced by Drugs and Herbal Supplements
4.1. Formation of Reactive Metabolites
4.2. Mitochondrial Dysfunction
4.3. Oxidative Stress
4.4. Immune-Mediated Injury
4.5. Disruption of Bile Salt Transport
4.6. Endoplasmic Reticulum (ER) Stress
4.7. Genetic Susceptibility and Polymorphisms
4.8. Disruption of Lipid Metabolism
5. Clinical Diagnosis of Drug and Herbal-Induced Hepatic Injury
- -
- Temporal association: exposure to the suspected agent must precede the onset of liver injury, though latency periods can vary widely.
- -
- Exclusion of alternative causes: common liver diseases (e.g., viral hepatitis, autoimmune hepatitis, biliary disorders, genetic conditions) must be ruled out.
- -
- De-challenge: liver injury typically improves upon discontinuation of the possible offending supplement or drug.
- -
- Rechallenge: a second exposure could lead to a more rapid and pronounced liver injury.
- (1)
- time to onset (+1 or +2).
- (2)
- course (−2, 0, +1, +2, or +3).
- (3)
- risk factors (2 scores: 0 or +1 each).
- (4)
- concomitant drugs (0, −1, −2, or −3).
- (5)
- nondrug causes of liver injury (−3, −2, 0, +1, or +2).
- (6)
- previous information on the hepatotoxicity of the drug (0, +1, or +2); and
- (7)
- response to rechallenge (−2, 0, +1, or +3).
- ≤0: Excluded
- 1–2: Unlikely
- 3–5: Possible
- 6–8: Probable
- 8: Highly probable
6. Histological Diagnosis and Morphological Patterns of Drug and Herbal-Induced Hepatic Injury
6.1. Cholestatic Injury
6.2. Hepatocellular Injury
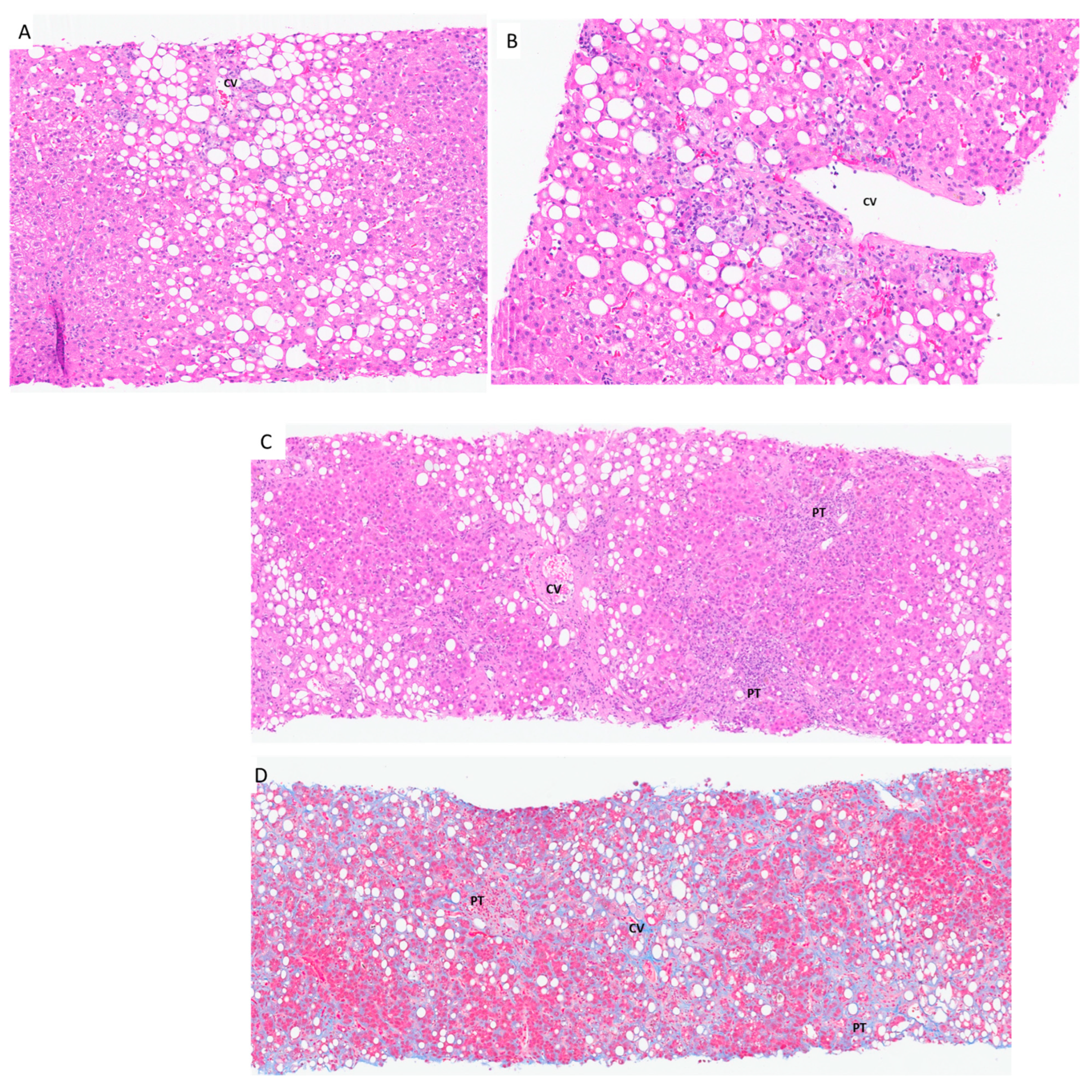
6.3. Steatosis and/or Steatohepatitis Due to Drug-Induced Liver Injury
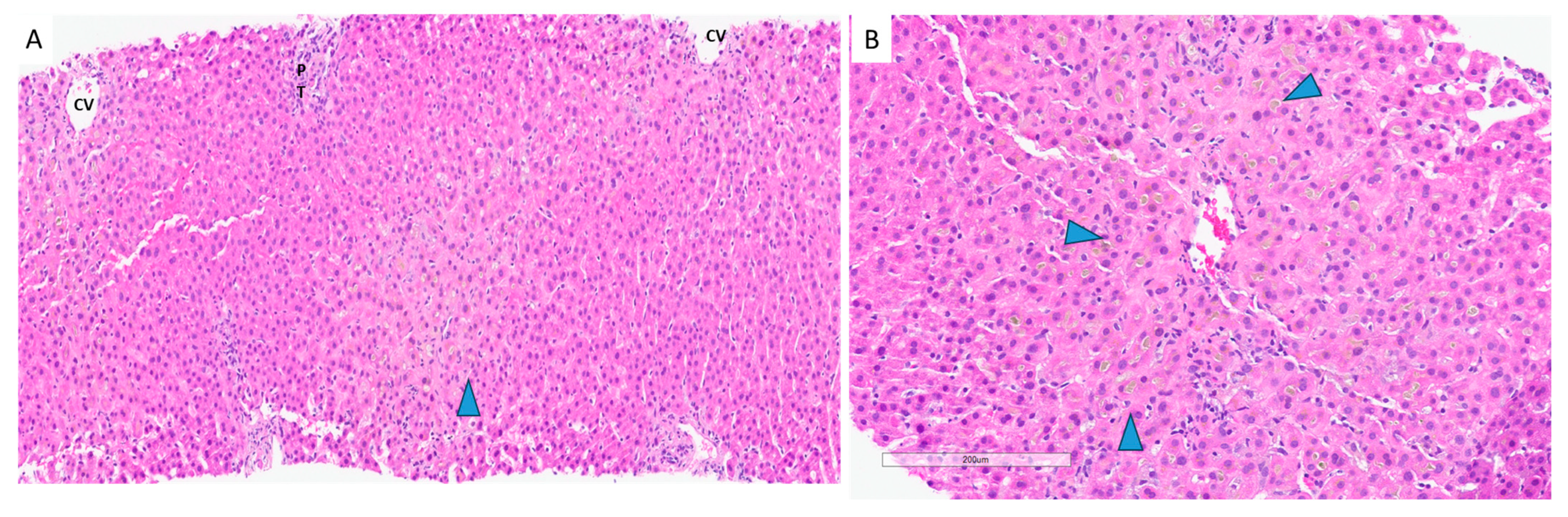
7. Hepatotoxicity Due to Bodybuilding Supplements: Selective Androgen Receptor Modulators (SARMs)
8. Hepatotoxicity Due to Weight Loss Supplements
9. Discussion
Funding
Data Availability Statement
Conflicts of Interest
References
- Navarro, V.J.; Khan, I.; Björnsson, E.; Seeff, L.B.; Serrano, J.; Hoofnagle, J.H. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373. [Google Scholar] [CrossRef]
- Bailey, R.L.; Gahche, J.J.; Lentino, C.V.; Dwyer, J.T.; Engel, J.S.; Thomas, P.R.; Betz, J.M.; Sempos, C.T.; Picciano, M.F. Dietary supplement use in the United States, 2003–2006. J. Nutr. 2011, 141, 261–266. [Google Scholar] [CrossRef] [PubMed]
- NIH DSHEA 1994. Available online: https://ods.od.nih.gov/About/DSHEA_Wording.aspx (accessed on 19 August 2025).
- Navarro, V.; Avula, B.; Khan, I.; Verma, M.; Seeff, L.; Serrano, J.; Stolz, A.; Fontana, R.; Ahmad, J. The Contents of Herbal and Dietary Supplements Implicated in Liver Injury in the United States Are Frequently Mislabeled. Hepatol Commun. 2019, 3, 792–794. [Google Scholar] [CrossRef] [PubMed]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/pubmed/NBK547852/ (accessed on 25 November 2023).
- Zhu, J.; Seo, J.E.; Wang, S.; Ashby, K.; Ballard, R.; Yu, D.; Ning, B.; Agarwal, R.; Borlak, J.; Tong, W.; et al. The Development of a Database for Herbal and Dietary Supplement Induced Liver Toxicity. Int. J. Mol. Sci. 2018, 19, 2955. [Google Scholar] [CrossRef]
- Avigan, M.I.; Mozersky, R.P.; Seeff, L.B. Scientific and Regulatory Perspectives in Herbal and Dietary Supplement Associated Hepatotoxicity in the United States. Int. J. Mol. Sci. 2016, 17, 331. [Google Scholar] [CrossRef]
- Navarro, V.J.; Barnhart, H.; Bonkovsky, H.L.; Davern, T.; Fontana, R.J.; Grant, L.; Reddy, K.R.; Seeff, L.B.; Serrano, J.; Sherker, A.H.; et al. Liver injury from herbals and dietary supplements in the U. S. Drug-Induced Liver Injury Network. Hepatology 2014, 60, 1399–1408. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, R. Review article: Herbal and dietary supplement hepatotoxicity. Aliment. Pharmacol. Ther. 2012, 37, 3–17. [Google Scholar] [CrossRef]
- Mehta, D.H.; Gardiner, P.M.; Phillips, R.S.; McCarthy, E.P. Herbal and dietary supplement disclosure to health care providers by individuals with chronic conditions. J. Altern. Complement. Med. 2008, 14, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Kessebohm, K.; Weimann, R.; Seitz, H.K. Review of liver injury associated with dietary supplements. Liver Int. 2011, 31, 595–605. [Google Scholar] [CrossRef]
- Navarro, V.J. Herbal and dietary supplement hepatotoxicity. Semin. Liver Dis. 2009, 29, 373–382. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Maddur, H.; Russo, M.W.; Wong, R.J.; Reddy, K.R. ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am. J. Gastroenterol. 2021, 116, 878–898. [Google Scholar] [CrossRef]
- Zheng, E.; Sandhu, N.; Navarro, V. Drug-induced Liver Injury Secondary to Herbal and Dietary Supplements. Clin. Liver Dis. 2020, 24, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Seeff, L.B.; Bonkovsky, H.L.; Navarro, V.J.; Wang, G.Q. Herbal Products and the Liver: A Review of Adverse Effects and Mechanisms. Gastroenterology 2015, 148, 517–532.e3. [Google Scholar] [CrossRef] [PubMed]
- Torgersen, J.; Mezochow, A.K.; Newcomb, C.W.; Carbonari, D.M.; Hennessy, S.; Rentsch, C.T.; Park, L.S.; Tate, J.P.; Bräu, N.; Bhattacharya, D.; et al. Severe Acute Liver Injury After Hepatotoxic Medication Initiation in Real-World Data. JAMA Intern. Med. 2024, 184, 943–952. [Google Scholar] [CrossRef]
- Stephens, C.; Lucena, M.I.; Andrade, R.J. Genetic variations in drug-induced liver injury (DILI): Resolving the puzzle. Front. Genet. 2012, 3, 253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- López-Pascual, E.; Rienda, I.; Perez-Rojas, J.; Rapisarda, A.; Garcia-Llorens, G.; Jover, R.; Castell, J.V. Drug-Induced Fatty Liver Disease (DIFLD): A Comprehensive Analysis of Clinical, Biochemical, and Histopathological Data for Mechanisms Identification and Consistency with Current Adverse Outcome Pathways. Int. J. Mol. Sci. 2024, 25, 5203. [Google Scholar] [CrossRef]
- Hayashi, P.H.; Hoofnagle, J.H. Diagnostic challenges in drug-induced liver injury. Clin. Liver Dis. 2024, 23, e0206. [Google Scholar] [CrossRef]
- Lin, J.K.-S.; Tujios, S.R. Hidden Dangers: Herbal and Dietary Supplement Induced Hepatotoxicity. Livers 2023, 3, 618–636. [Google Scholar] [CrossRef]
- Danan, G.; Benichou, C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 1993, 46, 1323–1330. [Google Scholar] [CrossRef]
- Benichou, C.; Danan, G.; Flahault, A. Causality assessment of adverse reactions to drugs--II. An original model for validation of drug causality assessment methods: Case reports with positive rechallenge. J. Clin. Epidemiol. 1993, 46, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Danan, G.; Teschke, R. Roussel Uclaf Causality Assessment Method for Drug-Induced Liver Injury: Present and Future. Front. Pharmacol. 2019, 10, 853. [Google Scholar] [CrossRef]
- Roussel Uclaf Causality Assessment Method (RUCAM) in Drug Induced Liver Injury. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bathesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548272/ (accessed on 19 August 2025).
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef]
- Miller, C.P.; Shomali, M.; Lyttle, C.R.; O’Dea, L.S.; Herendeen, H.; Gallacher, K.; Paquin, D.; Compton, D.R.; Sahoo, B.; Kerrigan, S.A.; et al. Design, Synthesis, and Preclinical Characterization of the Selective Androgen Receptor Modulator (SARM) RAD140. ACS Med. Chem. Lett. 2010, 2, 124–129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dobs, A.S.; Boccia, R.V.; Croot, C.C.; Gabrail, N.Y.; Dalton, J.T.; Hancock, M.L.; Johnston, M.A.; Steiner, M.S. Effects of enobosarm on muscle wasting and physical function in patients with cancer: A double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013, 14, 335–345. [Google Scholar] [CrossRef]
- The prohibited list. World Anti Doping Agency. Available online: https://www.wada-ama.org/en/prohibited-list?page=0&q=rad140&all=1#search-anchor (accessed on 20 June 2024).
- Leung, K.; Yaramada, P.; Goyal, P.; Cai, C.X.; Thung, I.; Hammami, M.B. RAD-140 Drug-Induced Liver Injury. Ochsner J. 2022, 22, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Barbara, M.; Dhingra, S.; Mindikoglu, A.L. Drug-Induced Liver Injury Associated with Alpha Bolic (RAD-140) and Alpha Elite (RAD-140 and LGD-4033). ACG Case Rep. J. 2020, 7, e00409. [Google Scholar] [CrossRef]
- Ladna, M.; Taylor, K.; Bhat, A.; Dideban, B. Idiosyncratic drug-induced liver injury related to use of novel selective androgen receptor modulator RAD140 (Testalone): A case report. J. Med. Case Rep. 2023, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Supplement Connect High Risk List: USADA. U.S. Anti-Doping Agency (USADA). Available online: https://www.usada.org/athletes/substances/supplement-connect/high-risk-list/ (accessed on 20 June 2024).
- Performance Enhancing Substance: MK-677 (Ibutamoren). Opss. Available online: https://www.opss.org/article/performance-enhancing-substance-mk-677-ibutamoren (accessed on 20 June 2024).
- Sigalos, J.T.; Pastuszak, A.W. The Safety and Efficacy of Growth Hormone Secretagogues. Sex Med. Rev. 2018, 6, 45–53. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Eichner, A.; Bhasin, S.; Deuster, P.A.; Eichner, D. Chemical Composition and Labeling of Substances Marketed as Selective Androgen Receptor Modulators and Sold via the Internet. JAMA 2017, 318, 2004–2010. [Google Scholar] [CrossRef]
- Patel, S.S.; Beer, S.; Kearney, D.L.; Phillips, G.; Carter, B.A. Green tea extract: A potential cause of acute liver failure. World J. Gastroenterol. 2013, 19, 5174–5177. [Google Scholar] [CrossRef]
- Surapaneni, B.K.; Le, M.; Jakobovits, J.; Vinayek, R.; Dutta, S. A Case of Acute Severe Hepatotoxicity and Mild Constriction of Common Bile Duct Associated with Ingestion of Green Tea Extract: A Clinical Challenge. Clin. Med. Insights Gastroenterol. 2018, 11, 1179552218779970. [Google Scholar] [CrossRef]
- Mazzanti, G.; Menniti-Ippolito, F.; Moro, P.A.; Cassetti, F.; Raschetti, R.; Santuccio, C.; Mastrangelo, S. Hepatotoxicity from green tea: A review of the literature and two unpublished cases. Eur. J. Clin. Pharmacol. 2009, 65, 331–341. [Google Scholar] [CrossRef]
- Molinari, M.; Watt, K.D.; Kruszyna, T.; Nelson, R.; Walsh, M.; Huang, W.Y.; Nashan, B.; Peltekian, K. Acute liver failure induced by green tea extracts: Case report and review of the literature. Liver Transpl. 2006, 12, 1892–1895. [Google Scholar] [CrossRef]
- Lynch, C.R.; Folkers, M.E.; Hutson, W.R. Fulminant hepatic failure associated with the use of black cohosh: A case report. Liver Transpl. 2006, 12, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choi, M.S.; Park, Y.B.; Kim, S.R.; Lee, M.K.; Jung, U.J. Garcinia Cambogia attenuates diet-induced adiposity but exacerbates hepatic collagen accumulation and inflammation. World J. Gastroenterol. 2013, 19, 4689–4701. [Google Scholar] [CrossRef]
- Pittler, M.H.; Ernst, E. Dietary supplements for body-weight reduction: A systematic review. Am. J. Clin. Nutr. 2004, 79, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Croce, N.; Pitaro, M.; Gallo, V.; Antonini, G. Toxicity of Usnic Acid: A Narrative Review. J. Toxicol. 2022, 2022, 8244340. [Google Scholar] [CrossRef] [PubMed]
- Favreau, J.T.; Ryu, M.L.; Braunstein, G.; Orshansky, G.; Park, S.S.; Coody, G.L.; Love, L.A.; Fong, T.L. Severe hepatotoxicity associated with the dietary supplement LipoKinetix. Ann. Intern Med. 2002, 136, 590–595. [Google Scholar] [CrossRef]
- Mahady, G.B.; Low Dog, T.; Barrett, M.L.; Chavez, M.L.; Gardiner, P.; Ko, R.; Marles, R.J.; Pellicore, L.S.; Giancaspro, G.I.; Sarma, D.N. United States Pharmacopeia review of the black cohosh case reports of hepatotoxicity. Menopause 2008, 15, 628–638. [Google Scholar] [CrossRef]
- Guzman, G.; Kallwitz, E.R.; Wojewoda, C.; Chennuri, R.; Berkes, J.; Layden, T.J.; Cotler, S.J. Liver Injury with Features Mimicking Autoimmune Hepatitis following the Use of Black Cohosh. Case Rep. Med. 2009, 2009, 918156. [Google Scholar] [CrossRef]
- Enbom, E.T.; Le, M.D.; Oesterich, L.; Rutgers, J.; French, S.W. Mechanism of hepatotoxicity due to black cohosh (Cimicifuga racemosa): Histological, immunohistochemical and electron microscopy analysis of two liver biopsies with clinical correlation. Exp. Mol. Pathol. 2014, 96, 279–283. [Google Scholar] [CrossRef]
- Sarri, G.; Pedder, H.; Dias, S.; Guo, Y.; Lumsden, M.A. Vasomotor symptoms resulting from natural menopause: A systematic review and network meta-analysis of treatment effects from the National Institute for Health and Care Excellence guideline on menopause. BJOG 2017, 124, 1514–1523. [Google Scholar] [CrossRef]
- Castelo-Branco, C.; Gambacciani, M.; Cano, A.; Minkin, M.J.; Rachoń, D.; Ruan, X.; Beer, A.M.; Schnitker, J.; Henneicke-von Zepelin, H.H.; Pickartz, S. Review & meta-analysis: Isopropanolic black cohosh extract iCR for menopausal symptoms—An update on the evidence. Climacteric 2021, 24, 109–119. [Google Scholar] [CrossRef]
- Luo, C.H.; Ma, L.L.; Liu, H.M.; Liao, W.; Xu, R.C.; Ci, Z.M.; Lin, J.Z.; Han, L.; Zhang, D.K. Research Progress on Main Symptoms of Novel Coronavirus Pneumonia Improved by Traditional Chinese Medicine. Front. Pharmacol. 2020, 11, 556885. [Google Scholar] [CrossRef]
- Gasmi, A.; Noor, S.; Dadar, M.; Semenova, Y.; Menzel, A.; Gasmi Benahmed, A.; Bjørklund, G. The Role of Traditional Chinese Medicine and Chinese Pharmacopoeia in the Evaluation and Treatment of COVID-19. Curr. Pharm. Des. 2024, 30, 1060–1074. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. Chinese herbal medicine: Fighting SARS-CoV-2 infection on all fronts. J. Ethnopharmacol. 2021, 270, 113869. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Alexander, G.J.; Davies, S.E. Ma huang associated acute liver failure requiring liver transplantation. Eur. J. Gastroenterol. Hepatol. 2005, 17, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Borum, M.L. Fulminant exacerbation of autoimmune hepatitis after the use of ma huang. Am. J. Gastroenterol. 2001, 96, 1654–1655. [Google Scholar] [CrossRef]
- Boozer, C.N.; Nasser, J.A.; Heymsfield, S.B.; Wang, V.; Chen, G.; Solomon, J.L. An herbal supplement containing Ma Huang-Guarana for weight loss: A randomized, double-blind trial. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Lobb, A. Science of weight loss supplements: Compromised by conflicts of interest? World J. Gastroenterol. 2010, 16, 4880–4882. [Google Scholar] [CrossRef] [PubMed]
- Haller, C.A.; Benowitz, N.L. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N. Engl. J. Med. 2000, 343, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- FDA. 2025. Available online: https://www.fda.gov/food/news-events-hfp/hfp-constituent-updates (accessed on 19 August 2025).
- EMA. HMPC Monographs & the Traditional Herbal Medicinal Products Directive. Off. J. Eur. Union 2004, L136, 85–90. [Google Scholar]
- WHO Traditional Medicine Strategy 2025–2034. Available online: https://cdn.who.int/media/docs/default-source/tci/draft-traditional-medicine-strategy-2025-2034.pdf?sfvrsn=dd350962_1 (accessed on 19 August 2025).
- Neff, G.W.; Rajender Reddy, K.; Durazo, F.A.; Meyer, D.; Marrero, R.; Kaplowitz, N. Severe hepatotoxicity associated with the use of weight loss diet supplements containing ma huang or usnic acid. J. Hepatol. 2004, 41, 1062–1064. [Google Scholar] [CrossRef]
- Araujo, J.L.; Worman, H.J. Acute liver injury associated with a newer formulation of the herbal weight loss supplement Hydroxycut. BMJ Case Rep. 2015, 2015, bcr2015210303. [Google Scholar] [CrossRef]
- Stevens, T.; Qadri, A.; Zein, N.N. Two patients with acute liver injury associated with use of the herbal weight-loss supplement hydroxycut. Ann. Intern Med. 2005, 142, 477–478. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.L.; Klontz, K.C.; Canas-Coto, A.; Casper, S.J.; Durazo, F.A.; Davern, T.J., 2nd; Hayashi, P.; Lee, W.M.; Seeff, L.B. Hepatotoxicity due to hydroxycut: A case series. Am. J. Gastroenterol. 2010, 105, 1561–1566. [Google Scholar] [CrossRef]
- Khetpal, N.; Mandzhieva, B.; Shahid, S.; Khetpal, A.; Jain, A.G. Not All Herbals are Benign: A Case of Hydroxycut-induced Acute Liver Injury. Cureus 2020, 12, e6870. [Google Scholar] [CrossRef]
- Foley, S.; Butlin, E.; Shields, W.; Lacey, B. Experience with OxyELITE pro and acute liver injury in active duty service members. Dig. Dis. Sci. 2014, 59, 3117–3121. [Google Scholar] [CrossRef]
- Heidemann, L.A.; Navarro, V.J.; Ahmad, J.; Hayashi, P.H.; Stolz, A.; Kleiner, D.E.; Fontana, R.J. Severe Acute Hepatocellular Injury Attributed to OxyELITE Pro: A Case Series. Dig. Dis. Sci. 2016, 61, 2741–2748. [Google Scholar] [CrossRef]
- Klontz, K.C.; DeBeck, H.J.; LeBlanc, P.; Mogen, K.M.; Wolpert, B.J.; Sabo, J.L.; Salter, M.; Seelman, S.L.; Lance, S.E.; Monahan, C. The Role of Adverse Event Reporting in the FDA Response to a Multistate Outbreak of Liver Disease Associated with a Dietary Supplement. Public Health Rep. 2015, 130, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.I.; Chang, A.; Viray, M.; Chatham-Stephens, K.; He, H.; Taylor, E.; Wong, L.L.; Schier, J.; Martin, C.; Fabricant, D.; et al. Hepatotoxicity associated with the dietary supplement OxyELITE Pro™-Hawaii, 2013. Drug Test. Anal. 2016, 8, 319–327. [Google Scholar] [CrossRef] [PubMed]



| Drug:________________________________Initial ALT:_____Initial ALP:________R Ratio = [ALT/ULN] ÷ [Alk P/ULN]: =__________ | |||||
|---|---|---|---|---|---|
| Injury type | Hepatocellular (R > 5) | Cholestatic (R < 2) or mixed (R= 2–5) | Assessment | ||
| 1. Time to onset | Initial treatment | Subsequent treatment | Initial treatment | Subsequent treatment | Score |
| From the beginning of the drug: | |||||
| -suggestive of DILI | 5–90 days | 1–15 days | 5–90 days | 1–90 days | +2 |
| -compatible with DILI | <5 or >90 days | >15 days | <5 or >90 days | >90 days | +1 |
| From cessation of the drug: | |||||
| -compatible | <15 days | <15 days | <30 days | <30 days | +1 |
| Note: If reaction begins before starting the medication, or >15 days after stopping (hepatocellular), or >30 days (cholestatic), the injury should be considered unrelated to medication and RUCAM score cannot be calculated. | |||||
| 2. Clinical course after stopping the drug | Change in ALT between peak value and ULN | Change in ALP between peak value and ULN | Score | ||
| -highly suggestive of DILI | Decrease > 50% within 8 days | Not applicable | +3 | ||
| -suggestive of DILI | Decrease > 50% within 30 days | Decrease > 50% within 180 days | +2 | ||
| -compatible with DILI | Not applicable | Decrease < 50% within 180 days | +1 | ||
| -inconclusive of DILI | No information, or decrease >50% after 30 days | Persistence; or increase; or no information | 0 | ||
| -not DILI | Decrease <50% after 30 days OR recurrent increase | Not applicable | −2 | ||
| Note: If drug is continued, RUCAM score cannot be calculated. | |||||
| 3. Risk factors | Alcohol | Alcohol or pregnancy | Score | ||
| Alcohol or pregnancy | Yes | Yes | +1 | ||
| No | No | 0 | |||
| Age | >55 | >55 | +1 | ||
| <55 | <55 | 0 | |||
| The interpretation of the final score: 0 or less indicate that the drug is “excluded” as a cause; 1 to 2 that it is “unlikely”; 3–5 “possible”; 6–8 “probable”; and >8, “highly probable”. When comparing RUCAM to other causality assessment instruments, other terms are sometimes used for “highly probable”, including “highly likely” and “definite.” | |||||
| Supplement Category | Active Agent | Commercial Name |
|---|---|---|
| Body building supplements | RAD-140 | Testolone Radarine |
| MK-2866 | Ostarine | |
| MK-677 | Ibutamoren | |
| Weight loss supplements | Epigallocatechin gallate, from leaves of Camellia sinensis | Metabolife 356, Xenadrine RFA-1, Muscletech Hydroxycut®, OxyELITE Pro, NOW Foods GTE 400 mg, Nature’s Bounty GTE 315 mg |
| hydroxycitric acid (HCA) from a tropical fruit | Garcinia cambogia included in multiple capsules: Pure Garcinia Cambogia Capsules—6in1 with Green Tea, Arjuna, Garlic Bulb, Turmeric & Black Pepper NatureWise Garcinia Cambogia Garcinia Plus | |
| Usnic acid (UA) from lichen species, including Usnea, Cladonia, and Evernia | LipoKinetix (withdrawn), some deodorants, creams, shampoos, and toothpaste | |
| Cimicifuga racemosa, perennial plant native to eastern North America |
| |
| Ephedra sinica/Ma Huang active ingredients Alkaloids (ephedrine and pseudoephedrine) | Metabolife 356 Xenadrine RFA-1 Bayer Herbal Ephedra Ma Huang Tea | |
| Multi-ingredient: botanicals and nutritional supplements | Herbalife Protein Shakes, Meal Replacements | |
| Initially Ephedra sinica Others: Garcinia cambogia, Cissus quadrangularis, caffeine, and green tea extract | Muscletech Hydroxycut® Hydroxycut® Original, Hydroxycut® Hardcore, Hydroxycut® Max | |
| Multi-ingredient | OxyELITE Pro OxyELITE Pro Original, OxyELITE Pro Super Thermogenic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marginean, E.C. Hepatotoxicity of Herbal and Dietary Supplements a Review of Diagnosis, Histologic Features, and Common Culprits: Bodybuilding and Weight Loss Supplements. Livers 2025, 5, 42. https://doi.org/10.3390/livers5030042
Marginean EC. Hepatotoxicity of Herbal and Dietary Supplements a Review of Diagnosis, Histologic Features, and Common Culprits: Bodybuilding and Weight Loss Supplements. Livers. 2025; 5(3):42. https://doi.org/10.3390/livers5030042
Chicago/Turabian StyleMarginean, Esmeralda Celia. 2025. "Hepatotoxicity of Herbal and Dietary Supplements a Review of Diagnosis, Histologic Features, and Common Culprits: Bodybuilding and Weight Loss Supplements" Livers 5, no. 3: 42. https://doi.org/10.3390/livers5030042
APA StyleMarginean, E. C. (2025). Hepatotoxicity of Herbal and Dietary Supplements a Review of Diagnosis, Histologic Features, and Common Culprits: Bodybuilding and Weight Loss Supplements. Livers, 5(3), 42. https://doi.org/10.3390/livers5030042




