The Role of Normothermic Machine Perfusion in Extended Criteria Donor Grafts: A New Direction in Liver Graft Assessment and Preservation
Abstract
1. Introduction
2. Mechanism of Organ Damage during Liver Transplantation
3. Normothermic Machine Perfusion
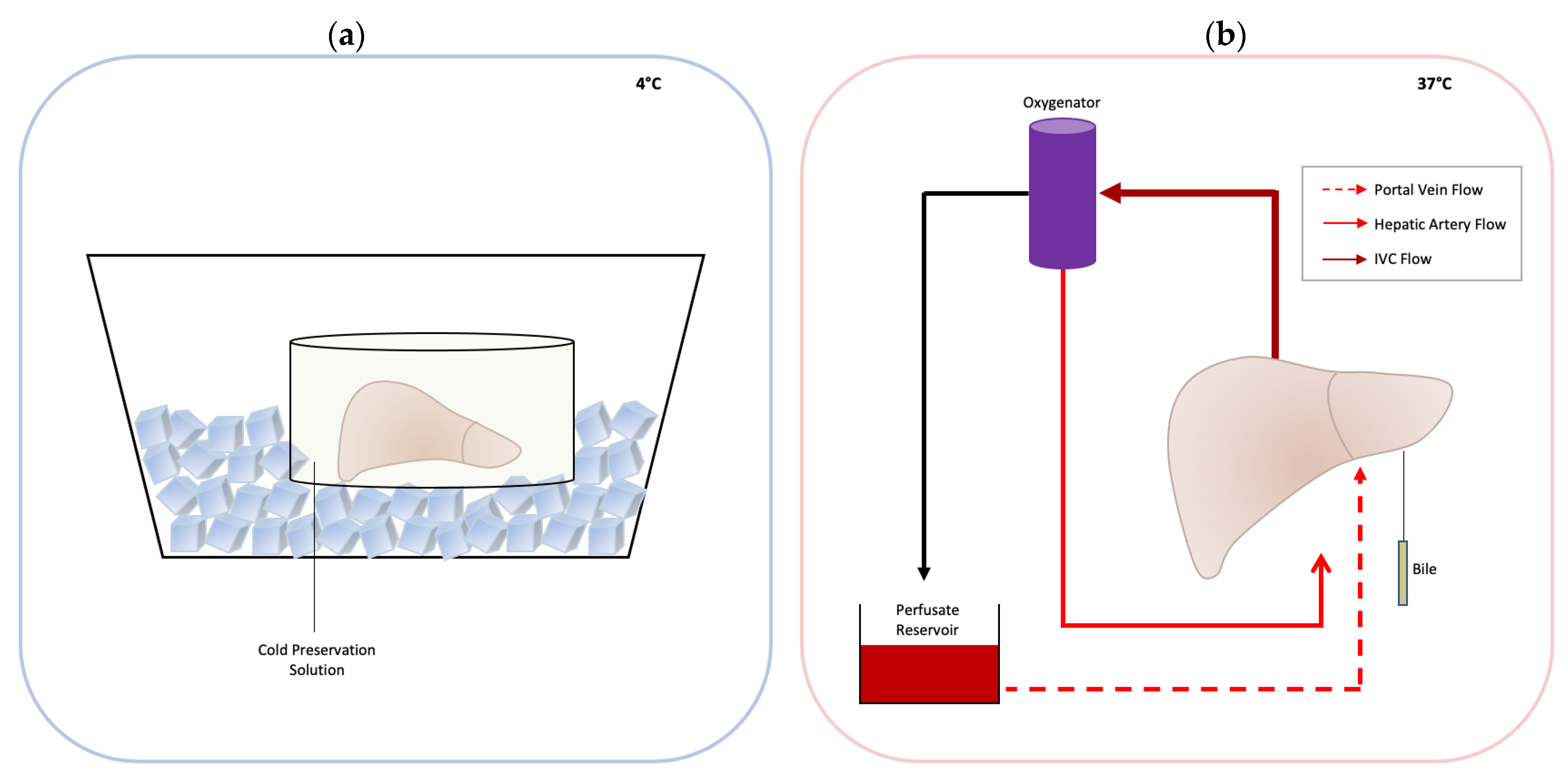
4. Commercially Available Machines
5. Outcomes of NMP versus SCS
5.1. Donor and Recipient Characteristics
5.2. Patient and Graft Survival
5.3. ICU and Hospital Stay
5.4. Liver Function and Biliary Complications
5.5. EAD, PNF, IRI, and Other Complications
6. Graft Viability Assessment: A Beneficial Tool in Extended Criteria Donors
7. Discussion
| Author (Year) | Experimental Groups | Donor Type (DCD/DBD) | Perfusion Device | Perfusate Characteristics | Perfusate | MP Time | Endpoints | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Ravikumar, et al. (2016) [66] | NMP (n = 20) vs. SCS (n = 40) | NMP (4/16) SCS (8/32) | OrganOx metra | HA: 60–75 mmHg PV: not recorded HA ≈ 0.2 L/min PV ≈ 0.8 L/min | 3 units of cross-matched PRBC + 1 unit of Gelofusine® (B Braun) | 9.3 h (3.5–18.5 h) | Primary: graft survival at 30 days Secondary: AST/ALT at 7 days and 6 months | Median peak aspartate aminotransferase in the first 7 days was significantly lower in the NMP group. Thirty-day graft survival was similar between NMP and SCS. |
| Selzner, et al. (2016) [73] | NMP (n = 10) vs. SCS (n = 30) | NMP (2/8) SCS (8/24) | OrganOx metra | Pressure: Not described HA: 0.3 L/min (0.2–0.4) PV: 1.25 L/min (1.2–1.3) | 3 units PRBC + Steen solution | 8 h (5.7–9.7 h) | Lactate, bile production, ALT/AST, ICU stay, hospital stay, complications | No significant difference in graft function, hospital stay, or complications |
| Mergental, et al. (2016) [44] | NMP (n = 5) | NMP (3/2) | Liver Assist and OrganOx | Not described | 3 units of the donor liver-specific blood group, Rhesus-negative, packed red blood cells, supplemented with 1000 mL human albumin solution 5%, 30 mL sodium bicarbonate 8.4%, and 10 mL calcium gluconate 10% | 332 min (318–564 min) | Hospital stay, 6-mon survival | Median in-hospital stay was 10 (range 6–14) days. All recipients were well, with normalized LFTs at median follow-up of 7 (range 6–19) months |
| Watson, et al. (2017) [74] | NMP (n = 12) (normoxic vs. hyperoxic) | NMP (9/3) | Liver Assist | PV: 660–1130 mL/min HA: 208–390 mL/min Oxygen: 621–671 mmHg or 153–187 mmHg | leucocyte-depleted washed red cells, succinylated gelatin, or Steen solution (cases 6 to 8 only) | 284 (122–530 min) | Post-reperfusion syndrome, vasoplegia, PNF, oxygen tension | Significantly decreased peak ALT in normoxic group at post-transplant day 7, significantly decreased post-reperfusion syndrome and vasoplegia in normoxic group |
| Bral, et al. (2017) [67] | NMP (n = 10) vs. SCS (n = 30) | NMP (4/6) SCS (8/22) | OrganOx metra | Pressure: Not described Flow: Not described | Gelofusine® (B Braun) + 3-unit type “O” PRBC | 11.5 h (3.3–22.5 h) | Primary: graft survival at 30 days Secondary: patient survival at 30 days, peak ALT/AST at 7 days, EAD at 7 days, liver biochem on days 1–7, 10 & 30, major complications defined by Clavien-Dindo score ≥ 3, patient and graft survival at 6 mo, biliary complications at 6 mo | No difference in graft survival at 30 days, prolonged hospital stays in NMP group, no difference in any other secondary endpoints |
| Nasralla, et al. (2018) [68] | NMP (n = 121) vs SCS (n = 101) | NMP (34/87) SCS (21/80) | OrganOx metra | HA ≈ 0.28 L/min PV ≈ 1.1 L/min | Gelofusine® (B Braun) + 3-unit donor-matched PRBC | 9.13 h (1.42–24 h) | Primary: peak AST at 7 days Secondary: organ discard rate, post-reperfusion syndrome, PNF, EAD, graft function, hospital stay, need for renal replacement therapy, cholangiopathy on MRCP at 6 months, graft and patient survival at 1 year | Significant reduction of peak AST, odds of early allograft dysfunction, and median bilirubin during first 7 days post-transplant in NMP vs. SCS. No significant difference in hospital stay, need for RRT in the first week, or 1-year survival |
| Ceresa, et al. (2019) [94] | NMP (n = 31) | NMP (8/23) | OrganOx metra | HA: 0.44 L/min (0.29–0.59) PV: 1.08 L/min (0.96–1.2) HA: 67 mmHg (64–70) | Gelofusine® (B Braun) + unspecified blood products | 8.4 h (4.3–12.5 h) | Primary: 30-day graft survival Secondary: AST/ALT, EAD, MEAF, PNF, PRS, RRT, hospital stay, adverse events, graft histology, adverse events, biliary complications and survival at 1 year | 94% 30-day graft survival. 13% developed EAD. PRS was observed in 10% of livers. Median duration of initial critical care stay was 3 days (1–20 days), and median hospital stay was 13 days (7–31 days). 23% developed complications of grade 3b severity or above. 6% developed biliary complications. 12-month overall graft survival rate (including death with a functioning graft) was 84% |
| Jassem, et al. (2019) [69] | NMP (n = 12) vs.SCS (n = 27) | DBD only | OrganOx metra | HA: 60–75 mmHg PV: not recorded HA≈0.2 L/min PV ≈ 0.8 L/min | 3 units of cross-matched PRBC + 1 unit of Gelofusine® (B Braun) | 9.3 h (3.5–18.5 h) | Peak AST, INR, ALP, bilirubin. AST, INR, ALP, bilirubin at 7 days. ICU stay length, rejection, graft survival at one year | Peak AST and INR within 7 days were significantly lower in the NMP group compared with the CS group. Alkaline phosphatase (ALP) and total bilirubin together with post-transplant clinical parameters such as the days of ITU stay, the rates of acute rejection and one-year graft and recipient survival, were comparable between the two groups. |
| Ghinolfi, et al. (2019) [70] | NMP (n = 10) vs. SCS (n = 10) All patients older than 70 yo | DBD only | LiverAssist | HA: 0.205–0.420 L/ min PV: 1.1–1.7 L/min | Gelofusine® (B Braun) + ABO-compatible RBC concentrate | 4.2 h (3.25–4.7 h) | Primary: graft and patient survival at 6 months Secondary: AST/ALT at 7 days, 6 mo biliary complications histology | No significant difference in graft and patient survival, lower lactate in NMP, decreased mitochondrial volume density at steatosis, and increased volume density of autophagic vacuoles in NMP |
| Watson, et al. (2019) [98] | NMP (n = 43) vs. non-NMP (n = 187) | DCD only | Medtronic, Cardiohelp or the Extra-Corporeal Organ Procurement System (ECOPS) or the Donor Assist | Abdominal flow = 2.5–3 L/min Thoracoabdominal flow = 4–6 L/min | Hartmann’s solution (Baxter Healthcare Ltd., Thetford, UK) and Gelofusine® (BBraun) | 2 h | Early allograft dysfunction, 30-day graft loss, freedom from ischemic cholangiopathy and anastomotic strictures | NRP was associated with a reduction in early allograft dysfunction, 30-day graft loss, freedom from ischemic cholangiopathy, and fewer anastomotic strictures |
| Mergental, et al. (2020) [71] | NMP (n = 22) vs. SCS (n = 44) | NMP (10/12) | OrganOx metra | Not described | Not described | 4–24 h | Primary: (A) feasibility of NMP in discarded organ recovery and (B) achievement of successful transplantation. Secondary: LFTs, 90-day graft survival, hospital stay, vascular complications, biliary strictures with MRCP at 6 months | Patient and graft survival is similar at 12 months. Higher rate of EAD in study group. Higher incidence on non-anastomotic biliary strictures was higher in study group. No differences in other parameters. |
| Chen et al. (2021) [99] | NMP (n = 2) | NMP (1/1) | Not described | Not described | Gelofusine® + cross-matched leukocyte depleted RBC, 5% NaHCO3, heparin, 10% Ca gluconate, 25% MgSO4, methylprednisone, compound AA injection, Impenem cilastatin, metronidazole | 7 h | Evaluate efficacy and safety of transplanting ECD directly under NMP without recooling | Continuous NMP without recooling is safe for LT with ECD livers |
| Seidita, et al. (2022) [100] | NMP (n = 17) | NMP (3/14) | Not described | Not described | Perfusion solution based on heparinized human plasma and red blood cells | 195–330 min | Kaplan–Meier survival estimates at 30, 90, 180, and 1 year after transplant, estimated 3-year survival | Overall survival rates did not differ from those of patients transplanted with non-perfused grafts from an ECD |
| Hann, et al. (2022) [72] | NMP (n = 26) vs. Historical CS (1) (n = 31) Contemporaneous CS (2) (n = 25) | DBD only | OrganOx metra | Not described | Gelofusine® + 3-unit O-negative red blood cells | Minimum of 4 h | Primary: graft and patient survival at 6 months | No difference at 6 months despite NMP group having significantly more steatotic grafts and previously declined grafts |
| Study Title | Study Type | Number Enrolled | Outcomes | Start Date | Device | Identifier | Group |
|---|---|---|---|---|---|---|---|
| OCS Liver PROTECT Trial: Preserving and Assessing Donor Livers for Transplantation | Randomized | 300 | Primary: incidence of EAD at 7 days, incidence of serious adverse events at 30 days | February 2016 | OCS™ Liver System (TransMedics, Andover, MA, USA) | NCT02522871 | TransMedics, Andover, MA, USA |
| Efficacy Evaluation of Normothermic Perfusion Machine Preservation in Liver Transplant Using Very Old Donors (CEFEMA) | Randomized pilot | 30 | Primary: 6-month graft survival rate Secondary: IRI through biopsy at 1 day and AST at 7 days, IRI through ischemic type biliary lesions | October 2016 | Liver Assist (Organ Assist, Groningen, The Netherlands) | NCT02940600 | UO Chirurgia Epatica e del Trapianto di Fegato Pisa, Italy |
| Normothermic Liver preservation Trial | Phase 2 | 50 | Primary: Graft survival rate at 30 days Secondary: patient survival rate at 30 days. EAD at 7 days, peak blood AST and lactate at 7 days, perfusate ALT, bilirubin and lactate levels | February 2017 | OrganOx metra (OrganOx Ltd., Oxford, UK) | NCT03089840 | University of Alberta Edmonton, Alberta, Canada |
| Post Static Cold Storage Normothermic Machine Liver Perfusion | Phase 2 | 30 | Primary: patient and graft survival at 30 days Secondary: peak AST at 7 days, EAD at 7 days, PNF at 10 days, adverse events., transplantation and organ discard rates, biliary investigation or intervention at 6 months, patient and graft survival at 6, 12 months | May 2017 | Not specified | NCT03176433 | University of Oxford |
| Efficacy of Ex-situ Normothermic Perfusion Versus Cold Storage in the Transplant With Steatotic Liver Graft. (ORGANOXLAFE) | RCT | 50 | Primary: peak AST & ALT at 1, 3, 5, 7 days post-transplant Secondary: primary graft failure at 10 days, patient and graft survival at 1, 6, 12 months, post-reperfusion syndrome, post-transplant bilirubin, GGT, AST, ALT and INR at 1, 3, 5, 7 days and 1, 6, 12 months, EAD at 7 days, ICU and hospital stay at 30 days, need for RRT at 1, 6, 12 months, intra-op thromboelastogram and reperfusion injury, biliary stenosis | April 2019 | OrganOx metra (OrganOx Ltd., Oxford, UK) | NCT03930459 | Hospital Universitario y Politécnico La Fe Valencia, Spain |
| APHP Plateform of Normothermic Perfusion for Rehabilitation of Hepatic Grafts (PENOFOR) | Single group assignment | 20 | Primary: portion of grafts that can be evaluated after evaluation by NMP with a 3 year survival rate >90% Secondary: proportion of grafts considered as not initially transplantable, proportion of non-eligible grafts eligible for NMP, proportion of grafts perfused, proportion transplanted, time until liver function recovery on NMP and post-transplant, EAD at 1 month, 1 month overall survival, 1 year graft survival, wait time, incidence of biliary stenosis | October 2019 | Not specified | NCT04154696 | AP-HP, Paul Brousse Hospital Villejuif, France |
| OCS Liver PROTECT Continued Access Protocol (CAP) Continuation Post-Approval Study | Observational | 74 | Primary: graft survival at 24 months post transplant | February 2020 | OCS™ Liver System (TransMedics, Andover, MA, USA) | NCT05096754 | TransMedics, Andover, MA, USA |
| Safety and Feasibility of Normothermic Machine Perfusion to Preserve and Evaluate Orphan Livers | Single group assignment | 30 | Primary: rate of patient survival and primary non function (PNF) at 30 days after transplantation Secondary: Early Allograft Dysfunction (EAD), 6 months patient and graft survival, peak liver function tests at 7 days after transplantation, surgical outcomes (operative time, transfusion requirement etc.), rate of post-transplant kidney failure, assessment of histological ischemia reperfusion (liver and bile duct), rate of vascular complications, rate of biliary complications, hospital and ICU length of stay, rejection rate, infection rate, the ability to predict function based on “on-pump” viability markers, and the incidence of adverse effect | March 2020 | Not specified | NCT03456284 | Cleveland Clinic Cleveland, Ohio, United States |
| OCS Liver DCD Trial | Single group assignment | 9 | Primary: graft survival 6 months post-transplant Secondary: rate of donor liver utilization after OCS perfusion, incidence of ischemic biliary cholangiopathy at 6 months, EAD or PNF at 7 days, patient and graft survival at 1, 6, 12, 24, 36, 48, 60 months | July 2020 | OCS™ Liver System (TransMedics, Andover, MA, USA) | NCT04194437 | TransMedics, Andover, MA, USA |
| RESTORE Declined Livers Study | Prospective, non-randomized | 25 | Primary: 6 month graft survival rate, patient survival rate at 6 months Secondary: graft function and survival at 3 months–1 year, 90 day and 1 year graft and patient survival, morbidity at 3 months–1 year, quality of life score, proportion of declined livers eligible for NMP | December 2020 | OrganOx metra (OrganOx Ltd., Oxford, UK) | NCT04483102 | Washington University School of Medicine Saint Louis, Missouri, United States |
| Comparison of Hypothermic Versus Normothermic Ex-vivo Preservation. (DCDNet) | Prospective randomized | 60 | Primary: graft loss at 6 months, ischemic type biliary lesions at 6 months Secondary: 1 year graft and patient survival, BCL-2/BAX livers after 2 h of perfusion, levels of soluble keratin 18 and HMGB1 in perfusate at 2 h | December 2020 | Not specified | NCT04744389 | UO Chirurgia Epatica e del Trapianto di Fegato Pisa, Italy |
| Hypothermic Oxygenated (HOPE) Versus Normothermic Machine Perfusion (NMP) in Human Liver Transplantation (HOPE-NMP) | Randomized control trial | 213 | Primary: post-op complications at 90 days Secondary: peak ALT, AST at 7 days, EAD and PNF anad 7 days, biliary complications at 6 months, organ utilization rate, total preservation time, duration and cost of ICU and hospital stay, post-op complications at 1 year, recipient and graft survival at 1 year | January 2021 | OrganOx metra (OrganOx Ltd., Oxford, UK) | NCT04644744 | Bridge to Life Ltd., Northbrook, IL USA |
| Sequential Hypo- and Normo-thermic Perfusion to Preserve Extended Criteria Donor Livers for Transplantation | Single group assignment | 15 | Primary: patient and graft survival at 1 month post-transplant Secondary: EAD at 7 days, patient and graft survival at 6 months, estimated blood loss during surgery, peak ALT and AST at 7 days, total bilirubin and INR at 7 days, hospital and ICU stay | May 2021 | Institutional-developed perfusion device | NCT04023773 | Cleveland Clinic Cleveland, Ohio, United States |
| OCS Liver Perfusion (OLP) Post-Approval Registry | Observational | 160 | Primary: patient survival at 1 year Secondary: graft and patient survival at 6 months, 1 and 2 years post-transplant | October 2021 | OCS™ Liver System (TransMedics, Andover, MA, USA) | NCT05074160 | TransMedics, Andover, MA, USA |
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Starzl, T.E.; Marchioro, T.L.; Porter, K.A.; Brettschneider, L. Homotransplantation of the liver. Transplantation 1967, 5, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Dutkowski, P.; De Rougemont, O.; Müllhaupt, B.; Clavien, P.A. Current and future trends in liver transplantation in Europe. Gastroenterology 2010, 138, 802–809.e4. [Google Scholar] [CrossRef] [PubMed]
- Dutkowski, P.; Linecker, M.; DeOliveira, M.L.; Müllhaupt, B.; Clavien, P.A. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology 2015, 148, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, A.; Vrakas, G.; Nasralla, D.; Ceresa, C.D.L. The future of organ perfusion and re-conditioning. Transpl. Int. 2019, 32, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Zanetto, A.; Russo, F.; Germani, G. Organ Preservation in Liver Transplantation. Semin. Liver Dis. 2018, 38, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Therneau, T.M.; Benson, J.T.; Kremers, W.K.; Rosen, C.B.; Gores, G.J.; Dickson, E.R. Deaths on the liver transplant waiting list: An analysis of competing risks. Hepatology 2006, 43, 345–351. [Google Scholar] [CrossRef]
- Tchilikidi, K.Y. Liver graft preservation methods during cold ischemia phase and normothermic machine perfusion. World J. Gastrointest. Surg. 2019, 11, 126–142. [Google Scholar] [CrossRef]
- Saidi, R.F.; Kenari, S.K.H. Liver ischemia/reperfusion injury: An overview. J. Investig. Surg. 2014, 27, 366–379. [Google Scholar] [CrossRef]
- Toniutto, P.; Zanetto, A.; Ferrarese, A.; Burra, P. Current challenges and future directions for liver transplantation. Liver Int. 2017, 37, 317–327. [Google Scholar] [CrossRef]
- Belzer, F.O.; Southard, J.H. Principles of solid-organ preservation by cold storage. Transplantation 1988, 45, 673–676. [Google Scholar] [CrossRef]
- Burlage, L.C.; Karimian, N.; Westerkamp, A.C.; Visser, N.; Matton, A.P.; van Rijn, R.; Adelmeijer, J.; Wiersema-Buist, J.; Gouw, A.S.; Lisman, T.; et al. Oxygenated hypothermic machine perfusion after static cold storage improves endothelial function of extended criteria donor livers. HPB 2017, 19, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Serifis, N.; Matheson, R.; Cloonan, D.; Rickert, C.G.; Markmann, J.F.; Coe, T.M. Machine Perfusion of the Liver: A Review of Clinical Trials. Front. Surg. 2021, 8, 625394. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.W.; Mergental, H.; Mirza, D.F. Normothermic ex-situ liver preservation: The new gold standard. Curr. Opin. Organ Transplant. 2017, 22, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Rauen, U.; de Groot, H. New insights into the cellular and molecular mechanisms of cold storage injury. J. Investig. Med. 2004, 52, 299–309. [Google Scholar] [CrossRef]
- Feng, X.N.; Xu, X.; Zheng, S.S. Current status and perspective of liver preservation solutions. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2006, 5, 490–494. [Google Scholar]
- Zhai, Y.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Liver ischemia and reperfusion injury: New insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am. J. Transplant. 2011, 11, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Sancho, J.; Casillas-Ramírez, A.; Peralta, C. Molecular pathways in protecting the liver from ischaemia/reperfusion injury: A 2015 update. Clin. Sci. 2015, 129, 345–362. [Google Scholar] [CrossRef]
- Czigany, Z.; Lurje, I.; Tolba, R.H.; Neumann, U.P.; Tacke, F.; Lurje, G. Machine perfusion for liver transplantation in the era of marginal organs—New kids on the block. Liver Int. 2019, 39, 228–249. [Google Scholar] [CrossRef]
- Tector, A.J.; Mangus, R.S.; Chestovich, P.; Vianna, R.; Fridell, J.A.; Milgrom, M.L.; Sanders, C.; Kwo, P.Y. Use of extended criteria livers decreases wait time for liver transplantation without adversely impacting posttransplant survival. Ann. Surg. 2006, 244, 439–450. [Google Scholar] [CrossRef]
- Durand, F.; Renz, J.F.; Alkofer, B.; Burra, P.; Clavien, P.-A.; Porte, R.J.; Freeman, R.B.; Belghiti, J. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transplant. 2008, 14, 1694–1707. [Google Scholar] [CrossRef]
- Czigany, Z.; Bleilevens, C.; Beckers, C.; Stoppe, C.; Möhring, M.; Fülöp, A.; Szijarto, A.; Lurje, G.; Neumann, U.P.; Tolba, R.H. Limb remote ischemic conditioning of the recipient protects the liver in a rat model of arterialized orthotopic liver transplantation. PLoS ONE 2018, 13, e0195507. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Schöning, W.; Ulmer, T.F.; Bednarsch, J.; Amygdalos, I.; Cramer, T.; Rogiers, X.; Popescu, I.; Botea, F.; Froněk, J.; et al. Hypothermic oxygenated machine perfusion (HOPE) for orthotopic liver transplantation of human liver allografts from extended criteria donors (ECD) in donation after brain death (DBD): A prospective multicentre randomised controlled trial (HOPE ECD-DBD). BMJ Open 2017, 7, e017558. [Google Scholar] [CrossRef] [PubMed]
- Czigány, Z.; Turóczi, Z.; Kleiner, D.; Lotz, G.; Homeyer, A.; Harsányi, L.; Szijártó, A. Neural elements behind the hepatoprotection of remote perconditioning. J. Surg. Res. 2015, 193, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Czigány, Z.; Turóczi, Z.; Ónody, P.; Harsányi, L.; Lotz, G.; Hegedüs, V.; Szijártó, A. Remote ischemic perconditioning protects the liver from ischemia-reperfusion injury. J. Surg. Res. 2013, 185, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Szijártó, A.; Czigány, Z.; Turóczi, Z.; Harsányi, L. Remote ischemic perconditioning—A simple, low-risk method to decrease ischemic reperfusion injury: Models, protocols and mechanistic background. A review. J. Surg. Res. 2012, 178, 797–806. [Google Scholar] [CrossRef]
- Doorschodt, B.M.; Schreinemachers, M.C.J.M.; Behbahani, M.; Florquin, S.; Weis, J.; Staat, M.; Tolba, R.H. Hypothermic machine perfusion of kidney grafts: Which pressure is preferred? Ann. Biomed. Eng. 2011, 39, 1051–1059. [Google Scholar] [CrossRef]
- Doorschodt, B.M.; Schreinemachers, M.-C.J.; Florquin, S.; Lai, W.; Sitzia, M.; Zernecke, A.; Tolba, R.H. Evaluation of a novel system for hypothermic oxygenated pulsatile perfusion preservation. Int. J. Artif. Organs 2009, 32, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Tolba, R.H.; Wei, L.; Doorschodt, B.M.; Büttner, R.; Yamamoto, Y.; Minor, T. Impact of polysol, a newly developed preservation solution, on cold storage of steatotic rat livers. Liver Transplant. 2007, 13, 114–121. [Google Scholar] [CrossRef]
- Kageyama, S.; Yagi, S.; Tanaka, H.; Saito, S.; Nagai, K.; Hata, K.; Fujimoto, Y.; Ogura, Y.; Tolba, R.; Shinji, U. Graft reconditioning with nitric oxide gas in rat liver transplantation from cardiac death donors. Transplantation 2014, 97, 618–625. [Google Scholar] [CrossRef]
- Kalenski, J.; Mancina, E.; Paschenda, P.; Beckers, C.; Bleilevens, C.; Tóthová, Ľ.; Boor, P.; Doorschodt, B.M.; Tolba, R.H. Improved preservation of warm ischemia-damaged porcine kidneys after cold storage in Ecosol, a novel preservation solution. Ann. Transplant. 2015, 20, 233–242. [Google Scholar] [CrossRef]
- Kalenski, J.; Mancina, E.; Paschenda, P.; Beckers, C.; Bleilevens, C.; Tóthová, Ľ.; Boor, P.; Gross, D.; Tolba, R.H.; Doorschodt, B.M. Comparison of Aerobic Preservation by Venous Systemic Oxygen Persufflation or Oxygenated Machine Perfusion of Warm-Ischemia-Damaged Porcine Kidneys. Eur. Surg. Res. 2016, 57, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Mancina, E.; Kalenski, J.; Paschenda, P.; Beckers, C.; Bleilevens, C.; Boor, P.; Doorschodt, B.M.; Tolba, R.H. Determination of the preferred conditions for the isolated perfusion of porcine kidneys. Eur. Surg. Res. 2015, 54, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.; Akbar, S.; Tolba, R.; Dombrowski, F. Cold preservation of fatty liver grafts: Prevention of functional and ultrastructural impairments by venous oxygen persufflation. J. Hepatol. 2000, 32, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.; Olschewski, P.; Tolba, R.H.; Akbar, S.; Kocálková, M.; Dombrowski, F. Liver preservation with HTK: Salutary effect of hypothermic aerobiosis by either gaseous oxygen or machine perfusion. Clin. Transplant. 2002, 16, 206–211. [Google Scholar] [CrossRef]
- Nagai, K.; Yagi, S.; Afify, M.; Bleilevens, C.; Uemoto, S.; Tolba, R.H. Impact of venous-systemic oxygen persufflation with nitric oxide gas on steatotic grafts after partial orthotopic liver transplantation in rats. Transplantation 2013, 95, 78–84. [Google Scholar] [CrossRef]
- Schreinemachers, M.-C.J.M.; Doorschodt, B.M.; Florquin, S.; Weerman, M.A.v.D.B.; Reitsma, J.B.; Lai, W.; Sitzia, M.; Minor, T.M.; Tolba, R.H.; van Gulik, T.M. Improved preservation and microcirculation with POLYSOL after transplantation in a porcine kidney autotransplantation model. Nephrol. Dial. Transplant. 2009, 24, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.K.; Yagi, S.; Nagai, K.; Afify, M.; Hata, K.; Uemoto, S.; Tolba, R.H. Impact of oxygen free radicals in rat partial liver transplantation. J. Surg. Res. 2014, 191, 469–475. [Google Scholar] [CrossRef]
- Yagi, S.; Doorschodt, B.M.; Afify, M.; Klinge, U.; Kobayashi, E.; Uemoto, S.; Tolba, R.H. Improved preservation and microcirculation with POLYSOL after partial liver transplantation in rats. J. Surg. Res. 2011, 167, e375–e383. [Google Scholar] [CrossRef]
- Yagi, S.; Nagai, K.; Kadaba, P.; Afify, M.; Teramukai, S.; Uemoto, S.; Tolba, R.H. A novel organ preservation for small partial liver transplantations in rats: Venous systemic oxygen persufflation with nitric oxide gas. Am. J. Transplant. 2013, 13, 222–228. [Google Scholar] [CrossRef]
- Vogel, T.; Brockmann, J.G.; Friend, P.J. Ex-vivo normothermic liver perfusion: An update. Curr. Opin. Organ. Transplant. 2010, 15, 167–172. [Google Scholar] [CrossRef]
- Marecki, H.; Bozorgzadeh, A.; Porte, R.J.; Leuvenink, H.G.; Uygun, K.; Martins, P.N. Liver ex situ machine perfusion preservation: A review of the methodology and results of large animal studies and clinical trials. Liver Transpl. 2017, 23, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.-J.; Li, J.-H.; Yu, H.; Nie, Y.; Jiang, L.; Li, H.-Y.; Zhou, L.; Zheng, S.-S. Machine perfusion for liver transplantation: A concise review of clinical trials. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Boteon, Y.L.; Afford, S.C. Machine perfusion of the liver: Which is the best technique to mitigate ischaemia-reperfusion injury? World J. Transplant. 2019, 9, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mergental, H.; Perera, M.; Laing, R.; Muiesan, P.; Isaac, J.; Smith, A.; Stephenson, B.; Cilliers, H.; Neil, D.; Hübscher, S.; et al. Transplantation of Declined Liver Allografts Following Normothermic Ex-Situ Evaluation. Am. J. Transplant. 2016, 16, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- Busuttil, R.W.; Tanaka, K. The utility of marginal donors in liver transplantation. Liver Transplant. 2003, 9, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Jing, L.; Yao, L.; Zhao, M.; Peng, L.P.; Liu, M. Organ preservation: From the past to the future. Acta Pharmacol. Sin. 2018, 39, 845–857. [Google Scholar] [CrossRef]
- Michel, S.G.; Madsen, J.C. Current perspectives in transplant medicine: Hypothermic oxygenated perfusion. Transpl. Res. Risk Manag. 2016, 8, 25–30. [Google Scholar] [CrossRef]
- Ogawa, S.; Koga, S.; Kuwabara, K.; Brett, J.; Morrow, B.; Morris, S.A.; Bilezikian, J.P.; Silverstein, S.C.; Stern, D. Hypoxia-induced increased permeability of endothelial monolayers occurs through lowering of cellular cAMP levels. Am. J. Physiol. 1992, 262, C546–C554. [Google Scholar] [CrossRef]
- Müller, A.L.; Freed, D.; Dhalla, N.S. Activation of proteases and changes in Na+-K+-ATPase subunits in hearts subjected to ischemia-reperfusion. J. Appl. Physiol. 2013, 114, 351–360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anderson, C.D.; Pierce, J.; Nicoud, I.; Belous, A.; Knox, C.D.; Chari, R.S. Modulation of mitochondrial calcium management attenuates hepatic warm ischemia-reperfusion injury. Liver Transplant. 2005, 11, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.S.; Andrade, C.F. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxid. Med. Cell Longev. 2015, 2015, 590987. [Google Scholar] [CrossRef] [PubMed]
- Jayant, K.; Reccia, I.; Virdis, F.; Shapiro, A.M.J. The Role of Normothermic Perfusion in Liver Transplantation (TRaNsIT Study): A Systematic Review of Preliminary Studies. HPB Surg. World J. Hepatic Pancreat. Biliary Surg. 2018, 2018, 6360423. [Google Scholar] [CrossRef]
- Carrel, A.; Lindbergh, C.A. The Culture of Whole Organs. Science 1935, 81, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Penn, I.; Halgrimson, C.G.; Starzl, T.E. Liver Transplantation in Man. Ann. N. Y. Acad. Sci. 1970, 170, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Starzl, T.E.; Groth, C.G.; Brettschneider, L.; Moon, J.B.; Fulginiti, V.A.; Cotton, E.K.; Porter, K.A. Extended survival in 3 cases of orthotopic homotransplantation of the human liver. Surgery 1968, 63, 549–563. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quillin, R.C., III; Guarrera, J.V. Hypothermic machine perfusion in liver transplantation. Liver Transpl. 2018, 24, 276–281. [Google Scholar] [CrossRef]
- Sanchez-Urdazpal, L.; Gores, G.J.; Ward, E.M.; Maus, T.P.; Wahlstrom, H.E.; Moore, S.B.; Wiesner, R.H.; Krom, R.A.F. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology 1992, 16, 49–53. [Google Scholar] [CrossRef]
- Carini, R.; De Cesaris, M.G.; Bellomo, G.; Albano, E. Intracellular Na+ accumulation and hepatocyte injury during cold storage. Transplantation 1999, 68, 294–297. [Google Scholar] [CrossRef]
- Beekum CJ van Vilz, T.O.; Glowka, T.R.; Websky MW von Kalff, J.C.; Manekeller, S. Normothermic Machine Perfusion (NMP) of the Liver—Current Status and Future Perspectives. Ann. Transplant. 2021, 26, e931664-1–e931664-8. [Google Scholar] [CrossRef]
- Adham, M.; Ducerf, C.; De La Roche, E.; Taibi, A.; Pouyet, M.; Baulieux, J.; Peyrol, S.; Chevallier, M.; Vernet, M.; Barakat, C.; et al. The isolated perfused porcine liver: Assessment of viability during and after six hours of perfusion. Transpl. Int. 1997, 10, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Op Den Dries, S.; Karimian, N.; Sutton, M.E.; Westerkamp, A.C.; Nijsten, M.W.N.; Gouw, A.S.H.; Wiersema-Buist, J.; Lisman, T.; Leuvenink, H.G.D.; Porte, R.J. Ex vivo Normothermic Machine Perfusion and Viability Testing of Discarded Human Donor Livers. Am. J. Transplant. 2013, 13, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Eshmuminov, D.; Becker, D.; Borrego, L.B.; Hefti, M.; Schuler, M.J.; Hagedorn, C.; Muller, X.; Mueller, M.; Onder, C.; Graf, R.; et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 2020, 38, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, R.; Leuvenink, H.; Friend, P.J. Normothermic liver preservation: A new paradigm? Transpl. Int. 2015, 28, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, R.; Jassem, W.; Mergental, H.; Heaton, N.; Mirza, D.; Perera, M.T.P.R.; Quaglia, A.; Holroyd, D.; Vogel, T.; Coussios, C.C.; et al. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am. J. Transplant. 2016, 16, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Bral, M.; Gala-Lopez, B.; Bigam, D.; Kneteman, N.; Malcolm, A.; Livingstone, S.; Andres, A.; Emamaullee, J.; Russell, L.; Coussios, C.; et al. Preliminary Single-Center Canadian Experience of Human Normothermic Ex Vivo Liver Perfusion: Results of a Clinical Trial. Am. J. Transplant. 2017, 17, 1071–1080. [Google Scholar] [CrossRef]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- Jassem, W.; Xystrakis, E.; Ghnewa, Y.G.; Yuksel, M.; Pop, O.; Martinez-Llordella, M.; Jabri, Y.; Huang, X.; Lozano, J.J.; Quaglia, A.; et al. Normothermic Machine Perfusion (NMP) Inhibits Proinflammatory Responses in the Liver and Promotes Regeneration. Hepatology 2019, 70, 682–695. [Google Scholar] [CrossRef]
- Ghinolfi, D.; Rreka, E.; De Tata, V.; Franzini, M.; Pezzati, D.; Fierabracci, V.; Masini, M.; Cacciatoinsilla, A.; Bindi, M.L.; Marselli, L.; et al. Pilot, Open, Randomized, Prospective Trial for Normothermic Machine Perfusion Evaluation in Liver Transplantation From Older Donors. Liver Transplant. 2019, 25, 436–449. [Google Scholar] [CrossRef]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.T.P.R.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11, 2939. [Google Scholar] [CrossRef] [PubMed]
- Hann, A.; Lembach, H.; Nutu, A.; Dassanayake, B.; Tillakaratne, S.; McKay, S.C.; Boteon, A.P.C.S.; Boteon, Y.L.; Mergental, H.; Murphy, N.; et al. Outcomes of normothermic machine perfusion of liver grafts in repeat liver transplantation (NAPLES initiative). Br. J. Surg. 2022, 109, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Selzner, M.; Goldaracena, N.; Echeverri, J.; Kaths, J.M.; Linares, I.; Selzner, N.; Serrick, C.; Marquez, M.; Sapisochin, G.; Renner, E.L.; et al. Normothermic ex vivo liver perfusion using steen solution as perfusate for human liver transplantation: First North American results. Liver Transpl. 2016, 22, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Kosmoliaptsis, V.; Randle, L.V.; Gimson, A.E.; Brais, R.; Klinck, J.R.; Hamed, M.; Tsyben, A.; Butler, A.J. Normothermic Perfusion in the Assessment and Preservation of Declined Livers Before Transplantation: Hyperoxia and Vasoplegia—Important Lessons From the First 12 Cases. Transplantation 2017, 101, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Michelotto, J.; Gassner, J.M.G.V.; Moosburner, S.; Muth, V.; Patel, M.S.; Selzner, M.; Pratschke, J.; Sauer, I.M.; Raschzok, N. Ex vivo machine perfusion: Current applications and future directions in liver transplantation. Langenbecks Arch. Surg. 2021, 406, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.E.; Dries, S.O.D.; Karimian, N.; Weeder, P.D.; de Boer, M.T.; Wiersema-Buist, J.; Gouw, A.S.H.; Leuvenink, H.G.D.; Lisman, T.; Porte, R.J. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS ONE 2014, 9, e110642. [Google Scholar] [CrossRef] [PubMed]
- Mergental, H.; Stephenson, B.T.F.; Laing, R.W.; Kirkham, A.J.; Neil, D.A.H.; Wallace, L.L.; Boteon, Y.L.; Widmer, J.; Bhogal, R.H.; Perera, M.T.P.R.; et al. Development of Clinical Criteria for Functional Assessment to Predict Primary Nonfunction of High-Risk Livers Using Normothermic Machine Perfusion. Liver Transplant. 2018, 24, 1453–1469. [Google Scholar] [CrossRef]
- van Leeuwen, O.B.; de Vries, Y.; Fujiyoshi, M.; Nijsten, M.W.N.; Ubbink, R.; Pelgrim, G.J.; Werner, M.J.M.; Reyntjens, K.M.E.M.; Berg, A.P.v.D.; de Boer, M.T.; et al. Transplantation of High-risk Donor Livers After Ex Situ Resuscitation and Assessment Using Combined Hypo- and Normothermic Machine Perfusion: A Prospective Clinical Trial. Ann. Surg. 2019, 270, 906–914. [Google Scholar] [CrossRef]
- Matton, A.P.; de Vries, Y.; Burlage, L.C.; van Rijn, R.; Fujiyoshi, M.; de Meijer, V.E.; de Boer, M.T.; de Kleine, R.H.; Verkade, H.J.; Gouw, A.S.H.; et al. Biliary Bicarbonate, pH, and Glucose Are Suitable Biomarkers of Biliary Viability During Ex Situ Normothermic Machine Perfusion of Human Donor Livers. Transplantation 2019, 103, 1405–1413. [Google Scholar] [CrossRef]
- Peter, S.D.; Imber, C.J.; Kay, J.; James, T.; Friend, P.J. Hepatic control of perfusate homeostasis during normothermic extrocorporeal preservation. Transplant. Proc. 2003, 35, 1587–1590. [Google Scholar] [CrossRef]
- Martins, P.N.; Buchwald, J.E.; Mergental, H.; Vargas, L.; Quintini, C. The role of normothermic machine perfusion in liver transplantation. Int. J. Surg. 2020, 82, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Brüggenwirth, I.M.A.; Porte, R.J.; Martins, P.N. Bile Composition as a Diagnostic and Prognostic Tool in Liver Transplantation. Liver Transpl. 2020, 26, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- NHS Blood and Transplant. ODT Clinical—NHS Blood and Transplant. Available online: https://www.odt.nhs.uk/ (accessed on 27 March 2022).
- Linares, I.; Hamar, M.; Selzner, N.; Selzner, M. Steatosis in Liver Transplantation: Current Limitations and Future Strategies. Transplantation 2019, 103, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Tullius, S.G.; Rabb, H. Improving the Supply and Quality of Deceased-Donor Organs for Transplantation. N. Engl. J. Med. 2018, 378, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Nardo, B.; Masetti, M.; Urbani, L.; Caraceni, P.; Montalti, R.; Filipponi, F.; Mosca, F.; Martinelli, G.; Bernardi, M.; Pinna, A.D.; et al. Liver transplantation from donors aged 80 years and over: Pushing the limit. Am. J. Transplant. 2004, 4, 1139–1147. [Google Scholar] [CrossRef]
- Spitzer, A.L.; Lao, O.B.; Dick, A.A.S.; Bakthavatsalam, R.; Halldorson, J.B.; Yeh, M.M.; Upton, M.P.; Reyes, J.D.; Perkins, J.D. The biopsied donor liver: Incorporating macrosteatosis into high-risk donor assessment. Liver Transplant. 2010, 16, 874–884. [Google Scholar] [CrossRef]
- Jia, J.-J.; Li, J.-H.; Jiang, L.; Lin, B.-Y.; Wang, L.; Su, R.; Zhou, L.; Zheng, S.-S. Liver protection strategies in liver transplantation. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 34–42. [Google Scholar] [CrossRef]
- Jia, J.-J.; Zhang, J.; Li, J.-H.; Chen, X.-D.; Jiang, L.; Zhou, Y.-F.; He, N.; Xie, H.-Y.; Zhou, L.; Zheng, S.-S. Influence of perfusate on liver viability during hypothermic machine perfusion. World J. Gastroenterol. WJG 2015, 21, 8848–8857. [Google Scholar] [CrossRef]
- Ceresa, C.D.L.; Nasralla, D.; Pollok, J.M.; Friend, P.J. Machine perfusion of the liver: Applications in transplantation and beyond. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 199–209. [Google Scholar] [CrossRef]
- Adam, R.; Bismuth, H.; Diamond, T.; Morino, M.; Astarcioglu, I.; Johann, M.; Azoulay, D.; Chiche, L.; Bao, Y.; Castaing, D.; et al. Effect of extended cold ischaemia with UW solution on graft function after liver transplantation. Lancet 1992, 340, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Bral, M.; Dajani, K.; Izquierdo, D.L.; Bigam, D.; Kneteman, N.; Ceresa, C.D.L.; Friend, P.J.; Shapiro, A.M.J. A Back-to-Base Experience of Human Normothermic Ex Situ Liver Perfusion: Does the Chill Kill? Liver Transplant. 2019, 25, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.D.L.; Nasralla, D.; Watson, C.J.E.; Butler, A.J.; Coussios, C.C.; Crick, K.; Hodson, L.; Imber, C.; Jassem, W.; Knight, S.R.; et al. Transient Cold Storage Prior to Normothermic Liver Perfusion May Facilitate Adoption of a Novel Technology. Liver Transplant. 2019, 25, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, M.; Mashayekhi, A.; Trevor, M.; Branagan-Harris, M.; Atkinson, J. Cost-utility analysis of normothermic liver perfusion with the OrganOx metra compared to static cold storage in the United Kingdom. J. Med. Econ. 2020, 23, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.N.; Lester, E.L.W.; Shapiro, A.M.J.; Eurich, D.T.; Bigam, D.L. Cost-utility analysis of normothermic machine perfusion compared to static cold storage in liver transplantation in the Canadian setting. Am. J. Transplant. 2022, 22, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Quintini, C.; Martins, P.N.; Shah, S.; Killackey, M.; Reed, A.; Guarrera, J.; Axelrod, D.A.; The American Society of Transplant Surgeons Standards Committee. Implementing an innovated preservation technology: The American Society of Transplant Surgeons’ (ASTS) Standards Committee White Paper on Ex Situ Liver Machine Perfusion. Am. J. Transplant. 2018, 18, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Hunt, F.; Messer, S.; Currie, I.; Large, S.; Sutherland, A.; Crick, K.; Wigmore, S.J.; Fear, C.; Cornateanu, S.; et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am. J. Transplant. 2019, 19, 1745–1758. [Google Scholar] [CrossRef]
- Chen, Z.; Hong, X.; Huang, S.; Wang, T.; Ma, Y.; Guo, Y.; Huang, C.; Zhao, Q.; Guo, Z.; He, X.; et al. Continuous Normothermic Machine Perfusion for Renovation of Extended Criteria Donor Livers Without Recooling in Liver Transplantation: A Pilot Experience. Front. Surg. 2021, 8, 638090. [Google Scholar] [CrossRef]
- Seidita, A.; Longo, R.; Di Francesco, F.; Tropea, A.; Calamia, S.; Panarello, G.; Barbara, M.; Gruttadauria, S. The use of normothermic machine perfusion to rescue liver allografts from expanded criteria donors. Updat. Surg. 2022, 74, 193–202. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Dutkowski, P. Hypothermic Machine Preservation of the Liver: State of the Art. Curr. Transplant. Rep. 2018, 5, 93–102. [Google Scholar] [CrossRef]
| Graft Risk | Definitions | Suggested Actions |
|---|---|---|
| Low-risk ECD grafts | DBD: donor age ≤ 80 yo, CIT ≤ 10 h, graft macrosteatosis ≤ 30% DCD: donor age ≤ 60 yo, CIT ≤ 6 h, WIT ≤ 20 min, graft macrosteatosis ≤ 5% | SCS is first line, can consider machine perfusion on a case-by-case basis |
| Intermediate-risk ECD grafts | DBD: donor age > 80 yo, CIT >10 h, graft macrosteatosis > 30% DCD: donor age 60–80 yo, CIT 6–8 h, WIT 20–30 min, graft macrosteatosis 5–20% | Machine perfusion |
| High-risk DCD grafts and declined overextended livers | Donor age > 80 yo, CIT > 8–10 h, WIT > 30 min, graft macrosteatosis > 30%, poor in situ perfusion, prolonged retrieval, significantly elevated LFTs, declined for reason other than nonvascular reason | Not possible without machine perfusion, requires viability assessment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malkawi, D.; Savsani, K.; Alfonso, A.; Lee, S.D.; James, N.; Sarkar, D.; Imai, D.; Khan, A.; Sharma, A.; Kumaran, V.; et al. The Role of Normothermic Machine Perfusion in Extended Criteria Donor Grafts: A New Direction in Liver Graft Assessment and Preservation. Livers 2023, 3, 709-726. https://doi.org/10.3390/livers3040046
Malkawi D, Savsani K, Alfonso A, Lee SD, James N, Sarkar D, Imai D, Khan A, Sharma A, Kumaran V, et al. The Role of Normothermic Machine Perfusion in Extended Criteria Donor Grafts: A New Direction in Liver Graft Assessment and Preservation. Livers. 2023; 3(4):709-726. https://doi.org/10.3390/livers3040046
Chicago/Turabian StyleMalkawi, Dima, Kush Savsani, Anjelica Alfonso, Seung Duk Lee, Nicholas James, Devanand Sarkar, Daisuke Imai, Aamir Khan, Amit Sharma, Vinay Kumaran, and et al. 2023. "The Role of Normothermic Machine Perfusion in Extended Criteria Donor Grafts: A New Direction in Liver Graft Assessment and Preservation" Livers 3, no. 4: 709-726. https://doi.org/10.3390/livers3040046
APA StyleMalkawi, D., Savsani, K., Alfonso, A., Lee, S. D., James, N., Sarkar, D., Imai, D., Khan, A., Sharma, A., Kumaran, V., Bruno, D., Cotterell, A., & Levy, M. F. (2023). The Role of Normothermic Machine Perfusion in Extended Criteria Donor Grafts: A New Direction in Liver Graft Assessment and Preservation. Livers, 3(4), 709-726. https://doi.org/10.3390/livers3040046






