Plasma Polyamines Decrease in Patients with Obstructive Cholecystitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Demographic Data
2.3. Surgical Intervention
2.4. Blood Collection and Sample Preparation
2.5. Polyamine Measures and Analysis
2.6. Biochemical Measurements
2.7. Statistical Analysis
3. Results
3.1. Basic Clinical and Biochemical Data Do Not Show the Characteristic Endophenotype in Patients with Obstructive Cholecystitis
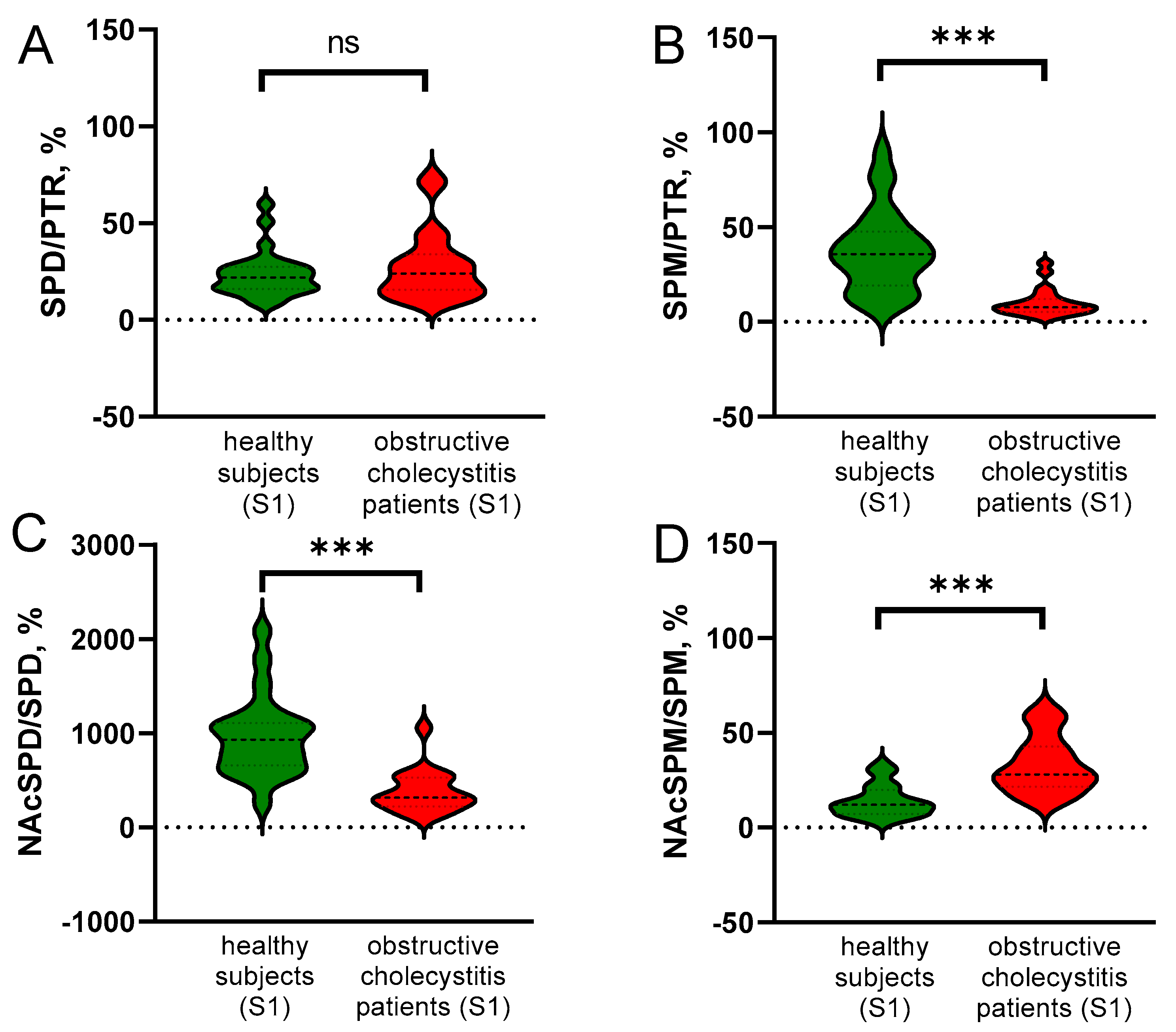
3.2. Obstructive Cholecystitis Leads to the Decrease in SPM and NAcSPD in Blood
3.3. PA Turnover Is Changed in Patients with Obstructive Cholecystitis
3.4. Plasma SPM Levels Decreased in Healthy Subjects Recovered from SARS-CoV-2
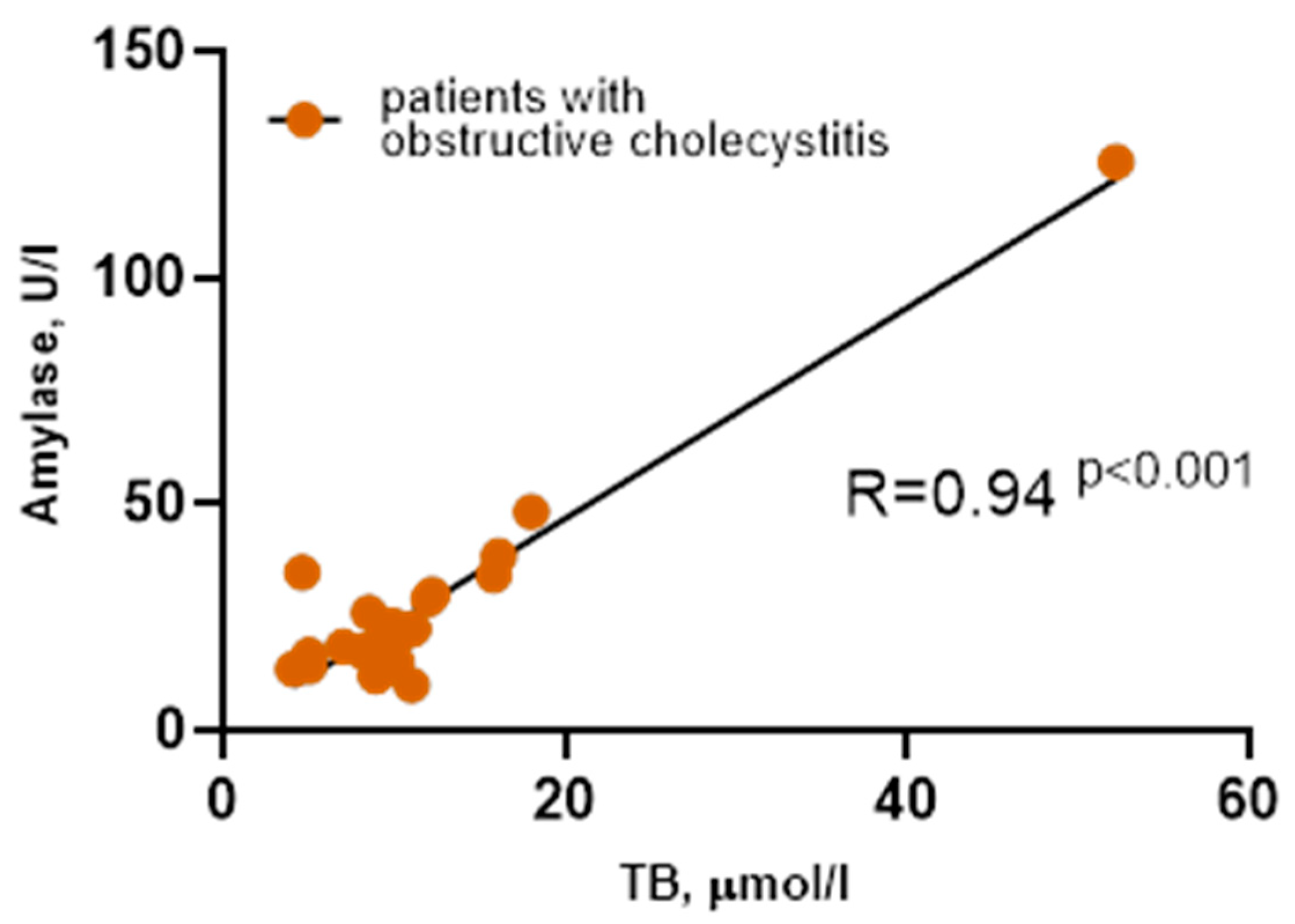
3.5. Candidate Regulatory Factors of Plasma PAs
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okamoto, K.; Suzuki, K.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Endo, I.; Iwashita, Y.; Hibi, T.; Pitt, H.A.; Umezawa, A.; et al. Tokyo Guidelines 2018: Flowchart for the Management of Acute Cholecystitis. J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Kessenich, C.R. Cholecystitis and HIDA Scan. Nurse Pract. 2011, 36, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Tavirani, M.; Abbasi, M.A.; Bagaee, M.; Tizmaghz, A.; Khavanin-Zadeh, M. Evaluation of Preoperative Liver Function Test Efficacy in Patients with Symptomatic Cholelithiasis. Gastroenterol. Hepatol. Bed Bench 2020, 13, 254–257. [Google Scholar] [PubMed]
- Zgheib, H.; Wakil, C.; Shayya, S.; Mailhac, A.; Al-Taki, M.; El Sayed, M.; Tamim, H. Utility of Liver Function Tests in Acute Cholecystitis. Ann. Hepato-Biliary-Pancreat. Surg. 2019, 23, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, J.M.; Goldin, R.D. Liver Changes Associated with Cholecystitis. J. Clin. Pathol. 1994, 47, 457–460. [Google Scholar] [CrossRef][Green Version]
- Barone, S.; Okaya, T.; Rudich, S.; Petrovic, S.; Tenrani, K.; Wang, Z.; Zahedi, K.; Casero, R.A.; Lentsch, A.B.; Soleimani, M. Distinct and Sequential Upregulation of Genes Regulating Cell Growth and Cell Cycle Progression during Hepatic Ischemia-Reperfusion Injury. Am. J. Physiol.-Cell Physiol. 2005, 289, C826–C835. [Google Scholar] [CrossRef]
- Zhang, H.; Simon, A.K. Polyamines Reverse Immune Senescence via the Translational Control of Autophagy. Autophagy 2020, 16, 181–182. [Google Scholar] [CrossRef]
- Koike, S.; Kabuyama, Y.; Obeng, K.A.; Sugahara, K.; Sato, Y.; Yoshizawa, F. An Increase in Liver Polyamine Concentration Contributes to the Tryptophan-Induced Acute Stimulation of Rat Hepatic Protein Synthesis. Nutrients 2020, 12, 2665. [Google Scholar] [CrossRef]
- Bjelaković, G.; Beninati, S.; Pavlović, D.; Sokolović, D.; Stojanović, I.; Jevtović, T.; Bjelaković, G.B.; Nikolić, J.; Basić, J. Selenomethionine Induces Polyamine Biosynthesis in Regenerating Rat Liver Tissue. Amino Acids 2007, 33, 525–529. [Google Scholar] [CrossRef]
- Alhonen, L.; Räsänen, T.-L.; Sinervirta, R.; Parkkinen, J.J.; Korhonen, V.-P.; Pietilä, M.; Jänne, J. Polyamines Are Required for the Initiation of Rat Liver Regeneration. Biochem. J. 2002, 362, 149–153. [Google Scholar] [CrossRef]
- Higaki, I.; Matsui-Yuasa, I.; Terakura, M.; Kinoshita, H.; Otani, S. Increased Spermidine or Spermine Level Is Essential for Hepatocyte Growth Factor-Induced DNA Synthesis in Cultured Rat Hepatocytes. Gastroenterology 1994, 106, 1024–1031. [Google Scholar] [CrossRef]
- Tse, R.T.-H.; Wong, C.Y.-P.; Chiu, P.K.-F.; Ng, C.-F. The Potential Role of Spermine and Its Acetylated Derivative in Human Malignancies. Int. J. Mol. Sci. 2022, 23, 1258. [Google Scholar] [CrossRef] [PubMed]
- Neborak, E.V.; Kaldybayeva, A.B.; Bey, L.; Malmakova, A.Y.; Tveritinova, A.S.; Hilal, A.; Yu, V.K.; Ploskonos, M.V.; Komarova, M.V.; Agostinelli, E.; et al. Anticancer Cytotoxic Activity of Bispidine Derivatives Associated with the Increasing Catabolism of Polyamines. Molecules 2022, 27, 3872. [Google Scholar] [CrossRef] [PubMed]
- Gilad, V.H.; Rabey, J.M.; Kimiagar, Y.; Gilad, G.M. The Polyamine Stress Response: Tissue-, Endocrine-, and Developmental-Dependent Regulation. Biochem. Pharmacol. 2001, 61, 207–213. [Google Scholar] [CrossRef]
- Raina, A.; Jänne, J. Physiology of the Natural Polyamines Putrescine, Spermidine and Spermine. Med. Biol. 1975, 53, 121–147. [Google Scholar]
- Sánchez-Jiménez, F.; Medina, M.Á.; Villalobos-Rueda, L.; Urdiales, J.L. Polyamines in Mammalian Pathophysiology. Cell Mol. Life Sci. 2019, 76, 3987–4008. [Google Scholar] [CrossRef]
- Ōyanagui, Y. Anti-Inflammatory Effect of Polyamines in Serotonin and Carrageenan Paw Edemata—Possible Mechanism to Increase Vascular Permeability Inhibitory Protein Level Which Is Regulated by Glucocorticoids and Superoxide Radical. Agents Actions 1984, 14, 228–237. [Google Scholar] [CrossRef]
- Jia, S.; Kang, Y.P.; Park, J.H.; Lee, J.; Kwon, S.W. Simultaneous Determination of 23 Amino Acids and 7 Biogenic Amines in Fermented Food Samples by Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2011, 1218, 9174–9182. [Google Scholar] [CrossRef]
- Minocha, R.; Long, S. Simultaneous Separation and Quantitation of Amino Acids and Polyamines of Forest Tree Tissues and Cell Cultures within a Single High-Performance Liquid Chromatography Run Using Dansyl Derivatization. J. Chromatogr. A 2004, 1035, 63–73. [Google Scholar] [CrossRef]
- Saaid, M.; Saad, B.; Hashim, N.H.; Mohamed Ali, A.S.; Saleh, M.I. Determination of Biogenic Amines in Selected Malaysian Food. Food Chem. 2009, 113, 1356–1362. [Google Scholar] [CrossRef]
- Castoldi, F.; Hyvönen, M.T.; Durand, S.; Aprahamian, F.; Sauvat, A.; Malik, S.A.; Baracco, E.E.; Vacchelli, E.; Opolon, P.; Signolle, N.; et al. Chemical Activation of SAT1 Corrects Diet-Induced Metabolic Syndrome. Cell Death Differ. 2020, 27, 2904–2920. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting Outliers When Fitting Data with Nonlinear Regression—A New Method Based on Robust Nonlinear Regression and the False Discovery Rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Popović, D.; Kocić, G.; Katić, V.; Zarubica, A.; Veličković, L.J.; Ničković, V.P.; Jović, A.; Veljković, A.; Petrović, V.; Rakić, V.; et al. Anthocyanins Protect Hepatocytes against CCl4-Induced Acute Liver Injury in Rats by Inhibiting Pro-Inflammatory Mediators, Polyamine Catabolism, Lipocalin-2, and Excessive Proliferation of Kupffer Cells. Antioxidants 2019, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.; Itani, H.; Richmond, B.; Arslanbaeva, L.; Vergeade, A.; Rahman, S.M.J.; Boutaud, O.; Blackwell, T.; Massion, P.P.; Harrison, D.G.; et al. Tobacco Smoking Induces Cardiovascular Mitochondrial Oxidative Stress, Promotes Endothelial Dysfunction, and Enhances Hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H639–H646. [Google Scholar] [CrossRef] [PubMed]
- Jothimani, D.; Venugopal, R.; Abedin, M.F.; Kaliamoorthy, I.; Rela, M. COVID-19 and the Liver. J. Hepatol. 2020, 73, 1231–1240. [Google Scholar] [CrossRef]
- Hoet, P.H.M.; Nemery, B. Polyamines in the Lung: Polyamine Uptake and Polyamine-Linked Pathological or Toxicological Conditions. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000, 278, L417–L433. [Google Scholar] [CrossRef]
- Cervelli, M.; Leonetti, A.; Duranti, G.; Sabatini, S.; Ceci, R.; Mariottini, P. Skeletal Muscle Pathophysiology: The Emerging Role of Spermine Oxidase and Spermidine. Med. Sci. 2018, 6, 14. [Google Scholar] [CrossRef]
- Fan, J.; Yang, X.; Li, J.; Shu, Z.; Dai, J.; Liu, X.; Li, B.; Jia, S.; Kou, X.; Yang, Y.; et al. Spermidine Coupled with Exercise Rescues Skeletal Muscle Atrophy from D-Gal-Induced Aging Rats through Enhanced Autophagy and Reduced Apoptosis via AMPK-FOXO3a Signal Pathway. Oncotarget 2017, 8, 17475–17490. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
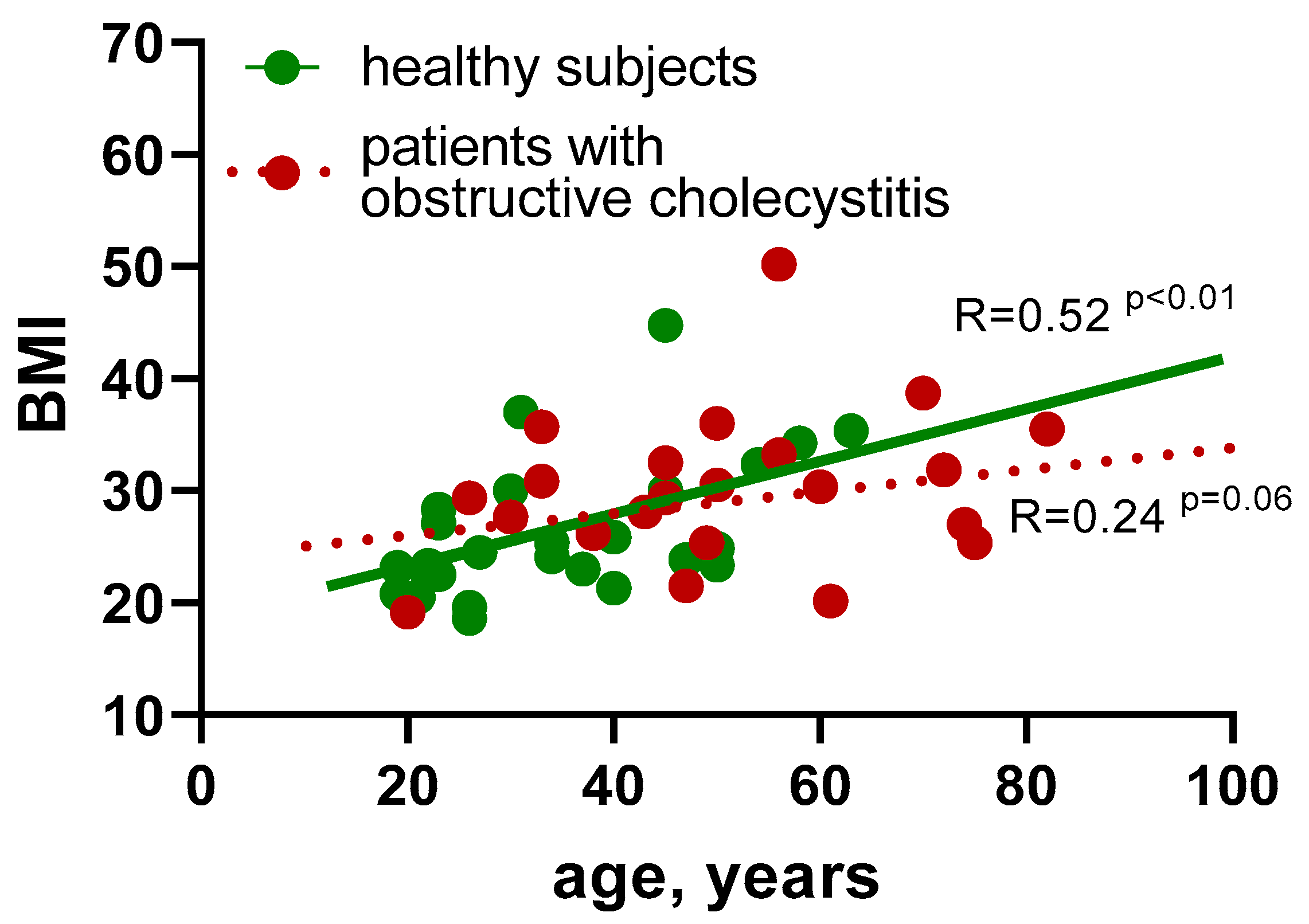


| Individual’s ID | SPM (<15.8 ng/mL) | SPM/PTR(<19.1%) | NAcSPD/SPD (<657.2%) | Additional Medical Conditions |
|---|---|---|---|---|
| 30 | 13,060 | 16,224 | regular crossfit training | |
| 31 | 4940 | 8949 | 547,891 | weight loss 12 kg/2 months |
| 32 | 12,920 | 14,629 | 547,287 | viral hepatitis C—recovered/ regular weightlifting |
| 74 | 265,006 | combined oral contraceptive use | ||
| 75 | 11,720 | 10,933 | using proton pump inhibitors for peptic ulcer disease |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbaraliev, A.A.; Akhvlediani, L.; Kavazashvili, A.; Diasamidze, E.; Surmanidze, O.; Gassen, N.C.; Anderzhanova, E.A. Plasma Polyamines Decrease in Patients with Obstructive Cholecystitis. Livers 2022, 2, 233-242. https://doi.org/10.3390/livers2030019
Akbaraliev AA, Akhvlediani L, Kavazashvili A, Diasamidze E, Surmanidze O, Gassen NC, Anderzhanova EA. Plasma Polyamines Decrease in Patients with Obstructive Cholecystitis. Livers. 2022; 2(3):233-242. https://doi.org/10.3390/livers2030019
Chicago/Turabian StyleAkbaraliev, Amaar A., Leila Akhvlediani, Ana Kavazashvili, Emzar Diasamidze, Omar Surmanidze, Nils C. Gassen, and Elmira A. Anderzhanova. 2022. "Plasma Polyamines Decrease in Patients with Obstructive Cholecystitis" Livers 2, no. 3: 233-242. https://doi.org/10.3390/livers2030019
APA StyleAkbaraliev, A. A., Akhvlediani, L., Kavazashvili, A., Diasamidze, E., Surmanidze, O., Gassen, N. C., & Anderzhanova, E. A. (2022). Plasma Polyamines Decrease in Patients with Obstructive Cholecystitis. Livers, 2(3), 233-242. https://doi.org/10.3390/livers2030019






