A Comprehensive Review on the Use of Herbal Dietary Supplements in the USA, Reasons for Their Use, and Review of Potential Hepatotoxicity
Abstract
:1. Introduction
2. Regulation of HDS
3. Hepatotoxic Effects
4. Genetic and Demographic Susceptibility
5. Pharmacokinetic and Pharmacodynamics Changes in Chronic Liver Disease
6. Interaction with Other Drugs
7. Clinical Diagnosis and Management
7.1. Clinical Diagnosis
7.1.1. Classifications
7.1.2. Hy’s Law
- The drug causes hepatocellular injury, demonstrated by a higher incidence of the upper limit of normal ALT or AST that is three-fold greater than the (non-hepatotoxic) control drug or placebo.
- Among trial subjects with AT elevations, one or more also show TBL serum elevations >2 × ULN without initial findings of cholestasis (elevated serum ALP).
- No other explanation can be found for the increased AT and TBL, such as viral hepatitis A, B, or C; preexisting or acute liver disease; or another drug capable of causing the injury.
7.2. Management
8. Drug-Induced Hepatotoxicity and Obesity
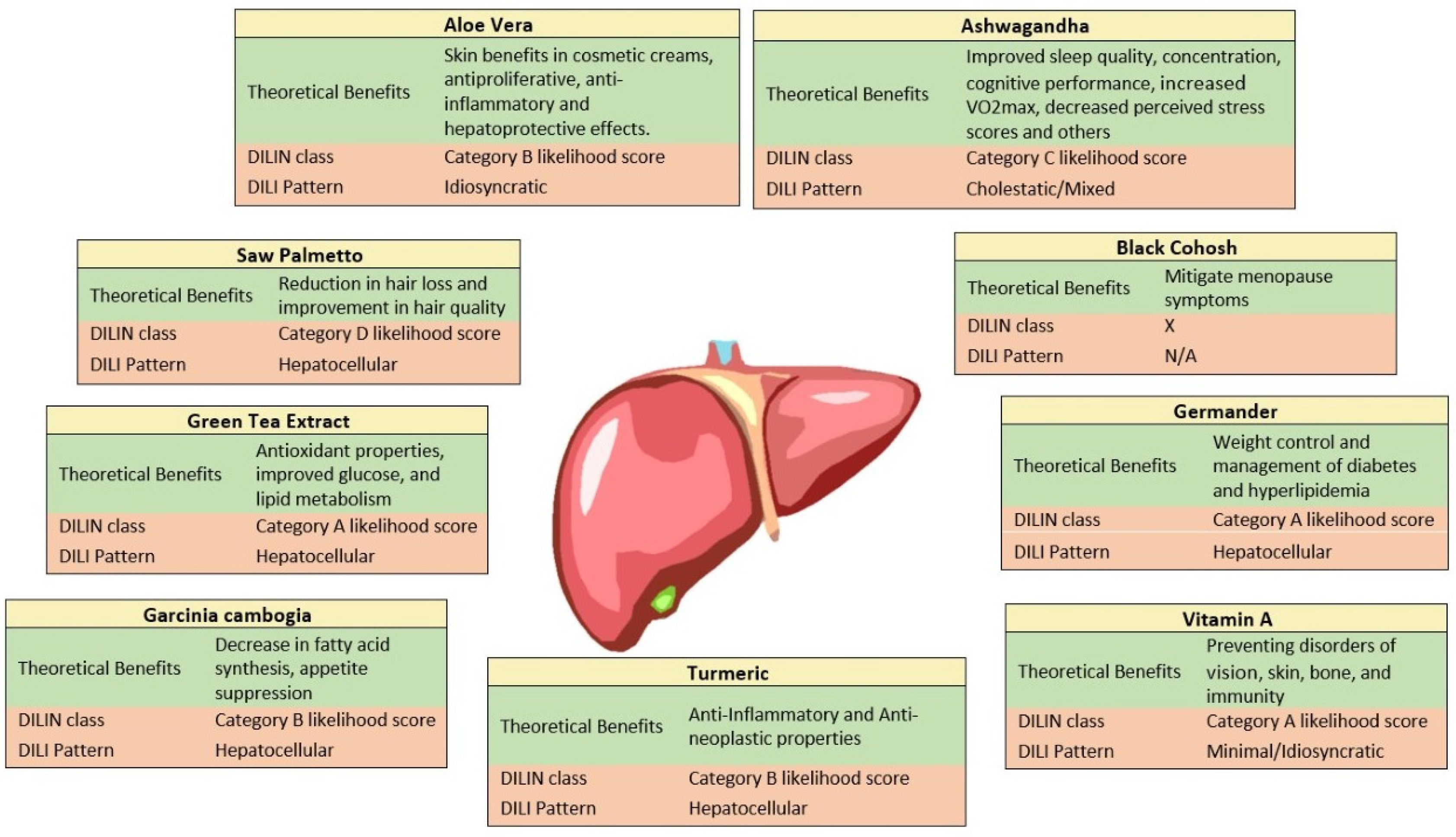
9. Common Hepatotoxic Agents on the HDS Market
9.1. Garcinia Cambogia
9.2. Saw Palmetto
9.3. Ashwagandha
9.4. Green Tea
9.5. Aloe Vera
9.6. Germander
9.7. Vitamin A
9.8. Black Cohosh
9.9. Turmeric
10. Limitations
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Boer, Y.S.; Sherker, A.H. Herbal and Dietary Supplement-Induced Liver Injury. Clin. Liver Dis. 2017, 21, 135–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Dietary Supplement Use Among Adults: United States, 2017–2018. In NCHS Data Brief; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021; pp. 1–8. [Google Scholar]
- Feng, Z.; Yang, J.; Xu, M.; Lin, R.; Yang, H.; Lai, L.; Wang, Y.; Wahner-Roedler, D.L.; Zhou, X.; Shin, K.M.; et al. Dietary supplements and herbal medicine for COVID-19: A systematic review of randomized control trials. Clin. Nutr. ESPEN 2021, 44, 50–60. [Google Scholar] [CrossRef]
- Council for Responsible Nutrition. 2020 CRN Consumer Survey on Dietary Supplements. Available online: https://www.crnusa.org/resources/2020-crn-consumer-survey-dietary-supplements (accessed on 4 May 2022).
- Zupo, R.; Castellana, F.; Sardone, R.; Sila, A.; Giagulli, V.A.; Triggiani, V.; Cincione, R.I.; Giannelli, G.; De Pergola, G. Preliminary Trajectories in Dietary Behaviors during the COVID-19 Pandemic: A Public Health Call to Action to Face Obesity. Int. J. Environ. Res. Public Health 2020, 17, 7073. [Google Scholar] [CrossRef] [PubMed]
- Aldwihi, L.A.; Khan, S.I.; Alamri, F.F.; AlRuthia, Y.; Alqahtani, F.; Fantoukh, O.I.; Assiri, A.; Almohammed, O.A. Patients’ Behavior Regarding Dietary or Herbal Supplements before and during COVID-19 in Saudi Arabia. Int. J. Environ. Res. Public Health 2021, 18, 5086. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Veronica, E.; Morton, R.C. Herbal Supplement Sales in US Increase by Record-Breaking 17.3% in 2020. Sales of immune health, stress relief, and heart health supplements grow during COVID-19 pandemic. Herb. J. Am. Bot. Counc. 2021, 131, 52–65. [Google Scholar]
- Hossain, M.M.; Tasnim, S.; Sultana, A.; Faizah, F.; Mazumder, H.; Zou, L.; McKyer, E.L.J.; Ahmed, H.U.; Ma, P. Epidemiology of mental health problems in COVID-19: A review. F1000Research 2020, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Questions and Answers on Dietary Supplements. 2022. Available online: https://www.fda.gov/food/information-consumers-using-dietary-supplements/questions-and-answers-dietary-supplements (accessed on 5 June 2022).
- Navarro, V.J.; Khan, I.; Bjornsson, E.; Seeff, L.B.; Serrano, J.; Hoofnagle, J.H. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Food and Drug Administration. FDA 101: Dietary Supplements. 2015. Available online: https://www.fda.gov/consumers/consumer-updates/fda-101-dietary-supplements (accessed on 15 July 2015).
- Larimore, W.L.; O’Mathuna, D.P. Quality assessment programs for dietary supplements. Ann. Pharmacother. 2003, 37, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.; Avula, B.; Khan, I.; Verma, M.; Seeff, L.; Serrano, J.; Stolz, A.; Fontana, R.; Ahmad, J. The Contents of Herbal and Dietary Supplements Implicated in Liver Injury in the United States Are Frequently Mislabeled. Hepatol. Commun. 2019, 3, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Larrey, D.; Faure, S. Herbal medicine hepatotoxicity: A new step with development of specific biomarkers. J. Hepatol. 2011, 54, 599–601. [Google Scholar] [CrossRef] [Green Version]
- Starr, R.R. Too little, too late: Ineffective regulation of dietary supplements in the United States. Am. J. Public Health 2015, 105, 478–485. [Google Scholar] [CrossRef] [PubMed]
- United States Government Accountability Office. Dietary Supplements: FDA May Have Opportunities to Expand Its Use of Reported Health Problems to Oversee Products U.S. Government Accountability Office Website—Reports & Testimonies. 2013. Available online: https://www.gao.gov/products/gao-13-244 (accessed on 4 May 2022).
- Mitra, V.; Jane, M. Metabolic functions of the liver. Anaesth. Intensive Care Med. 2009, 10, 334–335. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. Germander. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MA, USA, 2012.
- Anup Ramachandran, H.J. (Ed.) Drug-Induced Liver Injury; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Han, D.; Dara, L.; Win, S.; Than, T.A.; Yuan, L.; Abbasi, S.Q.; Liu, Z.X.; Kaplowitz, N. Regulation of drug-induced liver injury by signal transduction pathways: Critical role of mitochondria. Trends Pharmacol. Sci. 2013, 34, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewal, P.; Ahmad, J. Severe liver injury due to herbal and dietary supplements and the role of liver transplantation. World J. Gastroenterol. 2019, 25, 6704–6712. [Google Scholar] [CrossRef]
- Navarro, V.J.; Barnhart, H.; Bonkovsky, H.L.; Davern, T.; Fontana, R.J.; Grant, L.; Reddy, K.R.; Seeff, L.B.; Serrano, J.; Sherker, A.H.; et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology 2014, 60, 1399–1408. [Google Scholar] [CrossRef] [Green Version]
- Lunsford, K.E.; Bodzin, A.S.; Reino, D.C.; Wang, H.L.; Busuttil, R.W. Dangerous dietary supplements: Garcinia cambogia-associated hepatic failure requiring transplantation. World J. Gastroenterol. 2016, 22, 10071–10076. [Google Scholar] [CrossRef]
- Hoofnagle, J.H.; Bonkovsky, H.L.; Phillips, E.J.; Li, Y.J.; Ahmad, J.; Barnhart, H.; Durazo, F.; Fontana, R.J.; Gu, J.; Khan, I.; et al. HLA-B*35:01 and Green Tea-Induced Liver Injury. Hepatology 2021, 73, 2484–2493. [Google Scholar] [CrossRef]
- Rodighiero, V. Effects of liver disease on pharmacokinetics. An update. Clin. Pharmacokinet. 1999, 37, 399–431. [Google Scholar] [CrossRef] [PubMed]
- Bupsilondingen, F.V.; Gonzalez, D.; Tucker, A.N.; Derendorf, H. Relevance of Liver Failure for Anti-Infective Agents: From Pharmacokinetic Alterations to Dosage Adjustments. Ther. Adv. Infect. Dis. 2014, 2, 17–42. [Google Scholar] [PubMed]
- Katayama, K. Zinc and protein metabolism in chronic liver diseases. Nutr. Res. 2020, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elbekai, R.H.; Korashy, H.M.; El-Kadi, A.O. The effect of liver cirrhosis on the regulation and expression of drug metabolizing enzymes. Curr. Drug Metab. 2004, 5, 157–167. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Mannel, M. Drug interactions with St John’s wort: Mechanisms and clinical implications. Drug Saf. 2004, 27, 773–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.H.; Larrey, D.; Olsson, R.; Lee, W.M.; Frison, L.; Keisu, M. Utility of the Roussel Uclaf Causality Assessment Method (RUCAM) to analyze the hepatic findings in a clinical trial program: Evaluation of the direct thrombin inhibitor ximelagatran. Int. J. Clin. Pharmacol. Ther. 2008, 46, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Rochon, J.; Protiva, P.; Seeff, L.B.; Fontana, R.J.; Liangpunsakul, S.; Watkins, P.B.; Davern, T.; McHutchison, J.G. Drug-Induced Liver Injury Network. Reliability of the Roussel Uclaf Causality Assessment Method for assessing causality in drug-induced liver injury. Hepatology 2008, 48, 1175–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockey, D.C.; Seeff, L.B.; Rochon, J.; Freston, J.; Chalasani, N.; Bonacini, M.; Fontana, R.J.; Hayashi, P.H.; Network, U.S. Drug-Induced Liver Injury. Causality assessment in drug-induced liver injury using a structured expert opinion process: Comparison to the Roussel-Uclaf causality assessment method. Hepatology 2010, 51, 2117–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Cortes, M.; Stephens, C.; Lucena, M.I.; Fernandez-Castaner, A.; Andrade, R.J. Causality assessment methods in drug induced liver injury: Strengths and weaknesses. J. Hepatol. 2011, 55, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, P.H.; Lucena, M.I.; Fontana, R.J.; Bjornsson, E.S.; Aithal, G.P.; Barnhart, H.; Gonzalez-Jimenez, A.; Yang, Q.; Gu, J.; Andrade, R.J.; et al. A revised electronic version of RUCAM for the diagnosis of DILI. Hepatology 2022, 76, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Regev, A.; Bjornsson, E.S. Drug-induced liver injury: Morbidity, mortality, and Hy’s law. Gastroenterology 2014, 147, 20–24. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Guidance for Industry Drug-Induced Liver Injury: Premarketing Clinical Evaluation. 2009. Available online: https://www.fda.gov/media/116737/download (accessed on 4 May 2022).
- Chauhan, K.; Adnan, K.; Salil, C.; Ross, H.M.; Salinas, P.N.; Dina, H. A Comprehensive Review on the Risk of Metabolic Syndrome and Cardiovascular Disease after Liver Transplantation. Livers 2022, 2, 6. [Google Scholar] [CrossRef]
- Massart, J.; Begriche, K.; Moreau, C.; Fromenty, B. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J. Clin. Transl. Res. 2017, 3, 212–232. [Google Scholar] [PubMed] [Green Version]
- National Institute of Diabetes and Digestive and Kidney Diseases. Garcinia Cambogia. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MA, USA, 2012.
- Haber, S.L.; Awwad, O.; Phillips, A.; Park, A.E.; Pham, T.M. Garcinia cambogia for weight loss. Am. J. Health Syst. Pharm. 2018, 75, 17–22. [Google Scholar] [CrossRef]
- Han, J.H.; Jang, K.W.; Park, M.H.; Myung, C.S. Garcinia cambogia suppresses adipogenesis in 3T3-L1 cells by inhibiting p90RSK and Stat3 activation during mitotic clonal expansion. J. Cell Physiol. 2021, 236, 1822–1839. [Google Scholar] [CrossRef] [PubMed]
- Maia-Landim, A.; Ramirez, J.M.; Lancho, C.; Poblador, M.S.; Lancho, J.L. Long-term effects of Garcinia cambogia/Glucomannan on weight loss in people with obesity, PLIN4, FTO and Trp64Arg polymorphisms. BMC Complement. Altern. Med. 2018, 18, 26. [Google Scholar] [CrossRef] [Green Version]
- Chong, P.W.; Beah, Z.M.; Grube, B.; Riede, L. IQP-GC-101 reduces body weight and body fat mass: A randomized, double-blind, placebo-controlled study. Phytother. Res. 2014, 28, 1520–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasques, C.A.; Schneider, R.; Klein-Junior, L.C.; Falavigna, A.; Piazza, I.; Rossetto, S. Hypolipemic effect of Garcinia cambogia in obese women. Phytother. Res. 2014, 28, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Vasques, C.A.; Rossetto, S.; Halmenschlager, G.; Linden, R.; Heckler, E.; Fernandez, M.S.; Alonso, J.L. Evaluation of the pharmacotherapeutic efficacy of Garcinia cambogia plus Amorphophallus konjac for the treatment of obesity. Phytother. Res. 2008, 22, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Toromanyan, E.; Aslanyan, G.; Amroyan, E.; Gabrielyan, E.; Panossian, A. Efficacy of Slim339 in reducing body weight of overweight and obese human subjects. Phytother. Res. 2007, 21, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Opala, T.; Rzymski, P.; Pischel, I.; Wilczak, M.; Wozniak, J. Efficacy of 12 weeks supplementation of a botanical extract-based weight loss formula on body weight, body composition and blood chemistry in healthy, overweight subjects—A randomised double-blind placebo-controlled clinical trial. Eur. J. Med. Res. 2006, 11, 343–350. [Google Scholar] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I. Effect of orlistat alone or in combination with Garcinia cambogia on visceral adiposity index in obese patients. J. Intercult. Ethnopharmacol. 2016, 5, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.; Hung, S.K.; Perry, R.; Wider, B.; Ernst, E. The Use of Garcinia Extract (Hydroxycitric Acid) as a Weight loss Supplement: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. J. Obes. 2011, 2011, 509038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuppalanchi, R.; Bonkovsky, H.L.; Ahmad, J.; Barnhart, H.; Durazo, F.; Fontana, R.J.; Gu, J.; Khan, I.; Kleiner, D.E.; Koh, C.; et al. Garcinia cambogia, Either Alone or in Combination with Green Tea, Causes Moderate to Severe Liver Injury. Clin. Gastroenterol. Hepatol. 2022, 20, e1416–e1425. [Google Scholar] [CrossRef]
- Park, A.; Castineira, J.; Bournique, M.; Babcock, H.; Miller, R.T.; Thomas, M. 2442-Garcinia cambogia, Weight Loss at What Price? Am. J. Gastroenterol. 2019, 114, S1351–S1352. [Google Scholar] [CrossRef]
- Ferreira, V.; Mathieu, A.; Soucy, G.; Giard, J.M.; Erard-Poinsot, D. Acute Severe Liver Injury Related to Long-Term Garcinia cambogia Intake. ACG Case Rep. J. 2020, 7, e00429. [Google Scholar] [CrossRef] [PubMed]
- Ordeig, A.M.; Bordon Garcia, N. Hepatotoxicity caused by Garcinia cambogia. Gastroenterol. Hepatol. 2020, 43, 134–135. [Google Scholar] [CrossRef]
- McCarthy, R.E.; Bowen, D.G.; Strasser, S.I.; McKenzie, C. The dangers of herbal weight loss supplements: A case report of drug-induced liver injury secondary to Garcinia cambogia ingestion. Pathology 2021, 53, 545–547. [Google Scholar] [CrossRef]
- Calaquian, L.L.; Yau, I. Garcinia cambogia—A Supplement-Related Liver Injury. Cureus 2022, 14, e22225. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, G.; Lombardi, N.; Bettiol, A.; Marconi, E.; Risaliti, F.; Bertoni, M.; Menniti Ippolito, F.; Maggini, V.; Gallo, E.; Firenzuoli, F.; et al. Acute liver injury following Garcinia cambogia weight-loss supplementation: Case series and literature review. Intern. Emerg. Med. 2018, 13, 857–872. [Google Scholar] [CrossRef]
- Narda, M.; Sonia, A.; Enza, C.; Vincenzo, N. Efficacy and Safety of a Food Supplement Containing L-cystine, Serenoa repens Extract and Biotin for Hair Loss in Healthy Males and Females. A Prospective, Randomized, Double-blinded, Controlled Clinical Trial. J. Cosmetol. Trichol. 2017, 3, 127. [Google Scholar] [CrossRef]
- Koch, E. Extracts from fruits of saw palmetto (Sabal serrulata) and roots of stinging nettle (Urtica dioica): Viable alternatives in the medical treatment of benign prostatic hyperplasia and associated lower urinary tracts symptoms. Planta Med. 2001, 67, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Goepel, M.; Hecker, U.; Krege, S.; Rubben, H.; Michel, M.C. Saw palmetto extracts potently and noncompetitively inhibit human alpha1-adrenoceptors in vitro. Prostate 1999, 38, 208–215. [Google Scholar] [CrossRef]
- Oki, T.; Suzuki, M.; Nishioka, Y.; Yasuda, A.; Umegaki, K.; Yamada, S. Effects of saw palmetto extract on micturition reflex of rats and its autonomic receptor binding activity. J. Urol. 2005, 173, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Willetts, K.E.; Clements, M.S.; Champion, S.; Ehsman, S.; Eden, J.A. Serenoa repens extract for benign prostate hyperplasia: A randomized controlled trial. BJU Int. 2003, 92, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Bent, S.; Kane, C.; Shinohara, K.; Neuhaus, J.; Hudes, E.S.; Goldberg, H.; Avins, A.L. Saw palmetto for benign prostatic hyperplasia. N. Engl. J. Med. 2006, 354, 557–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, E. The risk-benefit profile of commonly used herbal therapies: Ginkgo, St. John’s Wort, Ginseng, Echinacea, Saw Palmetto, and Kava. Ann. Intern. Med. 2002, 136, 42–53. [Google Scholar] [CrossRef]
- Agbabiaka, T.B.; Pittler, M.H.; Wider, B.; Ernst, E. Serenoa repens (saw palmetto): A systematic review of adverse events. Drug Saf. 2009, 32, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, J.S.; Donovan, J.L.; Devane, C.L.; Taylor, R.M.; Ruan, Y.; Wang, J.S.; Chavin, K.D. Multiple doses of saw palmetto (Serenoa repens) did not alter cytochrome P450 2D6 and 3A4 activity in normal volunteers. Clin. Pharmacol. Ther. 2003, 74, 536–542. [Google Scholar] [CrossRef]
- Singh, Y.N.; Devkota, A.K.; Sneeden, D.C.; Singh, K.K.; Halaweish, F. Hepatotoxicity potential of saw palmetto (Serenoa repens) in rats. Phytomedicine 2007, 14, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Lapi, F.; Gallo, E.; Giocaliere, E.; Vietri, M.; Baronti, R.; Pieraccini, G.; Tafi, A.; Menniti-Ippolito, F.; Mugelli, A.; Firenzuoli, F.; et al. Acute liver damage due to Serenoa repens: A case report. Br. J. Clin. Pharmacol. 2010, 69, 558–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadood, R.; Waqas, N.; Hilary, H. 2421—A Case of Acute Liver Injury Caused by an Herbal Supplement Containing Saw Palmetto: “Good for the Prostate but Not for the Liver”. Am. J. Gastroenterol. 2019, 114, S134. [Google Scholar] [CrossRef]
- Jibrin, I.; Erinle, A.; Saidi, A.; Aliyu, Z.Y. Saw palmetto-induced pancreatitis. South Med. J. 2006, 99, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Wargo, K.A.; Allman, E.; Ibrahim, F. A possible case of saw palmetto-induced pancreatitis. South Med. J. 2010, 103, 683–685. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. Ashwagandha. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MA, USA, 2012.
- Smith, T.; Georgia, M.; Veronica, E.; Morton, R.C. US Sales of Herbal Supplements Increase by 8.6% in 2019. CBD, mushroom, and elderberry supplements continue to drive sales. Herb. J. Am. Bot. Counc. 2020, 127, 54–69. [Google Scholar]
- Deshpande, A.; Irani, N.; Balkrishnan, R.; Benny, I.R. A randomized, double blind, placebo controlled study to evaluate the effects of ashwagandha (Withania somnifera) extract on sleep quality in healthy adults. Sleep Med. 2020, 72, 28–36. [Google Scholar] [CrossRef]
- Kelgane, S.B.; Salve, J.; Sampara, P.; Debnath, K. Efficacy and Tolerability of Ashwagandha Root Extract in the Elderly for Improvement of General Well-being and Sleep: A Prospective, Randomized, Double-blind, Placebo-controlled Study. Cureus 2020, 12, e7083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salve, J.; Pate, S.; Debnath, K.; Langade, D. Adaptogenic and Anxiolytic Effects of Ashwagandha Root Extract in Healthy Adults: A Double-blind, Randomized, Placebo-controlled Clinical Study. Cureus 2019, 11, e6466. [Google Scholar] [CrossRef] [Green Version]
- Dimpfel, W.; Schombert, L.; Keplinger-Dimpfel, I.K.; Panossian, A. Effects of an Adaptogenic Extract on Electrical Activity of the Brain in Elderly Subjects with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled, Two-Armed Cross-over Study. Pharmaceuticals 2020, 13, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuladi, S.; Emami, S.A.; Mohammadpour, A.H.; Karimani, A.; Manteghi, A.A.; Sahebkar, A. Assessment of the Efficacy of Withania somnifera Root Extract in Patients with Generalized Anxiety Disorder: A Randomized Double-blind Placebo- Controlled Trial. Curr. Rev. Clin. Exp. Pharmacol. 2021, 16, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Langade, D.; Kanchi, S.; Salve, J.; Debnath, K.; Ambegaokar, D. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Insomnia and Anxiety: A Double-blind, Randomized, Placebo-controlled Study. Cureus 2019, 11, e5797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: A randomized, double-blind, placebo-controlled study. Medicine 2019, 98, e17186. [Google Scholar] [CrossRef]
- Perez-Gomez, J.; Villafaina, S.; Adsuar, J.C.; Merellano-Navarro, E.; Collado-Mateo, D. Effects of Ashwagandha (Withania somnifera) on VO2max: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhu, J.S.; Shah, B.; Shenoy, S.; Chauhan, S.; Lavekar, G.S.; Padhi, M.M. Effects of Withania somnifera (Ashwagandha) and Terminalia arjuna (Arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int. J. Ayurveda Res. 2010, 1, 144–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gannon, J.M.; Brar, J.; Rai, A.; Chengappa, K.N.R. Effects of a standardized extract of Withania somnifera (Ashwagandha) on depression and anxiety symptoms in persons with schizophrenia participating in a randomized, placebo-controlled clinical trial. Ann. Clin. Psychiatry 2019, 31, 123–129. [Google Scholar] [PubMed]
- Chengappa, K.N.R.; Brar, J.S.; Gannon, J.M.; Schlicht, P.J. Adjunctive Use of a Standardized Extract of Withania somnifera (Ashwagandha) to Treat Symptom Exacerbation in Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Psychiatry 2018, 79, 22496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjornsson, H.K.; Bjornsson, E.S.; Avula, B.; Khan, I.A.; Jonasson, J.G.; Ghabril, M.; Hayashi, P.H.; Navarro, V. Ashwagandha-induced liver injury: A case series from Iceland and the US Drug-Induced Liver Injury Network. Liver Int. 2020, 40, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Gerbes, A.L. Ashwagandha-Induced Liver Injury: Self-Reports on Commercial Websites as Useful Adjunct Tools for Causality Assessment. Am. J. Gastroenterol. 2021, 116, 2151–2152. [Google Scholar] [CrossRef] [PubMed]
- Ireland, P.J.; Hardy, T.; Burt, A.D.; Donnelly, M.C. Drug-induced hepatocellular injury due to herbal supplement ashwagandha. J. R. Coll. Phys. Edinb. 2021, 51, 363–365. [Google Scholar] [CrossRef]
- Suryawanshi, G.; Mohamed, A.; Mary, T.; Nidhi, D.; Aastha, C.; Nicholas, L. Ashwagandha-Associated Acute Liver Failure Requiring Liver Transplantation. Available online: https://journals.lww.com/americantherapeutics/Citation/9000/Ashwagandha_Associated_Acute_Liver_Failure.98012.aspx (accessed on 4 May 2022).
- Siddiqui, S.; Ahmed, N.; Goswami, M.; Chakrabarty, A.; Chowdhury, G. DNA damage by Withanone as a potential cause of liver toxicity observed for herbal products of Withania somnifera (Ashwagandha). Curr. Res. Toxicol. 2021, 2, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Ingawale, D.S.M.; Namdeo, A.G. Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. J. Diet. Suppl. 2021, 18, 183–226. [Google Scholar]
- Cho, T.; Wang, X.; Yeung, K.; Cao, Y.; Uetrecht, J. Liver Injury Caused by Green Tea Extract in PD-1(−/−) Mice: An Impaired Immune Tolerance Model for Idiosyncratic Drug-Induced Liver Injury. Chem. Res. Toxicol. 2021, 34, 849–856. [Google Scholar] [CrossRef]
- Tallei, T.E.; Niode, N.J.; Idroes, R.; Zidan, B.M.; Mitra, S.; Celik, I.; Nainu, F.; Agagunduz, D.; Emran, T.B.; Capasso, R.; et al. A Comprehensive Review of the Potential Use of Green Tea Polyphenols in the Management of COVID-19. Evid. Based Complement. Altern. Med. 2021, 2021, 7170736. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurgens, T.M.; Whelan, A.M.; Killian, L.; Doucette, S.; Kirk, S.; Foy, E. Green tea for weight loss and weight maintenance in overweight or obese adults. Cochrane Database Syst. Rev. 2012, 12, CD008650. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.J.; Bonkovsky, H.L.; Hwang, S.I.; Vega, M.; Barnhart, H.; Serrano, J. Catechins in dietary supplements and hepatotoxicity. Dig. Dis. Sci. 2013, 58, 2682–2690. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Diabetes and Digestive and Kidney Diseases. Green Tea. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MA, USA, 2012.
- Whitsett, M.; Marzio, D.H.; Rossi, S. SlimQuick-Associated Hepatotoxicity Resulting in Fulminant Liver Failure and Orthotopic Liver Transplantation. ACG Case Rep. J. 2014, 1, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Seeff, L.B.; Bonkovsky, H.L.; Navarro, V.J.; Wang, G. Herbal products and the liver: A review of adverse effects and mechanisms. Gastroenterology 2015, 148, 517–532.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halegoua-De Marzio, D.; Kraft, W.K.; Daskalakis, C.; Ying, X.; Hawke, R.L.; Navarro, V.J. Limited sampling estimates of epigallocatechin gallate exposures in cirrhotic and noncirrhotic patients with hepatitis C after single oral doses of green tea extract. Clin. Ther. 2012, 34, 2279–2285.e1. [Google Scholar] [CrossRef] [PubMed]
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. Aloe Vera. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MA, USA, 2012.
- Werawatganon, D.; Linlawan, S.; Thanapirom, K.; Somanawat, K.; Klaikeaw, N.; Rerknimitr, R.; Siriviriyakul, P. Aloe vera attenuated liver injury in mice with acetaminophen-induced hepatitis. BMC Complement. Altern. Med. 2014, 14, 229. [Google Scholar] [CrossRef] [Green Version]
- Klaikeaw, N.; Wongphoom, J.; Werawatganon, D.; Chayanupatkul, M.; Siriviriyakul, P. Anti-inflammatory and anti-oxidant effects of aloe vera in rats with non-alcoholic steatohepatitis. World J. Hepatol. 2020, 12, 363–377. [Google Scholar] [CrossRef]
- Sehitoglu, M.H.; Karaboga, I.; Kiraz, A.; Kiraz, H.A. The hepatoprotective effect of Aloe vera on ischemia-reperfusion injury in rats. North Clin. Istanb. 2019, 6, 203–209. [Google Scholar] [PubMed]
- Saka, W.A.; Akhigbe, R.E.; Ishola, O.S.; Ashamu, E.A.; Olayemi, O.T.; Adeleke, G.E. Hepatotherapeutic effect of Aloe vera in alcohol-induced hepatic damage. Pak. J. Biol. Sci. 2011, 14, 742–746. [Google Scholar] [CrossRef] [Green Version]
- Nahar, T.; Uddin, B.; Hossain, S.; Sikder, A.M.; Ahmed, S. Aloe vera gel protects liver from oxidative stress-induced damage in experimental rat model. J. Complement. Integr. Med. 2013, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Parlati, L.; Voican, C.S.; Perlemuter, K.; Perlemuter, G. Aloe vera-induced acute liver injury: A case report and literature review. Clin. Res. Hepatol. Gastroenterol. 2017, 41, e39–e42. [Google Scholar] [CrossRef] [PubMed]
- Ballotin, V.R.; Bigarella, L.G.; de Mello Brandao, A.B.; Balbinot, R.A.; Balbinot, S.S.; Soldera, J. Herb-induced liver injury: Systematic review and meta-analysis. World J. Clin. Cases 2021, 9, 5490–5513. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, T.C.H.; Patel, N.; Cook, N.A.; Williams, R.; Taylor-Robinson, S.D.; Lim, A.K.P. The Effect of Aloe Vera Juice on Liver Enzymes and Hepatic Structure in a Healthy Population. Integr. Med. 2020, 19, 30–34. [Google Scholar]
- Ross, D.A. Recommendations for vitamin A supplementation. J. Nutr. 2002, 132, 2902S–2906S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendia, L.E.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saadati, S.; Hatami, B.; Yari, Z.; Shahrbaf, M.A.; Eghtesad, S.; Mansour, A.; Poustchi, H.; Hedayati, M.; Aghajanpoor-Pasha, M.; Sadeghi, A.; et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019, 73, 441–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardi, N.; Crescioli, G.; Maggini, V.; Ippoliti, I.; Menniti-Ippolito, F.; Gallo, E.; Brilli, V.; Lanzi, C.; Mannaioni, G.; Firenzuoli, F.; et al. Acute liver injury following turmeric use in Tuscany: An analysis of the Italian Phytovigilance database and systematic review of case reports. Br. J. Clin. Pharmacol. 2021, 87, 741–753. [Google Scholar] [CrossRef]
- Nissen, T.; Wynn, R. The clinical case report: A review of its merits and limitations. BMC Res. Notes 2014, 7, 264. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; He, Y.; Zheng, G.; Zhang, W.; Yao, Z.; Xie, W. Meta-analysis of traditional herbal medicine in the treatment of nonalcoholic fatty liver disease. Cell Mol. Biol. 2016, 62, 88–95. [Google Scholar] [PubMed]
- Efsa Panel on Contaminants in the Food Chain; Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017, 15, e04908. [Google Scholar] [PubMed] [Green Version]
- Wang, X.; Zhang, W.; Yang, Y.; Chen, Y.; Zhuge, Y.; Xiong, A.; Yang, L.; Wang, Z. Blood microRNA Signatures Serve as Potential Diagnostic Biomarkers for Hepatic Sinusoidal Obstruction Syndrome Caused by Gynura japonica Containing Pyrrolizidine Alkaloids. Front. Pharmacol. 2021, 12, 627126. [Google Scholar] [CrossRef]
| Phase 1 Enzymes | |
|---|---|
| CYP Family | Typical Substrates |
| CYP1 | Hormones, various pharmaceuticals, procarcinogens, polycyclic aromatic hydrocarbons, and other environmental toxins. |
| CYP2A–E | Ketones, glycerol, fatty acids, drugs, xenobiotics, and hormones. |
| CYP3A | Caffeine, testosterone, progesterone, androstenedione, and polyaromatic hydrocarbons. |
| Phase 2 Enzymes | |
|---|---|
| Phase 2 Enzyme Class | Group Phase 2 Enzyme Adds to Target |
| UDP-glucuronosyltransferases | Glucuronic acid |
| Sulfotransferases | Sulfuryl |
| Glutathione S-Transferases | Glutathione |
| N-acetyl transferases | Acetyl |
| Methyltransferases | Methyl |
| Study | Claim | Major Limitation |
|---|---|---|
| Feng et al., 2021 [3] | Zinc sulfate shortens the duration of olfactory dysfunction in patients with COVID | Low quality of selected trials; only RCTs were included and had a wide variation with the targeted population, mode of delivering HDS, outcomes, and follow-up periods |
| Lunsford et al., 2016 [24] | Fulminant hepatic failure caused by Garcinia Cambogia 5:1 extract | No independent laboratory evaluation of supplement to identify contaminants/verify composition |
| Han et al., 2021 [43] | Garcinia cambogia extract suppresses adipogenesis in mouse 3T3-L1 preadipocytes via modulation of p90RSK and Stat3 | In vitro study |
| Maia-Landim et al., 2018 [44] | Garcinia cambogia and Glucomannan reduce weight and improve lipid and glucose blood profiles in overweight/obese individuals | Not randomized; combination therapy with GC and Glucomannan; included patients with dyslipidemias, hypertension and type 2 diabetes receiving treatment for 2 months to 4 years before study onset |
| Chong et al., 2014 [45] | IQP-GC-101 reduces body weight and body fat mass in overweight Caucasian adults | IQP-GC-101 includes 4 different extracts including GC; did not exclude subjects with regular caffeine intake; no strict control of daily caloric intake |
| Vasques et al., 2014 [46] | Short-term Garcinia cambogia treatment significantly reduces triglyceride levels in obese women without affecting anthropometric or calorimetric parameters | Small sample size; did not use intention-to-treat methods; did not describe dropouts; did not include p values for all comparisons |
| Vasques et al., 2008 [47] | Garcinia cambogia plus, Amorphophallus konjac treatment significantly reduces total cholesterol and LDL-c levels in patients with obesity compared to placebo without affecting anthropometric or calorimetric parameters | Combination therapy with GC and Amorphophallus konjac is relatively small sample size. |
| Toromanyan et al., 2007 [48] | Slim339 significantly reduces body weight in overweight and obese individuals | Slim339 contains 5 extracts and calcium pantothenate; relatively small sample size and short treatment period |
| Opala et al., 2006 [49] | Botanical extract-based weight loss formula produces significant change in the Body Composition Improvement Index and decrease in body fat in healthy, overweight subjects | Two different tablets with different combinations of herbal extracts (GC in one tablet); % body fat estimated with 4-skinfold method; more female than male subjects (77 vs. 21); smoking not in exclusion criteria (but monitored) |
| Tallei et al., 2021 [94] | Green tea extract may serve as a potential treatment against COVID-19 | In vivo studies have not been studied. |
| Peluso et al., 2017 [95] | The consumption of green tea can modulate the antioxidant capacity of individuals | No convincing evidence from long-term intervention studies in humans |
| Jurgens et al., 2012 [96] | Green tea preparations induce weight loss in overweight or obese adults | Some studies had incomplete reporting and short study period (12 weeks) |
| Whitsett et al., 2014 [99] | Case report of fulminant liver failure and orthotopic liver transplantation in a patient after Slimquick use | Danger of over-interpretation and publication bias. Not a powered clinical trial. |
| Isbrucker et al., 2006 [102] | EGCG caused dose-dependent hepatotoxicity in mice under dietary restriction | Studies were limited to animal trials |
| Peng et al., 2016 [119] | Eight randomized studies with 800 patients and showed positive effects on the levels of ALT, AST, TC and TG, LDL. | Limited to the duration of follow up and small sample size to confirm the efficacy and safety of the study |
| Deshpande et al., 2020 [75] | Ashwagandha Improved sleep quality in meta-analysis | Short trial period to establish long term effects, limited trials presented with a potential risk of publication bias |
| Dimpfel et al., 2020 [78] | Ashwagandha Improved cognitive and concentration performance | Insufficient details regarding preparation of the extract, treatment administration, and randomization procedures |
| Hoogenboom et al., 2020 [111] | hepatic injury caused by aloe vera appears to be idiosyncratic; no changes in the biochemical indices of liver function | Not all participants completed the trial, few missed doses for some participants, trial lasted 60 days, low number of participants |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Chauhan, K.; Ross, H.; Parra, N.S.; Magagna, J.; Wang, M.; Zhu, P.; Erwin, R.; Halegoua-DeMarzio, D. A Comprehensive Review on the Use of Herbal Dietary Supplements in the USA, Reasons for Their Use, and Review of Potential Hepatotoxicity. Livers 2022, 2, 119-138. https://doi.org/10.3390/livers2030011
Khan A, Chauhan K, Ross H, Parra NS, Magagna J, Wang M, Zhu P, Erwin R, Halegoua-DeMarzio D. A Comprehensive Review on the Use of Herbal Dietary Supplements in the USA, Reasons for Their Use, and Review of Potential Hepatotoxicity. Livers. 2022; 2(3):119-138. https://doi.org/10.3390/livers2030011
Chicago/Turabian StyleKhan, Adnan, Kashyap Chauhan, Heather Ross, Natalia Salinas Parra, John Magagna, Makala Wang, Patrick Zhu, Ryan Erwin, and Dina Halegoua-DeMarzio. 2022. "A Comprehensive Review on the Use of Herbal Dietary Supplements in the USA, Reasons for Their Use, and Review of Potential Hepatotoxicity" Livers 2, no. 3: 119-138. https://doi.org/10.3390/livers2030011
APA StyleKhan, A., Chauhan, K., Ross, H., Parra, N. S., Magagna, J., Wang, M., Zhu, P., Erwin, R., & Halegoua-DeMarzio, D. (2022). A Comprehensive Review on the Use of Herbal Dietary Supplements in the USA, Reasons for Their Use, and Review of Potential Hepatotoxicity. Livers, 2(3), 119-138. https://doi.org/10.3390/livers2030011






