A Comprehensive Review on the Risk of Metabolic Syndrome and Cardiovascular Disease after Liver Transplantation
Abstract
1. Introduction
2. Definition of Metabolic Syndrome and Its Prevalence in Liver Transplantation
3. Complications of Metabolic Syndrome Post-Liver Transplantation
4. Complications of CVD Post-Liver Transplantation
Differences in CV Outcomes in Liver Transplants Carried out for NASH vs. Any Other Indication
5. Components of Post-Liver Transplantation Metabolic Syndrome
5.1. Hypertension
5.2. Diabetes Mellitus
5.3. Obesity
5.4. Dyslipidemia
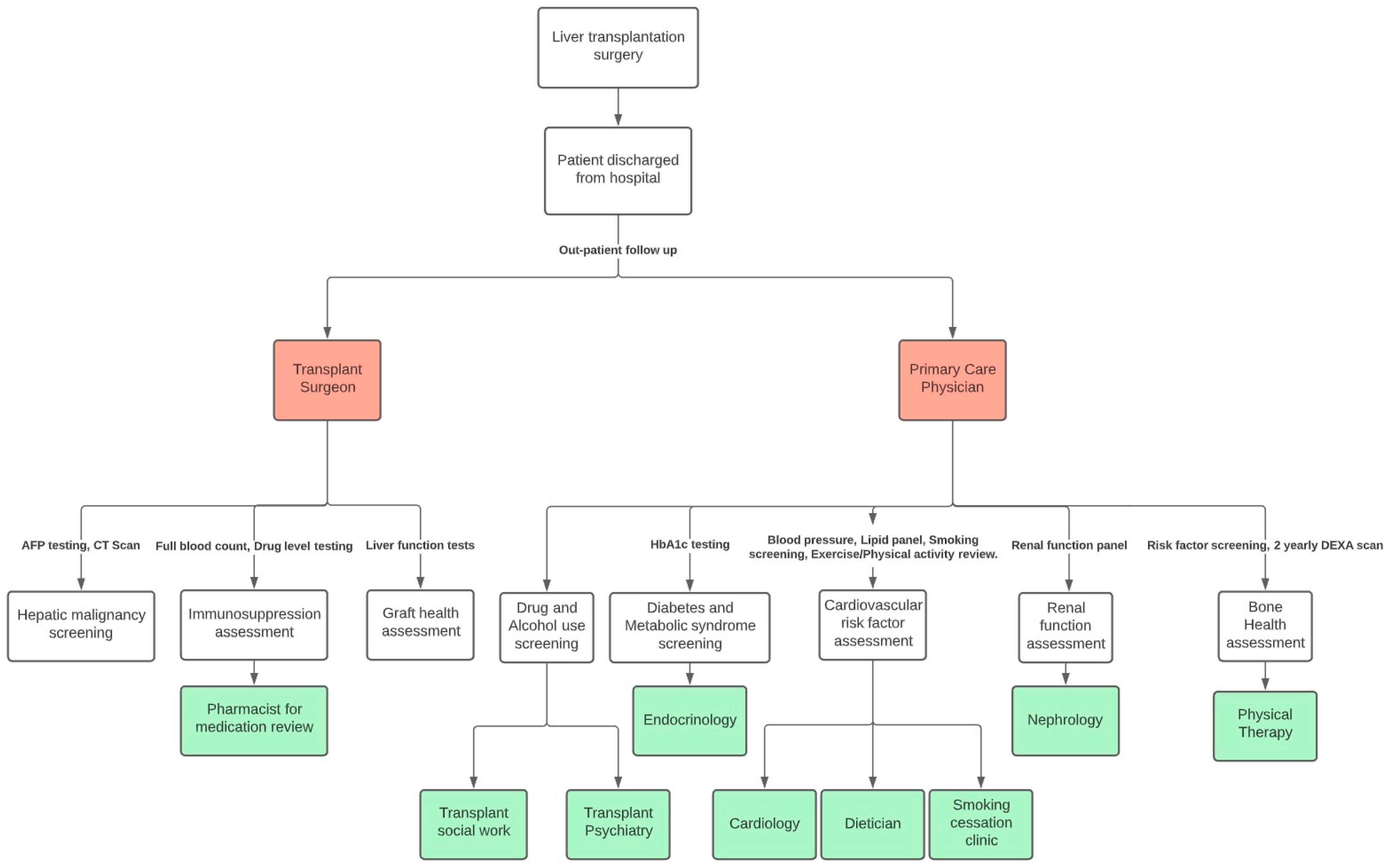
6. Strategies to Reduce Metabolic Syndrome and CVD Morbidity and Mortality Post-Liver Transplantation
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Merion, R.M.; Schaubel, D.E.; Dykstra, D.M.; Freeman, R.B.; Port, F.K.; Wolfe, R.A. The survival benefit of liver transplantation. Am. J. Transplant. 2005, 5, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Pacini, G.; Musso, G.; Gambino, R.; Mecca, F.; Depetris, N.; Cassader, M.; David, E.; Cavallo-Perin, P.; Rizzetto, M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: Further evidence for an etiologic association. Hepatology 2002, 35, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M. Obesity, hyperlipidemia, and metabolic syndrome. Liver Transpl. 2009, 15, S83–S89. [Google Scholar] [CrossRef]
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Kanwar, P.; Nelson, J.E.; Yates, K.; Kleiner, D.E.; Unalp-Arida, A.; Kowdley, K.V. Association between metabolic syndrome and liver histology among NAFLD patients without diabetes. BMJ Open Gastroenterol. 2016, 3, e000114. [Google Scholar] [CrossRef]
- Haukeland, J.W.; Konopski, Z.; Linnestad, P.; Azimy, S.; Loberg, E.M.; Haaland, T.; Birkeland, K.; Bjøro, K. Abnormal glucose tolerance is a predictor of steatohepatitis and fibrosis in patients with non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2005, 40, 1469–1477. [Google Scholar] [CrossRef]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Marzocchi, R.; Zannoni, C.; Vanni, E.; Manini, R.; Rizzetto, M.; Melchionda, N. Non-alcoholic steatohepatitis in patients cared in metabolic units. Diabetes Res. Clin. Pract. 2004, 63, 143–151. [Google Scholar] [CrossRef]
- Kallwitz, E.R. Metabolic syndrome after liver transplantation: Preventable illness or common consequence? World J. Gastroenterol. 2012, 18, 3627–3634. [Google Scholar] [CrossRef]
- Laish, I.; Braun, M.; Mor, E.; Sulkes, J.; Harif, Y.; Ari, Z.B. Metabolic syndrome in liver transplant recipients: Prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011, 17, 15–22. [Google Scholar] [CrossRef]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Van Wagner, L.B.; Gordon, E.; Adamski, L.; Kosirog, M.; Daud, A.; Finn, D.J.; Lloyd-Jones, D.M.; Holl, J.L. Liver Transplant Recipient, Caregiver, and Provider Perceptions of Cardiovascular Disease and Related Risk Factors After Transplant. Liver Transpl. 2021, 27, 668–683. [Google Scholar] [CrossRef] [PubMed]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Available online: https//pubmed.ncbi.nlm.nih.gov/12485966/ (accessed on 6 April 2022).
- Taneja, S.; Roy, A. Nonalcoholic steatohepatitis recurrence after liver transplant. Transl. Gastroenterol. Hepatol. 2020, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.M.; Devera, M.E.; Fontes, P.; Shaikh, O.; Sasatomi, E.; Ahmad, J. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transpl. 2009, 15, 1843–1851. [Google Scholar] [CrossRef]
- Marchesini, G.; Marzocchi, R. Metabolic syndrome and NASH. Clin. Liver Dis. 2007, 11, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Houlihan, D.D.; Armstrong, M.J.; Davidov, Y.; Hodson, J.; Nightingale, P.; Rowe, I.A.; Paris, S.; Gunson, B.K.; Bramhall, S.B.; Mutimer, D.J.; et al. Renal function in patients undergoing transplantation for nonalcoholic steatohepatitis cirrhosis: Time to reconsider immunosuppression regimens? Liver Transpl. 2011, 17, 1292–1298. [Google Scholar] [CrossRef]
- Spiritos, Z.; Abdelmalek, M.F. Metabolic syndrome following liver transplantation in nonalcoholic steatohepatitis. Transl. Gastroenterol. Hepatol. 2021, 6, 13. [Google Scholar] [CrossRef]
- Becchetti, C.; Dirchwolf, M.; Banz, V.; Dufour, J.F. Medical management of metabolic and cardiovascular complications after liver transplantation. World J. Gastroenterol. 2020, 26, 2138–2154. [Google Scholar] [CrossRef]
- Watt, K.D.; Pedersen, R.A.; Kremers, W.K.; Heimbach, J.K.; Charlton, M.R. Evolution of causes and risk factors for mortality post-liver transplant: Results of the NIDDK long-term follow-up study. Am. J. Transplant. 2010, 10, 1420–1427. [Google Scholar]
- Watt, K.D.; Charlton, M.R. Metabolic syndrome and liver transplantation: A review and guide to management. J. Hepatol. 2010, 53, 199–206. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Wilson, P.W.; Larson, M.G.; Beiser, A.; Leip, E.P.; D’Agostino, R.B.; Levy, D. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am. J. Cardiol. 2004, 94, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Thoefner, L.B.; Rostved, A.A.; Pommergaard, H.C.; Rasmussen, A. Risk factors for metabolic syndrome after liver transplantation: A systematic review and meta-analysis. Transplant. Rev. (Orlando) 2018, 32, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Laryea, M.; Watt, K.D.; Molinari, M.; Walsh, M.J.; McAlister, V.C.; Marotta, P.J.; Nashan, B.; Peltekian, K.M. Metabolic syndrome in liver transplant recipients: Prevalence and association with major vascular events. Liver Transpl. 2007, 13, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.D.; Morris, J.K.; Cramb, R.; Gunson, B.K.; Neuberger, J. Cardiovascular morbidity and mortality after orthotopic liver transplantation. Transplantation 2002, 73, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.K.; Vanatta, J.M.; Helmick, R.A.; Flowers, A.; Kedia, S.K.; Jiang, Y.; Ali, B.; Eason, J.; Nair, S.P.; Ibebuogu, U.N. Outcome of Liver Transplant Recipients with Revascularized Coronary Artery Disease: A Comparative Analysis with and Without Cardiovascular Risk Factors. Transplantation 2017, 101, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, P.; Mara, K.; Izzy, M.; Dierkhising, R.; Heimbach, J.; Allen, A.M.; Watt, K.D. Recurrent or De Novo Allograft Steatosis and Long-term Outcomes After Liver Transplantation. Transplantation 2019, 103, e14–e21. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Miranda, C.; Sanz, M.; dela Calle, A.; Loinaz, C.; Gomez, R.; Jimenez, C.; García, I.; Gómez de la Cámara, A.; Moreno, E. Cardiovascular risk factors in 116 patients 5 years or more after liver transplantation. Transpl. Int. 2002, 15, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Pfitzmann, R.; Nussler, N.C.; Hippler-Benscheidt, M.; Neuhaus, R.; Neuhaus, P. Long-term results after liver transplantation. Transpl. Int. 2008, 21, 234–246. [Google Scholar] [CrossRef]
- Stegall, M.D.; Everson, G.T.; Schroter, G.; Karrer, F.; Bilir, B.; Sternberg, T.; Shrestha, R.; Wachs, M.; Kam, I. Prednisone withdrawal late after adult liver transplantation reduces diabetes, hypertension, and hypercholesterolemia without causing graft loss. Hepatology 1997, 25, 173–177. [Google Scholar] [CrossRef]
- Di Maira, T.; Little, E.C.; Berenguer, M. Immunosuppression in liver transplant. Best Pract. Res. Clin. Gastroenterol. 2020, 46–47, 101681. [Google Scholar]
- Ascha, M.S.; Ascha, M.L.; Hanouneh, I.A. Management of immunosuppressant agents following liver transplantation: Less is more. World J. Hepatol. 2016, 8, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Gonwa, T.A. Hypertension and renal dysfunction in long-term liver transplant recipients. Liver Transpl. 2001, 7, S22–S26. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Levitsky, J.; Aqel, B.; O’Grady, J.; Hemibach, J.; Rinella, M.; Fung, J.; Ghabril, M.; Thomason, R.; Burra, P.; et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation 2018, 102, 727–743. [Google Scholar] [CrossRef]
- Hecking, M.; Sharif, A.; Eller, K.; Jenssen, T. Management of post-transplant diabetes: Immunosuppression, early prevention, and novel antidiabetics. Transpl. Int. 2021, 34, 27–48. [Google Scholar] [CrossRef]
- Pelaez-Jaramillo, M.J.; Cardenas-Mojica, A.A.; Gaete, P.V.; Mendivil, C.O. Post-Liver Transplantation Diabetes Mellitus: A Review of Relevance and Approach to Treatment. Diabetes Ther. 2018, 9, 521–543. [Google Scholar] [CrossRef]
- Pelletier, S.J.; Nadig, S.N.; Lee, D.D.; Ammori, J.B.; Englesbe, M.J.; Sung, R.S.; Magee, J.C.; Fontana, R.J.; Punch, J.D. A prospective, randomized trial of complete avoidance of steroids in liver transplantation with follow-up of over 7 years. HPB (Oxford) 2013, 15, 286–293. [Google Scholar] [CrossRef][Green Version]
- Lawendy, B.; Srinathan, S.; Kotha, S.; Gomes, C.; Misra, S.; Yu, J.; Orchanian-Cheff, A.; Tomlinson, G.; Bhat, M. Systematic review and meta-analysis of post-transplant diabetes mellitus in liver transplant recipients. Clin. Transplant. 2021, 35, e14340. [Google Scholar] [CrossRef]
- Jimenez-Perez, M.; Gonzalez-Grande, R.; Guzman, E.O.; Trillo, V.A.; Rodrigo Lopez, J.M. Metabolic complications in liver transplant recipients. World J. Gastroenterol. 2016, 22, 6416–6423. [Google Scholar] [CrossRef]
- Reuben, A. Long-term management of the liver transplant patient: Diabetes, hyperlipidemia, and obesity. Liver Transpl. 2001, 7, S13–S21. [Google Scholar] [CrossRef]
- Richards, J.; Gunson, B.; Johnson, J.; Neuberger, J. Weight gain and obesity after liver transplantation. Transpl. Int. 2005, 18, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.G.; Sharma, A.; Saab, S. Cardiovascular and metabolic disease in the liver transplant recipient. Best Pract Res. Clin. Gastroenterol. 2020, 46–47, 101683. [Google Scholar] [CrossRef] [PubMed]
- Moini, M.; Schilsky, M.L.; Tichy, E.M. Review on immunosuppression in liver transplantation. World J. Hepatol. 2015, 7, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Hoti, E. Liver transplantation: The current situation. Semin. Liver Dis. 2009, 29, 3–18. [Google Scholar] [CrossRef]
- Kuo, T.; McQueen, A.; Chen, T.C.; Wang, J.C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar]
- Epel, E.; Lapidus, R.; McEwen, B.; Brownell, K. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 2001, 26, 37–49. [Google Scholar] [CrossRef]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, R.B.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar] [CrossRef]
- Moctezuma-Velazquez, C.; Marquez-Guillen, E.; Torre, A. Obesity in the Liver Transplant Setting. Nutrients 2019, 11, 2552. [Google Scholar]
- Hillingso, J.G.; Wettergren, A.; Hyoudo, M.; Kirkegaard, P. Obesity increases mortality in liver transplantation--the Danish experience. Transpl. Int. 2005, 18, 1231–1235. [Google Scholar] [CrossRef]
- Nair, S.; Cohen, D.B.; Cohen, M.P.; Tan, H.; Maley, W.; Thuluvath, P.J. Postoperative morbidity, mortality, costs, and long-term survival in severely obese patients undergoing orthotopic liver transplantation. Am. J. Gastroenterol. 2001, 96, 842–845. [Google Scholar] [CrossRef]
- Nair, S.; Verma, S.; Thuluvath, P.J. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology 2002, 35, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Lassailly, G.; Caiazzo, R.; Ntandja-Wandji, L.C.; Gnemmi, V.; Baud, G.; Verkindt, H.; Ningarhari, M.; Louvet, A.; Leteurtre, E.; Raverdy, V.; et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology 2020, 159, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Diwan, T.S.; Rice, T.C.; Heimbach, J.K.; Schauer, D.P. Liver Transplantation and Bariatric Surgery: Timing and Outcomes. Liver Transpl. 2018, 24, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Syed, T.; Siddiqui, M.S. Atherogenic Dyslipidemia After Liver Transplantation: Mechanisms and Clinical Implications. Liver Transpl. 2021, 27, 1326–1333. [Google Scholar] [CrossRef]
- Gisbert, C.; Prieto, M.; Berenguer, M.; Breto, M.; Carrasco, D.; de Juan, M.; Berenguer, J. Hyperlipidemia in liver transplant recipients: Prevalence and risk factors. Liver Transpl. Surg. 1997, 3, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Heisel, O.; Heisel, R.; Balshaw, R.; Keown, P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: A systematic review and meta-analysis. Am. J. Transplant. 2004, 4, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.O.; Barr, M.L.; Radovancevic, B.; Renlund, D.G.; Mentzer, R.M., Jr.; Smart, F.W.; Tolman, D.E.; Frazier, O.H.; Young, J.B.; VanVeldhuisen, P. A randomized, multicenter comparison of tacrolimus and cyclosporine immunosuppressive regimens in cardiac transplantation: Decreased hyperlipidemia and hypertension with tacrolimus. J. Heart Lung Transplant. 1999, 18, 336–345. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Liang, K.; Azad, H. Effect of cyclosporine on HMG-CoA reductase, cholesterol 7alpha-hydroxylase, LDL receptor, HDL receptor, VLDL receptor, and lipoprotein lipase expressions. J. Pharmacol. Exp. Ther. 2000, 294, 778–783. [Google Scholar]
- Roy, A.; Kneteman, N.; Lilly, L.; Marotta, P.; Peltekian, K.; Scudamore, C.; Tchervenkov, J. Tacrolimus as intervention in the treatment of hyperlipidemia after liver transplant. Transplantation 2006, 82, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Seymen, P.; Yildiz, M.; Turkmen, M.F.; Titiz, M.I.; Seymen, H.O. Effects of cyclosporine-tacrolimus switching in posttransplantation hyperlipidemia on high-density lipoprotein 2/3, lipoprotein a1/b, and other lipid parameters. Transplant. Proc. 2009, 41, 4181–4183. [Google Scholar] [CrossRef]
- McKenna, G.J.; Trotter, J.F.; Klintmalm, E.; Ruiz, R.; Onaca, N.; Testa, G.; Saracino, G.; Levy, M.F.; Goldstein, R.M.; Klintmalm, G.B. Sirolimus and cardiovascular disease risk in liver transplantation. Transplantation 2013, 95, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, M.F.; Humar, A.; Stickel, F.; Andreone, P.; Pascher, A.; Barroso, E.; Neff, G.W.; Ranjan, D.; Toselli, L.T.; Gane, E.J.; et al. Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: A randomized trial. Am. J. Transplant. 2012, 12, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Terrec, F.; Malvezzi, P.; Rostaing, L. Adverse effects of immunosuppression after liver transplantation. Best Pract. Res. Clin. Gastroenterol. 2021, 54–55, 101762. [Google Scholar] [CrossRef] [PubMed]
- Lemahieu, W.P.; Hermann, M.; Asberg, A.; Verbeke, K.; Holdaas, H.; Vanrenterghem, Y.; Maes, B.D. Combined therapy with atorvastatin and calcineurin inhibitors: No interactions with tacrolimus. Am. J. Transplant. 2005, 5, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T.; VanWagner, L.B. Mind the Gap: Statin Underutilization and Impact on Mortality in Liver Transplant Recipients. Liver Transpl. 2019, 25, 1477–1479. [Google Scholar] [CrossRef]
- Holdaas, H.; Hagen, E.; Asberg, A.; Lund, K.; Hartman, A.; Vaidyanathan, S.; Prasad, P.; He, Y.L.; Yeh, C.M.; Bigler, H.; et al. Evaluation of the pharmacokinetic interaction between fluvastatin XL and cyclosporine in renal transplant recipients. Int. J. Clin. Pharmacol. Ther. 2006, 44, 163–171. [Google Scholar] [CrossRef]
- Asberg, A. Interactions between cyclosporin and lipid-lowering drugs: Implications for organ transplant recipients. Drugs 2003, 63, 367–378. [Google Scholar]
- Najeed, S.A.; Saghir, S.; Hein, B.; Neff, G.; Shaheen, M.; Ijaz, H.; Khan, I.A. Management of hypertension in liver transplant patients. Int. J. Cardiol. 2011, 152, 4–6. [Google Scholar] [CrossRef]
- Neal, D.A.; Brown, M.J.; Wilkinson, I.B.; Byrne, C.D.; Alexander, G.J. Hemodynamic effects of amlodipine, bisoprolol, and lisinopril in hypertensive patients after liver transplantation. Transplantation 2004, 77, 748–750. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Holl, J.L.; Montag, S.; Gregory, D.; Connolly, S.; Kosirog, M.; Campbell, P.; Pine, S.; Daud, A.; Finn, D.; et al. Blood pressure control according to clinical practice guidelines is associated with decreased mortality and cardiovascular events among liver transplant recipients. Am. J. Transplant. 2020, 20, 797–807. [Google Scholar] [CrossRef]
- Patel, S.S.; Rodriguez, V.A.; Siddiqui, M.B.; Faridnia, M.; Lin, F.P.; Chandrakumaran, A.; Laurenzano, J.; Clinton, J.; Kowlgi, G.N.; Kirkman, D.; et al. The Impact of Coronary Artery Disease and Statins on Survival After Liver Transplantation. Liver Transpl. 2019, 25, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, A.R.; Cockbain, A.J.; Raza, S.S.; Pollard, S.G.; Toogood, G.J.; Attia, M.A.; Ahmad, N.; Hidalgo, E.L.; Prasad, K.R.; Menon, K.V. Increased morbidity in overweight and obese liver transplant recipients: A single-center experience of 1325 patients from the United Kingdom. Liver Transpl. 2013, 19, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.; Glass, L.; Sharma, P.; Shannon, C.; Sonnenday, C.J.; Tincopa, M.A. Incidence and Risks for Nonalcoholic Fatty Liver Disease and Steatohepatitis Post-liver Transplant: Systematic Review and Meta-analysis. Transplantation 2019, 103, e345–e354. [Google Scholar] [CrossRef] [PubMed]
- Pajecki, D.; Cesconetto, D.M.; Macacari, R.; Joaquim, H.; Andraus, W.; de Cleva, R.; Santo, M.A.; Albuquerque, L.A.; Cecconello, I. Bariatric surgery (sleeve gastrectomy) after liver transplantation: Case report. Arq. Bras. Cir. Dig. 2014, 27 (Suppl. 1), 81–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elli, E.F.; Masrur, M.A.; Giulianotti, P.C. Robotic sleeve gastrectomy after liver transplantation. Surg. Obes. Relat. Dis. 2013, 9, e20–e22. [Google Scholar] [CrossRef]
- Tichansky, D.S.; Madan, A.K. Laparoscopic Roux-en-Y gastric bypass is safe and feasible after orthotopic liver transplantation. Obes. Surg. 2005, 15, 1481–1486. [Google Scholar] [CrossRef]
- Zamora-Valdes, D.; Watt, K.D.; Kellogg, T.A.; Poterucha, J.J.; Di Cecco, S.R.; Francisco-Ziller, N.M.; Taner, T.; Rosen, C.B.; Heimbach, J.K. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatology 2018, 68, 485–495. [Google Scholar] [CrossRef]
- Martin-Del-Campo, L.A.; Herrera, M.F.; Pantoja, J.P.; Sierra, M.; Iglesias, M.; Butron, P.; Herrera-Zamora, J.; Torres-Villalobos, G. Absence of an Additional Metabolic Effect of Body Contour Surgery in Patients with Massive Weight Loss after Laparoscopic Roux-En-Y Gastric Bypass. Ann. Plast. Surg. 2017, 79, 533–535. [Google Scholar] [CrossRef]
| Parameters | NCEP ATP3 2005 |
|---|---|
| Number of Abnormalities | ≥3 of: |
| Glucose | Fasting glucose ≥ 5.6 mmol/L (100 mg/dL) or drug treatment for elevated blood glucose |
| HDL cholesterol | <1.0 mmol/L (40 mg/dL) (men); <1.3 mmol/L (50 mg/dL) (women) or drug treatment for low HDL cholesterol § |
| Triglycerides | ≥1.7 mmol/L (150 mg/dL) or drug treatment for elevated triglycerides § |
| Obesity | Waist ≥ 102 cm (men) or ≥88 cm (women) * |
| Hypertension | ≥130/85 mmHg or drug treatment for hypertension |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, K.; Khan, A.; Chowdhury, S.; Ross, H.M.; Parra, N.S.; Halegoua-DeMarzio, D. A Comprehensive Review on the Risk of Metabolic Syndrome and Cardiovascular Disease after Liver Transplantation. Livers 2022, 2, 85-96. https://doi.org/10.3390/livers2020006
Chauhan K, Khan A, Chowdhury S, Ross HM, Parra NS, Halegoua-DeMarzio D. A Comprehensive Review on the Risk of Metabolic Syndrome and Cardiovascular Disease after Liver Transplantation. Livers. 2022; 2(2):85-96. https://doi.org/10.3390/livers2020006
Chicago/Turabian StyleChauhan, Kashyap, Adnan Khan, Salil Chowdhury, Heather M. Ross, Natalia Salinas Parra, and Dina Halegoua-DeMarzio. 2022. "A Comprehensive Review on the Risk of Metabolic Syndrome and Cardiovascular Disease after Liver Transplantation" Livers 2, no. 2: 85-96. https://doi.org/10.3390/livers2020006
APA StyleChauhan, K., Khan, A., Chowdhury, S., Ross, H. M., Parra, N. S., & Halegoua-DeMarzio, D. (2022). A Comprehensive Review on the Risk of Metabolic Syndrome and Cardiovascular Disease after Liver Transplantation. Livers, 2(2), 85-96. https://doi.org/10.3390/livers2020006






