A Review of Opportunities and Methods for Recovery of Rhodium from Spent Nuclear Fuel during Reprocessing
Abstract
Overview
- 1
- Introduction
- 2
- Rhodium in the Nuclear Fuel Cycle
- 2.1
- Rhodium Production by Fission
- 2.2
- Rhodium Speciation in Irradiated Spent Nuclear Fuel
- 2.3
- Rhodium Partitioning in Spent Nuclear Fuel Reprocessing
- 2.3.1
- Rh Head-End Behaviour and Speciation in Nitric Acid
- 2.3.2
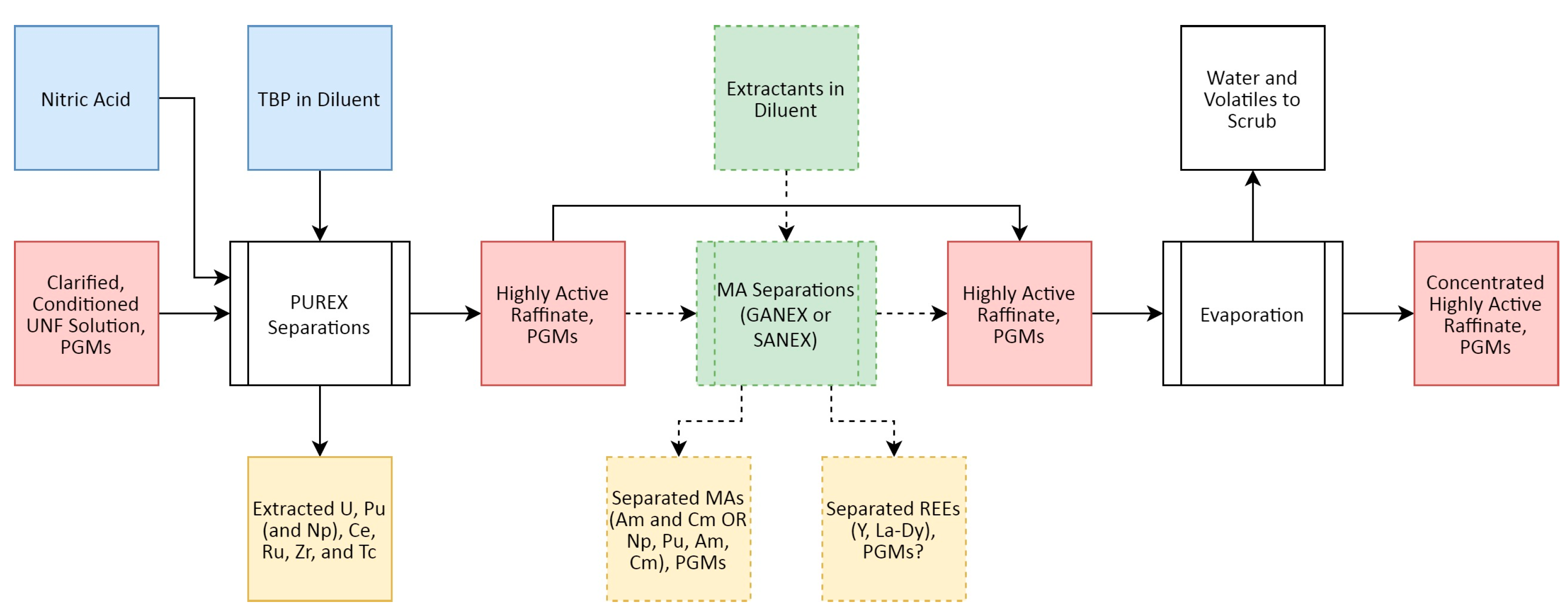
- Rh Behaviour in PUREX and Related Solvent Extraction Processes
- 2.3.3
- Rh Behaviour in the Back-End of SNF Reprocessing Operations
- 3
- Separating and Recovering Rh during SNF Reprocessing
- 3.1
- Heterogeneous Solid-Liquid Separations—Recovering Rh from Aqueous Feeds using Ion Exchange, Extraction Chromatography, and Related Techniques
- 3.1.1
- Ion Exchange and Solid Sorption Overview
- 3.1.2
- Ion Exchange Resins
- 3.1.3
- Inorganic Sorbents
- 3.1.4
- Ion Exchange and Solid Sorption Summary
- 3.2
- Extraction Chromatography and Functionalised Silica–Polymer Supports
- 3.2.1
- Extraction Chromatography Summary
- 3.3
- Homogeneous Liquid–Liquid Separations—Recovering Rh from Aqueous Feeds using Solvent Extraction and Ionic Liquids
- 3.3.1
- Phosphorus-Based Extractants
- 3.3.2
- Sulphur-Based Extractants
- 3.3.3
- Nitrogen-Based Extractants
- 3.3.4
- Solvent Extraction Summary
- 3.3.5
- Ionic Liquid Extraction
- 3.3.6
- Ionic Liquids Summary
- 3.4
- Other Heterogeneous Separations—Precipitation, Electrochemical Methods, Chemical Reduction, and Photoreduction Recovery of Rh from Aqueous Feeds
- 3.4.1
- Precipitation
- 3.4.2
- Electrochemical Methods
- 3.4.3
- Chemical Reduction and Photoreduction
- 3.5
- Recovery of Rh from Insoluble Dissolver Residue
- 3.5.1
- Secondary Dissolution
- 3.5.2
- Pyrochemistry
- 3.5.3
- Very High Temperature Processes
- 4
- Discussion and ConclusionsFundingAcknowledgementsAuthor ContributionsConflicts of InterestAppendixReferences
1. Introduction
2. Rhodium in the Nuclear Fuel Cycle
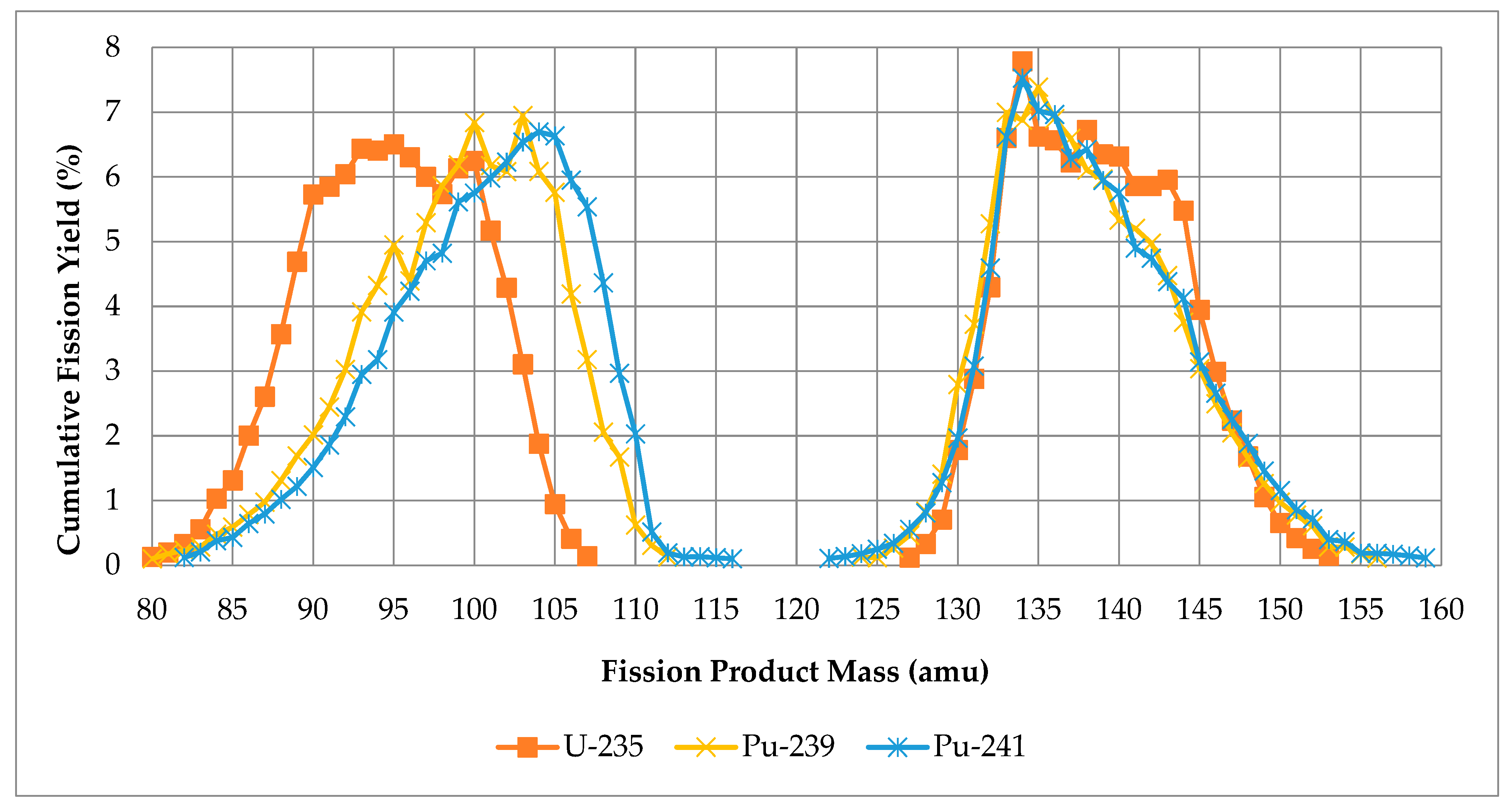
2.1. Rhodium Production by Fission
2.2. Rhodium Speciation in Irradiated Spent Nuclear Fuel
- Most of the ionic FPs and minor actinides (MAs—Np, Am, Cm) dope or dissolve into the fluorite crystal structure of fuel ceramic itself.
- Gaseous FPs (He, Kr, Xe) form bubbles within the fuel ceramic or migrate to He-filled the gap between the ceramic and the cladding.
- Some of the more volatile FPs migrate to the edge of the ceramic and form distinct crystalline phases, such as CsI, and Cs2MoO4.
- The lower reactivity metals and some nonmetals are reduced and form inert metallic inclusions within the fuel ceramic, commonly termed ε-particles. These consist primarily of Mo, Tc, Ru, Rh, Pd, Ag, Se, and Te and are typically under 1 μm in size. This is the most important phase when considering the recovery of PGMs.
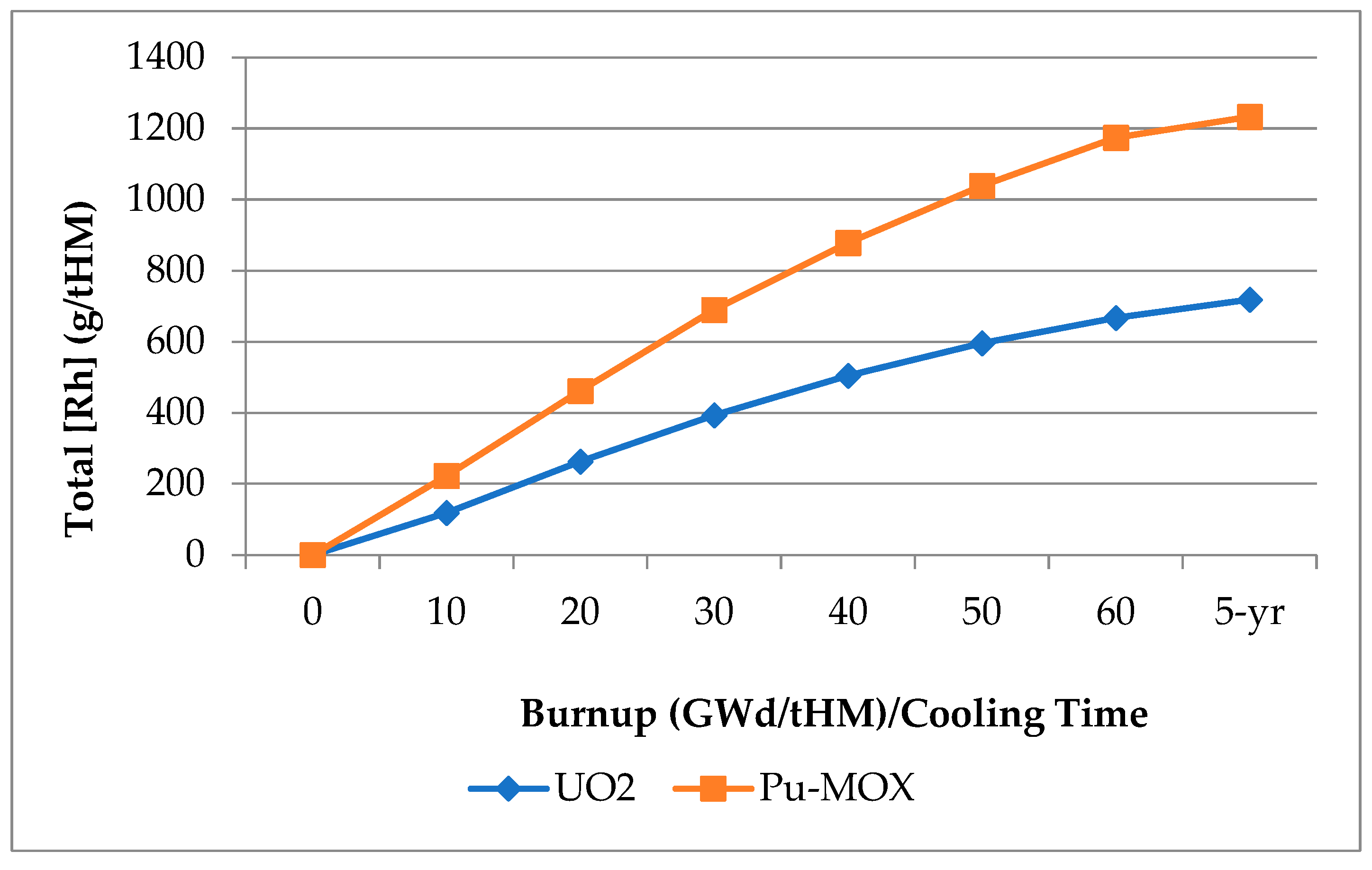
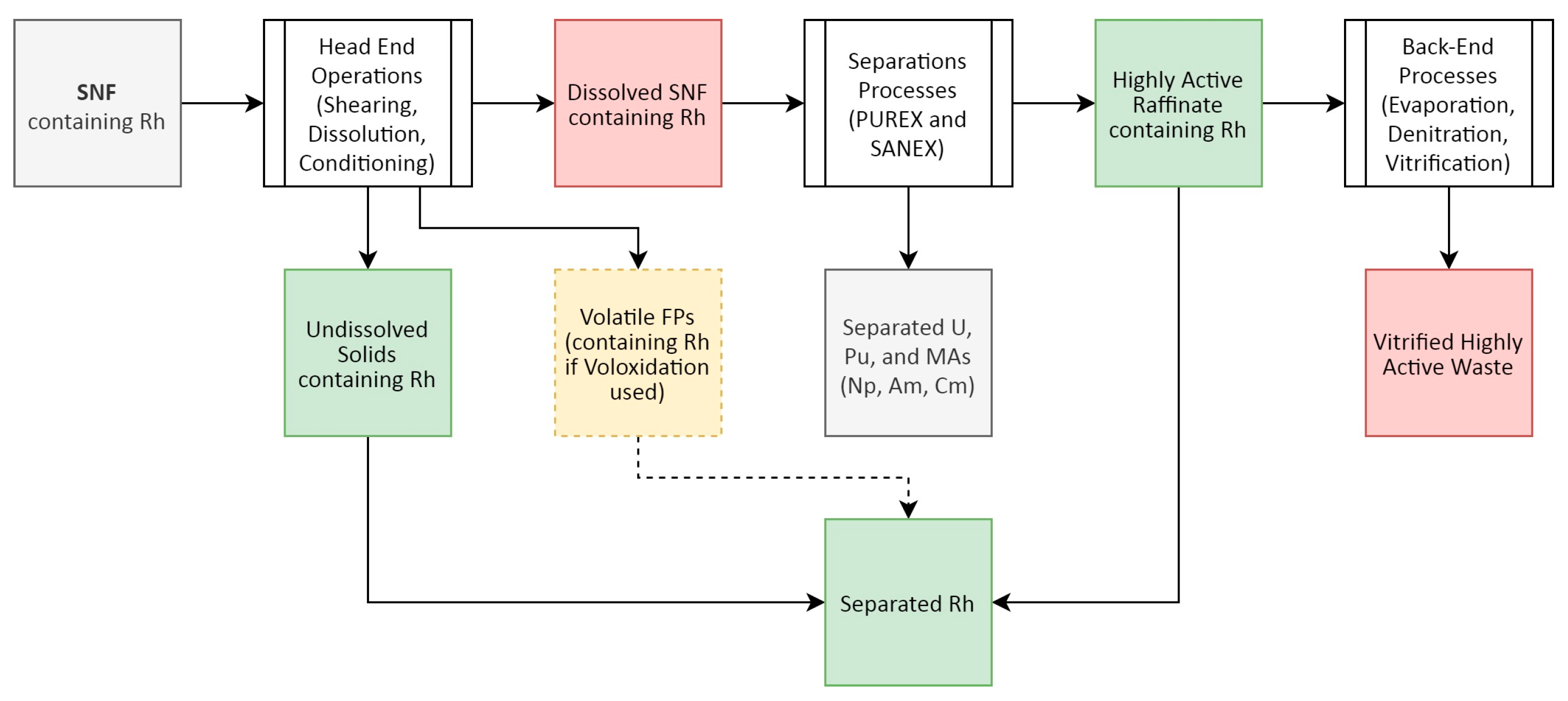
2.3. Rhodium Partitioning in Spent Nuclear Fuel Reprocessing
2.3.1. Rh Head-End Behaviour and Speciation in Nitric Acid
- Thermal pre-treatment of SNF before dissolution, which may or may not include chemical de-cladding, to oxidise the fuel ceramic to increase the rate and extent of dissolution and potentially drive off any volatile FPs, using steam, air, NO2, O2, or NF3.
- The addition of catalytic species to the dissolution step, such as AgII or CeIV to assist in the oxidation of SNF during dissolution.
- The initial Rh complex dissolved in solution, i.e., [Rh(NO3)3]3+, [Rh(H2O)6]3+, RhCl3, etc.
- Temperature, which increases ligand substitution rates.
- Equilibrium concentrations of [Rh,NO3−,H+] and also other solution components.
- The dissolved SNF feed containing U, Pu, and the bulk of the ionic FPs, from which Rh could be recovered using techniques such as solvent extraction, ion exchange, or electrochemical methods. These are discussed in Section 3.1, Section 3.2 and Section 3.3
- The UDS/IFP feed, which is normally sent to cementation/vitrification to be disposed of as waste alongside cladding fines and other insoluble species. This can/does represent the bulk of the Rh that was present in the initial SNF, and as such would be worthy of further processing to recover a greater proportion of the PGM value present, perhaps via the addition of a secondary dissolver. This is discussed in Section 3.4.
- Gas phase if voloxidation used. Given the relative immaturity of this concept, this is beyond the scope of this review and will not be discussed further.
2.3.2. Rh Behaviour in PUREX and Related Solvent Extraction Processes
2.3.3. Rh Behaviour in the Back-End of SNF Reprocessing Operations
3. Separating and Recovering Rh during SNF Reprocessing
3.1. Heterogeneous Solid–Liquid Separations—Recovering Rh from Aqueous Feeds Using Ion Exchange, Extraction Chromatography, and Related Techniques
3.1.1. Ion Exchange and Solid Sorption Overview
3.1.2. Ion Exchange Resins
3.1.3. Inorganic Sorbents
3.1.4. Ion Exchange and Solid Sorption Summary
3.2. Extraction Chromatography and Functionalised Silica–Polymer Supports
Extraction Chromatography Summary
3.3. Homogeneous Liquid–Liquid Separations—Recovering Rh from Aqueous Feeds Using Solvent Extraction and Ionic Liquids
3.3.1. Phosphorus-Based Extractants
- Undiluted TBP;
- 10–50% TBP/toluene;
- 25% TBP/CCl4;
- 5% trioctylphosphine oxide/xylene.
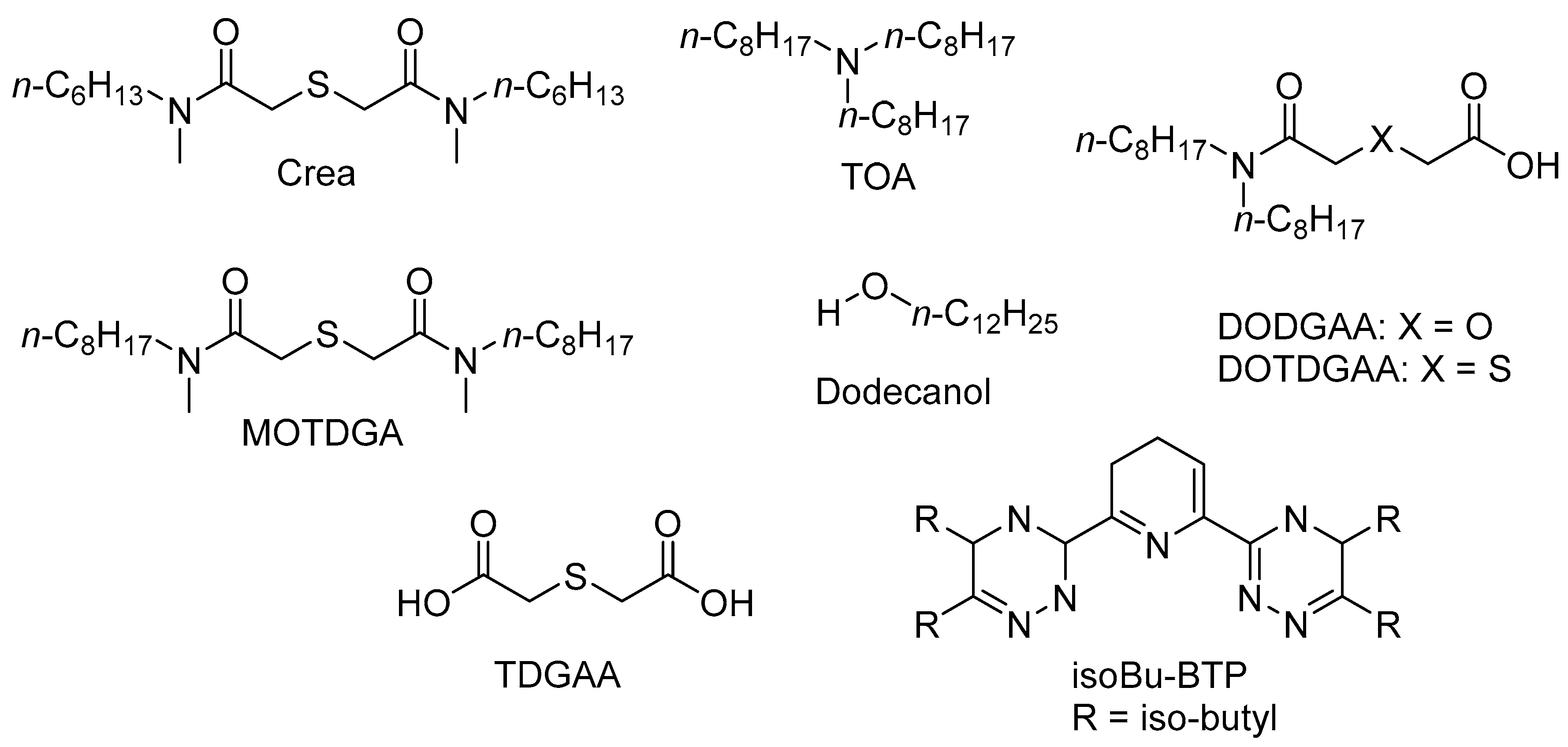
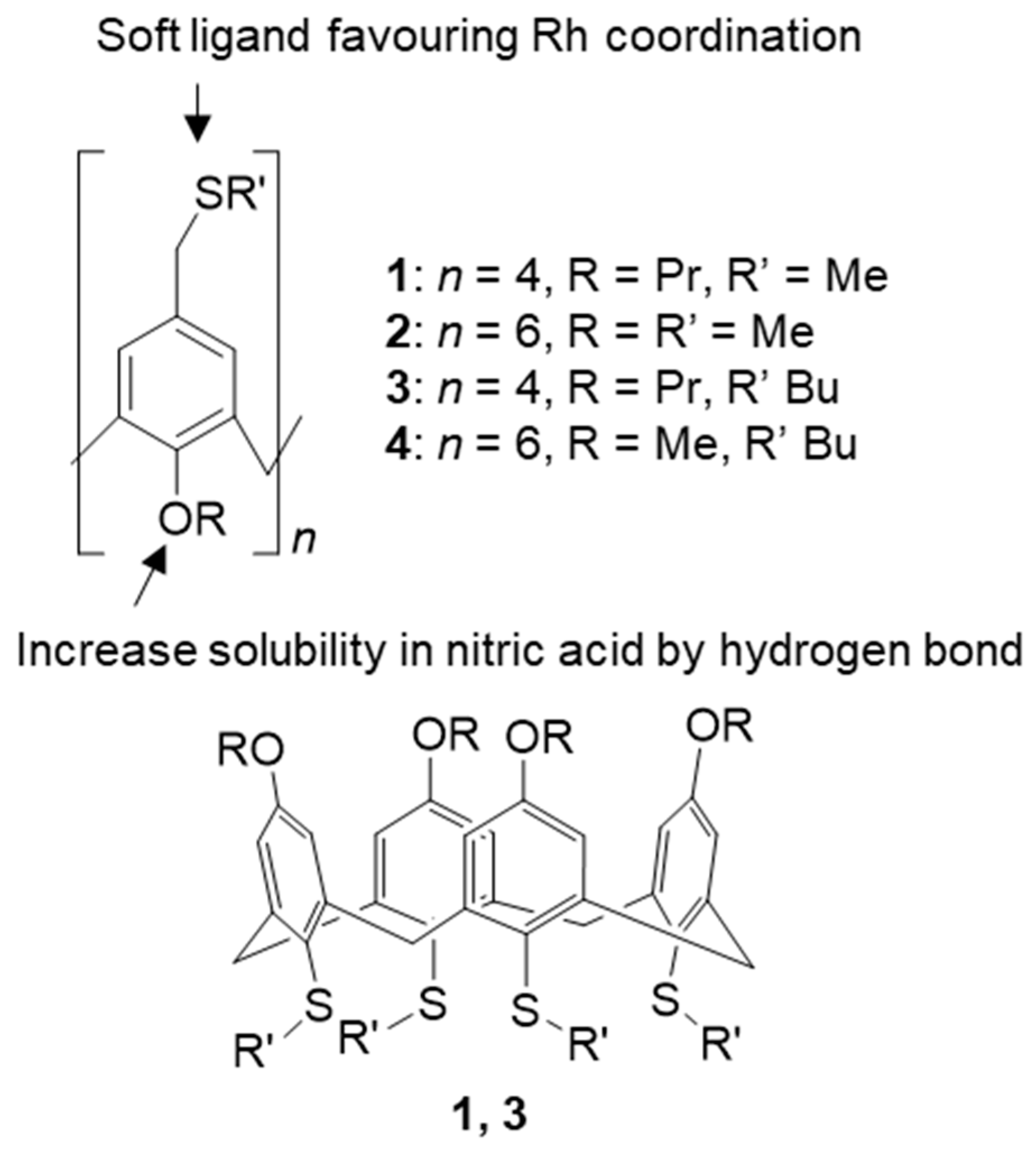
3.3.2. Sulphur-Based Extractants
3.3.3. Nitrogen-Based Extractants
3.3.4. Various Extractants
3.3.5. Solvent Extraction Summary
3.3.6. Ionic Liquid Extraction
3.3.7. Ionic Liquids Summary
3.4. Other Heterogeneous Separations—Precipitation, Electrochemical Methods, Chemical Reduction, and Photoreduction Recovery of Rh from Aqueous Feeds
3.4.1. Precipitation
3.4.2. Electrochemical Methods
- Rh(III)/Rh(0) = 0.528 V vs. saturated calomel electrode (SCE);
- RuNO(III)/Ru(0) = 0.230 V vs. SCE;
- Pd(II)/Pd(0) = 0.685 V vs. SCE.
3.4.3. Chemical Reduction and Photoreduction
- No reduction agents are required, which can degrade HNO3, limiting co-precipitation of other FPs;
- The reactions are induced by light, avoiding contamination of the reaction system and producing relatively high yields;
- The photocatalyst can be reused multiple times.
3.5. Recovery of Rh from Insoluble Dissolver Residue
3.5.1. Secondary Dissolution
3.5.2. Pyrochemistry
3.5.3. Very High-Temperature Processes
4. Discussion and Conclusions
- Recovery conditions should be kept as close to standard flowsheet conditions as reasonably possible and capable of continuous operation.
- The addition of extraneous species that can cause major changes to the carefully controlled solution chemistry should be minimised.
- Secondary waste generation should be avoided and minimised where possible. Reagents should ideally adhere to the CHON principle—i.e., only consist of carbon, nitrogen, oxygen, and hydrogen.
- The recovery technique should be quick, effective, and moderately selective. Methods that extract other species (primarily PGMs) along with Rh should not automatically be discounted, as secondary treatment steps could be used to separate coextracted Ru and/or Pd, or other valuable species.
- The chosen technological option must be able to function in high-radiation, highly acidic, oxidising environments and be able to be operated remotely.
- Solid sorption techniques, particularly those using extractant-impregnated and functionalised silica support materials.
- Ionic liquid extraction, with recent developments focused on TSILs.
- Solution phase speciation of Rh(III) in HNO3.
- Solvent extraction using micelle-forming extractants combined with sulphur-based ligands.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Alkyl anilines |
| AAHNO3 | Alkylanilium nitrate |
| AlHCF | Aluminium (hexa)ferrocyanide |
| CATE | Calyx(n)arenethiaethers |
| CEE | Catalytic electrolytic extraction |
| CMPO | Octyl-phenyl-N,N-diisobutylcarbamoyl methylphosphine oxide |
| Crea | N’,N’,-di-n-hexyl-thiodiglycolamide |
| DBTU | 1,3-dibutylthiourea |
| DBS | Dibutylsulphide |
| DHS | Dihexylsulphide |
| DHSO | Di-n-hexylsulphoxide |
| DMSO | Dimethylsulphoxide |
| DOTDGAA | N,N-di-n-octylthiodiglycolamic acid |
| DPDTPA | Diphenyldithiophosphinic acid |
| DPPA | Diphenylphosphinic acid |
| DTPA | Diethylenetriaminepentaacetic acid |
| EDA | Ethylenediamine |
| FP | Fission product |
| GANEX | Grouped actinide extraction |
| HLLW | High-level liquid waste |
| HSAB | Hard–soft acid–base (principle) |
| IFP | Insoluble fission product |
| IL | Ionic liquid |
| isoBu-BTP | 2,6- di(5, 6- diisobutyl-1,2,4-triazine-3-yl)pyridine |
| IX | Ion exchange |
| JAERI | Japan Atomic Energy Research Institute, now known as JAEA (Japan Atomic Energy Agency) |
| KAERI | Korean Atomic Energy Research Institute |
| i-SANEX | Innovative selective actinide extraction |
| MA | Minor actinide |
| MNTFMB | Metanitro(trifluoromethyl)benzene |
| MOTDGA | N,N′-dimethyl-N,N′-di-n-octyl-thiodiglycolamide |
| MOX | Mixed oxide (nuclear fuel) |
| MPE-TDGA | N,N’-dimethyl-N,N’-di-(2-phenylethyl)-thiodiglycolamide |
| NFC | Nuclear fuel cycle |
| OA | Para-n-octylaniline |
| OK | Odourless kerosene (diluent for SX) |
| PAN | Polyacrylonitrile |
| PGM | Platinum group metal |
| PUREX | Plutonium and uranium redox extraction (reprocessing process) |
| PNIPAAm | Poly(N-isopropylacrylamide) |
| REE | Rare earth element |
| SANEX | Selective actinide extraction |
| SBR | Sulphonic betaine resin |
| SCE | Saturated calomel electrode |
| SNF | Spent nuclear fuel |
| TBP | Tributylphosphate |
| TBPS | N,N’,N’’-tributyl phosphorothioic-triamide |
| TDGA | Thiodiglycolamide |
| TDGAA | Thiodiglycolamic acid |
| TEA | Triethylamine |
| THPS | N,N’,N’’-tri-n-hexyl phosphorothioic-triamide |
| TIOA | Triisooctylamine |
| TPPS | N,N’,N’’-triphenyl phosphorothioic-triamide |
| TOA | Trioctylamine |
| TOAN | Tetra-n-octylammonium nitrate |
| TODGA | N,N,N’,N’-tetraoctyldiglycolamide |
| Tren | Tris-(2-aminoethyl)-amine |
| TU | Thiourea |
| UCST | Upper critical solution temperature |
| UDS | Undissolved solids |
| UK | United Kingdom (of Great Britain and Northern Ireland) |
| USA | Unites States of America |
| USD | US dollars |
| UPD | Underpotential deposition |
Appendix A
| Ion Exchanger/Solid Sorbent | Functionality/Type | Highest Kd (mL/g) or Extraction (%) | Conditions | Comments | Ref. |
|---|---|---|---|---|---|
| Dowex 1X8 | Quaternary methylammonium | Kd = ~13 | 20 °C, in 2–3 M HNO3 | Kd > 10 in 0.1–4.5 M HNO3, peak at 2–3 M HNO3. Adsorption higher at 60 °C when tested in <0.5 M HNO3. | [100] |
| Amberlite IRN-78 | Conventional amine | Kd = ~8 | 20 °C, in 2–3 M HNO3 | Kd > 4–5 in 0.1–7 M HNO3, peak at 2–3 M HNO3 Adsorption higher at 60 °C when tested in <0.5 M HNO3. | [100] |
| Dowex 50W | Sulphonic group | Kd = 55 | 20 °C, in <0.5 M HNO3 | Sharp decrease in Kd from 0.5 M to ≥1 M HNO3. | [100] |
| AMP03 with additional amine ligands | N,N,N-trimethylglycine | Kd = 1240 | In 0.1 M HNO3 with 0.3 M NaNO3 | Kd = 1040 obtained in 0.4 M HNO3 with 0.3 M TEA added; extremely sensitive to [H+] and [NO3-]. Highest performance at low [H+] and high [NO3-]. | [104] |
| 99.2% adsorption | In 0.4 M HNO3 with 0.35 M TEA added | ||||
| AMP03 with TEA | N,N,N-trimethylglycine | Kd > 1000 | In 0.4 M HNO3 with 0.35 M TEA added | Addition of TEA drastically increases Kd in low [HNO3]. Attains equilibrium in ~15 min. Stepwise elution method proposed. 4.8 M NH3 eluted ~85% adsorbed Rh in column tests. | [105] |
| AV-17X8 | Quaternary methylammonium | Kd < 5 | 3 M HNO3, no other conditions known | Primary source unavailable. Referenced in [29]. | [106] |
| AN1-4 | Weak basic ammonium | Kd < 5 | 3 M HNO3, no other conditions known | Primary source unavailable. Referenced in [29]. | [106] |
| VP-1AP | Pyridinium | Kd < 5 | 3 M HNO3, no other conditions known | Primary source unavailable. Referenced in [29]. | [106] |
| KhFO | Phosphonium | Kd < 5 | 3 M HNO3, no other conditions known | Primary source unavailable. Referenced in [29]. | [106] |
| KU-2X8 | Sulphonic acid | Kd = ~0.5 | 3 M HNO3, no other conditions known | Primary source unavailable. Referenced in [29]. | [106] |
| KRF-20t-60 | Phosphoric acid | Kd < 3 | 3 M HNO3, no other conditions known | Primary source unavailable. Referenced in [29]. | [106] |
| VPK | Aminocarboxylic | Kd = ~230 | 3 M HNO3, no other conditions known | Primary source unavailable. Referenced in [29]. | [106] |
| ANKB-35 | Aminocarboxylic | Kd = ~24 | 3 M HNO3, no other conditions known | Primary source unavailable. Referenced in [29]. | [106] |
| MS-50 | Aminocarboxylic | Kd = ~5 | 3 M HNO3, no other conditions known | Primary source unavailable. Referenced in [29]. | [106] |
| Cu hexacyanoferrate/silica gel adsorbent (FS-14) | N/A | Kd = ~10 | 3 M HNO3, no other conditions known | Primary source unavailable. Referenced in [29]. | [106] |
| Ni hexacyanoferrate/silica gel adsorbent (FS-15) | |||||
| CuS adsorbent (GSM) | N/A | Kd = ~5 | 3 M HNO3, no other conditions known | Primary source unavailable. Referenced in [29]. | [106] |
| Hydrous TiO2:ZrO2 sorbent | N/A | Kd = ~5 | 3 M HNO3, no other conditions known | Primary source unavailable. Referenced in [29]. | [106] |
| Active carbon | N/A | ~16% from simulated HLLW | From 0.5 M HNO3 denitrated HLLW simulant | Study linked to JAERI partitioning process in ref. [108]. | [107] |
| AlHCF | N/A | 6% from irradiated MOX SNF, 1% (HLLW sim.) | 1.5 M HNO3, 1 h | Very poor adsorption from real SNF and simulant. | [109] |
| KCuFC-functionalised xerogel | N/A | 86% Rh from 29-component [2.6 M HNO3] HLLW sim. | Column operation, 15 h equilibration time at room temperature | Also adsorbed 69% Ru and 100% Pd from HLLW simulant. Co-adsorption of Ni, Zr, and Te. Pd was eluted using a mixed HNO3-TU solution. | [110] |
| Functionalised Silica Support | Highest Kd (mL/g) or Adsorption % | Conditions | Comments | Ref. |
|---|---|---|---|---|
| (Crea + TOA)/SiO2-P | ~65% adsorption | 25 °C, from 11-component [3 M HNO3] HLLW simulant, 72 h | Some co-adsorption of other PGMs, Zr, Mo, and Re (surrogate for Tc) from HLLW simulant. Almost no uptake of REEs from HLLW simulant. [H+] and [NO3−] solution concentrations had no effect on the adsorption—Rh adsorption increased from 0.1–5.0 M [HNO3]. | [112] |
| Kd = 5–6 in 5 M HNO3 | 25 °C, from 11-component [5 M HNO3] HLLW simulant, 72 h | |||
| (MOTDGA-TOA)/SiO2-P | Kd = ~3 | 25 °C, from 10 component [4–5 M HNO3] HLLW simulant, 8 h | Synergistic effect observed with the two extractants—larger than sum of extraction using both separately. Only ~20% extraction of Rh after 24 h in 3 M HNO3. | [111] |
| (TOA+Dodecanol)/SiO2-P | Kd < 1 | 25 °C, from 10 component [0.1–5 M HNO3] HLLW simulant, 8 h | Poor adsorption over entire HNO3 concentration range. | [111] |
| (MOTDGA+Dodecanol)/SiO2-P | Kd = ~1.5 | 25 °C, from 10 component [3.8–5 M HNO3] HLLW simulant, 8 h | Poor adsorption (Kd < 1 mL/g) below ~3.8 M [HNO3]. | [111] |
| (Crea+Dodec)/SiO2-P | ~65% adsorption | 25 °C, from 11 component [3 M HNO3] HLLW simulant, 72 h | Some co-adsorption of other PGMs, Zr, Mo, and Re (surrogate for Tc) from HLLW simulant. Almost no uptake of REEs from simulated HLLW sim. [H+] and [NO3-] solution concentrations had no effect on the adsorption—Rh adsorption increased from 0.1–5.0 M HNO3. | [114] |
| TDGAA-Si | Kd = ~12 | 25 °C, from 11-component [6 M HNO3] HLLW simulant, 8 h | Reasonable Kd (7–9 mL/g) in 11-component [2–3 M HNO3] HLLW simulant. Reasonably selective, only co-adsorbing Pd (~100%), Ru, Zr, Mo, and Ag from 26-component [2 M HNO3] HLLW. Adsorption increased with temperature, but also led to some degradation of adsorbent. | [116] |
| (DOTDGAA+Dodec)/SiO2-P | Kd = ~12 | 25 °C, from 11-component [6 M HNO3] HLLW simulant, 8 h | Worse adsorption than TDGAA-Si in 2–3 M HNO3, potentially due to lower hydrophilicity. Degradation at higher temperatures; leaking oil droplets. | [116] |
| isoBu-BTP/SiO2-P | 67.4% adsorption | 55 °C from 1 M HNO3, time unknown. ~50% adsorption in 2–3 M HNO3, same conditions. | Selective adsorption, producing a separation factor >40 for PGM adsorption against other FPs in simulated HLLW. Three days required to reach equilibrium, even at 55 °C. | [115] |
| isoBu-BTP/SiO2-P with NaNO3 | 89% adsorption | 55 °C from 1 M HNO3 and 3 M NaNO3 | Adsorption was 99% at 55 °C from 0.1 M HNO3 + 3 M NaNO3 solution. Equilibrium adsorption reached in 24 h. | [117] |
| Extractant/Diluent | Highest DRh or Extraction (%) | Conditions | Comments | Ref. |
|---|---|---|---|---|
| Organophosphine sulphides (R3PS) in heptanol THPS [(C6H13NH)3P=S] | DRh= 3 DRh > 50 * | 2 M HNO3; 64 °C, 8 h contact time. * 2 M HNO3; 64 °C; with excess NaNO2, where NO2−/Rh > 20. | DRh values insignificant below 40 °C. Increased [extractant], contact time and [NO2−] increased DRh. | [126] |
| DPPA in 1-pentanol DPDTPA acid in toluene | DPPA DRh = 4.25 DPDTPA DRh = 5.19 | pH = 3.32 ([HNO3]= ~0.5 mM), [NO3−] = 1.5 mM, (extr.) = 19 mM | Negligible Rh extraction when [H+] or [NO3−] ≥ 1 M | [18] |
| TBP (10, 25, 50%) in toluene | DRh = 0.1–0.01 | 1–15 M HNO3, 2 min contact time | Contact time likely too short for equilibrium. | [127] |
| Trioctylphosphine oxide (TOPO, 5%) in xylene | DRh = <0.01 | 1–15 M HNO3, 2 min contact time | Contact time likely too short for equilibrium. | [127] |
| Alkyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxides | DRh = <0.01 | N/A—primary source unavailable | Primary source unavailable. Referenced in [29] without DRh values but said to be similar to 5% TIOA in xylene from [127]. | [128] |
| Diisoamyl methylphosphonate (50%) in diethylbenzene | N/A—primary source unavailable | <5 M HNO3, high (extractant) (50%) and in the presence of salting out agents (1.6 M Al(NO3)3 + 1 M NH4NO3) | Primary source unavailable. Referenced in [29] without DRh values or indications to performance. | [129] |
| Dibutyl sulphide (1 M) in n-hexanol Dioctyl sulphide (1 M) in n-hexanol | DRh = ~5.6 DRh = ~10 * DRh ~25 ** | 3.5 M HNO3, 61 °C, 5–7 h contact time. * 3.5 M HNO3, 61 °C, 5–7 h contact time, with 10% v/v DMSO added. ** Simulant FP solution, 3.1 M [HNO3], 65 °C | Excess extractant concentration, adding DMSO and increasing temperature improved extraction kinetics and DRh values. A number of dialkyl- and diaryl sulphide extractants were able to extract Rh with DRh values ≥ 10 at 70 °C. Dioctyl sulphide (1 M) in n-hexanol with 2% v/v DMSO extracted Rh (DRh ~25), Ru (DRu ~10) and Pd (DPd ~200) from a simulated FP solution with 3.1 M [HNO3] at 65 °C, with good separation from Zn, Cu, Fe, Tc, and Pb. | [130] |
| Dinonylnaphthalene sulphonic acid in kerosene | DRh = ~5 | 0.13 M HNO3, 0.1 M (extractant) with added NO2− ions (optimal NO2−/Rh = 0.5–1.0) | Equilibrium reached in <15 min. Higher temp. increases DRh. Higher [H+] and [NO2−] decreases DRh. Co-extracts other species, not very selective. | [131] |
| Aliquat 336 (10%) in benzene | Extraction slightly > 60% from pH 0.3–6, 80–90% (DRh 4–9) at pH 7.5–8 | Equilibrium reached in 1–10 min, 24 °C. | Benzene, cyclohexane, and CCl4 also produced similar extraction %. Extraction % decreased with increasing temp.—highest at 9 °C = 99.2% (DRh ~ 125). Selective extraction and stripping from other species in HLLW. | [134] |
| Dihexyl sulphide (DHS, 10%) in dodecane | DRh = 0.001–0.002 | 30 min contact time. | Primary source unavailable. Referenced in [29] without DRh values or conditions. | [103] |
| TOA (10%) in dodecane modified with 5% v/v dodecanol | DRh = ~0.06 | 0.1 M HNO3, 30 min contact time | DRh decreased “monotonously” from ~0.06 at 0.1 M HNO3 to ~0.001 at 6 M HNO3. Primary source unavailable. Referenced in [29] without conditions. | [103] |
| TOA (0.5 M) in xylene | N/A—primary source unavailable | Appreciable DRh values only obtained pH > 2 (<0.01 M [HNO3]). | Primary source unavailable. Referenced in [29] without conditions. | [135] |
| Amberlite LA-1 (10%) in xylene | DRh = < 0.01 | 1–15 M HNO3, 2 min contact time | Contact time likely too short for equilibrium. | [127] |
| Triisoctylamine (5%)(TIOA) in xylene | DRh = <0.01–0.001 | 1–15 M HNO3, 2 min contact time | Contact time likely too short for equilibrium. | [127] |
| Calix(n)arenethiaethers | DRh = 500 | 4 M HNO3, 2 h contact time, 35 °C | Extraction performed on [Rh(NO2)3(H2O)3] starting compound. Extraction increased as HNO3 increased between 0.5–4 M. Quantitative extraction achieved under optimal conditions. | [132] |
| Tri-n-octylaminoxide (0.045 M) in nitrobenzene | >80% extraction | 0.5 M HNO3, 1 h contact time, 50 °C | Starting material is [Rh(NO2]3(H2O3)]. Higher extraction in 0.5 M HNO3 compared to 3 M, increasing temperature and phase contact time increased extraction. Alkyl anilines in triethylbenzene achieved 96–98% Rh extraction within 5 min at 35 °C and selectivity for PGMs, although only in the pH range 1.2–3.5 | [73] |
| Triphenylphosphine (0.045 M) in nitrobenzene | >80% extraction | 3 M HNO3, 24 h contact time, 22 °C | [73] | |
| Para-n-octylaniline (0.045 M) in nitrobenzene | >80% extraction | 0.5 M HNO3, 1 h contact time, 50 °C 3 M HNO3, 24 h contact time, 22 °C | [73] | |
| TBP (50% v/v), TOPO (0.045 M), DHS (0.045 M), di-n-hexylsulphoxide (0.045 M), in nitrobenzene or toluene | <20% extraction | 0.5–3 M HNO3, 1–24 h contact time, 22–50 °C | ≤10% extraction for TBP, ~0% extraction for TOPO in all conditions. Recovery increased with higher HNO3 concentration and lower temperature for S-based extractants. | [73] |
| Alkylanilium nitrate (AAHNO3, 1 M) + DHS (1 M) in 1,2,4-triethylbenzene | ~97% extraction | 3 M HNO3, 5 min contact time, 35 °C | Starting material is [Rh(NO2]3(H2O3)]. 98–99% Rh extraction using all mixed extractants from 0.06 M HNO3 within 5 min at 35 °C. No extraction using single extractants except AAHNO3. Mixed extractant reaction with AAHNO3 proceeds via a two-stage mechanism, where a colloidal chemical intermediate forms between the [Rh(NO2]3(H2O3)], HNO3 and (BHNO3)p (an associated form of the alkylanilium salt), which reacts with DHS. | [74] |
| AAHNO3 (1 M)+DHSO (1 M) in 1,2,4-triethylbenzene | ~58% extraction | 3 M HNO3, 5 min contact time, 35 °C | ||
| DHSO (1 M)+TBP (50% v/v) in 1,2,4-triethylbenzene | ~53% extraction | 3 M HNO3, 5 min contact time, 35 °C | ||
| DHS (1 M)+DHSO (1 M) in 1,2,4-triethylbenzene | ~33% extraction | 3 M HNO3, 5 min contact time, 35 °C | ||
| Alkylanilium nitrate (AAHNO3, 1 M) + DHS (1 M) in 1,2,4-triethylbenzene | 95–97% extraction | 3 M HNO3, 5 min contact time, 35 °C | Coextraction of ~100% Pd in all conditions. Rh extraction behaviour found to be different based on starting concentration. Increased Rh extraction with higher [Pd] as bis(alkyl sulphide) Pd(II) species forms and catalyses reaction between DHS and a Rh intermediate based on AA nitrate micelles. 85–90% Rh extraction when initial [Rh] = 0.3–1 g/L and initial [Pd] = 2 g/L. Substantial increase in Rh extraction when [Rh] increased from 0.01 M (~1 g/L) to ~0.02 M (~2 g/L), regardless of Pd being present or not, i.e., ~97% Rh extraction when [Rh] = 1.6–2 g/L. TU (1 M) was found to be a highly efficient strippant, recovering ~91% Rh and ~99% Pd from the organic phase [136]. | [67] |
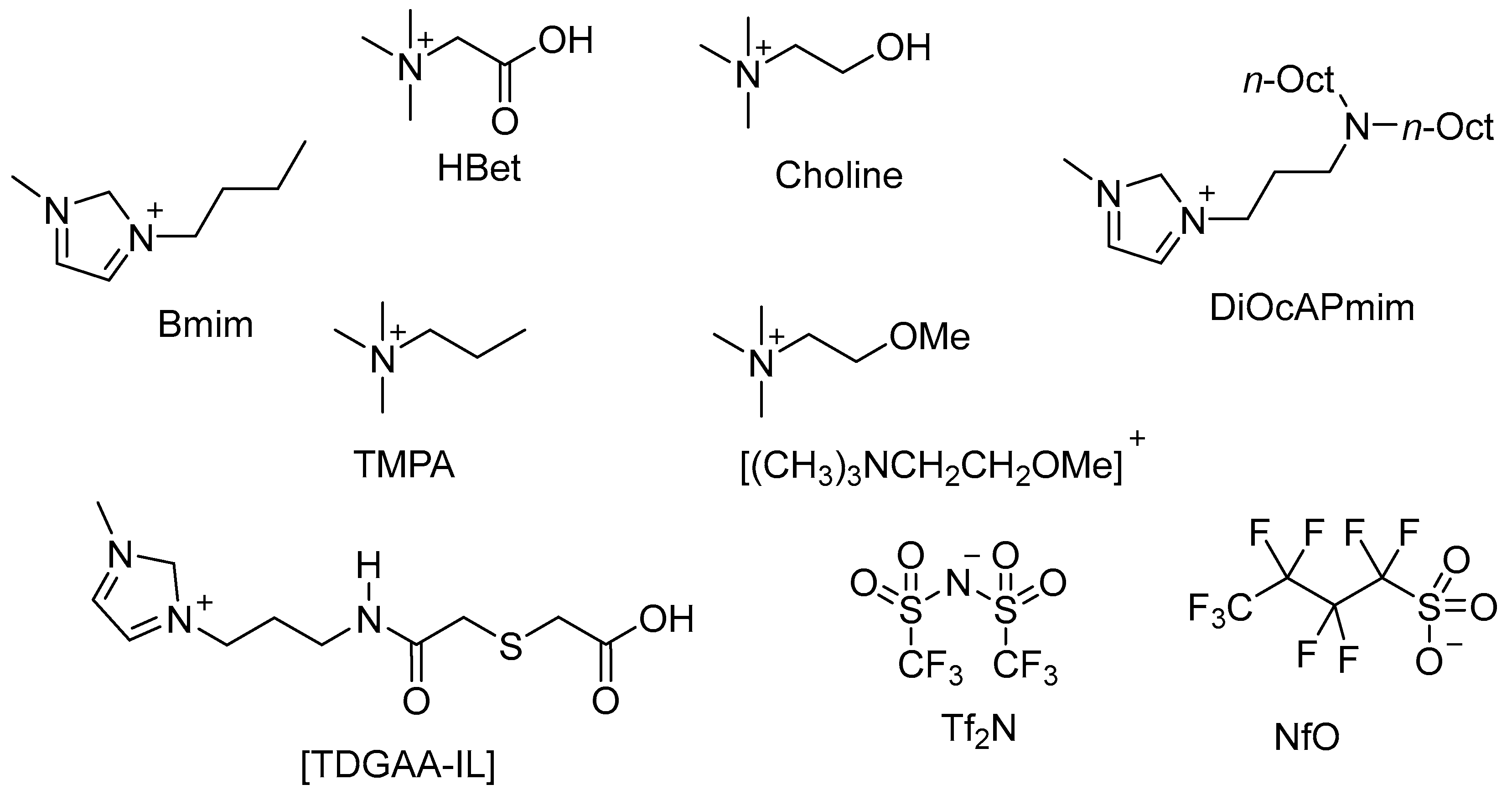
| Ionic Liquid | Highest DRh/Extraction (%) | Conditions | Comments | Ref. |
|---|---|---|---|---|
| [Hbet][Tf2N] | DRh = 2.12 | 25 °C in 0.3 M HNO3, 1 h. | Extraction decreased with increasing [HNO3]. DRh = 0.53 achieved in 2 M HNO3. | [141] |
| [Choline][Tf2N] | DRh =~0.13 | 25 °C in 0.4 M HNO3, 1 h. | Extraction independent of [HNO3]. DRh = ~0.3 achieved in 0.3–2 M HNO3. | [141] |
| [TMPA][Tf2N] | DRh =~0.04 | 25 °C in 0.3 M HNO3, 1 h. | Extraction independent of [HNO3]. DRh = ~0.3 achieved in 0.3–2 M HNO3. | [141] |
| [(CH3)3NCH2CH2OMe][Tf2N] mixed with TBP, dihexyl sulphide, Aliquat 336, CMPO, or TODGA | DRh = 16.9 | 25 °C in 0.01 M HNO3, with CMPO. | Very poor DRh in 6 M HNO3 in all cases. Three successive extractions on the aqueous phase using TODGA increased total DRh to 18.0 in 0.01 M HNO3. Only 0.01 M and 6 M HNO3 tested at 25 °C. | [142] |
| [DiOcAPmim][Tf2N] | DRh = ~9, ~90% extraction | 25 °C in 0.55 M HNO3 containing only Rh, Pd and Ru, 2 h contact time. | Extraction highest in 0.55 M HNO3, ~0% in 2.04 M HNO3, increased to ~10–15% between 3–6 M HNO3. Rh extraction of 13% from 25-component 2 M HNO3 HLLW simulant—higher than in PGM-only solution. DRh unaffected by temperature between 15–52 °C but tested in 2 M HNO3 where DRh ~ 0. | [138] |
| [TDGAA-IL][Tf2N] | DRh = 9–20 90–95% extraction | 50 °C in 2 M HNO3 and from 26-component 2 M HNO3 HLLW simulant, 8 h. | Equilibrium for Rh extraction reached in 24 h at 25 °C, 8 h at 50 °C. Good extraction performance for both Ru and Pd. Some coextraction of Ag(I), Zr(VI), Ba(II), Cs(I), and P(V) from simulated HLLW, but not as selective or efficient as for PGMs. Very little coextraction of trivalent lanthanides/REEs. | [139] |
| MPE-TDGA + [Bmim][Tf2N] | DRh = 0.2 20% extraction | 50 °C from 26-component 2 M HNO3 HLLW simulant, 2 h | Negligible Rh extraction in 0.1–6 M HNO3 solutions at 25 °C and 0.1–4 M HNO3 solutions at 50 °C. Rh extraction of ~20% from 6 M HNO3 at 50 °C, or from 26-component 2 M HNO3 HLLW simulant at 50 °C. No extraction observed with IL only. | [140] |
| MPE-TDGA + [Bmim][NfO] | DRh = 1.5–3 60–75% extraction | 50 °C from 26-component 2 M HNO3 HLLW simulant, 2 h | Up to 80% Rh extraction from 0.1 M or 6 M HNO3 at 50 °C, ~60% extraction n 0.5–4 M HNO3 under same conditions. Excellent separation of PGMs from 26-component 2 M HNO3 HLLW simulant. Coextraction of other metals, especially Ag(I), significantly decreased at 50 °C. Extraction observed with the IL which increased with addition of MPE-TDGA. Higher extraction from HLLW simulant compared to PGM-only solutions—attributed to salting-out effect. | [140] |
References
- Verma, P.K.; Mohapatra, P.K. Ruthenium speciation in radioactive wastes and state-of-the-art strategies for its recovery: A review. Sep. Purif. Tech. 2021, 275, 119148. [Google Scholar] [CrossRef]
- Bora, P.P.; Handa, S. Imides: A Special Chemical Entity in Rhodium Catalysis. In Developments in Organic Chemistry; Chapter 4; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–137. [Google Scholar] [CrossRef]
- Harjanto, S.; Cao, Y.; Shibayama, A.; Naitoh, I.; Nanami, T.; Kasahara, K.; Okumura, Y.; Liu, K.; Fujita, T. Leaching of Pt, Pd and Rh from Automotive Catalyst Residue in Various Chloride Based Solutions. Mater. Trans. 2006, 47, 129–135. [Google Scholar] [CrossRef]
- Zupanc, A.; Install, J.; Jereb, M.; Repo, T. Sustainable and Selective Modern Methods of Noble Metal Recycling. Angew. Chem. Int. Ed. 2023, 62, e202214453. [Google Scholar] [CrossRef]
- Shafiqul Alam, M.; Inoue, L. Extraction of rhodium from other platinum group metals with Kelex 100 from chloride media containing tin. Hydrometallurgy 1996, 46, 373–382. [Google Scholar] [CrossRef]
- National Minerals Information Center; United States Geological Survey. Platinum-Group Metals Statistics and Information. Available online: https://www.usgs.gov/centers/national-minerals-information-center/platinum-group-metals-statistics-and-information (accessed on 20 January 2023).
- European Commission. Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability; Report: COM(2020) 474 Final; European Commission: Brussels, Belgium, 2020; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0474 (accessed on 5 May 2023).
- U.S. Geological Survey. National News Release—U.S. Geological Survey Releases 2022 List of Critical Minerals. 2022. Available online: https://www.usgs.gov/news/national-news-release/us-geological-survey-releases-2022-list-critical-minerals#:~:text=The%202022%20list%20of%20critical%20minerals%20includes%20the,ceramics%2C%20glass%2C%20metallurgy%2C%20and%20polishing%20compounds%20More%20items (accessed on 20 January 2023).
- Glaister, B.J.; Mudd, G.M. The environmental costs of platinum–PGM mining and sustainability: Is the glass half-full or half-empty? Miner. Eng. 2010, 23, 438–450. [Google Scholar] [CrossRef]
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of platinum group metals from spent automotive catalysts: A review. Clean. Eng. Tech. 2021, 3, 100112. [Google Scholar] [CrossRef]
- Royal Society of Chemistry. Periodic Table—Rhodium. Available online: https://www.rsc.org/periodic-table/element/45/rhodium (accessed on 20 January 2023).
- GSI Exchange. Where is Rhodium Found in Nature? Available online: https://gsiexchange.com/learn/where-is-rhodium-found-in-nature/ (accessed on 20 January 2023).
- Patel, N.M. Speciation and Separation of Fission Product Rhodium. Ph.D. Thesis, Loughborough University, Loughborough, UK, 1985. Available online: https://hdl.handle.net/2134/7406 (accessed on 5 May 2023).
- Holdsworth, A.F.; Eccles, H.; Sharrad, C.A.; George, K. Spent Nuclear Fuel—Waste or Resource? The Potential of Strategic Materials Recovery during Recycle for Sustainability and Advanced Waste Management. Waste 2023, 1, 249–263. [Google Scholar] [CrossRef]
- Helmers, E.; Mergel, N. Platinum and rhodium in a polluted environment: Studying the emissions of automobile catalysts with emphasis on the application of CSV rhodium analysis’. Fres. J. Anal. Chem. 1998, 362, 522–528. [Google Scholar] [CrossRef]
- Shyam, T.; Dhruve, H. Comparative Analysis of Methods Employed in Rhodium Recovery. J. Chem. Rev. 2019, 1, 282–286. [Google Scholar] [CrossRef]
- Stanković, V.; Comninellis, C. Rhodium recovery and recycling from spent materials. In Proceedings of the 9th European Symposium on Electrochemical Engineering (9th ESEE), Chiana, Greece, 19–23 June 2011; Available online: https://www.researchgate.net/profile/Velizar-Stankovic/publication/284625081_RHODIUM_RECOVERY_AND_RECYCLING_FROM_SPENT_MATERIALS/links/5655851008ae1ef9297723bc/RHODIUM-RECOVERY-AND-RECYCLING-FROM-SPENT-MATERIALS.pdf (accessed on 5 May 2023).
- Samuels, A.C.; Victor, E.M.; Clark, A.E.; Wall, N.A. Rh(III) Extraction by Phosphinic Acids from Nitrate Media. Solv. Extr. Ion Exch. 2015, 33, 418–428. [Google Scholar] [CrossRef]
- Jayakumar, J.; Parthasarathy, K.; Cheng, C.H. One-Pot Synthesis of Isoquinolinium Salts by Rhodium-Catalyzed C-H Bond Activation: Application to the Total Synthesis of Oxychelerythrine. Angew. Chem. Int. Ed. 2012, 51, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Hyster, T.K.; Rovis, T. An improved catalyst architecture for rhodium (III) catalyzed C–H activation and its application to pyridone synthesis. Chem. Sci. 2011, 2, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I.; Vidal, E.S. Rhodium-catalyzed cyclohydrocarbonylation: Application to the synthesis of (+)-prosopinine and (−)- deoxoprosophylline. J. Org. Chem. 1998, 63, 7999–8003. [Google Scholar] [CrossRef]
- Fagnou, K.; Lautens, M. Rhodium-catalyzed carbon-carbon bond formation reactions of organometallic compounds. Chem. Rev. 2003, 103, 169–196. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, A.M. Rhodium Plating. Metal Finish. 1999, 1, 297–301. [Google Scholar]
- Pushpavanam, M.; Raman, V.; Shenoi, B.A. Rhodium—Electrodeposition and Applications. Surf. Tech. 1981, 12, 351–360. [Google Scholar]
- Kolarik, B.Z.; Renard, E.V. Potential Applications of Fission Platinoids in Industry. Platin. Met. Rev. 2005, 49, 79–90. [Google Scholar] [CrossRef]
- Pokhitonov, Y.A.; Tananaev, I.G. Prospects for the Use of Palladium from NPP Spent Nuclear Fuel and Ways to Design the Technology of its Recovery at a Radiochemical Enterprise. Radiochemistry 2022, 64, 270–279. [Google Scholar] [CrossRef]
- Pokhitonov, Y.A. Recovery of Platinoids from NPP Spent Nuclear Fuel and Outlook for Their Use. At. Energy 2020, 127, 367–374. [Google Scholar] [CrossRef]
- Bourg, S.; Poinssot, C. Could spent nuclear fuel be considered as a non-conventional mine of critical raw materials? Progr. Nucl. Ener. 2017, 94, 222–228. [Google Scholar] [CrossRef]
- Kolarik, Z.; Renard, E.V. Recovery of Value Fission Platinoids from Spent Nuclear Fuel Part I: General Considerations and Basic Chemistry. Plat. Met. Rev. 2003, 47, 74–87. [Google Scholar]
- Kolarik, Z.; Renard, E.V. Recovery of Value Fission Platinoids from Spent Nuclear Fuel Part II: Separation Processes. Plat. Met. Rev. 2003, 47, 123–131. [Google Scholar]
- Kayanuma, Y.; Okabe, T.H.; Maeda, M. Metal vapor treatment for enhancing the dissolution of platinum group metals from automotive catalyst scrap. Metal. Mater. Trans. B 2004, 35, 817–824. [Google Scholar] [CrossRef]
- Benson, M.; Bennett, C.R.; Harry, J.E.; Patel, M.K.; Cross, M. The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Res. Conserv. Recyc. 2000, 31, 1–7. [Google Scholar] [CrossRef]
- Jimenez de Aberasturi, D.; Pinedo, R.; Ruiz de Larramendi, I.; Ruiz de Larramendi, J.I.; Rojo, T. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner. Eng. 2011, 24, 503–513. [Google Scholar] [CrossRef]
- Panda, R.; Jha, M.K.; Pathak, D.D. Commercial Processes for the Extraction of Platinum Group Metals (PGMs). In Rare Metal Technology; Kim, H., Wesstrom, B., Alam, S., Ouchi, T., Azimi, G., Neelameggham, N.R., Wang, S., Guan, X., Eds.; Springer: New York, NY, USA, 2018; pp. 119–130. [Google Scholar] [CrossRef]
- United States Geological Survey (USGS). Available online: http://minerals.usgs.gov/minerals/pubs/mcs (accessed on 19 January 2023).
- International Atomic Energy Agency (IAEA). Feasibility of Separation and Utilization of Ruthenium, Rhodium and Palladium from High Level Wastes; Technical Report Series No. 308; IAEA: Vienna, Austria, 1989; pp. 18–19. Available online: https://www.iaea.org/publications/1411/feasibility-of-separation-and-utilization-of-ruthenium-rhodium-and-palladium-from-high-level-wastes (accessed on 5 May 2023).
- Rohrmann, C.A. Values in Spent Fuel from Power Reactors; Report: BNWL-25; Battelle Pacific Northwest National Laboratory: Washington, DC, USA, 1965. [Google Scholar] [CrossRef]
- Narita, H.; Morisaku, K.; Tanaka, M. The first effective extractant for trivalent rhodium in hydrochloric acid solution. Chem. Commun. 2008, 45, 5921. [Google Scholar] [CrossRef] [PubMed]
- Narita, H.; Kasuya, R.; Suzuki, T.; Motokawa, R.; Tanaka, M. Precious Metal Separations. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons: New Jersey, NJ, USA, 2020; pp. 1–28. [Google Scholar] [CrossRef]
- Kuczynski, R.J.; Atkinson, G.B.; Dolinar, W.J. Recovery of platinum group metals from automobile catalysts—Pilot plant operation. In Proceedings of the International Symposium of Recycling of Metals and Engineered Materials, Point Clear, AL, USA, 12–16 November 1995; Available online: https://www.osti.gov/biblio/197270 (accessed on 5 May 2023).
- Suoranta, T.; Zugazua, O.; Niemelä, M.; Perämäki, P. Recovery of palladium, platinum, rhodium and ruthenium from catalyst materials using microwave-assisted leaching and cloud point extraction. Hydrometallurgy 2015, 154, 56–62. [Google Scholar] [CrossRef]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Chen, M.; Li, S.; Luo, G.; Fan, M.; Chen, J.; Huang, Z.; Xie, X. Selective recovery of palladium and rhodium by combined extraction and photocatalytic reduction. Sep. Purif. Tech. 2021, 274, 119006. [Google Scholar] [CrossRef]
- Gupta, B.; Singh, I. Extraction and separation of platinum, palladium and rhodium using Cyanex 923 and their recovery from real samples. Hydrometallurgy 2013, 134–135, 11–18. [Google Scholar] [CrossRef]
- Mhaske, A.A.; Dhadke, P.M. Extraction separation studies of Rh, Pt and Pd using Cyanex 921 in toluene—A possible application to recovery from spent catalysts. Hydrometallurgy 2001, 61, 145–150. [Google Scholar] [CrossRef]
- Nowottny, C.; Halwachs, W.; Schügerl, K. Recovery of platinum, palladium and rhodium from industrial process leaching solutions by reactive extraction. Sep. Purif. Tech. 1997, 12, 135–144. [Google Scholar] [CrossRef]
- Barakat, M.A.; Mahmoud, M.H.H. Recovery of platinum from spent catalyst. Hydrometallurgy 2004, 72, 179–184. [Google Scholar] [CrossRef]
- Lanaridi, O.; Platzer, S.; Nischkauer, W.; Betanzos, J.H.; Iturbe, A.U.; Gaztelurrutia, C.D.R.; Sanchez-Cupido, L.; Siriwardana, A.; Schnürch, M.; Limbeck, M.; et al. Benign recovery of platinum group metals from spent automotive catalysts using choline-based deep eutectic solvents. Green Chem. Lett. Rev. 2022, 15, 405–415. [Google Scholar] [CrossRef]
- George, K.; Masters, A.J.; Livens, F.R.; Sarsfield, M.J.; Taylor, R.J.; Sharrad, C.A. A review of technetium and zirconium extraction into tributyl phosphate in the PUREX process. Hydrometallurgy 2022, 211, 105892. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Live Chart of Nuclides. Available online: https://www-nds.iaea.org/relnsd/vcharthtml/VChartHTML.html (accessed on 22 March 2023).
- Ando, Y.; Takano, H. Estimation of LWR Spent Fuel Composition; Report: JAERI-Research 99-004; Japan Atomic Energy Research Institute (JAERI); Center for Neutron Science; Tokai Research Establishment: Tokyo, Japan, 1999; Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:30019847 (accessed on 5 May 2023).
- Holdsworth, A.F.; George, K.; Adams, S.J.S.; Sharrad, C.A. An accessible statistical regression approach for the estimation of spent nuclear fuel compositions and decay heats to support the development of nuclear fuel management strategies. Progr. Nucl. Ener. 2021, 141, 103935. [Google Scholar] [CrossRef]
- Bush, R.P. Recovery of Platinum Group Metals from High Level Radioactive Waste: Possibilities of Separation and Use Re-evaluated. Platin. Metal. Rev. 1991, 35, 202–208. [Google Scholar]
- International Atomic Energy Agency (IAEA). Clearance of Materials Resulting from the Use of Radionuclides in Medicine, Industry and Research; Report IAEA-TECDOC-1000; IAEA: Vienna, Austria, 1998; p. 54. [Google Scholar]
- Allison, W. We Should Stop Running Away from Radiation. Philos. Tech. 2011, 24, 193–195. [Google Scholar] [CrossRef]
- Kleykamp, H. The Chemical State of Fission Products in Oxide Fuels at Different Stages of the Nuclear Fuel Cycle. Nucl. Tech. 1988, 80, 412–422. [Google Scholar] [CrossRef]
- Baron, P.; Cornet, S.M.; Collins, E.D.; DeAngelis, G.; Del Cul, G.; Fedorov, Y.; Glatz, J.P.; Ignatiev, V.; Inoue, T.; Khaperskaya, A.; et al. A review of separation processes proposed for advanced fuel cycles based on technology readiness level assessments. Progr. Nucl. Ener. 2019, 117, 103091. [Google Scholar] [CrossRef]
- Chen, H.; Taylor, R.J.; Jobson, M.; Woodhead, D.A.; Boxall, C.; Masters, A.J.; Edwards, S. Simulation of Neptunium Extraction in an Advanced PUREX Process—Model Improvement. Solv. Extr. Ion Exch. 2017, 35, 1–18. [Google Scholar] [CrossRef]
- Taylor, R.J.; Gregson, C.R.; Carrott, M.J.; Mason, C.; Sarsfield, M.J. Progress towards the Full Recovery of Neptunium in an Advanced PUREX Process. Solv. Extr. Ion Exch. 2013, 31, 442–462. [Google Scholar] [CrossRef]
- Taylor, R. The Chemical Basis for Separating Recycling Materials by Hydro-Processes. In Encyclopedia of Nuclear Energy; Elsevier: Amsterdam, The Netherlands, 2021; pp. 450–464. [Google Scholar] [CrossRef]
- Collins, E.D.; Del Cul, G.D.; Moyer, B.A. Advanced reprocessing for fission product separation and extraction. In Advanced Separation Techniques for Nuclear Fuel Reprocessing and Radioactive Waste Treatment; Chapter 8; Nash, K.L., Lumetta, G.J., Eds.; Woodhead: Cambridge, UK, 2011; pp. 201–228. [Google Scholar] [CrossRef]
- Samuels, A.C.; Boele, C.A.; Bennett, K.T.; Clark, S.B.; Wall, N.A.; Clark, A.E. Integrated Computational and Experimental Protocol for Understanding Rh(III) Speciation in Hydrochloric and Nitric Acid Solutions. Inorg. Chem. 2014, 53, 12315–12322. [Google Scholar] [CrossRef] [PubMed]
- Miguirditchian, M.; Vanel, V.; Marie, C.; Pacary, V.; Charbonnel, M.-C.; Berthon, L.; Hérès, X.; Montuir, M.; Sorel, C.; Bollesteros, M.-J.; et al. Americium Recovery from Highly Active PUREX Raffinate by Solvent Extraction: The EXAm Process. A Review of 10 Years of R&D. Solv. Extr. Ion Exch. 2020, 38, 365–387. [Google Scholar] [CrossRef]
- Bush, R.P.; Acres, C.J.K. Prospects for the Separation and Utilisation of Valuable Fission Products from High Level Wastes; Report: AERE R 12830; United Kingdom Atomic Energy Authority: Abingdon, UK, 1987. [Google Scholar]
- Hoffman, W.A., Jr. Rhodium Species in Radioactive Waste Solutions; Report: ARH-732; Atlantic Richfield Hanford Company: Richland, WA, USA, 1968. [Google Scholar]
- Belyaev, A.V.; Renard, E.V.; Khranenko, S.P.; Emel’yanov, V.A.; Fedotov, M.A. State of Radiorhodium in High-Level Liquid Waste from Regeneration of Spent Nuclear Fuel. Radiochemistry 2002, 44, 546–558. [Google Scholar] [CrossRef]
- Tatarchuk, V.V.; Druzhinina, I.A.; Korda, T.M. Rhodium and palladium joint extraction by dihexyl sulfide and alkylanilinium nitrate mixtures from nitrate solutions. Russ. J. Inorg. Chem. 2009, 54, 1332–1338. [Google Scholar] [CrossRef]
- Watanabe, S.; Sato, T.; Harigai, M.; Inaba, Y.; Takeshita, K.; Onoe, J. Chemical forms of rhodium ion in pure water and nitric acid solution studied using ultraviolet-visible spectroscopy and first-principles calculations. IOP Conf. Ser. Mater. Sci. Eng. 2020, 835, 12001. [Google Scholar] [CrossRef]
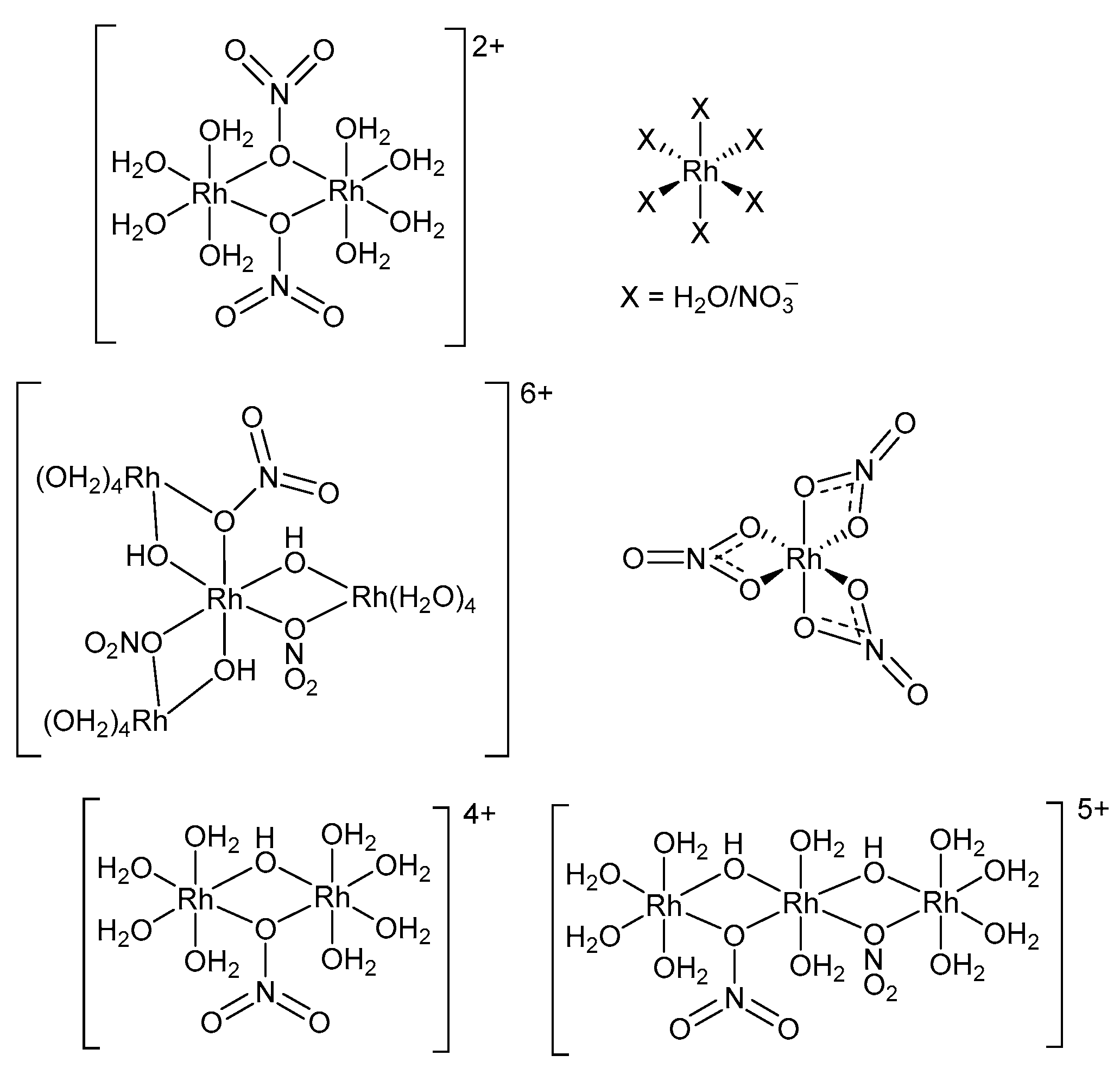
- Vasilchenko, D.; Vorob’eva, S.; Tkachev, S.; Baidina, I.; Balyaev, A.; Korenev, S.; Solovyov, L.; Vasilliev, A. Rhodium(III) Speciation in Concentrated Nitric Acid Solutions. Eur. J. Inorg. Chem. 2016, 23, 3822–3828. [Google Scholar] [CrossRef]
- Caminiti, R.; Atzei, D.; Cucca, P.; Anedda, A.; Bongiovanni, G. Structure of Rhodium(III) Nitrate Aqueous Solutions. An Investigation by X-ray Diffraction and Raman Spectroscopy. J. Phys. Chem. 1986, 90, 238–243. [Google Scholar] [CrossRef]
- Belyaev, A.V.; Fedotov, M.A.; Khranenko, S.P.; Emel’yanov, V.A. Forms of Rh(III) in Nitric Acid Solutions. Russ. J. Coord. Chem. 2001, 27, 855–864. [Google Scholar] [CrossRef]
- Berdyugin, S.N.; Vasilchenko, D.B.; Baidina, I.A.; Korenev, S.V.; Korolkov, I.V. Crystal Structure and Properties of [Rh2(H2O)8(μ-OH)2](NO3)4.4H2O. J. Struct. Chem. 2018, 59, 664–668. [Google Scholar] [CrossRef]
- Tatarchuk, V.V.; Korda, T.M.; Tatarchuk, A.N.; Torgov, V.G. Solvent Extraction of Differently Charged Aquanitro Forms of Rhodium (III) with Reference to the Recovery of the Fission Rhodium from Nitrate-Nitrite Solutions. Chem. Sust. Dev. 2003, 11, 755–764. [Google Scholar]
- Tatarchuk, V.V.; Druzhinina, I.A.; Korda, T.M.; Tatarchuk, A.N. Kinetics of rhodium extraction from nitric acid solutions with a mixture of dihexyl sulfide and alkylanilinium nitrate. Russ. J. Inorg. Chem. 2006, 51, 1977–1987. [Google Scholar] [CrossRef]
- Vorobyeva, S.N.; Shekhovstov, N.A.; Baidina, I.A.; Sukhikh, T.S.; Tkachev, S.V.; Bushuev, M.B.; Belyaev, A.V. The saga of rhodium(III) nitrate complexes and their speciation in solution: An integrated experimental and quantum chemical study. Polyhedron 2022, 211, 115564. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Vorobieva, S.; Baidina, I.; Piryazev, D.; Tsipis, A.; Korenev, S. Structure and properties of a rhodium(III) pentanitrato complex embracing uni- and bidentate nitrato ligands. Polyhedron 2018, 147, 69–74. [Google Scholar] [CrossRef]
- Yoshida, Z.; Aoyagi, H.; Mutoh, H.; Takeishi, H.; Sasaki, Y.; Uno, S.; Tachikawa, E. Spent fuel reprocessing based on electrochemical extraction process (SREEP). J. Alloy. Comp. 1994, 213–214, 435–455. [Google Scholar] [CrossRef]
- Baldwin, A.G.; Bridges, N.J.; Braley, J.C. Distribution of Fission Products into Tributyl Phosphate under Applied Nuclear Fuel Recycling Conditions. Ind. Eng. Chem. Res. 2016, 55, 13114–13119. [Google Scholar] [CrossRef]
- Ishimori, T.; Watanabe, K. Inorganic Extraction Studies on the System of Tri-n-butyl Phosphate—Nitric Acid. Bull. Chem. Soc. Jap. 1960, 33, 1443. [Google Scholar] [CrossRef]
- Wilden, A.; Schreinemachers, C.; Sypula, M.; Modolo, G. Direct Selective Extraction of Actinides (III) from PUREX Raffinate using a Mixture of CyMe4BTBP and TODGA as 1-cycle SANEX. Solv. Extr. Ion Exch. 2011, 29, 190–212. [Google Scholar] [CrossRef]
- Zalupski, P.R.; Ensor, D.D.; Riddle, C.L.; Peterman, D.R. Complete Recovery of Actinides from UREX-like Raffinates using a Combination of Hard and Soft Donor Ligands. Solv. Extr. Ion Exch. 2013, 31, 430–441. [Google Scholar] [CrossRef]
- Zalupski, P.R.; Klaehn, J.R.; Peterman, D.R. Complete Recovery of Actinides from UREX-like Raffinates Using a Combination of Hard and Soft Donor Ligands. II. Soft Donor Structure Variation. Solv. Extr. Ion Exch. 2015, 33, 523–539. [Google Scholar] [CrossRef]
- Bond, G.; Eccles, H.; Kavi, P.C.; Holdsworth, A.F.; Rowbotham, D.; Mao, R. Removal of Cesium from Simulated Spent Fuel Dissolver Liquor. J. Chromatog. Sep. Tech. 2019, 10, 417. [Google Scholar]
- Paul, N. Characterisation of Highly Active Nuclear Waste Simulants. Ph.D. Thesis, University of Leeds, Leeds, UK, 2014. [Google Scholar]
- Belyaev, A.V. Technological problems of platinum metals in nuclear fuel waste disposal. J. Struct. Chem. 2003, 44, 29–36. [Google Scholar] [CrossRef]
- Hartmann, T.; Pentinghaus, H. The ternary system palladium–rhodium–tellurium: A Study to understand phase formation in the vitrification process of high-level waste concentrates (HLWC). J. Nucl. Mater. 2012, 422, 124–130. [Google Scholar] [CrossRef]
- Nakamura, H.; Yamaguchi, I.; Kubota, M. Effect of Platinum Group Elements on Denitration of High-Level Liquid Waste with Formic Acid. J. Nucl. Sci. Tech. 1978, 15, 760–764. [Google Scholar] [CrossRef][Green Version]
- Laurin, C. Redox behavior of ruthenium in nuclear glass melt: Ruthenium dioxide reduction reaction. J. Nucl. Mater. 2021, 545, 152650. [Google Scholar] [CrossRef]
- Puyou, M.; Jacquet-Francillon, N.; Moncouyoux, J.P.; Sombret, C.; Teulon, F. Vitrification of Fission Product Solutions: Investigation of the Effects of Noble Metals on the Fabrication and Properties of R7T7 Glass. Nucl. Tech. 1995, 111, 163–168. [Google Scholar] [CrossRef]
- Gossé, S.; Bordier, S.; Guéneau, C.; Brackx, E.; Domenger, R.; Rogez, J. Thermodynamic assessment of the rhodium-ruthenium-oxygen (Rh-Ru-O) system. J. Nucl. Mater. 2018, 500, 252–264. [Google Scholar] [CrossRef]
- Sugawara, T.; Ohira, T.; Minami, K.; Komamine, S.; Ochi, E. Phase equilibrium experiments on the simulated high-level waste glass containing platinum group elements. J. Nucl. Sci. Tech. 2016, 53, 380–390. [Google Scholar] [CrossRef]
- Hames, A.L.; Tkac, P.; Paulenova, A.; Willit, J.L.; Williamson, M.A. Investigation of molybdate melts as an alternative method of reprocessing used nuclear fuel. J. Nucl. Mater. 2017, 486, 158–166. [Google Scholar] [CrossRef]
- Singh, R.; Sajan, C.P.; Naik, A. Chemical Analysis of High-Level Nuclear Waste Elements Fixed in Sodium Zirconium Phosphate (NaZr2P3O12) Matrix. J. Pollut. 2018, 1, 109. [Google Scholar]
- Okamoto, Y.; Kobayashi, H.; Shiwaku, H.; Sasage, K.; Hatakeyama, K.; Nagai, T. XAFS analysis of ruthenium in simulated iron phosphate radioactive waste glass. J. Non-Cryst. Sol. 2021, 551, 120393. [Google Scholar] [CrossRef]
- Hrudananda, J.; Sudha, R.; Venkatesh, P.; Reddy, B.P.; Kutty, K.V.G. Removal of Ru from Simulated High-Level Waste Prior to the Final Vitrification into Borosilicate Glass Using Tin as the Alloying Element: Feasibility Study. J. Haz. Tox. Radioact. Waste 2018, 22, 4018014. [Google Scholar] [CrossRef]
- Navratil, J.D. Ion Exchange Technology in Spent Fuel Reprocessing. J. Nucl. Sci. Tech. 1989, 26, 735–743. [Google Scholar] [CrossRef]
- Stevenson, C.E.; Gresky, A.T.; Mason, E.A. Progress in Nuclear Energy; Series 3: Process Chemistry; Pergamon Press: Oxford, UK, 1970; Volume 4. [Google Scholar]
- Gerber, M.S. The Plutonium Production Story at the Hanford Site: Processes and Facilities History; Report: WHC-MR-0521, Revision 0, UC-900; Hanford: Richlanw, WA, USA, 1996. [Google Scholar]
- Holdsworth, A.F.; Eccles, H.; Rowbotham, D.; Brookfield, A.; Collison, D.; Bond, G.; Kavi, P.C.; Edge, R. The Effect of Gamma Irradiation on the Physiochemical Properties of Caesium-Selective Ammonium Phosphomolybdate–Polyacrylonitrile (AMP–PAN) Composites. Clean Tech. 2019, 1, 294–310. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, H. Ion Exchange Characteristics of Palladium and Rhodium from a Simulated Radioactive Liquid Waste. J. Nucl. Sci. Tech. 2000, 37, 281–287. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoo, J.H.; Kim, J.H. Ion Exchange Characteristics of Rhodium and Ruthenium from a Simulated Radioactive Liquid Waste. Kor. J. Chem. Eng. 2004, 21, 1038–1043. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, H. Ion Exchange Characteristics of Palladium and Ruthenium from a Simulated Radioactive Liquid Waste. Sep. Sci. Tech. 2003, 38, 3459–3472. [Google Scholar] [CrossRef]
- Kirishima, K.; Shibayama, H.; Nakahira, H.; Shimauchi, H.; Myochin, M.; Wada, Y.; Kawase, K.; Kishimoto, Y. Recovery and utilization of valuable metals from spent nuclear fuel. 3: Mutual separation of valuable metals. In Proceedings of the 1993 International Conference on Nuclear Waste Management and Environmental Remediation, Prague, Czech Republic, 5–11 September 1993; p. 667. [Google Scholar]
- Suzuki, T.; Morita, K.; Sasaki, Y.; Matsumara, T. Recovery of rhodium(III) from nitric acid solutions using adsorbent functionalized with N,N,N-trimethylglycine. Bull. Chem. Soc. Jap. 2016, 89, 608–616. [Google Scholar] [CrossRef]
- Suzuki, T.; Morita, K.; Sasaki, Y.; Matsumara, T. Separation of Ru(III), Rh(III) and Pd(II) from nitric acid solutions using ion-exchange resins bearing carboxylic betaine. Sep. Sci. Tech. 2016, 51, 2815–2822. [Google Scholar] [CrossRef]
- Milyutin, V.V.; Peskishev, S.B.; Gelis, V.M. Investigation of sorption of palladium, ruthenium and rhodium ions from nitric acid solutions sorbents of different sorts. Radiokhimiya 1994, 36, 25–28. [Google Scholar]
- Yamagishi, I.; Kubota, M. Recovery of Technetium with Active Carbon Column in Partitioning Process of High-Level Liquid Waste. J. Nuc. Sci. Tech. 1993, 30, 717–719. [Google Scholar] [CrossRef]
- Kondo, Y.; Kubota, M. Precipitation Behavior of Platinum Group Metals from Simulated High Level Liquid Waste in Sequential Denitration Process. J. Nucl. Sci. Tech. 1992, 29, 140–148. [Google Scholar] [CrossRef]
- Onishi, T.; Sekioka, K.; Suto, M.; Tanak, K.; Koyama, S.-I.; Inaba, Y.; Takahashi, H.; Harigai, M.; Takeshita, K. Adsorption of platinum-group metals and molybdenum onto aluminum ferrocyanide in spent fuel solution. Ener. Proc. 2017, 131, 151–156. [Google Scholar] [CrossRef]
- Onishi, T.; Koyama, S.; Mimura, H. Adsorption of Ruthenium, Rhodium and Palladium from Simulated High-Level Liquid Waste by Highly Functional Xerogel—13286. In Proceedings of the WM2013 Conference, Phoenix, AZ, USA, 24–28 February 2013. [Google Scholar]
- Ito, T.; Kim, S.-Y.; Xu, Y.; Hitomi, K.; Ishii, K.; Nagaishi, K.; Kimura, T. Adsorption Behaviors of Platinum Group Metals in Simulated High Level Liquid Waste Using Macroporous (MOTDGA-TOA)/SiO2-P Silica-based Absorbent. Sep. Sci. Tech. 2013, 48, 2616–2625. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, S.-Y.; Ito, T.; Tada, T.; Hitomi, K.; Ishii, K. Adsorption properties and behavior of the platinum group metals onto a silica-based (Crea + TOA)/SiO2–P adsorbent from simulated high level liquid waste of PUREX reprocessing. J. Radioanal. Nucl. Chem. 2013, 297, 41–48. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, S.-Y.; Ito, T.; Tokuda, H.; Hitomi, K.; Ishii, K. Chromatographic separation of platinum group metals from simulated high level liquid waste using macroporous silica-based adsorbents. J. Chromatog. A 2013, 1312, 37–41. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, S.-Y.; Ito, T.; Tokuda, H.; Hitomi, K.; Ishii, K. Adsorption Behavior of Platinum Group Metals onto a Silica-based (Crea+Dodec)/SiO2-P Extraction Resin from Simulated High Level Liquid Waste. Sep. Sci. Tech. 2015, 50, 260–266. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Ning, S.-Y.; Zhou, J.; Wang, S.-Y.; Zhang, W.; Wang, X.-P.; Wei, Y.-Z. New insight into the adsorption of ruthenium, rhodium, and palladium from nitric acid solution by a silica-polymer adsorbent. Nucl. Sci. Tech. 2020, 31, 34. [Google Scholar] [CrossRef]
- Ito, T.; Kim, S.-Y. Study on Separation of Platinum Group Metals from High-level Liquid Waste Using Sulfur-containing Amic Acid-functionalized Silica. J. Ion Exch. 2018, 29, 97–103. [Google Scholar] [CrossRef]
- Ning, S.; Zhang, S.; Zhang, W.; Zhou, J.; Wang, S.; Wang, X.; Wei, Y. Separation and recovery of Rh, Ru and Pd from nitrate solution with a silica-based IsoBu-BTP/SiO2-P adsorbent. Hydrometallurgy 2020, 191, 105207. [Google Scholar] [CrossRef]
- Tateno, H.; Park, K.C.; Tsukahara, T. Direct Extraction of Platinum Group Metals from Nitric Acid Solution Using the Phase Transition of Poly(N-isopropylacrylamide). Chem. Lett. 2018, 47, 318–321. [Google Scholar] [CrossRef]
- Moore, R.H. Recovery of Fission-Produced Technetium, Palladium, Rhodium and Ruthenium. U.S. Patent 3,848,048, 12 November 1974. [Google Scholar]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Alfarra, A.; Frackowiak, E.; Béguin, F. The HSAB concept as a means to interpret the adsorption of metal ions onto activated carbons. Appl. Surf. Sci. 2004, 228, 84–92. [Google Scholar] [CrossRef]
- Fitoussi, R.; Lours, S.; Musikas, C. Ruthenium Recovery Process by Solvent Extraction. U.S. Patent 4,282,112, 4 August 1981. [Google Scholar]
- Chen, J.; Jiao, R.; Zhu, Y. The extraction of Tc(VII), Fe(III), Ru(II), Pd(II) and Mo(VI) from nitric acid solution by bis(2,4,4-trimethylpentyl) dithiophosphinic acid (HBTMPDTP). Radiochim. Acta 1999, 86, 151–154. [Google Scholar] [CrossRef]
- Swain, P.; Mallika, C.; Srinivasan, R.; Mudali, U.K.; Natarajan, R. Separation and recovery of ruthenium: A review. J. Radioanal. Nucl. Chem. 2013, 298, 781–796. [Google Scholar] [CrossRef]
- Khaperskaya, A.V.; Renard, E.V.; Koltunov, V.S. About the Extraction Recovery of Fission Rhodium from Radioactive Wastes. In Proceedings of the International Conference Scientific Research on the Back-End of the Fuel Cycle for the 21 Century, Avignon, France, 24–26 October 2000. [Google Scholar]
- Longden, I.; Patel, N.M.; Thornback, J.R.; Miles, J.H. The Extraction of Rhodium from Aqueous Nitric Acid by Organophosphine Sulphides. Solv. Extr. Ion Exch. 1986, 4, 421–433. [Google Scholar] [CrossRef]
- Ishimori, T.; Kobayashi, Y.; Usuba, Y. Solvent-Extraction Behavior of Carrier-Free Rhodium. Bull. Chem. Soc. Jap. 1968, 41, 1458–1459. [Google Scholar] [CrossRef]
- Horwitz, E.P.; Kalina, D.C.; Diamond, H.; Vandegrift, G.F.; Schulz, W.W. TRUEX process—A process for the extraction of the transuranic elements from nitric acid wastes utilizing modified PUREX solvent. Solv. Ext. Ion Exch. 1985, 3, 75–109. [Google Scholar] [CrossRef]
- Lunichkina, K.P.; Renard, E.V. On the extraction of rhodium (III) from nitrite solutions. Radiokhimiya 1974, 16, 268. [Google Scholar]
- Fritsch, E.; Gorski, B.; Beer, M. Extraction of Rhodium from HNO3 Solutions with Organic Sulphides. In Proceedings of the International Solvent Extraction Conference, Aiche Isec ′83, Denver, CO, USA, 26 August–2 September 1983; p. 199. [Google Scholar]
- Patel, N.M.; Thornback, J.R. The Extraction of Rhodium from Aqueous Nitric Acid by Dinonylnaphthalene Sulphonic Acid. Sol. Extr. Ion Exch. 1987, 5, 633–647. [Google Scholar] [CrossRef]
- Torgov, V.G.; Tatarchuk, V.V.; Druzhinina, I.A.; Korda, T.M. Extraction of triaquatrinitrorhodium form with calix[n]arenethiaethers from nitric acid nitrite–nitrate solutions. Russ. J. Inorg. Chem. 2016, 61, 1054–1059. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Cesium separation from radioactive waste by extraction and adsorption based on crown ethers and calixarenes. Nucl. Eng. Tech. 2020, 52, 328–336. [Google Scholar] [CrossRef]
- Campbell, M.H. A rapid determination of rhodium and palladium using liquid-liquid extraction with tricapryl monomethyl ammonium chloride and flame photometry. Anal. Chem. 1968, 40, 6–9. [Google Scholar] [CrossRef]
- Gorski, B.; Beer, M.; Ruβ, L. Über die Extraktion von Spaltrhodium aus salpetersauren Lösungen I. Die Extraktion anionischer Rhodiumnitritkomplexe. Isotopenpraxis 1988, 24, 200–204. [Google Scholar] [CrossRef]
- Tatarchuk, V.V.; Druzhinina, I.A.; Korda, T.M.; Malkova, V.I.; Sheludyakova, L.A.; Plyusnin, P.E. Thiourea stripping of rhodium from organic phases resulting from extraction with a mixture of dialkyl sulfide and alkylanilinium nitrate from acid nitrate-nitrite aqueous solutions of triaquatrinitrorhodium(III). Russ. J. Inorg. Chem. 2012, 57, 1398–1404. [Google Scholar] [CrossRef]
- Domínguez de María, P. Ionic Liquids, Switchable Solvents, and Eutectic Mixtures. In The Application of Green Solvents in Separation Processes; Chapter 6; Elsevier: Amsterdam, The Netherlands, 2017; pp. 139–154. [Google Scholar]
- Wu, H.; Kim, S.-Y.; Takahashi, T.; Oosugi, H.; Ito, T.; Kanie, K. Extraction behaviors of platinum group metals in simulated high-level liquid waste by a hydrophobic ionic liquid bearing an amino moiety. Nucl. Eng. Tech. 2021, 53, 1218–1223. [Google Scholar] [CrossRef]
- Ito, T.; Oosugi, H.; Osawa, N.; Takahashi, T.; Kim, S.-Y.; Nagaishi, R. Extraction Behavior of a Novel Functionalized Ionic Liquid for Separation of Platinum Group Metals from Aqueous Nitric Acid Solution. Anal. Sci. 2022, 38, 91–97. [Google Scholar] [CrossRef]
- Oosugi, H.; Ito, T.; Takahashi, T.; Wu, H.; Kim, S.-Y. Extraction behaviors of platinum group metals from an aqueous HNO3 solution using ionic liquids containing a novel thiodiglycolamide-type extractant. J. Radioanal. Nucl. Chem. 2022, 331, 4577–4585. [Google Scholar] [CrossRef]
- Sasaki, K.; Takao, K.; Suzuki, T.; Mori, T.; Arai, T.; Ikeda, Y. Extraction of Pd(ii), Rh(iii) and Ru(iii) from HNO3 aqueous solution to betainium bis(trifluoromethanesulfonyl)imide ionic liquid. Dalton Trans. 2014, 34, 5648–5651. [Google Scholar] [CrossRef]
- Bell, T.J.; Ikeda, Y. Efficient extraction of Rh(iii) from nitric acid medium using a hydrophobic ionic liquid. Dalton Trans. 2012, 41, 4303–4305. [Google Scholar] [CrossRef]
- Essington, M.E. Soil Water Chemistry. In Soil and Water Chemistry: An Integrative Approach, 2nd ed.; Chapter 5; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Takahashi, T. Ph.D. Thesis, Tokohu University, Sendai, Japan, 2019.
- Oosugi, H.; Takahashi, T.; Kim, S.-Y.; Ito, T. Separation of Platinum Group Metals from Nitric Acid Solution using Ionic Liquids containing TDGA Extractant. In Proceedings of the Atomic Energy Society of Japan 2019 Annual Meeting, Mito, Ibaraki, Japan, 20 March–22 February 2019. [Google Scholar]
- Hausman, E.A. Recovery of Rhodium from Fission Products. U.S. Patent 3,166,404, 25 February 1965. [Google Scholar]
- Tomiyasu, H.; Asano, Y. New reprocessing method overcoming environmental problems. Progr. Nucl. Ener. 1995, 29, 227–234. [Google Scholar] [CrossRef]
- Asano, Y.; Yamamura, T.; Tomiyasu, H.; Mizumachi, K.; Ikeda, Y.; Wada, Y. Recovery of noble metals from high-level liquid waste by precipitation method. In Proceedings of the International Topical Meeting on Nuclear and Hazardous Waste Management (SPECTRUMë94), Atlanta, GA, USA, 14–18 August 1994; p. 836. [Google Scholar]
- Jayakumar, M.; Venkatesan, K.A.; Srinivasan, T.G.; Vasudeva Rao, P.R. Feasibility studies on the electrochemical recovery of fission platinoids from high-level liquid waste. J. Radioanal. Nucl. Chem. 2010, 284, 79–85. [Google Scholar] [CrossRef]
- Koizumi, K.; Ozawa, M.; Kawata, T. Electrolytic Extraction of Platinum Group Metals from Dissolver Solution of Purex Process. J. Nucl. Sci. Tech. 1993, 30, 1195–1197. [Google Scholar] [CrossRef]
- Jayakumar, M.; Venkatesan, K.A.; Srinivasan, T.G.; Rao, P.R.V. Electrolytic extraction of palladium from nitric acid and simulated high-level liquid waste. Desal. Water Treatm. 2009, 12, 34–39. [Google Scholar] [CrossRef]
- Carlin, W.W.; Darlington, W.B.; Dubois, D.W. Recovery of Fission Products from Acidic Waste Solutions Thereof. U.S. Patent 3,891,741, 24 November 1972. [Google Scholar]
- Jayakumar, M.; Venkatesan, K.A.; Sudha, R.; Srinivasan, T.G.; Vasudeva Rao, P.R. Electrodeposition of ruthenium, rhodium and palladium from nitric acid and ionic liquid media: Recovery and surface morphology of the deposits. Mater. Chem. Phys. 2011, 128, 141–150. [Google Scholar] [CrossRef]
- Ozawa, M.; Suzuki, S.; Takeshita, K. Advanced Hydrometallurgical Separation of Actinides and Rare Metals in Nuclear Fuel Cycle. Solvent Extr. Res. Dev. Jpn. 2010, 17, 19–34. [Google Scholar] [CrossRef][Green Version]
- McKibben, J.M. Chemistry of the Purex Process. Radiochim. Acta 1984, 36, 3–16. [Google Scholar] [CrossRef]
- Sinharoy, P.; Banerjee, D.; Manohar, S.; Kaushik, C.P. Separation of radio-chemically pure 106Ru from radioactive waste for the preparation of brachytherapy sources: An insight of process development study. Sep. Sci. Tech. 2021, 56, 1450–1456. [Google Scholar] [CrossRef]
- Venkatesan, K.A.; Sukumaran, V.; Antony, M.P.; Srinivasan, T.G. Studies on the feasibility of using crystalline silicotitanates for the separation of cesium-137 from fast reactor high-level liquid waste. J. Radioanal. Nucl. Chem. 2009, 280, 129–136. [Google Scholar] [CrossRef]
- Nishi, T.; Uetake, N.; Kawamura, F.; Yusa, H. Recovery of noble metals from HLLW using photocatalytic reduction. Trans. Am. Nucl. Soc. 1987, 55, 242–243. [Google Scholar]
- Lee, S.H.; Jung, C.-H.; Chon, J.S.; Chung, H. Separation of Palladium from a Simulated Radioactive Liquid Waste by Precipitation Using Ascorbic Acid. Sep. Sci. Tech. 2000, 35, 411–420. [Google Scholar] [CrossRef]
- Kim, E.-H.; Yoo, J.-H.; Choi, C.-S. Removal of Palladium Precipitate from a Simulated High-level Radioactive Liquid Waste by Reduction by Ascorbic Acid. Radiochim. Acta. 1998, 80, 53–57. [Google Scholar] [CrossRef]
- Kanert, G.A.; Chow, A. The separation of rhodium and iridium by anion-exchange. Analyt. Chim. Acta 1975, 78, 375–382. [Google Scholar] [CrossRef]
- Moriyama, H.; Kinoshita, K.; Seshimo, T.; Asoaka, Y.; Moritani, K.; Ito, Y. RECOD ′91, Proceedings of the 3rd International Conference on Nuclear Fuel Reprocessing and Waste Management, Sendai, Japan, 14–18 April 1991; Japan Atomic Industrial Forum: Tokyo, Japan, 1991; p. 639. [Google Scholar]
- Jensen, G.A.; Platt, A.M.; Mellinger, G.B.; Bjorklund, W.J. Recovery of Noble Metals from Fission Products. Nucl. Tech. 1984, 65, 305–324. [Google Scholar] [CrossRef]
- Naito, K.; Matsui, T.; Tanaka, Y. Recovery of Noble Metals from Insoluble Residue of Spent Fuel. J. Nucl. Sci. Tech. 1986, 23, 540–549. [Google Scholar] [CrossRef]
- Naito, K.; Matsui, T.; Nakahira, H.; Kitagawa, M.; Okada, H. Recovery and mutual separation of noble metals from the simulated insoluble residue of spent fuel. J. Nucl. Mater. 1991, 184, 30–38. [Google Scholar] [CrossRef]
- Uno, M.; Kadotani, Y.; Kinoshita, H.; Miyaki, C. Processing High-Level Liquid Waste by Super-High-Temperature Method, (IV) Reducing Reactions and Alloy Formation by Platinum Group Elements, Molybdenum and Corrosion Products Taking Place in Simulated HLLW. J. Nucl. Sci. Tech. 1996, 33, 973–980. [Google Scholar] [CrossRef]
- Smith, F.J.; McDuffie, J.F. Recovery of Nonradioactive Palladium and Rhodium from Radioactive Waste. Sep. Sci. Tech. 1981, 16, 1071–1079. [Google Scholar] [CrossRef]
- Bunn, M.G.; Zhang, H.; Kang, L. Report: The Cost of Reprocessing in China; Belfer Center for Science and International Affairs, Harvard Kennedy School: Cambridge, MA, USA, 2016. [Google Scholar]




| Isotope | Content (wt.%) | Half-Life | Decay Mode |
|---|---|---|---|
| Rh-101 | Trace | 3.3 y | Electron capture |
| Rh-102 | Trace | 2.9 years | γ, electron capture |
| Rh-102m | Trace | 207 days | βγ, electron capture |
| Rh-103 | ~100 | Stable | -- |
| Rh-106 * | Trace | 30 s | βγ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodgson, B.J.; Turner, J.R.; Holdsworth, A.F. A Review of Opportunities and Methods for Recovery of Rhodium from Spent Nuclear Fuel during Reprocessing. J. Nucl. Eng. 2023, 4, 484-534. https://doi.org/10.3390/jne4030034
Hodgson BJ, Turner JR, Holdsworth AF. A Review of Opportunities and Methods for Recovery of Rhodium from Spent Nuclear Fuel during Reprocessing. Journal of Nuclear Engineering. 2023; 4(3):484-534. https://doi.org/10.3390/jne4030034
Chicago/Turabian StyleHodgson, Ben J., Joshua R. Turner, and Alistair F. Holdsworth. 2023. "A Review of Opportunities and Methods for Recovery of Rhodium from Spent Nuclear Fuel during Reprocessing" Journal of Nuclear Engineering 4, no. 3: 484-534. https://doi.org/10.3390/jne4030034
APA StyleHodgson, B. J., Turner, J. R., & Holdsworth, A. F. (2023). A Review of Opportunities and Methods for Recovery of Rhodium from Spent Nuclear Fuel during Reprocessing. Journal of Nuclear Engineering, 4(3), 484-534. https://doi.org/10.3390/jne4030034






