Association Between Age at Menarche and Gestational Diabetes: A Retrospective Case–Control Study
Abstract
1. Introduction
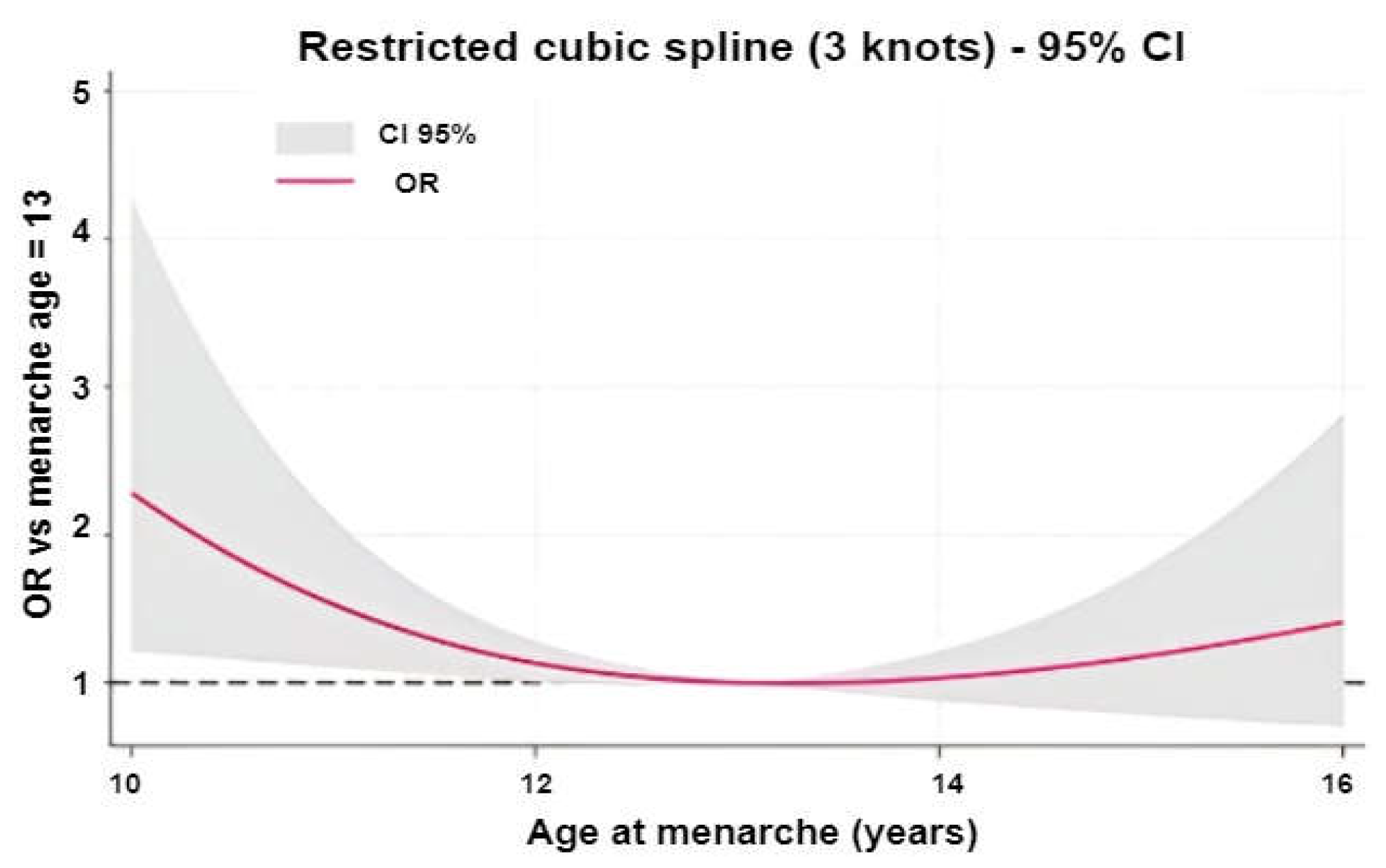
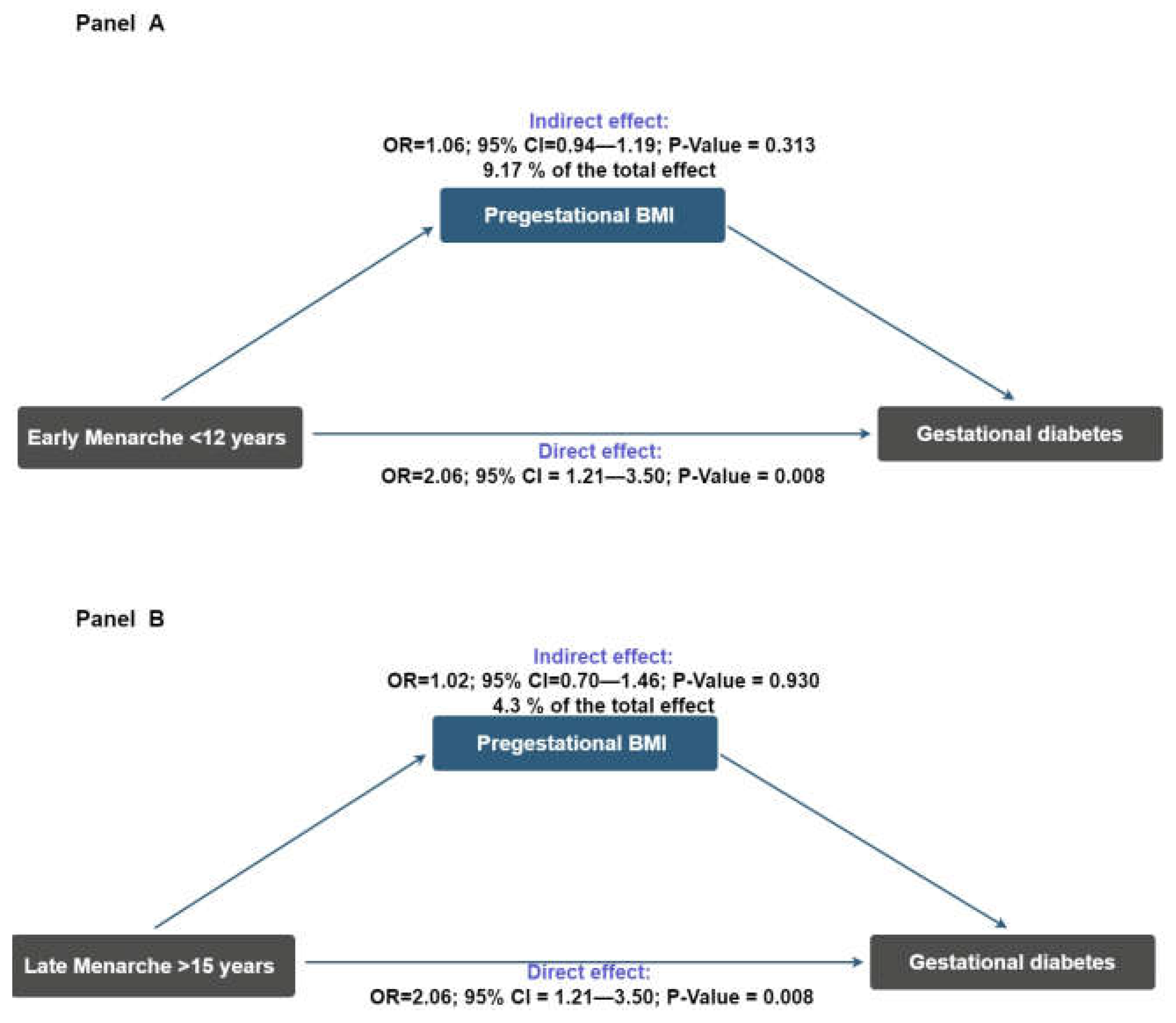
2. Results
3. Discussion
Limitations
4. Materials and Methods
4.1. Design and Study Population
4.2. Study Variables
4.3. Data Collection
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GDM | Gestational diabetes mellitus |
| OR | Odds ratio |
| CI | Confidence interval |
| DAG | Directed acyclic graphs |
| IQR | Interquartile range |
References
- American Diabetes Association Professional Practice Committee. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47, S282–S294. [Google Scholar] [CrossRef] [PubMed]
- Kunarathnam, V.; Vadakekut, E.S.; Mahdy, H. Gestational Diabetes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sheiner, E. Gestational Diabetes Mellitus: Long-Term Consequences for the Mother and Child Grand Challenge: How to Move on Towards Secondary Prevention? Front. Clin. Diabetes Healthc. 2020, 1, 546256. [Google Scholar] [CrossRef]
- Garza-Martínez, M.J.; Á Hernández-Mariano, J.; Hurtado-Salgado, E.M.; Cupul-Uicab, L.A. Maternal Diabetes during Pregnancy and Offspring’s Risk of Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2025, 182, 100–115. [Google Scholar] [CrossRef]
- Guan, J.; Qiu, J.; Li, L.; Fu, M.; Zhang, M.; Wu, Y.; Xu, Y.; Ding, H.; Gao, Q. A Meta-Analysis of Adverse Offspring Health Outcomes in Patients with Gestational Diabetes Mellitus. Diabetes Obes. Metab. 2025, 27, 3555–3567. [Google Scholar] [CrossRef]
- Leth-Møller, M.; Hulman, A.; Kampmann, U.; Hede, S.; Ovesen, P.G.; Knorr, S. Effect of Gestational Diabetes on Fetal Growth Rate and Later Overweight in the Offspring. J. Clin. Endocrinol. Metab. 2025, 110, 1350–1357. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Zhou, J. Impact of Gestational Diabetes Mellitus on Neonatal Birth Outcomes. Br. J. Hosp. Med. 2024, 85, 1–12. [Google Scholar] [CrossRef]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef]
- Blanco, E.; Marin, M.; Nuñez, L.; Retamal, E.; Ossa, X.; Woolley, K.E.; Oludotun, T.; Bartington, S.E.; Delgado-Saborit, J.M.; Harrison, R.M.; et al. Adverse Pregnancy and Perinatal Outcomes in Latin America and the Caribbean: Systematic Review and Meta-Analysis. Rev. Panam. Salud Publica (Pan Am. J. Public Health) 2022, 46, e21. [Google Scholar] [CrossRef]
- Herández-Ruíz, S.; Solano-Ceh, A.; Villarreal-Ríos, E.; Curiel Pérez, M.O.; Galicia-Rodríguez, L.; Elizarrarás-Rivas, J.; Jiménez-Reyes, O.H.; Herández-Ruíz, S.; Solano-Ceh, A.; Villarreal-Ríos, E.; et al. Prevalence of gestational diabetes and gestational hypertension in pregnant women with pregestational obesity. Ginecol. Obstet. México 2023, 91, 85–91. [Google Scholar] [CrossRef]
- Font-López, K.C.; Gutiérrez-Castañeda, M.R.; Font-López, K.C.; Gutiérrez-Castañeda, M.R. Diagnóstico de diabetes gestacional en población mexicana. Ginecol. Obstet. México 2017, 85, 116–124. [Google Scholar]
- Zhong, J.; Zhang, H.; Wu, J.; Zhang, B.; Lan, L. Analysis of Risk Factors Associated with Gestational Diabetes Mellitus: A Retrospective Case-Control Study. Int. J. Gen. Med. 2024, 17, 4229–4238. [Google Scholar] [CrossRef]
- Lee, H.S. Why Should We Be Concerned about Early Menarche? Clin. Exp. Pediatr. 2020, 64, 26–27. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Rigon, F.; Bernasconi, S.; Bianchin, L.; Bona, G.; Bozzola, M.; Buzi, F.; De Sanctis, C.; Tonini, G.; Radetti, G.; et al. Age at Menarche and Menstrual Abnormalities in Adolescence: Does It Matter? The Evidence from a Large Survey among Italian Secondary Schoolgirls. Indian J. Pediatr. 2019, 86, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A.; Derraik, J.G.B.; Rowe, D.L.; Hofman, P.L.; Cutfield, W.S. Earlier Menarche Is Associated with Lower Insulin Sensitivity and Increased Adiposity in Young Adult Women. PLoS ONE 2015, 10, e0128427. [Google Scholar] [CrossRef]
- Elks, C.E.; Ong, K.K.; Scott, R.A.; van der Schouw, Y.T.; Brand, J.S.; Wark, P.A.; Amiano, P.; Balkau, B.; Barricarte, A.; Boeing, H.; et al. Age at Menarche and Type 2 Diabetes Risk. Diabetes Care 2013, 36, 3526–3534. [Google Scholar] [CrossRef]
- Cheng, T.S.; Day, F.R.; Lakshman, R.; Ong, K.K. Association of Puberty Timing with Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLoS Med. 2020, 17, e1003017. [Google Scholar] [CrossRef]
- Nichols, A.R.; Chavarro, J.E.; Oken, E. Reproductive Risk Factors across the Female Lifecourse and Later Metabolic Health. Cell Metab. 2024, 36, 240–262. [Google Scholar] [CrossRef]
- Jung, H.; Sung, Y.-A.; Hong, Y.S.; Song, D.K.; Hong, S.-H.; Lee, H. Relationship between Age at Menarche and Metabolic Diseases in Korean Postmenopausal Women: The Korea National Health and Nutrition Examination Survey 2016–2018. PLoS ONE 2023, 18, e0280929. [Google Scholar] [CrossRef]
- Baek, T.-H.; Lim, N.-K.; Kim, M.-J.; Lee, J.; Ryu, S.; Chang, Y.; Choi, Y.; Park, H.-Y. Age at Menarche and Its Association with Dysglycemia in Korean Middle-Aged Women. Menopause 2015, 22, 542–548. [Google Scholar] [CrossRef][Green Version]
- Kheradmand, M.; Hamzehgardeshi, Z.; Shahhosseini, Z.; Mirjalili, R.; Moosazadeh, M. The Association between Early Menarche and Higher-Risk Cardiometabolic Profile: A Dose-Response Analysis of the Tabari Cohort at Enrollment Phase. Front. Cardiovasc. Med. 2023, 10, 1241179. [Google Scholar] [CrossRef]
- Mueller, N.T.; Duncan, B.B.; Barreto, S.M.; Chor, D.; Bessel, M.; Aquino, E.M.; Pereira, M.A.; Schmidt, M.I. Earlier Age at Menarche Is Associated with Higher Diabetes Risk and Cardiometabolic Disease Risk Factors in Brazilian Adults: Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Cardiovasc. Diabetol. 2014, 13, 22. [Google Scholar] [CrossRef]
- Gupta, I.; Kondal, D.; Mohan, S.; Deepa, M.; Anjana, R.M.; Ali, M.K.; Narayan, K.M.V.; Mohan, V.; Tandon, N.; Prabhakaran, D.; et al. Association of Age at Menarche with Type 2 Diabetes Mellitus among Urban Indian Women: Results from the CARRS Study. Int. J. Epidemiol. 2025, 54, dyaf049. [Google Scholar] [CrossRef]
- Saquib, N.; Kritz-Silverstein, D.; Barrett-Connor, E. Age at Menarche, Abnormal Glucose Tolerance and Type 2 Diabetes Mellitus: The Rancho Bernardo Study. Climacteric J. Int. Menopause Soc. 2005, 8, 76–82. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.M.; Ma, C.G.; Shim, Y.S. Impact of Age at Menarche on Glycosylated Hemoglobin Levels in Korean Non-Diabetic Women: The Korean National Health and Nutrition Examination Surveys. Int. J. Clin. Exp. Med. 2017, 10, 16415–16424. [Google Scholar]
- Schoenaker, D.A.J.M.; Mishra, G.D. Association Between Age at Menarche and Gestational Diabetes Mellitus: The Australian Longitudinal Study on Women’s Health. Am. J. Epidemiol. 2017, 185, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Ergin, A.; Türkay, Ü.; Özdemir, S.; Taşkın, A.; Terzi, H.; Özsürmeli, M. Age at Menarche: Risk Factor for Gestational Diabetes. J. Obstet. Gynaecol. 2022, 42, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, B.; Shi, X.; Song, H.; Su, W.; Huang, B.; Zhang, Y.; Wang, S.; Lv, F.; Lin, M.; et al. Age at Menarche and Risk of Gestational Diabetes Mellitus: A Population-Based Study in Xiamen, China. BMC Pregnancy Childbirth 2019, 19, 138. [Google Scholar] [CrossRef] [PubMed]
- Dishi, M.; Enquobahrie, D.A.; Abetew, D.F.; Qiu, C.; Rudra, C.B.; Williams, M.A. Age at Menarche, Menstrual Cycle Characteristics and Risk of Gestational Diabetes. Diabetes Res. Clin. Pract. 2011, 93, 437–442. [Google Scholar] [CrossRef]
- Farahmand, M.; Tehrani, F.R.; Dovom, M.R.; Azizi, F.; Tehrani, F.R.; Dovom, M.R.; Azizi, F. Menarcheal Age and Risk of Type 2 Diabetes: A Community-Based Cohort Study. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 156–162. [Google Scholar] [CrossRef]
- Zárate, C.M.R.; Caballero, C.A.Z.; Hernández, F.V.M.; Robles, C.M.F.; Sánchez, A.M.R.; Ambe, A.K. Secular changes in onset of menarche among Mexican women. Ginecol. Obstet. México 2024, 92, 359–363. [Google Scholar]
- Marván, M.L.; Catillo-López, R.L.; Alcalá-Herrera, V.; Callejo, D.D. The Decreasing Age at Menarche in Mexico. J. Pediatr. Adolesc. Gynecol. 2016, 29, 454–457. [Google Scholar] [CrossRef]
- Shamah-Levy, T.; Gaona-Pineda, E.B.; Cuevas-Nasu, L.; Valenzuela-Bravo, D.G.; Morales-Ruan, C.; Rodríguez-Ramírez, S.; Méndez-Gómez-Humarán, I.; Ávila-Arcos, M.A.; Álvarez-Sánchez, C.; Ávila-Curiel, A.; et al. Sobrepeso y obesidad en población escolar y adolescente. Salud Pública México 2024, 66, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Shalitin, S.; Phillip, M. Role of Obesity and Leptin in the Pubertal Process and Pubertal Growth—A Review. Int. J. Obes. 2003, 27, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, Z.; Saki, N.; Cheraghian, B.; Sarvandian, S.; Hashemi, S.J.; Kaabi, J.; Saki Malehi, A.; Shahriari, A.; Nasehi, N. Association between Age at Menarche and Metabolic Syndrome in Southwest Iran: A Population-Based Case-Control Study. J. Res. Health Sci. 2022, 22, e00558. [Google Scholar] [CrossRef] [PubMed]
- Benny, P.; Yang, Q.; Wong, B.W.-X.; Zhang, C.; Yong, E.-L.; Li, L.-J.; Huang, Z. Exploring the Link between Age at Menarche, Anthropometry and Body Fat Composition with Type II Diabetes in a Singapore Multi-Ethnic Cohort. BMC Med. 2025, 23, 306. [Google Scholar] [CrossRef]
- Nieuwenhuis, D.; Pujol-Gualdo, N.; Arnoldussen, I.A.C.; Kiliaan, A.J. Adipokines: A Gear Shift in Puberty. Obes. Rev. 2020, 21, e13005. [Google Scholar] [CrossRef]
- Tzounakou, A.-M.; Stathori, G.; Paltoglou, G.; Valsamakis, G.; Mastorakos, G.; Vlahos, N.F.; Charmandari, E. Childhood Obesity, Hypothalamic Inflammation, and the Onset of Puberty: A Narrative Review. Nutrients 2024, 16, 1720. [Google Scholar] [CrossRef]
- Landgraf, K.; Rockstroh, D.; Wagner, I.V.; Weise, S.; Tauscher, R.; Schwartze, J.T.; Löffler, D.; Bühligen, U.; Wojan, M.; Till, H.; et al. Evidence of Early Alterations in Adipose Tissue Biology and Function and Its Association with Obesity-Related Inflammation and Insulin Resistance in Children. Diabetes 2014, 64, 1249–1261. [Google Scholar] [CrossRef]
- Farahmand, M.; Mousavi, M.; Momenan, A.A.; Azizi, F.; Ramezani Tehrani, F. The Association between Arterial Hypertension and Menarcheal Age. Maturitas 2023, 174, 14–22. [Google Scholar] [CrossRef]
- Canoy, D.; Beral, V.; Balkwill, A.; Wright, F.L.; Kroll, M.E.; Reeves, G.K.; Green, J.; Cairns, B.J.; for the Million Women Study Collaborators. Age at Menarche and Risks of Coronary Heart and Other Vascular Diseases in a Large UK Cohort. Circulation 2015, 131, 237–244. [Google Scholar] [CrossRef]
- Santos, M.P.; Bazzano, L.; Carmichael, O.; O’Bryant, S.; Hsia, D.S.; He, J.; Ley, S.H. Association of Age at Menarche with Inflammation and Glucose Metabolism Biomarkers in US Adult Women: NHANES 1999–2018. J. Clin. Endocrinol. Metab. 2025, 110, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Wang, M.; Lakatta, E.G.; Ungvari, Z. Inflammation and Endothelial Dysfunction during Aging: Role of NF-κB. J. Appl. Physiol. 2008, 105, 1333–1341. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial dysfunction and chronic inflammation: The cornerstones of vascular alterations in age-related diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Digitale, J.C.; Martin, J.N.; Glymour, M.M. Tutorial on Directed Acyclic Graphs. J. Clin. Epidemiol. 2022, 142, 264–267. [Google Scholar] [CrossRef]
- Tennant, P.W.G.; Murray, E.J.; Arnold, K.F.; Berrie, L.; Fox, M.P.; Gadd, S.C.; Harrison, W.J.; Keeble, C.; Ranker, L.R.; Textor, J.; et al. Use of Directed Acyclic Graphs (DAGs) to Identify Confounders in Applied Health Research: Review and Recommendations. Int. J. Epidemiol. 2021, 50, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; VanderWeele, T.J. Sensitivity Analysis Without Assumptions. Epidemiology 2016, 27, 368–377. [Google Scholar] [CrossRef] [PubMed]
| Features | Gestational Diabetes | |||
|---|---|---|---|---|
| n = 426 | Yes (Cases) n = 71 | No (Controls) n = 355 | p-Value a | |
| Age (in years) | ||||
| Median (IQR) | 25 (10) | 30 (13) | 25 (10) | 0.001 |
| Marital status, f (%) | ||||
| No partner | 34 (8.0) | 6 (8.5) | 28 (7.9) | 0.873 |
| With partner | 392 (92.0) | 65 (91.5) | 327 (92.1) | |
| Education level, f (%) | ||||
| Primary education | 40 (9.4) | 7 (9.9) | 33 (9.3) | 0.138 |
| Lower secondary education | 215 (50.6) | 30 (42.3) | 185 (52.3) | |
| Upper secondary education | 89 (20.9) | 22 (31.0) | 67 (18.9) | |
| Tertiary education | 81 (19.1) | 12 (16.9) | 69 (19.5) | |
| Monthly household income b | ||||
| Median (IQR) | 459 (74.0) | 442.3 (67.3) | 460.1 (74.8) | 0.218 |
| Parity, f (%) | ||||
| Nulliparous | 172 (40.4) | 19 (26.6) | 153 (43.1) | 0.030 |
| 1–2 | 202 (47.4) | 43 (60.6) | 159 (44.8) | |
| ≥3 | 52 (12.2) | 9 (12.7) | 43 (12.1) | |
| Previous gestational diabetes | ||||
| No | 382 (89.7) | 45 (63.4) | 337 (94.9) | 0.001 |
| Yes | 44 (10.3) | 26 (36.6) | 18 (5.1) | |
| Alcohol consumption, f (%) | ||||
| No | 401 (94.1) | 68 (95.8) | 333 (93.8) | 0.519 |
| Yes | 25 (5.9) | 3 (12.0) | 22 (6.2) | |
| Cigarette smoking, f (%) | ||||
| No | 375 (88.0) | 61 (85.9) | 314 (88.5) | 0.548 |
| Yes | 51 (12.0) | 10 (14.1) | 41 (11.5) | |
| Family history of diabetes, f (%) | ||||
| No | 314 (73.7) | 53 (74.6) | 261 (73.5) | 0.844 |
| Yes | 112 (26.3) | 18 (25.4) | 94 (26.5) | |
| Age of menarche, f (%) | ||||
| <12 years | 125 (29.3) | 30 (42.2) | 95 (26.8) | 0.021 |
| 12–15 years | 258 (60.6) | 33 (46.5) | 225 (63.4) | |
| >15 years | 43 (10.1) | 8 (11.3) | 35 (9.8) | |
| Age at Menarche | Gestational Diabetes | |||
|---|---|---|---|---|
| OR (CI 95%) | p-Value | OR (CI 95%) a | p-Value | |
| 12–15 years | Ref. | - | Ref. | - |
| <12 years | 2.31 (1.29, 4.13) | 0.005 | 2.51 (1.40, 4.50) | 0.002 |
| >15 years | 1.55 (0.69, 3.50) | 0.286 | 1.84 (0.75, 4.49) | 0.179 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solis-Gómez, X.; Cureño-Díaz, M.A.; Olguín-Montiel, M.; Jiménez, A.; Gómez-Zamora, E.; Leyva-Lopez, A.G.; Orbe-Orihuela, Y.C.; Trujillo-Martínez, M.; Castrejón-Salgado, R.; Hernández-Mariano, J.Á. Association Between Age at Menarche and Gestational Diabetes: A Retrospective Case–Control Study. Women 2025, 5, 43. https://doi.org/10.3390/women5040043
Solis-Gómez X, Cureño-Díaz MA, Olguín-Montiel M, Jiménez A, Gómez-Zamora E, Leyva-Lopez AG, Orbe-Orihuela YC, Trujillo-Martínez M, Castrejón-Salgado R, Hernández-Mariano JÁ. Association Between Age at Menarche and Gestational Diabetes: A Retrospective Case–Control Study. Women. 2025; 5(4):43. https://doi.org/10.3390/women5040043
Chicago/Turabian StyleSolis-Gómez, Ximena, Mónica Alethia Cureño-Díaz, Maximiliano Olguín-Montiel, Adriana Jiménez, Erika Gómez-Zamora, Ahidée Guadalupe Leyva-Lopez, Yaneth Citlalli Orbe-Orihuela, Miguel Trujillo-Martínez, Ricardo Castrejón-Salgado, and José Ángel Hernández-Mariano. 2025. "Association Between Age at Menarche and Gestational Diabetes: A Retrospective Case–Control Study" Women 5, no. 4: 43. https://doi.org/10.3390/women5040043
APA StyleSolis-Gómez, X., Cureño-Díaz, M. A., Olguín-Montiel, M., Jiménez, A., Gómez-Zamora, E., Leyva-Lopez, A. G., Orbe-Orihuela, Y. C., Trujillo-Martínez, M., Castrejón-Salgado, R., & Hernández-Mariano, J. Á. (2025). Association Between Age at Menarche and Gestational Diabetes: A Retrospective Case–Control Study. Women, 5(4), 43. https://doi.org/10.3390/women5040043






