Oxytocin: From Biomarker to Therapy for Postmenopausal Osteoporosis
Abstract
1. Introduction
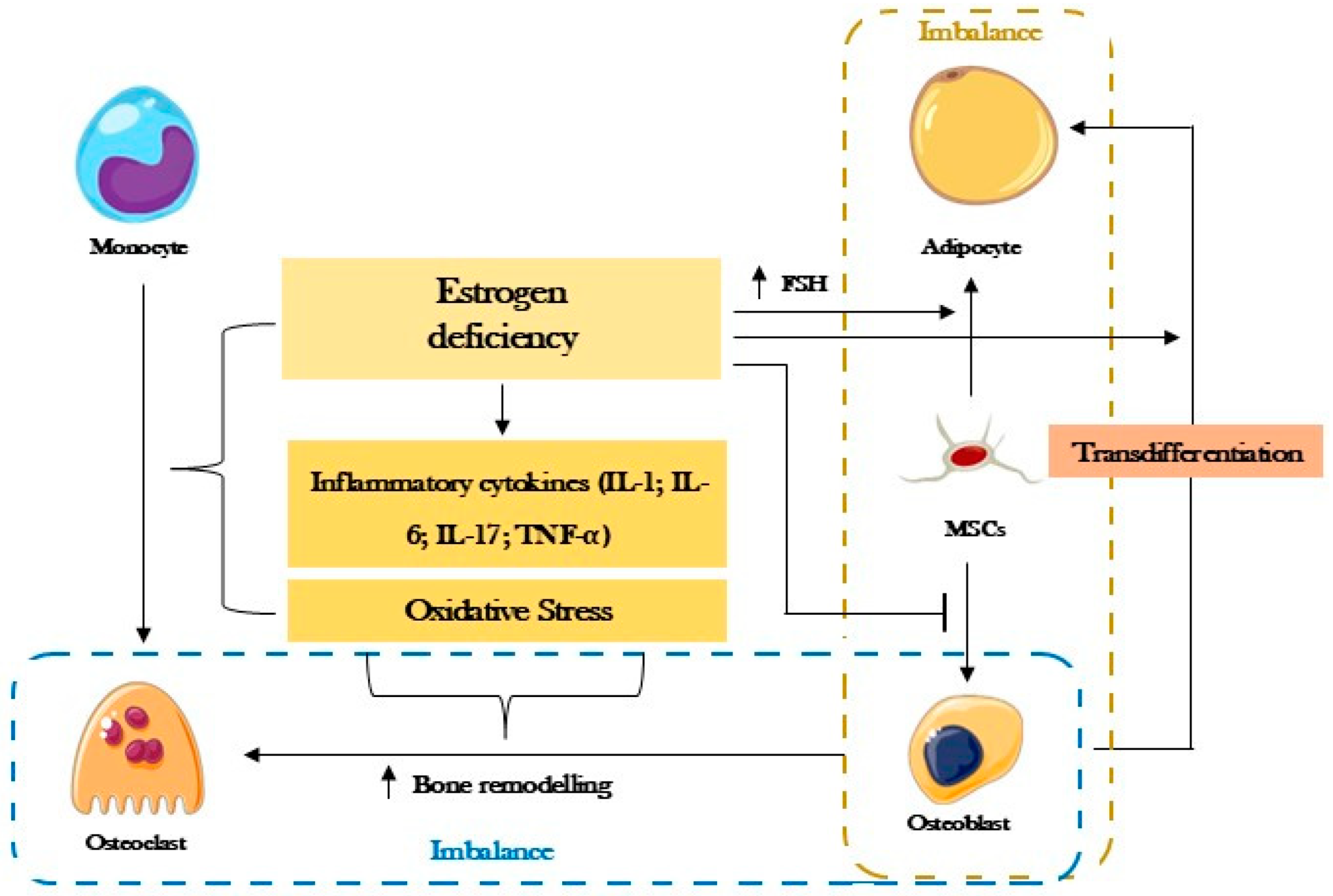
2. Postmenopausal Osteoporosis
3. Oxytocin
4. Oxytocin in Osteoporosis
4.1. Oxytocin as a Biomarker
4.2. Oxytocin as a Treatment
4.2.1. In Vitro Studies: Human and Animal Cells
4.2.2. In Vivo Studies
5. Discussion
5.1. Human Cross-Sectional Studies
5.2. Animal Model Studies
5.3. Therapeutic Approach
5.4. Final Considerations and Future Perspectives
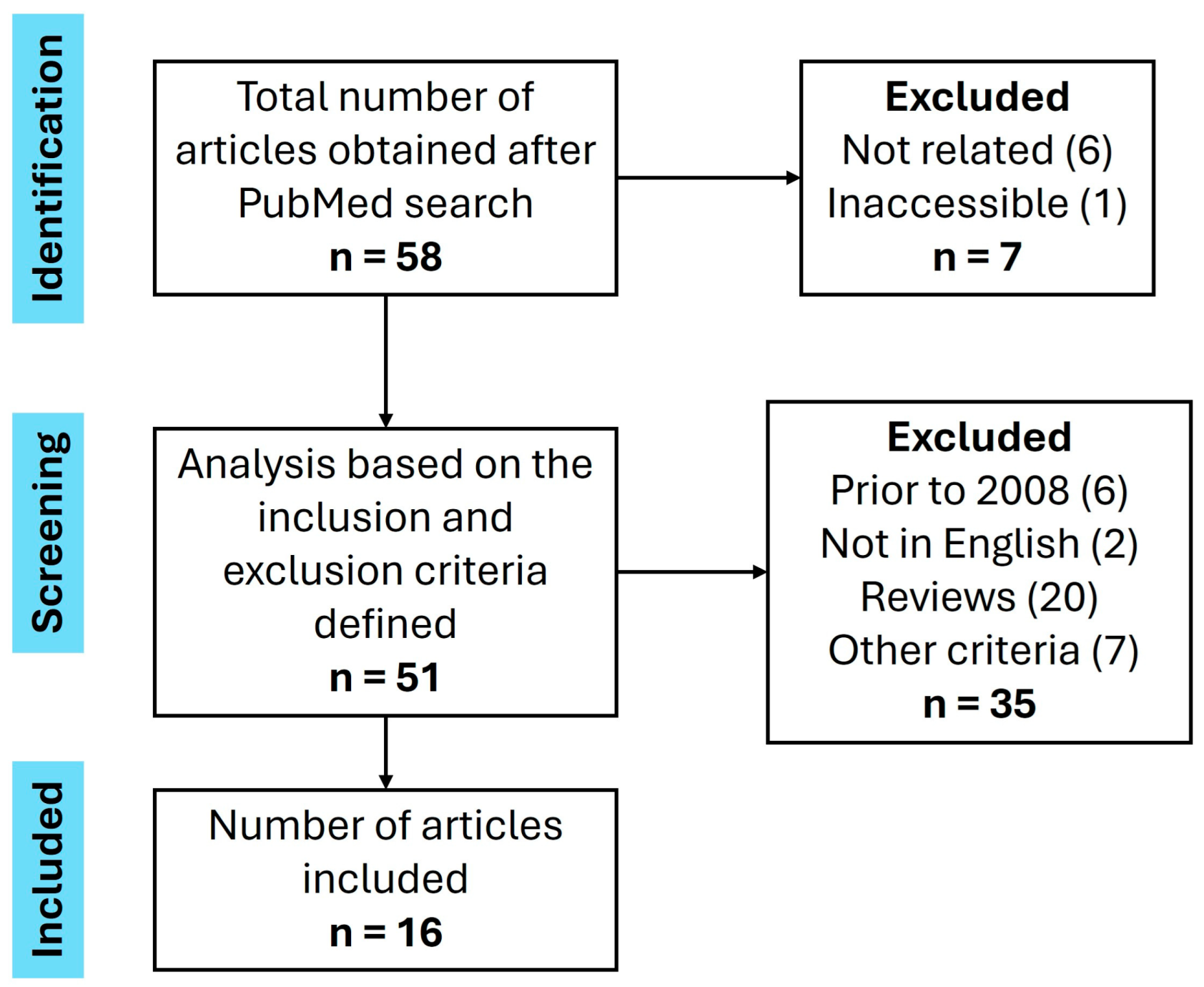
6. Methods
- -
- Articles published from 2008 inclusive to June 2024;
- -
- Articles written in English;
- -
- Original articles;
- -
- Articles with postmenopausal women;
- -
- Articles with an animal population in a life cycle corresponding to the human postmenopause period;
- -
- Articles in which Oxytocin is studied as an option for diagnosing postmenopausal Osteoporosis;
- -
- Articles in which Oxytocin is presented as an option for the treatment of postmenopausal Osteoporosis.
- -
- Articles published before 2008 and after June 2024;
- -
- Articles written in a language other than English;
- -
- Non-original articles;
- -
- Articles with an exclusively male population;
- -
- Articles with animal populations at a stage in their life cycle that is not equivalent to the human postmenopause period;
- -
- Articles in which Oxytocin is presented as useful for situations other than the diagnosis and/or treatment of postmenopausal Osteoporosis.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| BMD | Bone mineral density |
| BMI | Body mass index |
| BMMSCs | Bone marrow mesenchymal cells |
| BV/TV | Trabecular bone volume fraction to total volume ratio |
| CB | Carbetocin |
| FSH | Follicle-stimulating hormone |
| hBMSC | Human mesenchymal stromal cells |
| hMADS | Adipose tissue-derived multipotent cells |
| IL | Interleukin |
| MSCs | Mesenchymal cells |
| OP | Osteoporosis |
| OPG | Osteoprotegerin |
| OT | Oxytocin |
| P1NP | Procollagen Type I N-terminal Propeptide |
| RANK | Receptor activator of nuclear factor kappa-B |
| RANK-L | Receptor activator of nuclear factor kappa-B ligand |
| SMI | Structural model index |
| Tb.N | Trabeculae per unit length |
| Tb.Sp | Trabecular spacing |
| Tb.Th | Trabecular thickness |
| TNF-α | Tumor necrosis factor-α |
| TRAP | Tartrate-resistant acid phosphatase |
References
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Breuil, V.; Trojani, M.C.; Ez-Zoubir, A. Oxytocin and Bone: Review and Perspectives. Int. J. Mol. Sci. 2021, 22, 8551. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Shoback, D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1595–1622. [Google Scholar] [CrossRef]
- Gregson, C.L.; Armstrong, D.J.; Bowden, J.; Cooper, C.; Edwards, J.; Gittoes, N.J.L.; Harvey, N.; Kanis, J.; Leyland, S.; Low, R.; et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2022, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Feixiang, L.; Yanchen, F.; Xiang, L.; Yunke, Z.; Jinxin, M.; Jianru, W.; Zixuan, L. The mechanism of oxytocin and its receptors in regulating cells in bone metabolism. Front. Pharmacol. 2023, 14, 1171732. [Google Scholar] [CrossRef]
- Moghazy, H.; Abdeen Mahmoud, A.; Elbadre, H.; Abdel Aziz, H.O. Protective Effect of Oxytocin Against Bone Loss in a Female Rat Model of Osteoporosis. Rep. Biochem. Mol. Biol. 2020, 9, 147–155. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Lu, L.; Yu, X. The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis. Cytokine Growth Factor Rev. 2020, 52, 88–98. [Google Scholar] [CrossRef]
- Breuil, V.; Amri, E.Z.; Panaia-Ferrari, P.; Testa, J.; Elabd, C.; Albert-Sabonnadiere, C.; Roux, C.H.; Ailhaud, G.; Dani, C.; Carle, G.F.; et al. Oxytocin and bone remodelling: Relationships with neuropituitary hormones, bone status and body composition. Jt. Bone Spine 2011, 78, 611–615. [Google Scholar] [CrossRef]
- Breuil, V.; Panaia-Ferrari, P.; Fontas, E.; Roux, C.; Kolta, S.; Eastell, R.; Ben Yahia, H.; Faure, S.; Gossiel, F.; Benhamou, C.L.; et al. Oxytocin, a new determinant of bone mineral density in post-menopausal women: Analysis of the OPUS cohort. J. Clin. Endocrinol. Metab. 2014, 99, E634–E641. [Google Scholar] [CrossRef]
- Du, Y.; Xu, C.; Shi, H.; Jiang, X.; Tang, W.; Wu, X.; Chen, M.; Li, H.; Zhang, X.; Cheng, Q. Serum concentrations of oxytocin, DHEA and follistatin are associated with osteoporosis or sarcopenia in community-dwelling postmenopausal women. BMC Geriatr. 2021, 21, 542. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.J.; Shi, H.L.; Wu, X.Q.; Du, Y.P.; Li, H.L.; Tang, W.J.; Chen, M.M.; Zhang, X.M.; Shen, L.; Cheng, Q. Association between Serum Oxytocin, Bone Mineral Density and Body Composition in Chinese Adult Females. Medicina 2022, 58, 1625. [Google Scholar] [CrossRef] [PubMed]
- Elabd, C.; Basillais, A.; Beaupied, H.; Breuil, V.; Wagner, N.; Scheideler, M.; Zaragosi, L.E.; Massiera, F.; Lemichez, E.; Trajanoski, Z.; et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells 2008, 26, 2399–2407. [Google Scholar] [CrossRef]
- Fallahnezhad, S.; Piryaei, A.; Darbandi, H.; Amini, A.; Ghoreishi, S.K.; Jalalifirouzkouhi, R.; Bayat, M. Effect of low-level laser therapy and oxytocin on osteoporotic bone marrow-derived mesenchymal stem cells. J. Cell. Biochem. 2018, 119, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Fallahnezhad, S.; Jajarmi, V.; Shahnavaz, S.; Amini, A.; Ghoreishi, S.K.; Kazemi, M.; Chien, S.; Bayat, M. Improvement in viability and mineralization of osteoporotic bone marrow mesenchymal stem cell through combined application of photobiomodulation therapy and oxytocin. Lasers Med. Sci. 2020, 35, 557–566. [Google Scholar] [CrossRef]
- Tamma, R.; Colaianni, G.; Zhu, L.L.; DiBenedetto, A.; Greco, G.; Montemurro, G.; Patano, N.; Strippoli, M.; Vergari, R.; Mancini, L.; et al. Oxytocin is an anabolic bone hormone. Proc. Natl. Acad. Sci. USA 2009, 106, 7149–7154. [Google Scholar] [CrossRef]
- Beranger, G.E.; Pisani, D.F.; Castel, J.; Djedaini, M.; Battaglia, S.; Amiaud, J.; Boukhechba, F.; Ailhaud, G.; Michiels, J.F.; Heymann, D.; et al. Oxytocin reverses ovariectomy-induced osteopenia and body fat gain. Endocrinology 2014, 155, 1340–1352. [Google Scholar] [CrossRef]
- Qiu, Y.; Yao, J.; Wu, X.; Zhou, B.; Shao, H.; Hua, T.; Xiong, Z.; Tang, G. Longitudinal assessment of oxytocin efficacy on bone and bone marrow fat masses in a rabbit osteoporosis model through 3.0-T magnetic resonance spectroscopy and micro-CT. Osteoporos. Int. 2015, 26, 1081–1092. [Google Scholar] [CrossRef]
- Qiu, Y.; Tang, C.; Serrano-Sosa, M.; Hu, J.; Zhu, J.; Tang, G.; Huang, C.; Huang, M. Bone microarchitectural parameters can detect oxytocin induced changes prior to bone density on mitigating bone deterioration in rabbit osteoporosis model using micro-CT. BMC Musculoskelet. Disord. 2019, 20, 560. [Google Scholar] [CrossRef]
- Wang, M.; Lan, L.; Li, T.; Li, J.; Li, Y. The effect of oxytocin on osseointegration of titanium implant in ovariectomized rats. Connect. Tissue Res. 2016, 57, 220–225. [Google Scholar] [CrossRef]
- Fernandes, F.; Stringhetta-Garcia, C.T.; Peres-Ueno, M.J.; Fernandes, F.; Nicola, A.C.; Castoldi, R.C.; Ozaki, G.; Louzada, M.J.Q.; Chaves-Neto, A.H.; Ervolino, E.; et al. Oxytocin and bone quality in the femoral neck of rats in periestropause. Sci. Rep. 2020, 10, 7937. [Google Scholar] [CrossRef]
- Santos, L.F.G.; Fernandes-Breitenbach, F.; Silva, R.A.S.; Santos, D.R.; Peres-Ueno, M.J.; Ervolino, E.; Chaves-Neto, A.H.; Dornelles, R.C.M. The action of oxytocin on the bone of senescent female rats. Life Sci. 2022, 297, 120484. [Google Scholar] [CrossRef]
- Fernandes-Breitenbach, F.; Peres-Ueno, M.J.; Santos, L.F.G.; Brito, V.G.B.; Castoldi, R.C.; Louzada, M.J.Q.; Chaves-Neto, A.H.; Oliveira, S.H.P.; Dornelles, R.C.M. Analysis of the femoral neck from rats in the periestropause treated with oxytocin and submitted to strength training. Bone 2022, 162, 116452. [Google Scholar] [CrossRef]
- Elabd, S.; Sabry, I. Two Birds with One Stone: Possible Dual-Role of Oxytocin in the Treatment of Diabetes and Osteoporosis. Front. Endocrinol. 2015, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Stampfli, D.; Dommrich, R.; Orbach-Zinger, S.; Burden, A.M.; Heesen, M. An explorative analysis of pharmacovigilance data of oxytocin and its analogue carbetocin, with a focus on haemodynamic adverse effects. Int. J. Clin. Pharm. 2023, 45, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Passoni, I.; Leonzino, M.; Gigliucci, V.; Chini, B.; Busnelli, M. Carbetocin is a Functional Selective Gq Agonist That Does Not Promote Oxytocin Receptor Recycling After Inducing beta-Arrestin-Independent Internalisation. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef]
- Hodgins, S. New Evidence on Carbetocin: Another Arrow in Our Quiver. Glob. Health Sci. Pract. 2018, 6, 405–407. [Google Scholar] [CrossRef]
- Beranger, G.E.; Djedaini, M.; Battaglia, S.; Roux, C.H.; Scheideler, M.; Heymann, D.; Amri, E.Z.; Pisani, D.F. Oxytocin reverses osteoporosis in a sex-dependent manner. Front. Endocrinol. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed]
| Year Published | Author | Country | Study Design | Population | Oxytocin Values | Main Conclusions | |
|---|---|---|---|---|---|---|---|
| Women Without OP | Women with OP | ||||||
| 2011 [9] | Breuil et al. | France | Cross-sectional | Postmenopausal women | 110 pg/mL | 50 pg/mL | Women with OP have lower serum OT than healthy women. |
| 2014 [10] | Breuil et al. | France; Germany; England | Cross-sectional | Postmenopausal women | - | - | Low OT levels are associated with a decline in bone mass and fractures. |
| 2021 [11] | Du et al. | China | Cross-sectional | Postmenopausal women | 612 pg/mL | 425 pg/mL | Women with OP have lower OT values. History of fractures associated with lower OT levels. |
| 2022 [12] | Yu et al. | China | Cross-sectional | Postmenopausal women | 777 pg/mL | 364 pg/mL | OT protects against loss of bone mass in women. |
| 2008 [13] | Elabd et al. | France | Pre-clinical | Postmenopausal women | 110.6 ± 19.8 pg/mL | 50.2 ± 8.8 pg/mL | OT is inversely correlated with the development of OP |
| Year Published | Authors | Country | Species/Cells | Dose of OT Administered | Main Conclusions |
|---|---|---|---|---|---|
| 2008 [13] | Elabd et al. | France | hMADS hBMSC C57Bl/6J mice | 30 nM 1 mg/kg | OT and CB stimulate osteoblastic differentiation. OT makes the bone less prone to deformation and fracture and improves strength at the cortical level. |
| 2017 [14] | Fallahnezhad et al. | Iran | Rat BMMSCs | - | OT alone has limited effects on bone recovery after severe bone loss. |
| 2019 [15] | Fallahnezhad et al. | Iran | Rat BMMSCs | - | OT induces a mineralizing phenotype and increases the concentration of osteocalcin and OPG. |
| 2009 [16] | Tamma et al. | Italy USA | C57BL/6 and 129 SvEv mice mutant or wild-type for OT or Oxtr | - | OT deprivation induces OP. OT induces a pattern of osteoblastic mineralization. |
| 2014 [17] | Beranger et al. | France | C57Bl/6J mice | 0.1 mg/kg and 1 mg/kg | OT can normalize femoral trabecular parameters. |
| 2014 [18] | Qiu et al. | China | New Zealand white rabbits | 1 mg/kg | OT prevents a decrease in BMD and maintains the quality of trabecular bone. OT reduces medullary adiposity. |
| 2019 [19] | Qiu et al. | China | New Zealand white rabbits | 1 mg/kg | OT slows down bone deterioration and restores the quality of bone microarchitecture. BV/TV and Tb.Sp are sensitive to follow-up therapy with OT. |
| 2016 [20] | Wang et al. | China | Sprague Dawley rats | 1 mg/kg | OT improves the osseointegration of femoral implants. OT maintains the structural quality of trabecular bone. |
| 2020 [7] | Moghazy et al. | Egypt | Sprague-Dawley rats | 0.1 mg/kg | Bone loss related to gonadal decline is dependent on serum OT levels, with OT having the capacity to recover the structural deficit. |
| 2020 [21] | Fernandes et al. | Brazil | Rattus norvegicus albinus | 134 μg/kg | OT favors bone tissue formation. The likelihood of fracture is lower due to the improvement in trabecular and cortical bone. |
| 2022 [22] | Santos et al. | Brazil | Wistar rats | 134 μg/kg | OT improves the histomorphometry of the femoral neck. The group exposed to AT showed high bone deterioration. |
| 2022 [23] | Fernandes-Breitenbach et al. | Brazil | Rattus norvegicus albinus | 134 μg/kg | OT and strength training are synergistic for gains in bone properties. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franca, T.; Ferreira, J.F.; Mariana, M.; Cairrao, E. Oxytocin: From Biomarker to Therapy for Postmenopausal Osteoporosis. Women 2025, 5, 27. https://doi.org/10.3390/women5030027
Franca T, Ferreira JF, Mariana M, Cairrao E. Oxytocin: From Biomarker to Therapy for Postmenopausal Osteoporosis. Women. 2025; 5(3):27. https://doi.org/10.3390/women5030027
Chicago/Turabian StyleFranca, Tiago, Joana Fonseca Ferreira, Melissa Mariana, and Elisa Cairrao. 2025. "Oxytocin: From Biomarker to Therapy for Postmenopausal Osteoporosis" Women 5, no. 3: 27. https://doi.org/10.3390/women5030027
APA StyleFranca, T., Ferreira, J. F., Mariana, M., & Cairrao, E. (2025). Oxytocin: From Biomarker to Therapy for Postmenopausal Osteoporosis. Women, 5(3), 27. https://doi.org/10.3390/women5030027







