Improved Myocardial Function in Autoimmune-Mediated Fetal Complete Atrioventricular Block Following Dexamethasone and Intravenous Immunoglobulin: A Case Report
Abstract
1. Introduction
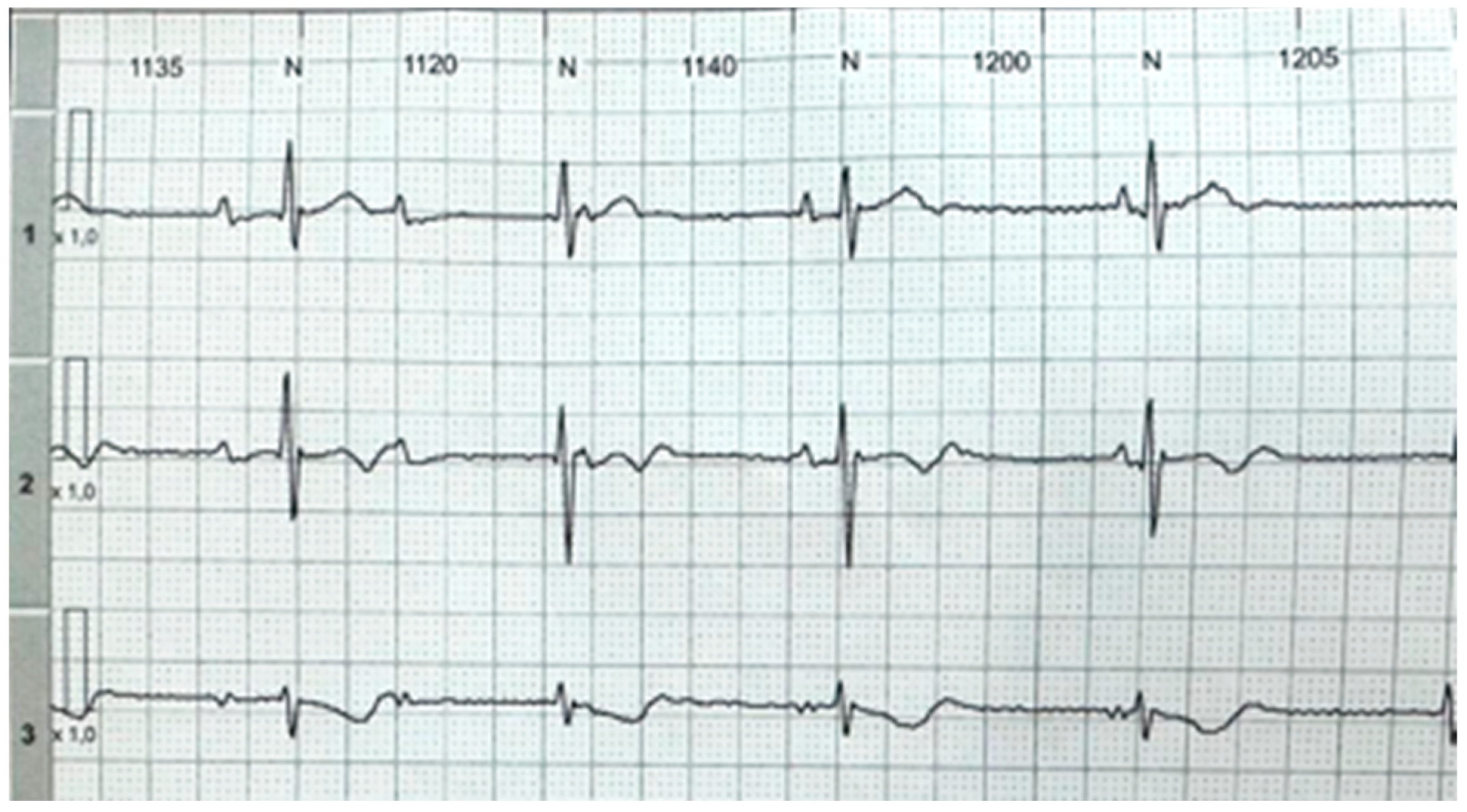
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bordachar, P.; Zachary, W.; Ploux, S.; Labrousse, L.; Haissaguerre, M.; Thambo, J.B. Pathophysiology, clinical course, and management of congenital complete atrioventricular block. Heart Rhythm. 2013, 10, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Thorlacius, G.E.; Sonesson, S.E.; Wahren-Herlenius, M. Interferons and innate immune activation in autoimmune congenital heart block. Scand. J. Immunol. 2021, 93, e12995. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, B.; Bhan, R.; Trad, C.; Cohen, R.; Saxena, A.; Buyon, J.; Izmirly, P. Autoimmune-mediated congenital heart block. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 64, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Melita, H.; Manolis, A.S. Congenital heart block: Pace earlier (Childhood) than later (Adulthood). Trends Cardiovasc. Med. 2020, 30, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.Y.; Hua, Y.M. Autoimmune-associated Congenital Heart Block: A New Insight in Fetal Life. Chin. Med. J. 2017, 130, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Wahren-Herlenius, M.; Sonesson, S.E. Specificity and effector mechanisms of autoantibodies in congenital heart block. Curr. Opin. Immunol. 2006, 18, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, H.; Sonesson, S.E.; Sharland, G.; Granath, F.; Simpson, J.M.; Carvalho, J.S.; Jicinska, H.; Tomek, V.; Dangel, J.; Zielinsky, P.; et al. Isolated atrioventricular block in the fetus: A retrospective, multinational, multicenter study of 175 patients. Circulation 2011, 124, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Kaplinski, M.; Cuneo, B.F. Novel approaches to the surveillance and management of fetuses at risk for anti-Ro/SSA mediated atrioventricular block. Semin. Perinatol. 2022, 46, 151585. [Google Scholar] [CrossRef] [PubMed]
- Brucato, A. Prevention of congenital heart block in children of SSA-positive mothers. Rheumatology 2008, 47 (Suppl. S3), iii35–iii37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, Y.; Wang, C.; Li, Y.; Zhu, Q.; Hua, Y.; Qiao, L.; Wu, J.; Zhou, K. Successful Prevention of Fetal Autoimmune-Mediated Heart Block by Combined Therapies with Hydroxychloroquine and Intravenous Immunoglobulin: A Case Report. Front. Cardiovasc. Med. 2021, 8, 759260. [Google Scholar] [CrossRef] [PubMed]
- Batra, A.S.; Silka, M.J.; Borquez, A.; Cuneo, B.; Dechert, B.; Jaeggi, E.; Kannankeril, P.J.; Tabulov, C.; Tisdale, J.E.; Wolfe, D.; et al. Pharmacological Management of Cardiac Arrhythmias in the Fetal and Neonatal Periods: A Scientific Statement from the American Heart Association: Endorsed by the Pediatric & Congenital Electrophysiology Society (PACES). Circulation 2024, 149, e996. [Google Scholar]
- Ruffatti, A.; Marson, P.; Svaluto-Moreolo, G.; Marozio, L.; Tibaldi, M.; Favaro, M.; Calligaro, A.; Grava, C.; Hoxha, A.; Pengo, V.; et al. A combination therapy protocol of plasmapheresis, intravenous immunoglobulins and betamethasone to treat anti-Ro/La-related congenital atrioventricular block. A case series and review of the literature. Autoimmun. Rev. 2013, 12, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Mawad, W.; Hornberger, L.; Cuneo, B.; Raboisson, M.; Moon-Grady, A.J.; Lougheed, J.; Diab, K.; Parkman, J.; Silverman, E.; Jaeggi, E. Outcome of Antibody-Mediated Fetal Heart Disease with Standardized Anti-Inflammatory Transplacental Treatment. J. Am. Heart Assoc. 2022, 11, e023000. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, E.T.; Fouron, J.C.; Silverman, E.D.; Ryan, G.; Smallhorn, J.; Hornberger, L.K. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation 2004, 110, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Skoutelis, N.; Zhou, Y.; Kawanami, R.; Charras, A.; Occhigrossi, F.; Agarwal, U.; Khan, S.; Donegan, S.; Hawcutt, D. Treatment options for preventing autoimmune-mediated congenital heart block: A systematic review. Arch Dis Child. 2025. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.E.M.; de Miranda, N.R.D.C.R.; Giuberti, M.; Araujo Júnior, E. Fetal total atrioventricular block in transgender man with systemic lupus erythematosus—Literature review and establishment of a protocol with management and treatment with terbutaline. Ceska Gynekol. 2025, 90, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef] [PubMed]
- Veduta, A.; Panaitescu, A.M.; Ciobanu, A.M.; Neculcea, D.; Popescu, M.R.; Peltecu, G.; Cavoretto, P. Treatment of Fetal Arrhythmias. J. Clin. Med. 2021, 10, 2510. [Google Scholar] [CrossRef] [PubMed]
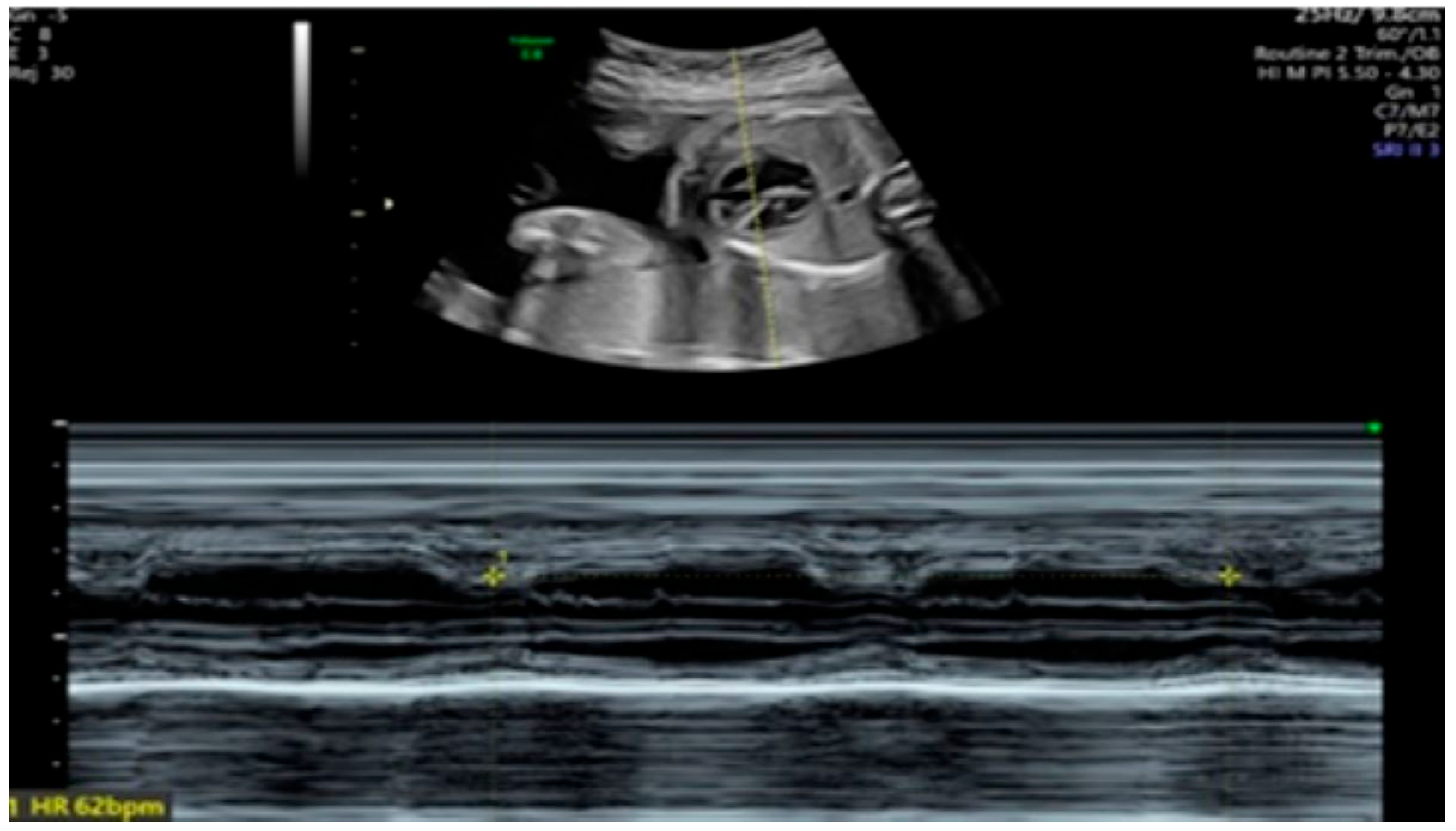
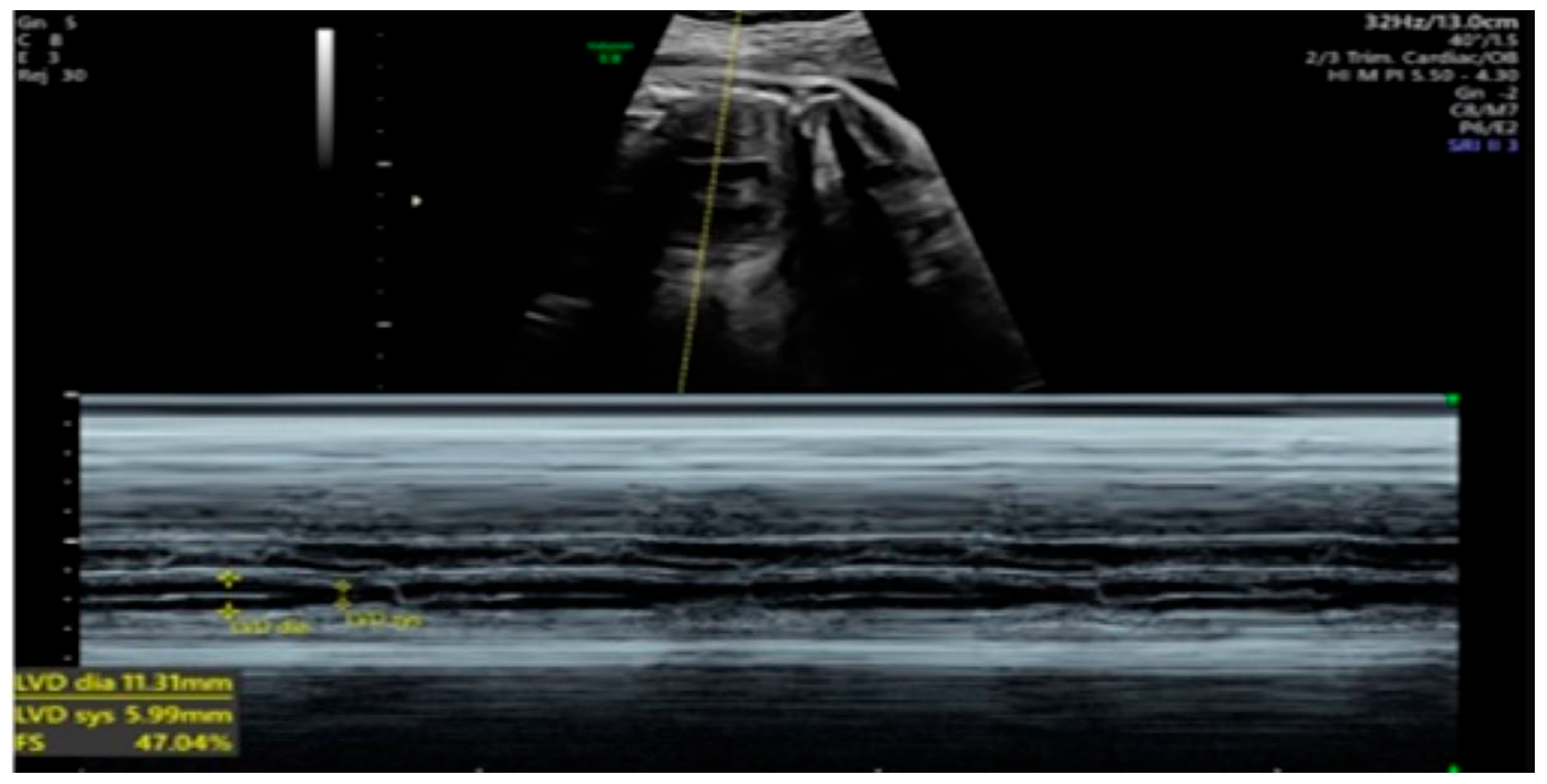

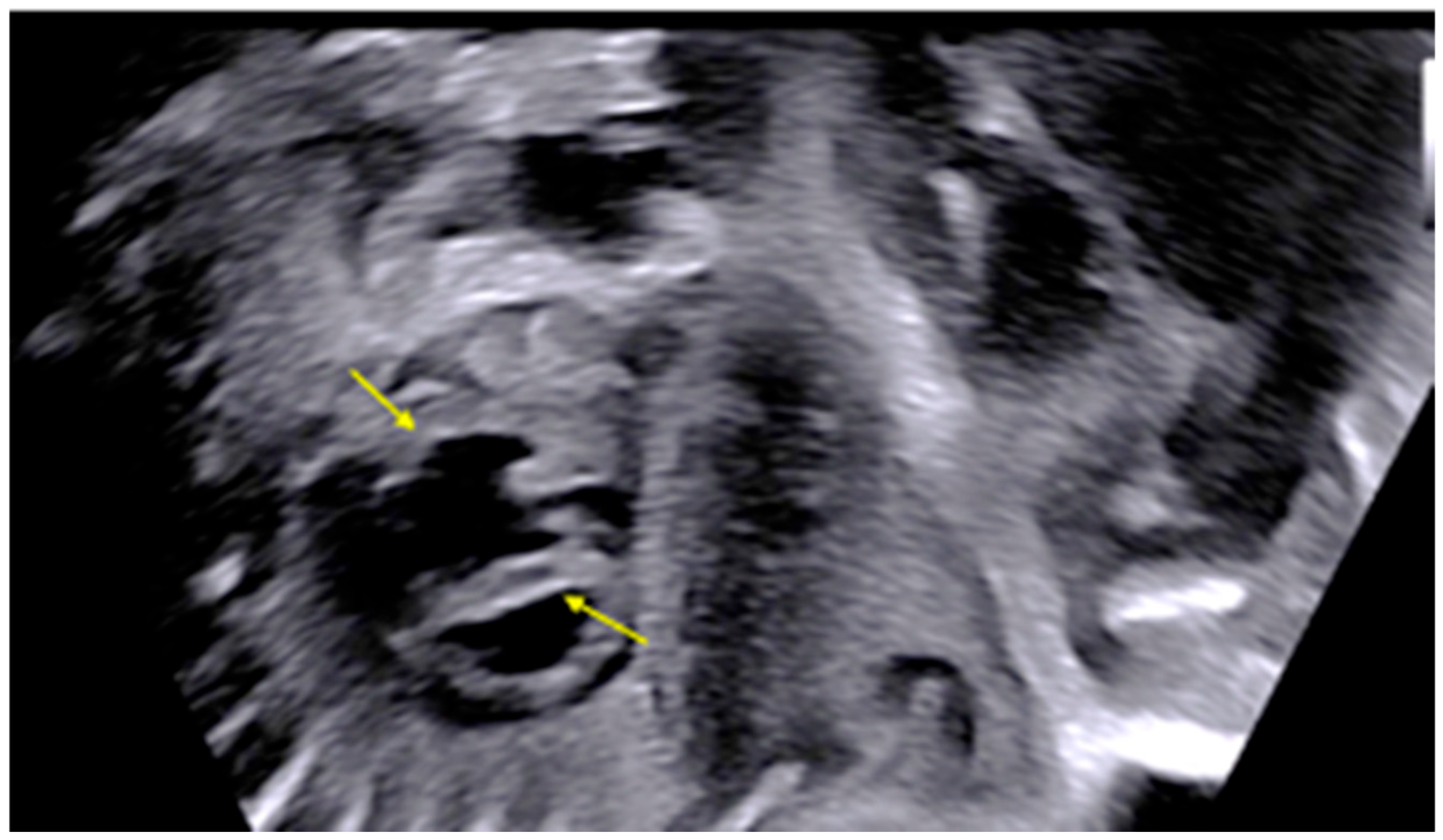
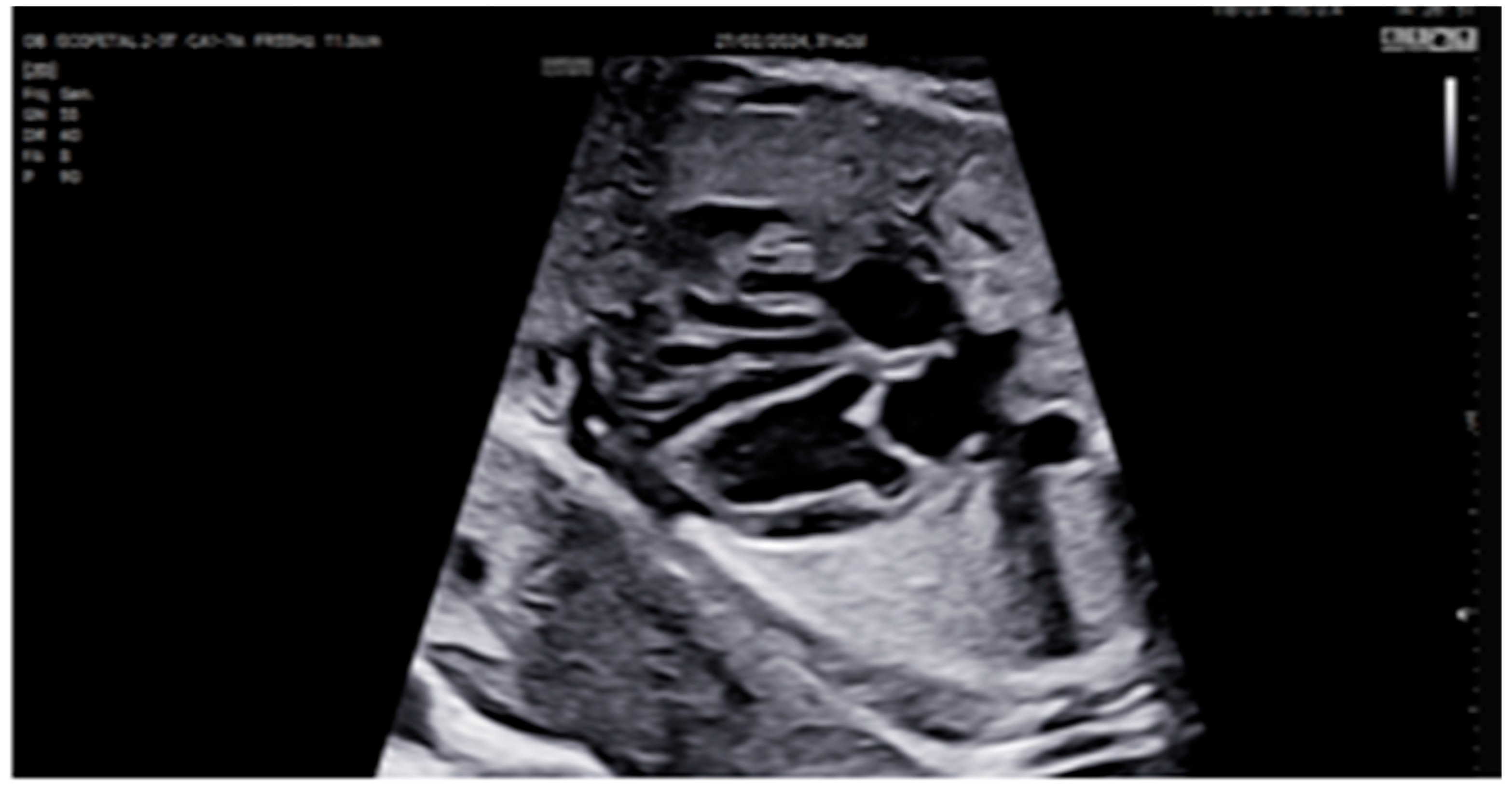
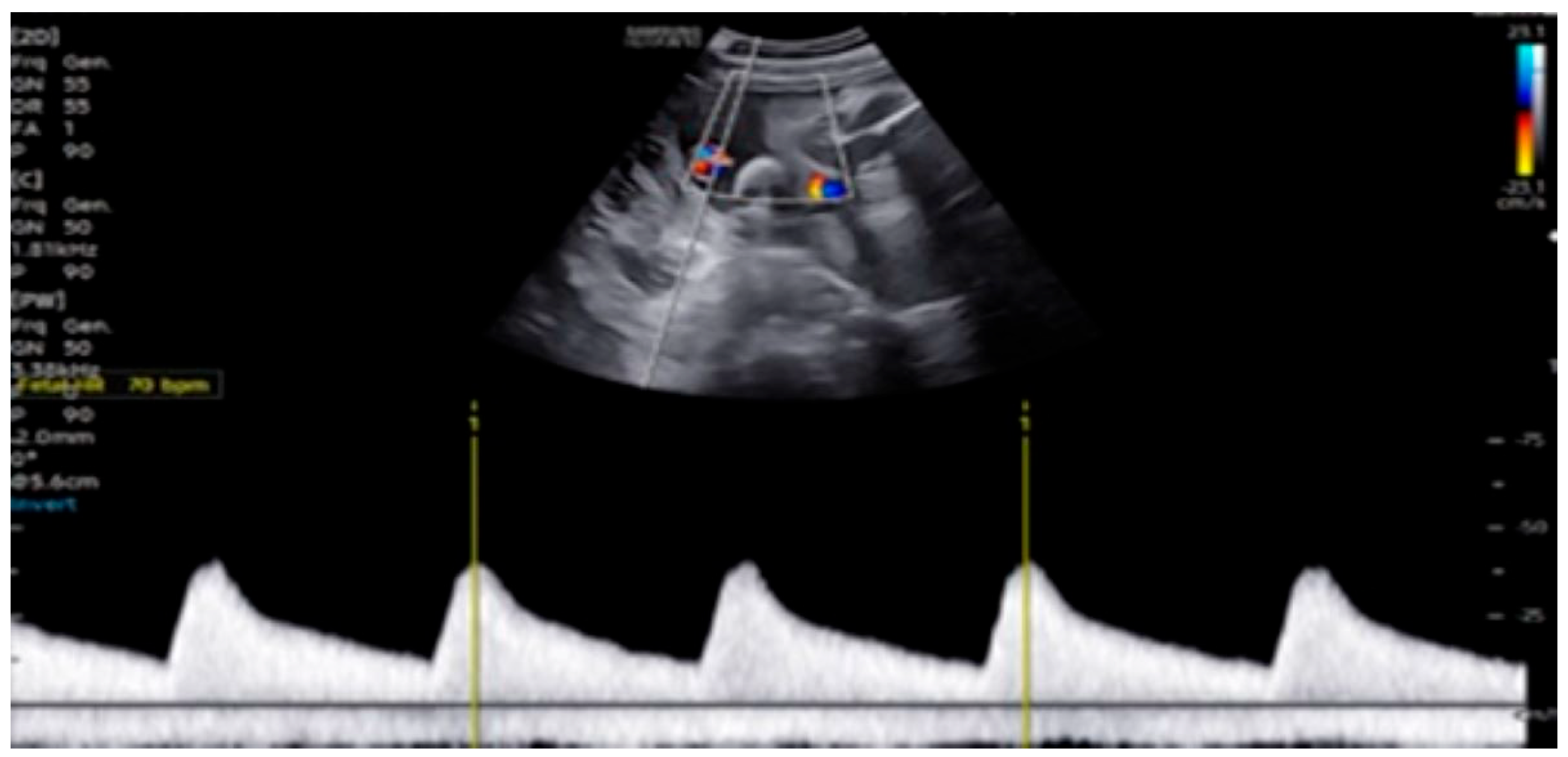


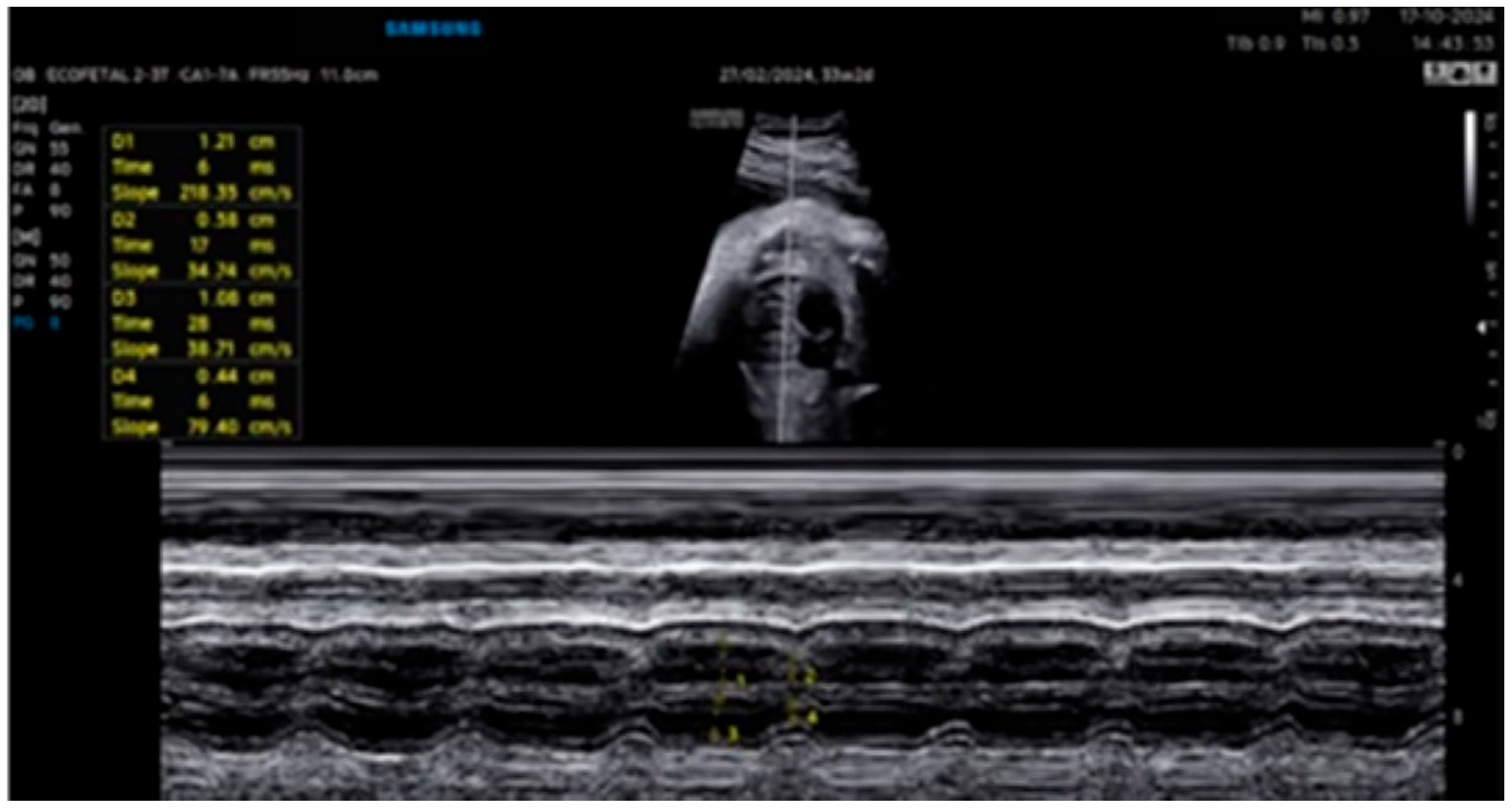
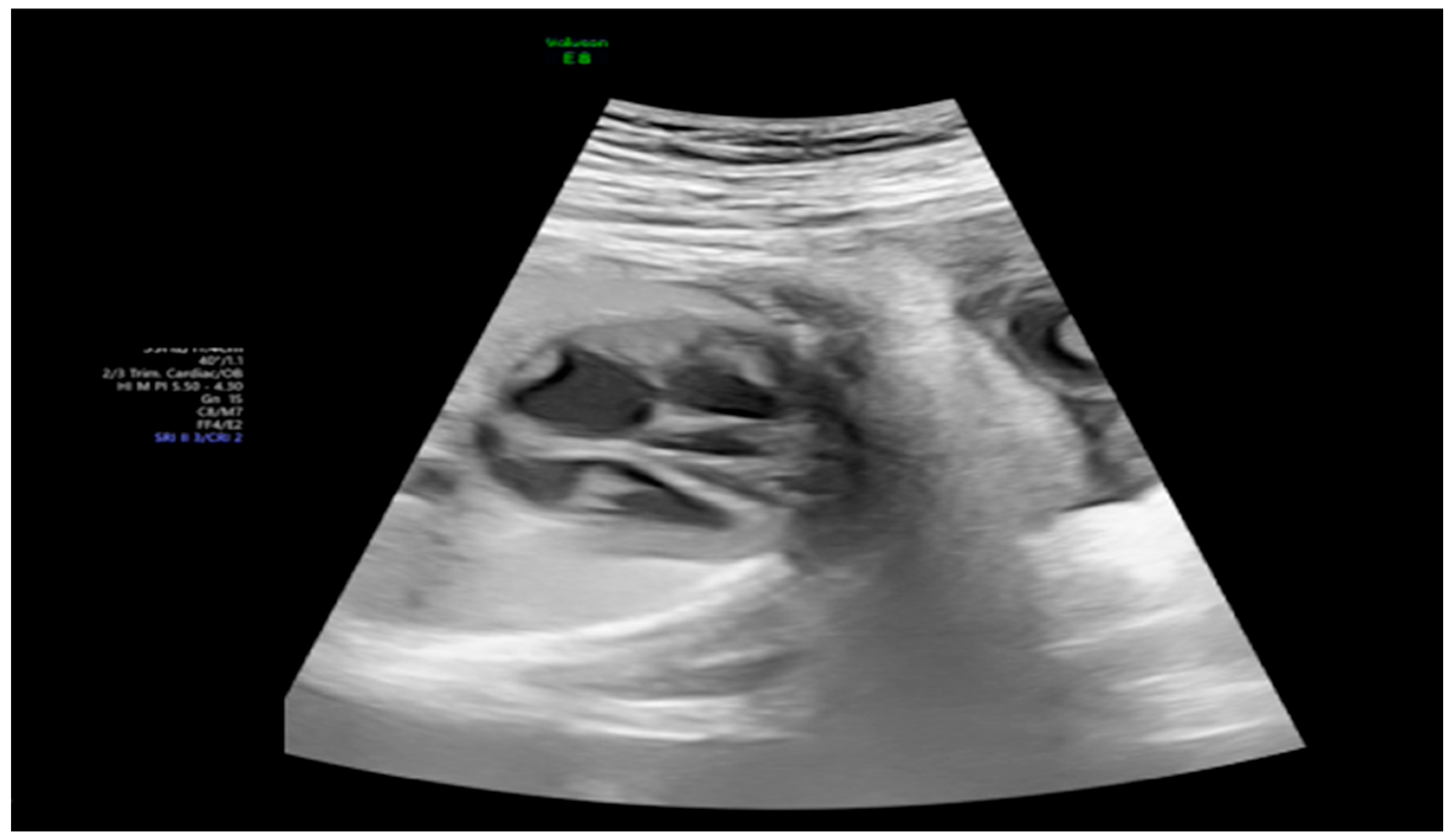


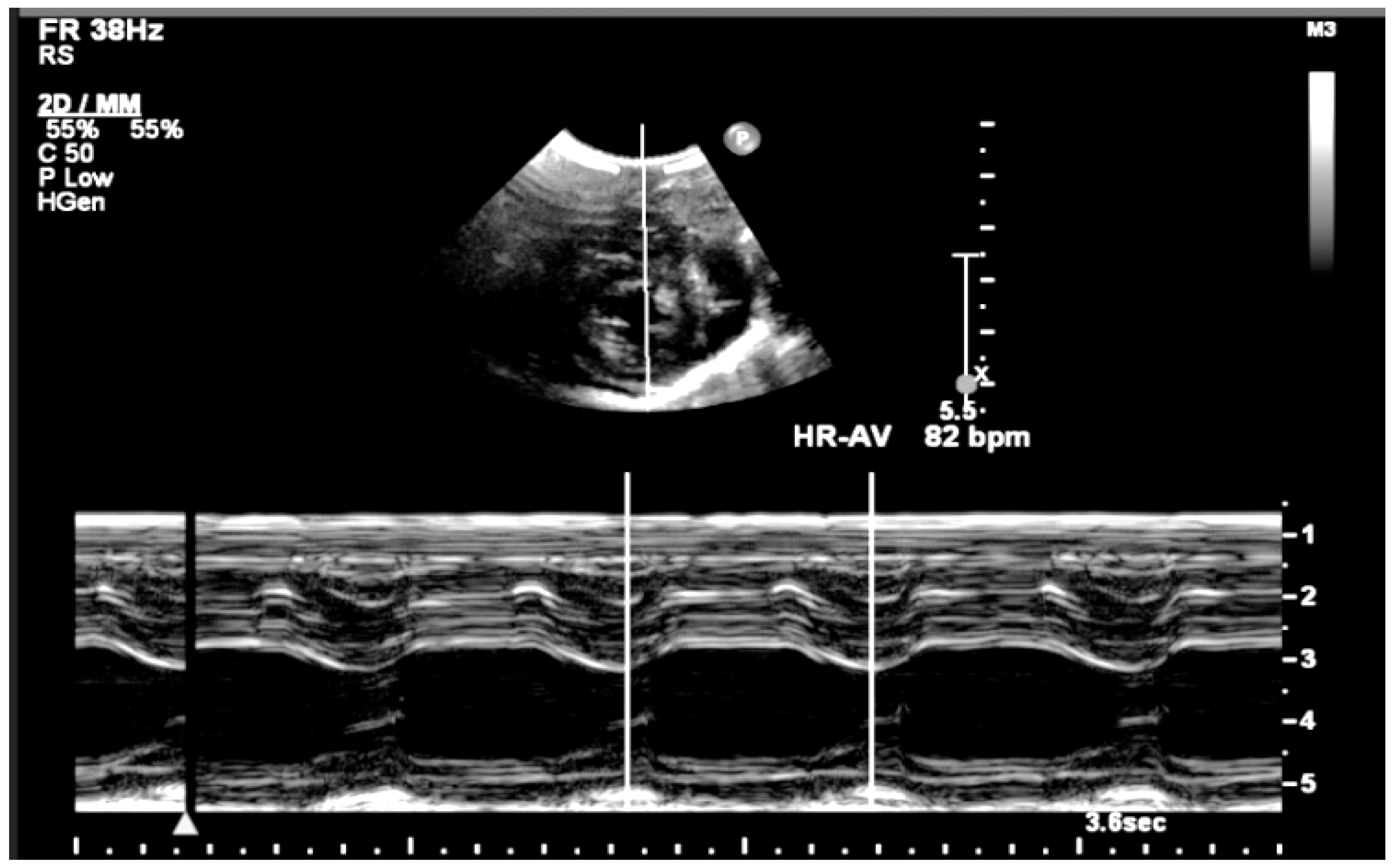
| GA (Weeks + Days) | Intervention | VHR (bpm) | Cardiac Function Response | Notes |
|---|---|---|---|---|
| 26 + 0 | Start of dexamethasone 4 mg/day | 60 | SF increased from 34% to 47%, but qualitative contractility decreased | Mild edema; no beta- agonists used |
| 28 + 0 | First IVIG cycle (65 g total) | 75 | SF increased to 52%; mild improvement in contractility | Initiated due to signs of fibroelastosis |
| 31 + 2 | Post-IVIG peak response | 70 | SF: 59% (LV), 52% (RV); marked biventricular improvement | Aortic isthmus 3 mm (Z-score –1.65) |
| 33 + 0 | Second IVIG cycle | 68 | Stable function maintained | Repeated full protocol; no complications |
| 36 + 3 | Delivery | 84 | – | Stable neonate; no pacemaker needed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albrecht, M.E.M.; Bathomarco, M.G.; Callado, G.Y.; Bravo-Valenzuela, N.J.; Araujo Júnior, E. Improved Myocardial Function in Autoimmune-Mediated Fetal Complete Atrioventricular Block Following Dexamethasone and Intravenous Immunoglobulin: A Case Report. Women 2025, 5, 20. https://doi.org/10.3390/women5020020
Albrecht MEM, Bathomarco MG, Callado GY, Bravo-Valenzuela NJ, Araujo Júnior E. Improved Myocardial Function in Autoimmune-Mediated Fetal Complete Atrioventricular Block Following Dexamethasone and Intravenous Immunoglobulin: A Case Report. Women. 2025; 5(2):20. https://doi.org/10.3390/women5020020
Chicago/Turabian StyleAlbrecht, Maria Elisa Martini, Milena Giuberti Bathomarco, Gustavo Yano Callado, Nathalie Jeanne Bravo-Valenzuela, and Edward Araujo Júnior. 2025. "Improved Myocardial Function in Autoimmune-Mediated Fetal Complete Atrioventricular Block Following Dexamethasone and Intravenous Immunoglobulin: A Case Report" Women 5, no. 2: 20. https://doi.org/10.3390/women5020020
APA StyleAlbrecht, M. E. M., Bathomarco, M. G., Callado, G. Y., Bravo-Valenzuela, N. J., & Araujo Júnior, E. (2025). Improved Myocardial Function in Autoimmune-Mediated Fetal Complete Atrioventricular Block Following Dexamethasone and Intravenous Immunoglobulin: A Case Report. Women, 5(2), 20. https://doi.org/10.3390/women5020020








