The Impact of COVID-19 Pandemic Waves on Maternal Health and Infant Outcomes—A Retrospective Cohort Study
Abstract
1. Introduction
2. Results
2.1. General Demographics and Patient Characteristics
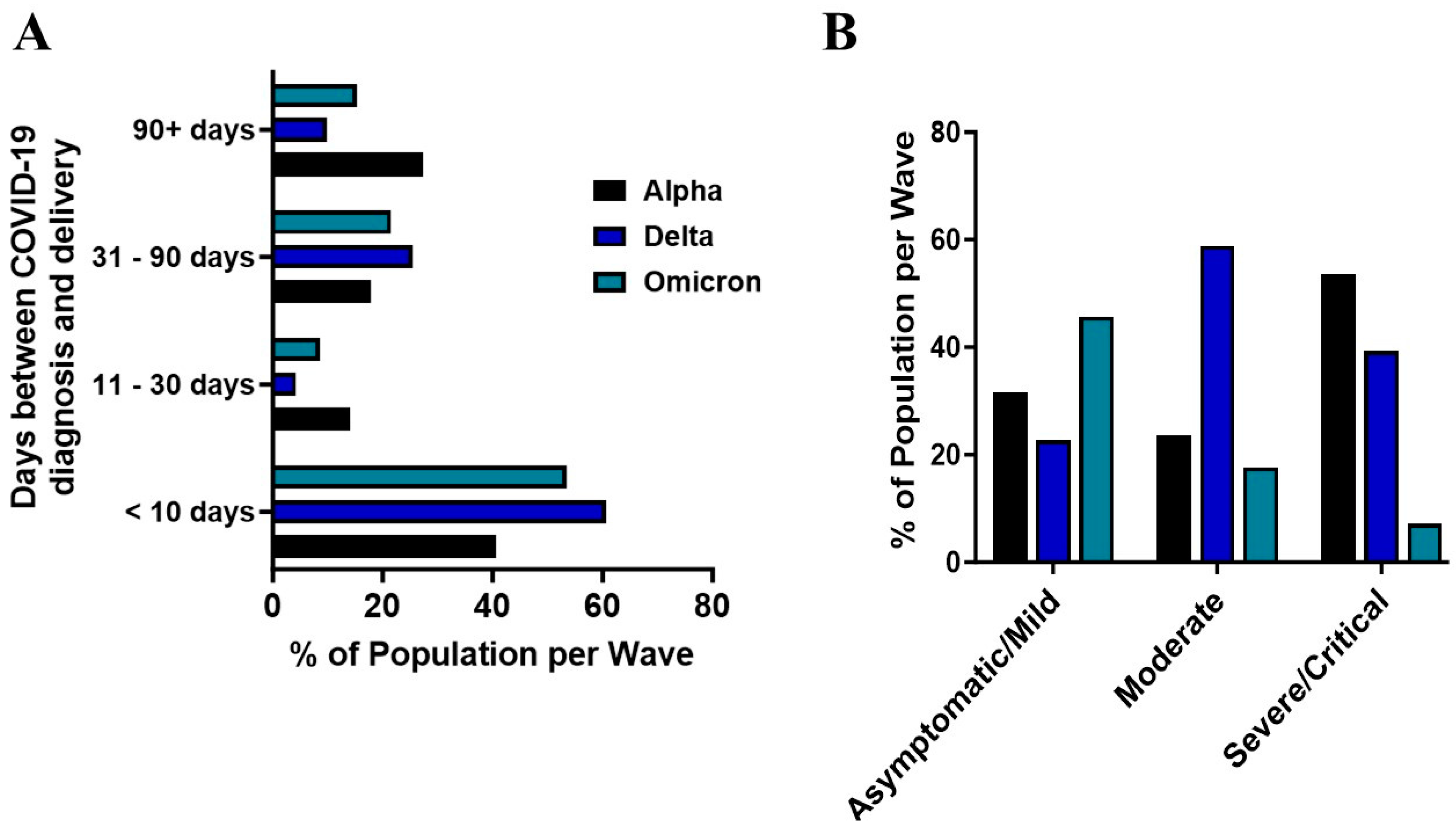
2.2. COVID-19 Pandemic Waves and Maternal Outcomes
2.3. Infant Outcomes
3. Discussion
4. Materials and Methods
4.1. Inclusion Criteria and Data Extraction
4.2. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ala, A.; Wilder, J.; Jonassaint, N.L.; Coffin, C.S.; Brady, C.; Reynolds, A.; Schilsky, M.L. COVID-19 and the Uncovering of Health Care Disparities in the United States, United Kingdom and Canada: Call to Action. Hepatol. Commun. 2021, 5, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Bourdin, S.; Levratto, N. Regional implications of COVID-19. Int. Reg. Sci. Rev. 2023, 46, 515–522. [Google Scholar] [CrossRef]
- Ward, I.L.; Bermingham, C.; Ayoubkhani, D.; Gethings, O.J.; Pouwels, K.B.; Yates, T.; Khunti, K.; Hippisley-Cox, J.; Banerjee, A.; Walker, A.S.; et al. Risk of COVID-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): Retrospective cohort study. BMJ 2022, 378, e070695. [Google Scholar] [CrossRef] [PubMed]
- Bager, P.; Wohlfahrt, J.; Bhatt, S.; Stegger, M.; Legarth, R.; Møller, C.H.; Skov, R.L.; Valentiner-Branth, P.; Voldstedlund, M.; Fischer, T.K.; et al. Risk of hospitalisation associated with infection with SARS-CoV-2 omicron variant versus delta variant in Denmark: An observational cohort study. Lancet Infect. Dis. 2022, 22, 967–976. [Google Scholar] [CrossRef]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Dashraath, P.; Wong, J.L.J.; Lim, M.X.K.; Lim, L.M.; Li, S.; Biswas, A.; Choolani, M.; Mattar, C.; Su, L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 521–531. [Google Scholar] [CrossRef]
- Wong, S.; Chow, K.; de Swiet, M. Severe acute respiratory syndrome and pregnancy. BJOG 2003, 110, 641–642. [Google Scholar] [CrossRef]
- Assiri, A.; Abedi, G.; Al Masri, M.; Saeed, A.; Gerber, S.; Watson, J. Middle East respiratory syndrome coronavirus infection during pregnancy: A Report of 5 cases from Saudia Arabia. Clin. Infect. Dis. 2016, 63, 951–953. [Google Scholar] [CrossRef]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef]
- Allotey, J.; Chatterjee, S.; Kew, T.; Gaetano, A.; Stallings, E.; Fernández-García, S.; Yap, M.; Sheikh, J.; Lawson, H.; Coomar, D.; et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: Living systematic review and meta-analysis. BMJ 2022, 376, e067696. [Google Scholar] [CrossRef]
- Ouma, J.; Hookham, L.; Akera, L.A.; Rukundo, G.; Kyohere, M.; Kakande, A.; Nakyesige, R.; Musoke, P.; Le Doare, K. Using electronic medical records to understand the impact of SARS-CoV-2 lockdown measures on maternal and neonatal outcomes in Kampala, Uganda. PLoS Glob. Public Health 2023, 3, 30002022. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Moustafa, A.; Kassahun-Yimer, W.; Novotny, S.; Billsby, B.; Abbas, A.; Wallace, K. COVID-19 not hypertension or diabetes increases the risk of preeclampsia among a high-risk population. Int. J. Environ. Res. Public Health 2022, 19, 16631. [Google Scholar] [CrossRef] [PubMed]
- de Montalvao Franca, A.P.F.; Paixo, J.T.R.; de Souza Fonseca, R.R.; Laurentino, R.V.; de Montalvao Leite, L.G.F.; Veras, A.S.F.; da Silva Feitosa Ribeiro, F.J.; das Neves, P.F.M.; Falcao, L.F.M.; de Montalvao Serrao, A.C.F.; et al. Clinical characteristics of pregnant women with COVID-19 and infection outcomes in one of the largest cities in the Brazilian Amazon. BMC Infect. Dis. 2024, 24, 1175. [Google Scholar] [CrossRef] [PubMed]
- Ilter, P.; Prasad, S.; Mutlu, M.; Tekin, A.; O’Brien, P.; Von Dadelszen, P.; Magee, L.; Tekin, S.; Tug, N.; Kalafat, E.; et al. Maternal and perinatal outcomes of SARS-CoV-2 infection in unvaccinated pregnancies during Delta and Omicron waves. Ultrasound Obstet. Gynecol. 2022, 60, 96–102. [Google Scholar] [CrossRef]
- Günther, J.; Ziert, Y.; Andresen, K.; Pecks, U.; von Versen-Höynck, F. Variability in COVID-19 symptom presentation during pregnancy and its impact on maternal and infant outcomes across the pandemic. Int. J. Infect. Dis. 2024, 146, 107157. [Google Scholar] [CrossRef]
- Shuai, H.; Chan, J.F.; Hu, B.; Chai, Y.; Yuen, T.T.; Yin, F.; Huang, X.; Yoon, C.; Hu, J.C.; Liu, H.; et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature 2022, 603, 693–699. [Google Scholar] [CrossRef]
- Malik, Y.A. COVID-19 variants: Impact on transmissibility and virulence. Malays. J. Pathol. 2022, 44, 387–396. [Google Scholar]
- Orlic, N.K.; Mandic-Markovic, V.; Jankovic, S.; Lukic, R.; Milovanovic, Z.; Maglic, D.; Popov, D.; Stankovic, M.; Drobnjak, S.; Preradovic, D.; et al. Comparison of Perinatal Outcome of Delta and Omicron Variant of COVID-19 Infection—A Retrospective Observational Study. Medicina 2024, 60, 935. [Google Scholar] [CrossRef]
- Stock, S.J.; Moore, E.; Calvert, C.; Carruthers, J.; Denny, C.; Donaghy, J.; Hillman, S.; Hopcroft, L.E.M.; Hopkins, L.; Goulding, A.; et al. Pregnancy outcomes after SARS-CoV-2 infection in periods dominated by delta and omicron variants in Scotland: A population-based cohort study. Lancet Respir. Med. 2022, 10, 1129–1136. [Google Scholar] [CrossRef]
- Seaton, C.L.; Cohen, A.; Henninger, E.M.; Gendlina, I.; Hou, W.; Bernstein, P.S.; Duong, T.Q. Coronavirus Disease 2019 (COVID-19) Perinatal Outcomes across the pandemic at an academic medical center in New York City. Obstet. Gynecol. 2023, 141, 144–151. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Romero, R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2022, 226, 68–89.e63. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Romero, R.; Tarca, A.L.; Iliodromiti, S.; Rehal, A.; Banerjee, A.; Yu, C.; Peeva, G.; Palaniappan, V.; Tan, L.; et al. SARS-CoV-2 and the subsequent development of preeclampsia and preterm birth: Evidence of a dose-response relationship supporting causality. Am. J. Obstet. Gynecol. 2021, 225, 689–693.e681. [Google Scholar] [CrossRef] [PubMed]
- Papageorghiou, A.T.; Deruelle, P.; Gunier, R.B.; Rauch, S.; García-May, P.K.; Mhatre, M.; Usman, M.A.; Abd-Elsalam, S.; Etuk, S.; Simmons, L.E.; et al. Preeclampsia and COVID-19: Results from the INTERCOVID prospective longitudinal study. Am. J. Obstet. Gynecol. 2021, 225, 289.e1–289.e17. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.A.; Melnick, E.R.; Savitz, S.T.; Jeffery, M.M.; Nath, B.; Janke, A.T. National trends in emergency conditions through the Omicron COVID-19 wave in commercial and Medicare Advantage enrollees. J. Am. Coll. Emerg. Physicians Open 2023, 4, e13023. [Google Scholar] [CrossRef]
- Thompson, J.A.; Mudaranthakam, D.P.; Chollet-Hinton, L. The rural mortality penalty in U.S. hospital patients with COVID-19. Popul. Health Metr. 2024, 22, 20. [Google Scholar] [CrossRef]
- Khalil, E.M.; Madney, Y.M.; Hassan, M.; Fahmy, A.M.; Alshammari, S.O.; Alshammari, A.Q.; Abou-Taleb, H.A.; Taha, A.A.; Elgendy, M.O.; Ali, H.A.A. Maternal and Fetal Outcome of COVID-19 Infection among Pregnant Women. Medicina 2024, 60, 1676. [Google Scholar] [CrossRef]
- Al-Husban, N.; Di’bas, R.M.; Karadsheh, S.S.; Alananzeh, L.A.; Aolymat, I.; Kilani, A.; Obeidat, A.; Alhusban, A.E.; Al-Husban, H. Maternal and Fetal Outcomes of COVID-19 according to the trimester of diagnosis: A cross-sectional prospective study in a tertiary university hospital. J. Clin. Med. 2024, 13, 5262. [Google Scholar] [CrossRef]
- Qin, C.Q.; Wilkins, K.J.; Jones, S.E.; Bradwell, K.R.; Chan, L.E.; Sun, J.; Anzalone, J.; Zheng, Q.; Liebman, M.; Mariona, F.; et al. Evaluating COVID-19 vaccine effectiveness during pre-Delta and Omicron dominant periods among pregnant people in the U.S.: Retrospective cohort analysis from a nationally sampled cohort in National COVID collaborative cohort (N3C). BMJ Public Health 2024, 2, e000770. [Google Scholar] [CrossRef]
- Harris, P.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- National Institutes of Health COVID-19 Treatment Guidelines. Anti-SARS-CoV-2 Antibody Products. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 21 December 2020).
| Characteristic | Alpha (n = 136) | Delta (n = 95) | Omicron (n = 143) | p Value |
|---|---|---|---|---|
| Maternal Age at Diagnosis (years) | 28.42 ± 5.86 | 27.46 ± 6.49 | 27.12 ± 5.63 | 0.39 |
| Gestational Age at Diagnosis (weeks) | 27.44 ± 9.59 | 30.05 ± 7.05 | 30.03 ± 9.56 | 0.05 |
| Gravida | 2.91 ± 1.84 | 3.34 ± 2.36 | 3.24 ± 2.03 | 0.29 |
| BMI (kg/m2) at Diagnosis | 0.04 | |||
| Underweight (<18.5) | 0/136 (0) | 3/95 (3.16) | 0/143 (0) | |
| Normal (18.5–24.9) | 14/136 (10.3) | 8/95 (8.42) | 19/143 (13.23) | |
| 88Overweight (25.0–29.9) | 35/136 (25.73) | 20/95 (21.05) | 38/143 (26.57) | |
| Class I Obesity (30.0–34.9) | 36/136 (26.47) | 19/95 (20.00) | 29/143 (20.28) | |
| Class II Obesity (35.0–39.9) | 26/136 (19.12) | 18/95 (18.95) | 27/143 (18.88) | |
| Class III Obesity (40 and Above) | 25/136 (18.38) | 27/95 (28.42) | 30/143 (21.04) | |
| Maternal Race | 0.31 | |||
| Black | 78/136 (56.34) | 59/95 (62.11) | 101/143 (70.68) | |
| White | 34/136 (25.00) | 21/95 (22.11) | 18/143 (12.56) | |
| Hispanic | 20/136 (14.71) | 12/95 (12.63) | 20/143 (13.98) | |
| Asian | 1/136 (0.74) | 0/95 (0) | 1/143 (0.69) | |
| NAI/PI/Hawaiian | 0/136 (0) | 0/95 (0) | 1/143 (0.69) | |
| Other Race | 3/136 (2.21) | 3/95 (3.16) | 2/143 (1.40) | |
| Trimester of Diagnosis | 0.24 | |||
| First | 13/132 (9.83) | 4/93 (4.30%) | 12/143 (8.39%) | |
| Second | 46/132 (34.85) | 36/93 (38.71%) | 39/143 (27.27%) | |
| Third | 73/132 (55.30) | 53/93 (56.99%) | 92/143 (64.34%) | |
| COVID-19 Severity | 0.001 | |||
| Asymptomatic | 54/110 (49.1) | 30/86 (34.9) | 68/136 (50) | |
| Mild | 37/110 (33.6) | 35/86 (40.1) | 63/136 (46.3) | |
| Moderate | 4/110 (3.6) | 10/86 (11.6) | 3/136 (2.2) | |
| Severe | 10/110 (9.1) | 4/86 (4.7) | 2/136 (1.5) | |
| Critical | 5/110 (4.6) | 7/86 (8.1) | 0/136 (0) | |
| Development of PreE | 0.47 | |||
| Yes | 12/97 (12.4) | 14/77 (18.2) | 23/124 (18.5) | |
| No | 85/97 (87.6) | 63/77 (81.8) | 101/124 (81.5) | |
| Gestational Age at Delivery (weeks; n = 298) | 36.54 ± 4.15 | 35.62 ± 4.31 | 37.28 ± 2.69 | 0.008 |
| Preterm birth | 0.08 | |||
| Yes | 36/97 (37.1) | 27/77 (35.1) | 30/124 (24.2) | |
| No | 61/97 (62.9) | 50/77 (64.9) | 94/124 (75.8) | |
| Infant Birthweight (grams) | 2861.72 ± 838.64 | 2699.38 ± 882.72 | 2946.73 ± 623.90 | 0.09 |
| COVID-19 Vaccination | 0.76 | |||
| Not Applicable | 56/136 (41.18) | 0/95 (0) | 0/143 (0) | |
| Unknown | 74/136 (54.42) | 90/95 (94.74) | 97/143 (67.83) | |
| At Least 1 Shot | 6/136 (4.4) | 5/95 (5.26) | 46/143 (32.17) |
| Complication at Delivery | Alpha (n = 97) | Delta (n = 77) | Omicron (n = 124) | p Value |
|---|---|---|---|---|
| 0.36 | ||||
| None | 70/97 (72.2) | 58/77 (75.3) | 97/124 (78.2) | |
| Postpartum Hemorrhage | 10/97 (10.3) | 4/77 (5.2) | 8/124 (6.5) | |
| Placental Abruption | 1/97 (1.0) | 2/77 (2.6) | 1/124 (0.8) | |
| PPROM | 2/97 (2.1) | 3/77 (3.9) | 9/124 (7.3) | |
| Other | 12/97 (12.4) | 7/77 (9.1) | 6/124 (4.8) | |
| Multiple | 2/97 (2.1) | 3/77 (3.9) | 3/124 (2.4) |
| Maternal Deaths | |||
|---|---|---|---|
| Alpha (n = 5) | Delta (n = 6) | p Value | |
| Maternal age at diagnosis (years) | 26.60 ± 2.88 | 34.00 ± 5.29 | 0.02 |
| Maternal race | 1 | ||
| Black | 4/5 (80) | 3/6 (50) | |
| Hispanic | 0/5 (0) | 2/6 (33.3) | |
| White | 1/5 (20) | 1/6 (16.7) | |
| BMI at diagnosis (kg/m2) | 42.31 ± 11.01 | 44.24 ± 18.57 | 0.84 |
| Gestational age at diagnosis (weeks) | 23.28 ± 11.88 | 27.18 ± 5.24 | 0.48 |
| Maternal comorbidities | |||
| Diabetes | 2/5 (40) | 2/6 (33.3) | 1 |
| Chronic hypertension | 2/5 (40) | 2/6 (33.3) | 1 |
| COVID-19 severity classification range | 0.45 | ||
| Asymptomatic/mild | 1/5 (20) | 0/6 (0) | |
| Moderate | 0/5 (0) | 0/6 (0) | |
| Severe/critical | 4/5 (80) | 6/6 (100) | |
| Hospital length of stay (days) | 19.80 ± 29.47 | 17.33 ± 12.36 | 0.86 |
| Gestational age at delivery (weeks) | 37.03 ± 2.64 | 28.08 ± 4.63 | 0.009 |
| Average infant birthweight (grams) | 2680 ± 489.1 | 972 ± 358.5 | 0.0005 |
| Infant Deaths | |||
| Alpha (n = 6) | Delta (n = 2) | ||
| Maternal age at diagnosis (years) | 31.83 ± 4.54 | 34.00 ± 4.24 | - |
| Maternal race | - | ||
| Black | 2/6 (33.3) | 2/2 (100) | |
| Hispanic | 2/6 (33.3) | 0/2 (0) | |
| White | 2/6 (33.3) | 0/2 (0) | |
| Gestational age at diagnosis (weeks) | 26.02 ± 5.91 | 32.90 ± 9.19 | - |
| BMI at diagnosis (kg/m2) | 40.72 ± 14.94 | 48.53 ± 13.79 | - |
| Maternal comorbidities | - | ||
| Diabetes | 0/6 (0) | 1/2 (50) | |
| Chronic hypertension | 3/6 (50) | 1/2 (50) | |
| COVID-19 severity classification range | - | ||
| Asymptomatic/mild | 5/6 (83.3) | 2/2 (100) | |
| Moderate | 0/6 (0) | 0/2 (0) | |
| Severe/critical | 1/6 (16.7) | 0/2 (0) | |
| Gestational age at delivery (weeks) | 32.87 ± 5.24 | 33.40 ± 9.62 | - |
| Preterm birth | - | ||
| Yes | 4/6 (66.7) | 1/2 (50) | |
| No | 2/6 (33.3) | 1/2 (50) | |
| Intrauterine growth restriction | - | ||
| Yes | 5/6 (83.3) | 1/2 (50) | |
| No | 1/6 (16.7) | 1/2 (50) | |
| Average infant birthweight (grams) | 1745 ± 911.5 | 1782 ± 1813 | - |
| Infant gender | - | ||
| Male sex | 4/6 (66.7) | 1/2 (50) | |
| Female sex | 2/6 (33.3) | 1/2 (50) | |
| NIUC admission | - | ||
| Not applicable | 2/6 (33.3) | 1/2 (50) | |
| Yes | 4/6 (66.7) | 1/2 (50) | |
| Length of stay (days) | 29.75 ± 39.84 | 167 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohaegbulam, G.; Wallace, K.; Yimer, W.K.; Moustafa, A.S.Z.; Morris, R. The Impact of COVID-19 Pandemic Waves on Maternal Health and Infant Outcomes—A Retrospective Cohort Study. Women 2024, 4, 469-479. https://doi.org/10.3390/women4040035
Ohaegbulam G, Wallace K, Yimer WK, Moustafa ASZ, Morris R. The Impact of COVID-19 Pandemic Waves on Maternal Health and Infant Outcomes—A Retrospective Cohort Study. Women. 2024; 4(4):469-479. https://doi.org/10.3390/women4040035
Chicago/Turabian StyleOhaegbulam, Gail, Kedra Wallace, Wondwosen K. Yimer, Ahmed S. Z. Moustafa, and Rachael Morris. 2024. "The Impact of COVID-19 Pandemic Waves on Maternal Health and Infant Outcomes—A Retrospective Cohort Study" Women 4, no. 4: 469-479. https://doi.org/10.3390/women4040035
APA StyleOhaegbulam, G., Wallace, K., Yimer, W. K., Moustafa, A. S. Z., & Morris, R. (2024). The Impact of COVID-19 Pandemic Waves on Maternal Health and Infant Outcomes—A Retrospective Cohort Study. Women, 4(4), 469-479. https://doi.org/10.3390/women4040035







