GC-MS Analysis of Liposoluble Components from Six Kinds of Bast Fibers and Correlative Study on Their Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. GC-MS Analysis
3. Results and Discussion
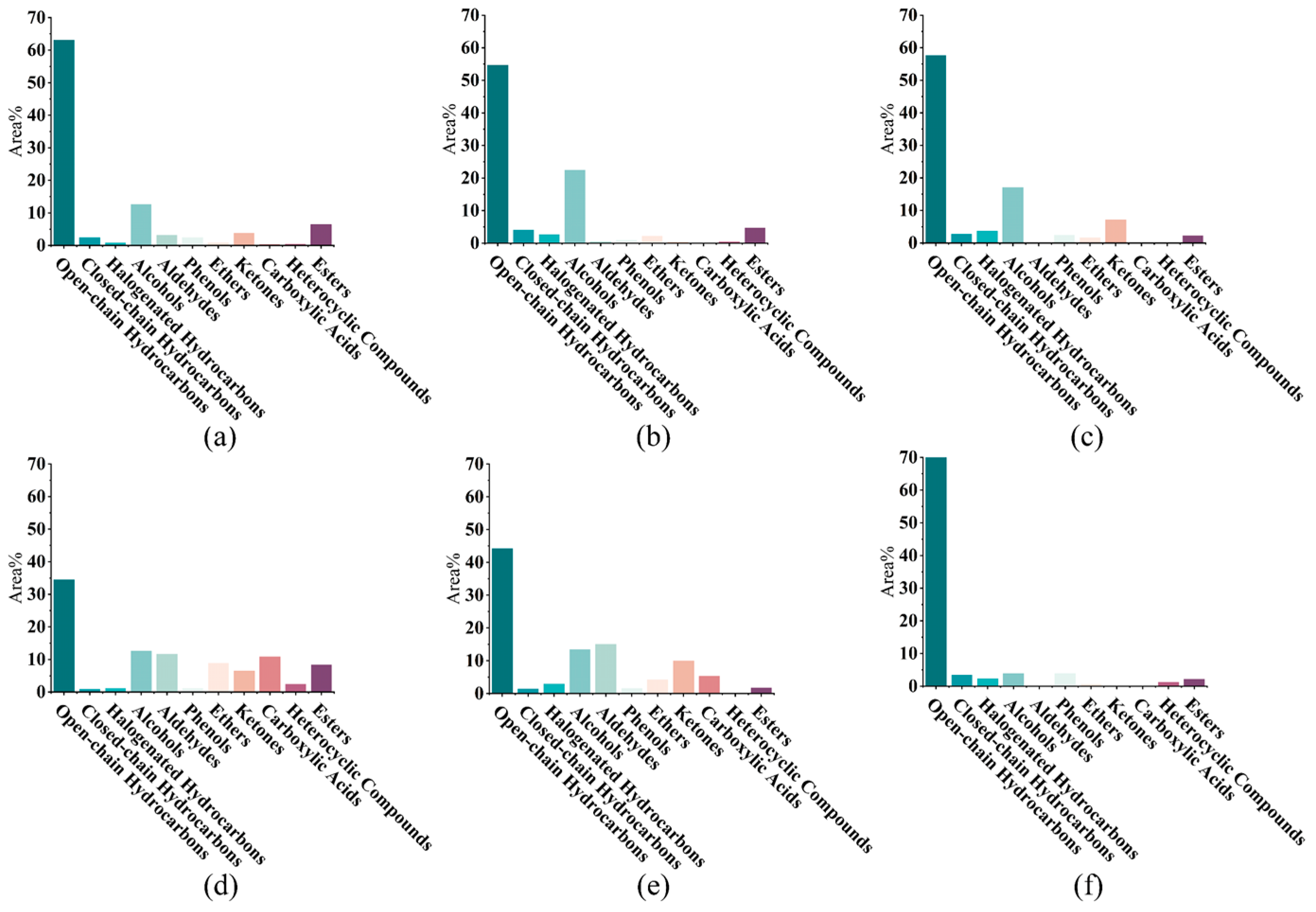
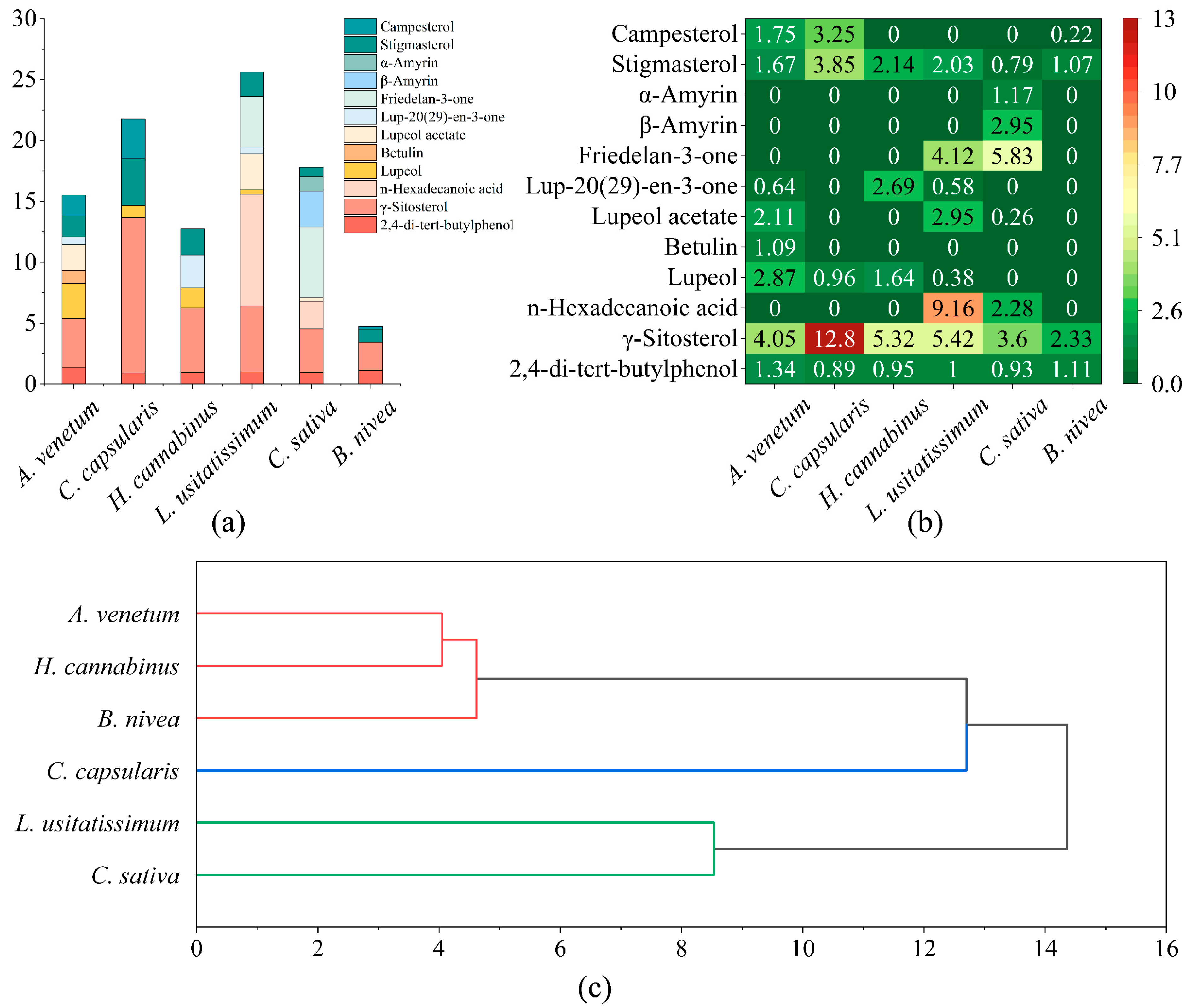
3.1. Comparative Analysis of Liposoluble Components in Bast Fibers of Six Varieties
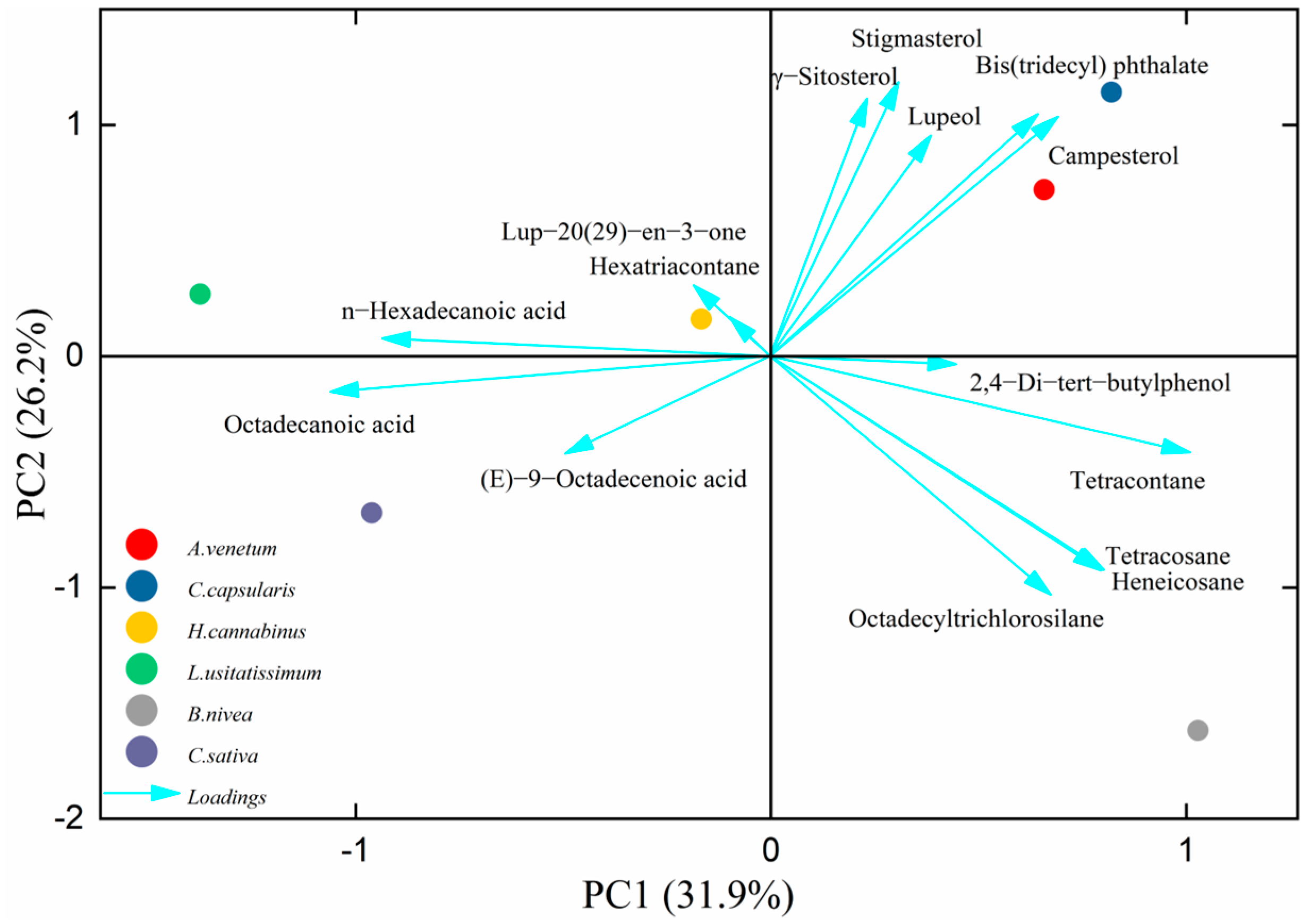
3.2. Correlation Between Liposoluble Components and Antibacterial Activity
3.3. Literature Overview of Antibacterial Activities and Comparative Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zimniewska, M. Hemp fibre properties and processing target textile: A review. Materials 2022, 15, 1901. [Google Scholar] [CrossRef] [PubMed]
- Atmakuri, A.; Palevicius, A.; Griskevicius, P.; Janusas, G. Investigation of mechanical properties of hemp and flax fibers hybrid composites for biomedical applications. Mechanics 2019, 25, 149–155. [Google Scholar] [CrossRef]
- Liu, X.; Yang, C.; Sun, M.; Quan, G.; Gong, B.; Kang, Q.; Xiao, L.; Zhu, W.; Wang, H. Preparation of new natural hemp fiber-based antibacterial hydrogel dressing and its performance in promoting wound healing of bacterial infection. Colloids Surf. A Physicochem. Eng. Asp. 2025, 719, 137008. [Google Scholar] [CrossRef]
- Vaquero, M.R.; Alberto, M.R.; De Nadra, M.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Mudalungu, C.M.; Mokaya, H.O.; Tanga, C.M. Beneficial sterols in selected edible insects and their associated antibacterial activities. Sci. Rep. 2023, 13, 10786. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef] [PubMed]
- Seenivasan, A.; Manikkam, R.; Kaari, M.; Sahu, A.K.; Said, M.; Dastager, S.G. 2,4-Di-tert-butylphenol (2,4-DTBP) purified from Streptomyces sp. KCA1 from Phyllanthus niruri: Isolation, characterization, antibacterial and anticancer properties. J. King Saud Univ.-Sci. 2022, 34, 102088. [Google Scholar] [CrossRef]
- Duke, J. Duke’s Ethanobotanical and Phytochemistry Database. Available online: https://phytochem.nal.usda.gov (accessed on 1 September 2025).
- Abdel-Karim, M.; Weam, A.M.P.; Yousif, M.; Inas, O.P. GC-MS Analysis and antimicrobial activity of sudanese Brassica nigra L. (Brassicaceae) fixed oil. Int. J. Sci. Eng. App. Sci. 2017, 3, 74–81. [Google Scholar]
- Kim, H.; Lee, D.G. Lupeol-induced nitric oxide elicits apoptosis-like death within Escherichia coli in a DNA fragmentation-independent manner. Biochem. J. 2021, 478, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, Z.F.; Zhang, J.F.; Gong, J.X.; Meng, F.J. A Study on the Antibacterial Property of the Extract from Apocynum venetum. Adv. Mater. Res. 2011, 332, 164–167. [Google Scholar] [CrossRef]
- Ilhan, S.; Savaroğlu, F.; Çolak, F. Antibacterial and Antifungal Activity of Corchorus olitorius L. (Molokhia) Extracts. Int. J. Nat. Eng. Sci. 2007, 1, 59–61. [Google Scholar]
- Birhanie, Z.M.; Xiao, A.; Yang, D.; Huang, S.; Zhang, C.; Zhao, L.; Liu, L.; Li, J.; Chen, A.; Tang, H.; et al. Polysaccharides, total phenolic, and flavonoid content from different kenaf (Hibiscus cannabinus L.) genotypes and their antioxidants and antibacterial properties. Plants 2021, 10, 1900. [Google Scholar] [CrossRef] [PubMed]
- Nand, P.; Drabu, S.; Gupta, K.R. Antimicrobial investigation of Linum usitatissimum for the treatment of acne. Nat. Prod. Commun. 2011, 6, 1934578X1100601133. [Google Scholar] [CrossRef]
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, purification, and antimicrobial characterization of cannabidiolic acid and cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Wang, X.; Lee, D.G.; Kim, Y.M.; Jung, Y.S.; Kim, H.B.; Kim, H.Y.; Cho, E.J.; Lee, S. Various biological activities of ramie (Boehmeria nivea). J. Appl. Biol. Chem. 2014, 57, 279–286. [Google Scholar] [CrossRef]
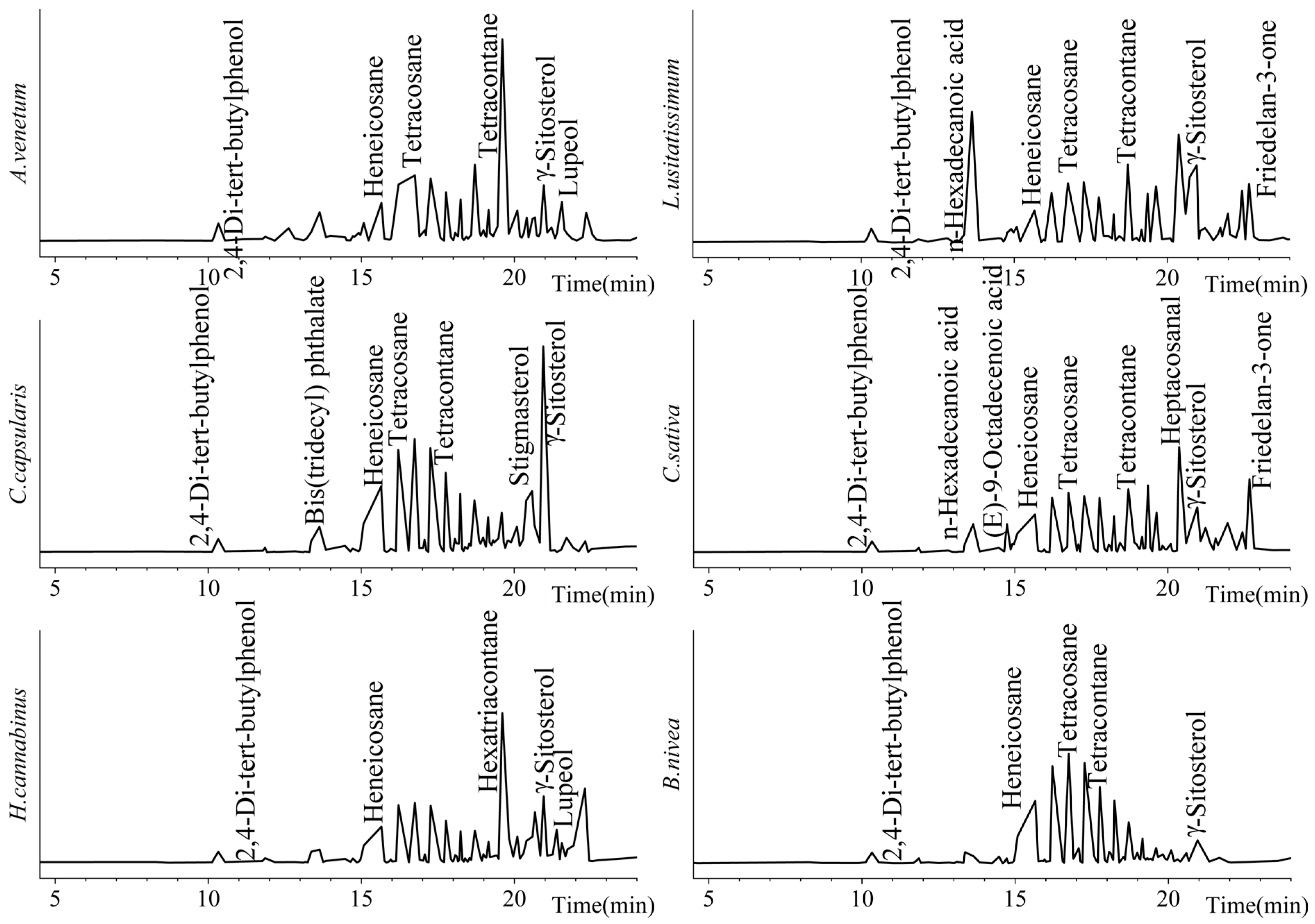
| Species | A. venetum | C. capsularis | H. cannabinus | L. usitatissimum | C. sativa | B. nivea | |
|---|---|---|---|---|---|---|---|
| Contents (%) | |||||||
| Compounds | |||||||
| Tetracontane | 40.39 | 32.07 | 22.47 | 20.24 | 23.23 | 45.42 | |
| γ-Sitosterol | 4.05 | 12.8 | 5.32 | 5.42 | 3.6 | 2.33 | |
| Tetracosane | 4.4 | 6.81 | 4.96 | 3.49 | 5.25 | 10.09 | |
| Heneicosane | 4.55 | 6.81 | 4.99 | 3.89 | 5.16 | 10.13 | |
| n-Hexadecanoic acid | 0 | 0 | 0 | 9.16 | 2.28 | 0 | |
| Stigmasterol | 1.67 | 3.85 | 2.14 | 2.03 | 0.79 | 1.07 | |
| Lupeol | 2.87 | 0.96 | 1.64 | 0.38 | 0 | 0 | |
| 2,4-Di-tert-butylphenol | 1.34 | 0.89 | 0.95 | 1 | 0.93 | 1.11 | |
| Bis(tridecyl) phthalate | 2.14 | 1.64 | 0 | 0 | 0 | 0 | |
| Campesterol | 1.75 | 3.25 | 0 | 0 | 0 | 0.22 | |
| Hexatriacontane | 1.77 | 0 | 15.93 | 0.36 | 0.65 | 0 | |
| Lup-20(29)-en-3-one | 0.64 | 0 | 2.69 | 0.58 | 0 | 0 | |
| Octadecanoic acid | 0 | 0 | 0 | 0.97 | 0.72 | 0 | |
| (E)-9-Octadecenoic acid | 0.38 | 0 | 0 | 0 | 2.28 | 0 | |
| Octadecyltrichlorosilane | 0.74 | 0.74 | 0.96 | 0.59 | 0.75 | 1.16 | |
| Species | A. venetum | C. capsularis | H. cannabinus | L. usitatissimum | C. sativa | B. nivea | |
|---|---|---|---|---|---|---|---|
| Contents (%) | |||||||
| Compounds | |||||||
| 2,4-di-tert-butylphenol | 1.34 | 0.89 | 0.95 | 1 | 0.93 | 1.11 | |
| γ-Sitosterol | 4.05 | 12.8 | 5.32 | 5.42 | 3.6 | 2.33 | |
| n-Hexadecanoic acid | 0 | 0 | 0 | 9.16 | 2.28 | 0 | |
| Lupeol | 2.87 | 0.96 | 1.64 | 0.38 | 0 | 0 | |
| Betulin | 1.09 | 0 | 0 | 0 | 0 | 0 | |
| Lupeol acetate | 2.11 | 0 | 0 | 2.95 | 0.26 | 0 | |
| Lup-20(29)-en-3-one | 0.64 | 0 | 2.69 | 0.58 | 0 | 0 | |
| Friedelan-3-one | 0 | 0 | 0 | 4.12 | 5.83 | 0 | |
| β-Amyrin | 0 | 0 | 0 | 0 | 2.95 | 0 | |
| α-Amyrin | 0 | 0 | 0 | 0 | 1.17 | 0 | |
| Stigmasterol | 1.67 | 3.85 | 2.14 | 2.03 | 0.79 | 1.07 | |
| Campesterol | 1.75 | 3.25 | 0 | 0 | 0 | 0.22 | |
| Variety | Target Microorganism | Activity Strength | References |
|---|---|---|---|
| A. venetum | Escherichia coli | ** | [12] |
| Staphylococcus aureus | ** | [12] | |
| Saccharomyces cerevisiae | * | [12] | |
| Aspergillus niger | - | [12] | |
| C. capsularis | Escherichia coli | *** | [13] |
| Staphylococcus aureus | *** | [13] | |
| Yersinia enterocolitica | *** | [13] | |
| Geotrichum candidum | *** | [13] | |
| Botrytis cinerea | ** | [13] | |
| H. cannabinus | Staphylococcus aureus | ** | [14] |
| Escherichia coli | ** | [14] | |
| L. usitatissimum | Staphylococcus aureus | *** | [15] |
| Propionibacterium acnes | *** | [15] | |
| Staphylococcus epidermidis | ** | [15] | |
| C. sativa | Staphylococcus aureus | **** | [16] |
| Staphylococcus epidermidis | **** | [16] | |
| Escherichia coli | - | [16] | |
| Pseudomonas aeruginosa | - | [16] | |
| B. nivea | Escherichia coli | *** | [17] |
| Staphylococcus aureus | *** | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Feng, X.; Cheng, L.; Xi, G.; Hu, Y.; Tan, S.; Zhou, W.; Chen, Z.; Peng, Z.; Duan, S.; et al. GC-MS Analysis of Liposoluble Components from Six Kinds of Bast Fibers and Correlative Study on Their Antibacterial Activity. Polysaccharides 2025, 6, 107. https://doi.org/10.3390/polysaccharides6040107
Zhou X, Feng X, Cheng L, Xi G, Hu Y, Tan S, Zhou W, Chen Z, Peng Z, Duan S, et al. GC-MS Analysis of Liposoluble Components from Six Kinds of Bast Fibers and Correlative Study on Their Antibacterial Activity. Polysaccharides. 2025; 6(4):107. https://doi.org/10.3390/polysaccharides6040107
Chicago/Turabian StyleZhou, Xiang, Xiangyuan Feng, Lifeng Cheng, Guoguo Xi, Yuqin Hu, Si Tan, Wei Zhou, Zishu Chen, Zhenghong Peng, Shengwen Duan, and et al. 2025. "GC-MS Analysis of Liposoluble Components from Six Kinds of Bast Fibers and Correlative Study on Their Antibacterial Activity" Polysaccharides 6, no. 4: 107. https://doi.org/10.3390/polysaccharides6040107
APA StyleZhou, X., Feng, X., Cheng, L., Xi, G., Hu, Y., Tan, S., Zhou, W., Chen, Z., Peng, Z., Duan, S., & Yang, Q. (2025). GC-MS Analysis of Liposoluble Components from Six Kinds of Bast Fibers and Correlative Study on Their Antibacterial Activity. Polysaccharides, 6(4), 107. https://doi.org/10.3390/polysaccharides6040107






