Abstract
Yeast protein (YP) offers nutritional and sustainable benefits; however, its poor gelation properties limit its use in soft material formulations. This study investigates the rheological behavior and the formation of crosslinked networks using YP–polysaccharide mixtures for extrusion-based 3D printing. Binary bioink blends with alginate (Alg) or xanthan gum (XG) showed enhanced viscosity and exhibited shear-thinning properties. However, a high concentration of Alg negatively affected the material’s thixotropic recovery. On the other hand, YP–XG bioink displayed more pronounced elastic behavior and demonstrated thixotropic recovery, though they lacked the capacity for ionic crosslinking. A triple bioink formulation consisting of 8% (w/v) YP, 2% (w/v) Alg, and 0.5% (w/v) XG effectively combined the advantages of both polysaccharides. Alg provided structural stability through calcium crosslinking, while XG offered rheological flexibility. These bioinks were successfully printed using embedded 3D printing and maintained their shape fidelity after printing. The crosslinked triple hydrogel exhibited good mechanical strength, volume retention after crosslinking, structural integrity under compression of up to 70%, and recovery after deformation that indicates high structural stability. This research presents an effective strategy to enhance the application of yeast-derived proteins in sustainable, animal-free 3D printed food products and other soft biomaterials.
1. Introduction
With rising global demand for sustainable proteins, yeast-derived proteins have emerged as promising alternatives to traditional sources [1]. Yeast protein (YP) is derived from the biomass of yeast species like Saccharomyces cerevisiae and Yarrowia lipolytica produced in controlled fermentation. It makes up 40–60% of the yeast’s dry weight and contains a complete amino acid profile, including all essential amino acids [1,2,3]. Its nutritional quality generally exceeds that of most plant proteins but remains slightly below animal proteins due to lower digestibility [1,4,5]. Notably, YP is virtually non-allergenic, odorless, and often obtained as a byproduct of food industries like brewing and baking, thus its utilization supports waste valorization and sustainable protein production [1,6]. Although YP is gaining increasing attention as a sustainable, high-value ingredient in the food industry, its utilization as a functional food ingredient and biomaterial remains limited.
Gelation ability is a key property influencing the formation and stability of structured protein-based materials, significantly affecting the texture, stability, and functional properties of food [7]. Apart from their crucial role in the food industry, protein gels are increasingly utilized for advanced applications such as controlled drug release, sensors, and diagnostics [8]. However, YP has a relatively low gel-forming ability, attributed to its low sulfhydryl group and disulfide bond content compared to other proteins [9]. As a result, fabrication of YP gels often requires either crosslinking or formulating with gel-forming macromolecules. Several studies have attempted to address this limitation through different strategies. For instance, Bodenberger et al. (2016) developed hydrogels for 3D cell culture using YP and tetrakis (hydroxymethyl) phosphonium chloride (THPC) as a crosslinking agent [10]. While THPC covalently reacts with primary and secondary amines, its use may alter the natural properties and nutritional profile of proteins. Wang et al. developed an edible scaffold composed of proanthocyanidins, chitosan, collagen, and varying proportions of YP to partially replace collagen in cultured meat [11] and Kong et al. (2025) used YP to enhance the 3D printability of tapioca starch bioinks [12]. Furthermore, studies have presented protein-based meat analogues via high-moisture extrusion by combining YP with soy protein [13] or konjac glucomannan [14]. Despite the innovative studies, the integration of YP into functional soft material systems is still limited and underexplored, particularly in extrusion-based 3D printing systems where interaction with polysaccharides could enhance performance.
To address this gap, we explored various combinations of YP with two polysaccharides, alginate (Alg) and Xanthan gum (XG), and evaluated their suitability as bioinks for 3D printing applications. Both Alg and XG are natural, biocompatible, and non-toxic polysaccharides used in food and biomedical applications [15]. Alg, an anionic polysaccharide from brown seaweed, consists of β-D-mannuronic (M) and α-L-guluronic (G) acid units and forms ionotropic gels by crosslinking with divalent cations (e.g., Ca2+) [16,17]. Its fast and easy crosslinking makes Alg popular for extrusion-based printing requiring high structural integrity [18,19,20]. XG, produced by fermenting sugars with Xanthomonas campestris, is a high molecular weight anionic polysaccharide with a (1–4)-linked D-glucose backbone and trisaccharide side chains forming a right-handed helix [21,22]. Its rigid conformation imparts shear-thinning behavior and excellent rheological properties, making XG a highly effective stabilizer in water-based systems for food and agricultural products [23]. The choice of Alg and XG was guided by complementary combination with protein molecules: Alg’s ionic gelation with calcium can reinforce the weak self-gelation of YP, while XG’s pronounced shear-thinning behavior supports smooth extrusion and shape fidelity [24]. Together, these characteristics make Alg and XG ideal candidates to enhance the rheological and structural performance of YP-based bioinks.
Protein–polysaccharide blends are widely used due to their complementary functions. In 3D printing, they serve as effective bioinks and support materials in both food [25,26] and tissue engineering [19,20,27,28], where the selection of their source significantly impacts functional and structural properties. Typical proteins used in these applications include isolates from soy, pea, and whey, as well as gelatin. These proteins are often combined with polysaccharides such as Alg, XG, pectin, and carboxymethyl cellulose [27]. Proteins primarily contribute to network formation, emulsification, bioactivity, and cell support, while also enhancing nutritional value. On the other hand, polysaccharides help adjust rheological properties like viscosity, shear-thinning, and viscoelasticity, ensuring structural integrity during and after the printing process. The synergistic interactions between proteins and polysaccharides, through mechanisms such as complexation or covalent bonding, enhance the printability and stability of the inks used in printed formulations [25,26]. These interactions have also been shown to induce structural and functional changes in proteins, such as pea protein, for example, through the exposure of internal sulfhydryl (SH) groups [29,30].
This study aimed to investigate varying compositions of YP and Alg or XG used as bioinks for 3D printing, with a special emphasis on exploring how polysaccharides affect the rheological properties as well as understanding the effect of calcium-induced ionic crosslinking.
We hypothesize that integrating YP with Alg or XG in various compositional ratios will produce composite formulations with adjustable rheological properties. Alg is expected to enhance stability during and after the printing process, while XG will improve viscosity and shear-thinning behavior. Therefore, we anticipate that combining Alg and XG together will create an advanced bioink, as both stability and viscosity are crucial for extrusion-based 3D printing. Overall, these effects are expected to yield bio-based materials with improved printability and structural integrity, improving their use in applications in food and biomedical engineering. To the best of our knowledge, this study is the first to characterize bioinks of YP with Alg or XG for enhanced 3D printing performance, highlighting a novel application of YP as a promising protein substitute.
2. Materials and Methods
2.1. Materials
Yeast protein isolate (YP, Lot: KT-13) powder was kindly provided by Nextferm Technologies Ltd. (Yokneam, Israel). According to the supplier’s specifications, YP was composed of 75 g/100 g protein, 14 g/100 g fat, 8 g/100 g ash, and 6 g/100 g moisture.
Sodium alginate (Alg, Lot: SLCF1476) with medium viscosity was purchased from Sigma-Aldrich (Rehovot, Israel). According to the supplier’s specifications, a 2% (w/v) solution of Alg in water at 25 °C exhibited a viscosity of 3240 cP, with a molecular weight range of 250,000–350,000 Da. Xanthan gum (XG, Lot: 031M0239V) was also obtained from Sigma-Aldrich (Rehovot, Israel). As per the supplier’s data, a 1% (w/v) solution of XG in water at 25 °C had a viscosity of 978 cP. The molecular weight of XG was determined via high-performance liquid chromatography (HPLC) (Waters, MA, USA) and was found to be 3.8 × 106 Da (see Section S1 in the Supplementary Material).
Hydrochloric acid (HCl, 32%), sodium hydroxide (NaOH), and phosphate-buffered saline (PBS) 10X (Ca2+/Mg2+-free) were obtained from Bio-Lab Ltd. (Jerusalem, Israel). Calcium chloride (CaCl2) was purchased from Fisher Scientific (Loughborough, UK). κ-Carrageenan (Lot: BCCB7583), Span 80 (Lot: MKXL875) and paraffin oil (Lot: STBJ4943) were purchased from Sigma-Aldrich. Potassium chloride (KCl) and nylon filter (60 µm pore) were purchased from Merck. Canola oil was purchased at a local supermarket. Double-distilled water (DDW) was used in all the aqueous solutions.
2.2. Preparation and Characterization of YP Solutions
Yeast protein isolate (YP) powder was dissolved in DDW to the required concentration, stirred for 2 h at room temperature, and stored at 4 °C overnight to enhance dissolution. The following day, the solution was adjusted to pH 9–10 with 1M NaOH, mixed for 2 h on a stirrer, and vortexed before adding Alg or XG. 0.5% (w/v) YP at pH 9 solution were visualized by light microscopy using an Olympus BX53M microscope equipped with a CMOS color camera (Tokyo, Japan).
2.2.1. Zeta Potential Analysis
Dynamic light scattering (DLS) was used to determine the YP zeta potential across pH levels, using a Zetasizer Nano Series (Malvern Instruments Ltd., Worcestershire, UK) equipped with a He–Ne laser (633 nm) at 25 °C and an angle of 173°. The analysis was conducted on 0.1% (w/v) YP solutions as described at 2.2, as the pH adjusted to 9–10, 6–7 or 2–3 with 1M NaOH and 1M HCl.
2.2.2. SDS-PAGE
To prepare the sample, 15 μg of YP was dissolved in 40 µL of DDW and mixed with 10 µL of 5X sample buffer, then denatured at 95 °C for 5 min. An 8% SDS polyacrylamide gel was run for 2 h at 80–120 V and washed three times with Milli-Q water. Imperial Protein Stain was applied, with overnight agitation at 4 °C. The gel was washed again and analyzed using the ChemiDoc System (Bio-Rad Laboratories, Hercules, CA, USA).
2.3. Preparation of the YP–Alg, YP–XG and YP–Alg–XG Bioink Blends
Alg and XG were dissolved separately in PBS to the desired concentrations and mixed overnight at room temperature using a magnetic stirrer to ensure complete dissolution. For the binary mixtures, each polysaccharide was combined with YP solution (pH 9–10) by repeated pipetting for approximately 2–3 min until a uniform and homogenous mixture with a consistent color was obtained. For the triple bioink mixtures, Alg and XG were first blended in the specified ratios under gentle stirring for an hour, followed by the addition of YP solution (pH 9–10) and mixing by pipetting until homogeneity was achieved. The pH of all mixtures was then adjusted to 7 using 0.1 M HCl. All preparations were carried out under standard laboratory conditions (non-sterile environment) in sealed glass vials to minimize contamination. The final bioink were stored at 4 °C for up to 24 h before use.
2.4. Preparation of the YP–Alg, YP–XG–YP and YP–Alg–XG Cross-Linked Hydrogels
To crosslink the bioink, 210 µL of the mixture was placed in an 8 mm diameter and 3.2 mm thick mold. A 0.45 μm filter paper was positioned above and below for uniform crosslinking, and the mold was submerged in 2 mL of 0.1 M CaCl2 for 30 min (see Figure S1). Following crosslinking, the hydrogels were extracted for characterization. Control gels containing only Alg or Alg–XG underwent the same procedure. Hydrogels dimensions were measured post-extraction, and the volume compared to the mold volume was calculated based on at least five independent samples to evaluate the change in volume during the crosslinking process.
2.5. Fourier Transform Infrared (FTIR)
Fourier transform infrared (FTIR) spectra were recorded using a Bruker Tensor 27 FT-IR (Bruker, Billerica, MA, USA) containing a high-sensitivity LN-cooled MCT detector to examine the IR spectra between 400 and 4000 cm−1. The spectra of Alg, XG, or YP solutions, which were dried using freeze-drying, were compared to those of the dried bioink mixtures of YP–Alg, YP–XG and YP–Alg–XG.
2.6. Rheological Properties
Rheology measurements were conducted using an MCR 302 rheometer (Anton Paar, PrimeLab, Kefar Sava, Israel) featuring a 25 mm parallel plate geometry with a 0.5 mm gap. The bioink samples were vortexed prior to measurement.
Steady shear flow measurements were used to study the shear thinning behavior of the bioink. The viscosity was measured in a wide range of shear rates (0.01–1000 1/s) using a logarithmic ramp and the zero shear viscosity (η0) was determined as the average viscosity at the lowest shear rates, where the viscosity reaches a plateau. The linear decay of the viscosity curves was fitted to a power law model:
where is the viscosity of the sample, m is a constant, is the shear rate, and n is the flow behavior index. An n < 1 typical for shear-thinning fluids and n ≈ 1 for Newtonian fluids [31].
Strain amplitude measurements of the bioink were performed by measuring the storage (G′) and loss (G″) moduli at 0.1–1000% strain under an angular frequency of ω = 10 rad/s. The average G′ was calculated within the linear viscoelastic region (LVR, 1–10% strain) and the crossover point was determined as the strain at which G′ = G″ using the rheometer software (RheoCompass V1.35.1394, Anton Paar).
A 3-stage thixotropy test (3-ITT) based on viscosity measurements was conducted to assess the recovery of the bioink’s viscosity after exposure to high shear strains.
Viscosities were measured during low (0.5%) and high (300%) strain cycles to evaluate material properties and thixotropic recovery. The ratio of the post-cycle viscosity to the initial, δ, is calculated as follows:
where 1 is the average viscosity during the first cycle before high strain, and 2 is the viscosity after 120 s within the stabilization period after high strain.
2.7. Mechanical Properties
The mechanical properties of the crosslinked hydrogels were evaluated using a Llyod LF-plus machine (Llyod Instruments, Fareham, UK) with a 10 N cell and two 5 cm diameter circular plates. For compression testing, hydrogels prepared as described in Section 2.4 were compressed between two circular plates at a rate of 1 mm/min. Young’s modulus was calculated from the initial slope of the stress–strain curve, typically within the 5–10% strain range. For cyclic compression testing, samples were preloaded to 0.1 N and then subjected to four loading–unloading cycles at a rate of 3 mm/min. Each test was performed at a constant maximum strain of either 10%, 20%, 50%, or 70%, applied uniformly across all four cycles within that test. Thus, each sample underwent four cycles at a fixed strain level. The maximum stress is determined as the highest stress in each cycle [32,33]. The diameter and height of all hydrogels were measured prior to testing.
2.8. Water Uptake and Stability Experiments
Water uptake and dissolution kinetics were evaluated gravimetrically by monitoring the weight changes in crosslinked hydrogels over time under various conditions. In the first experiment, hydrogels were immersed in PBS at pH 7 and 4 °C to assess water absorption and stability during storage. In the second experiment, samples were exposed sequentially to buffers simulating gastrointestinal conditions: first, an acidic buffer (pH 3) at 37 °C for 2 h to represent gastric conditions, followed by PBS (pH 6.8) at 37 °C to mimic intestinal conditions. The buffer compositions are detailed in Section S2 of the Supplementary Material. For both conditions, pre-weighed hydrogel discs were placed on a stainless-steel grid in a covered Petri dish with 20 mL of medium. All incubations at 37 °C were conducted in an NB-T207 incubator (N-BIOTEK, Korea) without shaking. Water uptake kinetics were measured gravimetrically by weighing each hydrogel after blotting excess water. The relative weight (RW) was calculated as the ratio of the current weight to the initial weight at each time point. Dissolution behavior was inferred from the progressive decrease in sample weight and loss of structural integrity over time. All experiments were performed in triplicate, and fresh buffer was used for each replicate to maintain constant pH and ionic strength.
2.9. Embedded 3D Bioprinting
A granular support material (CarGrow), composed of κ-carrageenan microgels suspended in CaCl2 solution, was fabricated as previously described [34,35] and detailed in Section S3 of the Supplementary Materials. YP–based bioink blends were printed into the support bath to evaluate their shape fidelity and structural integrity under embedded conditions. Four compositions of YP, Alg and XG bioink blends were prepared according to Table 1.

Table 1.
Bioink compositions and component concentrations.
Prepared bioinks were transferred into 3 mL printing cartridges (Nordson EFD) fitted with 640 µm inner diameter blunt-end needle (CML supply). The printing cartridge was then loaded into a printing tool of 6 axis extrusion bioprinter BioAssemblyBot (Advanced Solutions, Louisville, KY, USA). The design of the printed structures was performed using TSIM (Advanced Solutions, Louisville, KY, USA). The printed structures included a square cube (10 × 10 × 2 mm) and a cylinder (5 mm diameter and 3 mm length). The object was sliced to the desired slice thickness (80% of the needle’s inner diameter), and the file was sent to the bioprinter. The printing was performed with 6–7 PSI pressure and 10–12 mm/s speed parameters, using CarGrow granular hydrogel as the support material. The printed structures were subsequently cross linked within the support matrix and gently extracted by pipetting.
The printing fidelity of the triple blend (8% w/v YP + 2% w/v Alg + 0.5% w/v XG) was evaluated by quantifying the deviation between the printed construction and its computer-aided design (CAD) dimensions. Pore size analysis was performed using ImageJ version 1.54g (National Institutes of Health, Bethesda, MD, USA), and the results were expressed as normalized pore area (Ar) and normalized pore perimeter (Pr), where values close to 1 indicate higher fidelity and deviations from 1 reflect reduced accuracy. Each parameter was calculated from at least 32 different pores.
2.10. Statistical Analysis
Quantitative results were obtained from at least three independent samples. Statistical analyses were performed using GraphPad Prism 9. A two-tailed Student’s t-test was used for two-group comparisons. Data is presented as mean ± SD. In graphs, significance is indicated as * p < 0.05, ** p < 0.01, *** p < 0.001, and ns (not significant). In tables, different superscript letters in the same column indicate significant differences (p < 0.05), with the lowest value defined as ‘a’.
3. Results and Discussion
3.1. YP Characterization
The molecular weights distribution of YP isolate was analyzed using SDS-PAGE (Figure 1A), revealing bands at approximately 11 kDa, 25 kDa, 35–40 kDa, and 50–75 kDa. These correspond to previous studies which have been documented to range from 14 kDa to over 180 kDa, with the most abundant proteins falling between 14 kDa and 75 kDa [36,37]. YP exhibits a molecular size within the same range of other common plant-derived proteins, e.g., pea protein isolates (10–100 kDa) [38], sesame protein isolates (15–45 kDa), flaxseed protein isolates (25–48 kDa), and canola protein isolates (16–65 kDa) [39]. This result indicates that the YPs are significantly smaller, by one to two orders of magnitude, compared to the molecules of Alg and XG. The size difference between proteins and polysaccharides shapes their mutual interactions: the larger polysaccharides form networks that restrict mobility and can entrap smaller protein molecules or complexes, which also become integrated between the polysaccharide chains [40].
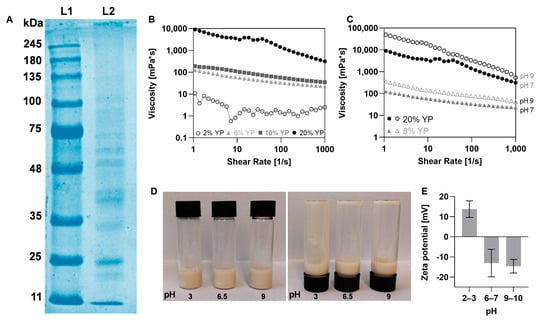
Figure 1.
Yeast protein (YP) insulates characterization. (A) Molecular weight distribution of YP through sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). L1 represents the size marker and L2 represents YP. (B + C) Viscosity vs. shear rate curves of YP at different (B) concentrations and (C) pH conditions. (D) Images of 8% (w/v) YP solutions at different pH conditions. (E) Zeta-potential of YP at different pH conditions.
Next, we investigated the flow properties of YP at different concentrations and pH conditions. As shown in Figure 1B, the viscosity of YP solutions decreased with increasing shear rate, indicating shear-thinning behavior. The highest viscosity was observed for the 20% (w/v) YP solution with approximately 10,000 mPa·s at a low shear rate of 1 1/s. In addition, increasing the pH from 7 to 9 led to an increase in the viscosity at a shear rate of 1 1/s: from 124 to 350 mPa·s for the 8% (w/v) YP solutions and from 10,000 to 50,000 mPa·s for the 20% (w/v) solution (Figure 1C). This effect is attributed to improved protein solubility at higher pH levels. This findings align with Lee et al.’s 2023 [36] research, which demonstrated that YP solubility was higher at pH ≥ 8 compared to pH ≤ 7. Across all tested concentrations and pH levels, the YP remained in a liquid state and did not form a gel, as shown in Figure 1D.
The surface charge of YP is affected by pH levels, primarily due to the presence of α-carboxyl (–COOH) groups in the proteins [36]. As shown in Figure 1E the zeta potential is positive at an acidic pH of 2–3 (13 ± 4 mV) and becomes negative at pH levels of 6–7 and 9–10 (−13 ± 6 mV and −14 ± 3 mV), in accordance with previous research [36].
3.2. YP–Alg Bioink Characterization
To fabricate 3D-printed constructs with post printing structural integrity, we sought to combine YP with biocompatible, food-grade polysaccharides that undergo physical gelation in the presence of biocompatible cross linkers. Alg was selected for its ability to undergo calcium ion-mediated cross-linking via the egg-box mechanism [17], ensuring post-printing structural integrity. Its carboxyl groups possess a negative charge at neutral and basic pH levels, while its hydroxyl groups promote hydrogen bonding [17]. The YP–Alg bioink was prepared under basic pH conditions to improve protein solubility and facilitate molecular interactions between the two macromolecules. Acidic conditions were avoided, although they are reported to enhance protein solubility, as they can cause Alg to collapse due to reduced ionization of its carboxyl groups [17].
Binary mixtures were tested with a fixed YP concentration of 8% (w/v) and Alg concentrations ranging from 0.5% (w/v) to 2% (w/v), as shown at Figure 2 and Table 2. Alg solutions alone at varying concentrations exhibited a nearly Newtonian behavior, characterized by constant viscosity, across shear rates of up to 50 1/s (Figure 2A), consistent with previous studies [41,42]. At higher shear rates the viscosity decreased, indicating shear-thinning behavior only at high deformation rates. The flow behavior index n, calculated from this region, ranged from 0.59 to 0.89 and decreased with an increasing Alg concentration (Table 2). When combined with YP, the binary bioink displayed shear-thinning behavior characterized by viscosity reduction at relatively low shear rates, which is essential for 3D bioprinting, with n values ranging from 0.59 to 0.80 (Figure 2B). Moreover, higher Alg concentrations in the blends resulted in higher n values implying on the role of both components in the shear thinning behavior. These n values are higher than those reported for other protein–polysaccharide systems, such as the tapioca–YP ink (n = 0.14–0.21) [12] and Pea Protein—ĸ-Carrageenan emulsion (n = 0.1–0.24) [43]. In addition, to assess the effect of combining components, we compared the viscosities of the individual components with that of the mixture. The zero viscosity (η0) of the bioink composed of 8% (w/v) YP and 2% (w/v) Alg was around 14,056 mPa·s—much higher than the combined viscosities of the individual components (770 mPa·s for 2% (w/v) Alg and less than 1000 mPa·s for 8% (w/v) YP). This synergistic phenomenon has also been reported in other systems, such as mixtures of cellulose with guar gum [44] and Alg with various hydrocolloids [45]. Synergism was observed across the entire shear rate range (Figure 2C), suggesting possible intermolecular interactions between YP and Alg.
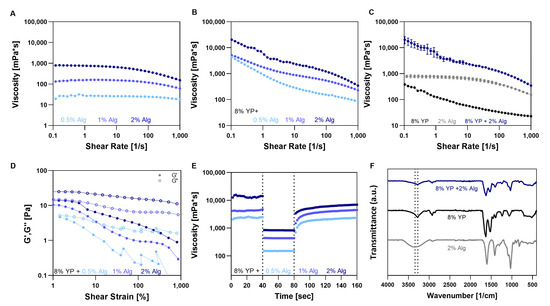
Figure 2.
Alginate (Alg) and Yeast protein (YP) bioink characterization. (A–C) Viscosity vs. shear rate curves of (A) Alg at different concentrations (B) 8% (w/v) YP blends with Alg at different concentrations, and (C) YP, Alg, and their blend. (D) Strain sweeps curves and (E) three-iteration thixotropy test of 8% (w/v) YP blends with Alg at different concentrations. (F) FTIR spectra of YP, Alg, and their blend.

Table 2.
Parameters for the binary mixtures YP–Alg and YP–XG.
Next, we characterized the viscoelastic properties of the YP–Alg bioinks using oscillatory shear experiments. In all mixtures, the storage modulus (G′) was smaller than the loss modulus (G′′) across the entire strain range (Figure 2D), indicating viscous behavior. A liquid-like behavior prior to crosslinking is crucial for extrusion-based bioprinting. This trend was also observed in the Alg solution alone, as shown in Figure S2 in the Supplementary Material.
Viscosity-based thixotropy measurements were conducted to assess the ability of the bioink formulations to regain their rheological properties after shear, under conditions relevant to extrusion-based 3D printing. Using a 3-stage thixotropy test (3-ITT), viscosities were recorded during alternating low (0.5%) and high (300%) strain cycles, simulating shear during extrusion and subsequent rest. A drop in viscosity under high shear followed by thixotropic recovery at low shear indicates favorable flowability and re-establishment of the original viscosity, important for maintaining shape fidelity during printing [46]. The 3-ITT results showed decreasing recovery values with increasing Alg concentration (Figure 2E). Bioinks containing 8% (w/v) YP with 0.5% and 1% (w/v) Alg recovered 89 ± 4% and 97 ± 9% of their initial viscosity, respectively, whereas bioinks with 2% (w/v) Alg demonstrated reduced recovery of 63 ± 9%. This reduction may result from increased self-association of Alg chains at higher concentrations, limiting reversible protein–polysaccharide interactions that support thixotropic recovery. FTIR analysis showed that the characteristic peaks of Alg and YP are preserved in their mixture (Figure 2F). However, a shift in the 3200–3600 cm−1 region (O–H of hydroxyl groups [47]) was observed in the blend, which may indicate hydrogen bonding between the components [48]. Despite both Alg and YP being negatively charged, hydrogen bonding between Alg’s hydroxyl to carboxyl groups and polar regions of YP may help minimize electrostatic repulsion and potentially lead to the observed increase in viscosity and altered rheological behavior.
In conclusion, increasing Alg concentration makes the bioink blend more rigid without forming a gel network, but it also reduces its ability to flow under shear. This highlights a trade-off between structural integrity and printability, as higher rigidity comes at the cost of less favorable rheological properties for extrusion.
3.3. YP–XG Bioink Characterization
Based on the previous results, we hypothesized that combining YP with a polysaccharide exhibiting superior rheological properties is essential for its suitability as a bioink in extrusion bioprinting. XG was selected due to its appropriate flow properties [21,49], which enhance printability [50], and due to its partial cross-linking ability in salt environments [22]. At neutral and basic pH, XG’s carboxyl groups carry a negative charge, while its hydroxyl and ether groups facilitate hydrogen bonding. In addition, despite its hydrophilic nature, XG’s acetyl and pyruvyl side chains can interact with hydrophobic protein regions [21]. As with Alg (Section 3.2), XG was mixed with YP at basic pH to improve protein solubility and promote interactions with XG chains.
YP–XG binary bioink mixtures were tested with a fixed YP concentration of 8% (w/v) and XG concentrations ranging from 0.5% (w/v) to 1.5% (w/v), as shown at Figure 3 and Table 2. XG solutions alone at different concentrations displayed pronounced shear-thinning behavior throughout the entire shear rate range studied, with decreasing flow index n values ranging between 0.13 and 0.34 as their concentration increased (Figure 3A), in agreement with previous studies [51,52]. When mixed with YP, the bioinks retained their shear-thinning nature, with n values ranging from 0.21 to 0.4 (Figure 3B). In addition, increasing XG concentration results in higher zero viscosity η0, with 1.5% XG demonstrating the highest viscosity (about 133,185 mPa·s for XG alone and 448,147 mPa·s with 8% (w/v) YP). The incorporation of YP into the XG network significantly increased the η0 (18,676–448,147 mPa·sec) compared to YP–Alg blend (1292–14,056 mPa·sec), further highlighting the advantage of using XG to enhance bioink stability at the border of the pressure range.
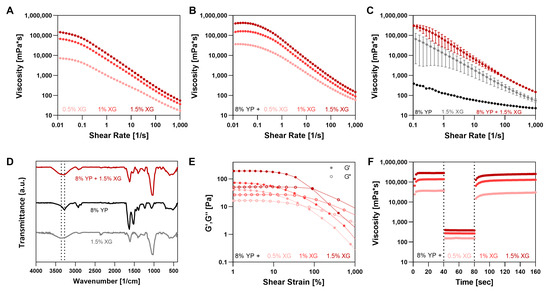
Figure 3.
Characterization of xanthan gum (XG) and yeast protein (YP) bioink. (A–C) Viscosity vs. shear rate for: (A) XG at varying concentrations, (B) 8% (w/v) YP with varying XG concentrations, and (C) YP, XG, and their blend. (D) FTIR spectra of YP, XG, and their blend. (E) Strain sweeps and (F) three-cycle thixotropy tests of 8% (w/v) YP with varying XG concentrations.
The zero viscosity (η0) of the 8% (w/v) YP and 0.5% (w/v) XG bioink mixture was not significantly higher than the sum of the individual components (Figure 3C), unlike the YP–Alg mixture (Figure 2D), indicating a lack of synergistic effect. Similarly, FTIR analysis showed that the characteristic peaks of each component were maintained in the mixture (Figure 3D), with no changes that could suggest significant interactions.
In all mixtures, as well as in XG alone, the storage modulus (G′) within the linear viscoelastic region (1–10% strain) was higher than the loss modulus (G″) before the crossover points (Figure 3E and Figure S2), meeting the formal definition of physical gel network formation although defined as soft due to the small differences between the loss and storage moduli. Moreover, a higher XG concentration led to an increase in both the G′ and the cross over point of the mixture (Table 2). Among all the tested samples, the bioink formulation with 8% (w/v) YP and 0.5% (w/v) XG showed the highest storage modulus and crossover point values (184 ± 7 Pa and 105 ± 13%) indicating an enhanced elastic nature of this blend.
The 3-ITT results demonstrated that the YP–XG mixtures maintained high thixotropic recovery after shear, indicating effective re-establishment of their rheological properties, a key requirement for maintaining shape fidelity during 3D printing. However, as XG concentration increased, a gradual decline in recovery was observed (Figure 3F). The mixture of 8% (w/v) YP with 0.5% (w/v) XG showed complete recovery of 100 ± 1%, while recoveries decreased slightly to 94 ± 4% and 89 ± 5% for blends with 1% and 1.5% (w/v) XG, respectively. Compared to the corresponding Alg–based mixtures, which showed a sharper decline in recovery with dropping from 97 ± 9% at 1% (w/v) Alg to 63 ± 9% at 2% (w/v) Alg, the XG mixtures exhibited more stable thixotropic recovery under increasing polysaccharide concentrations. In comparison, in the tapioca–YP system [12], recovery was evaluated based on the restoration of the storage modulus G′ after high shear (500%) rather than viscosity, and the bioinks exhibited lower recovery levels of 30–80%.
Combining XG with protein enhances printability due to XG’s inherent shear-thinning and gel-forming properties, which support smooth extrusion and structural stability, consistent with findings from previous studies [53,54]. Even at the lowest tested concentration (0.5% w/v), XG provides the desired rheological behavior, including high recovery (100 ± 1%) and a dominant storage modulus, indicating gel-like characteristics and making it effective for printing applications.
3.4. YP–Alg–XG Triple Bioink Characterization
To maximize the complementary benefits of each polysaccharide in combination with YP, where Alg primarily enhances structural stability after cross linking with Ca2+ and XG improves rheological properties, we explored a triple mixture containing Alg, XG, and YP. This approach is supported by the findings of Cofelice et al. (2023) who demonstrated that incorporating 0.4% (w/v) XG to 1% (w/v) Alg significantly improves rheological properties [50]. Specifically, they showed that the addition of XG increased viscosity and storage modulus (G′), which led to a stronger shear-thinning network and an extended linear viscoelastic region. These enhancements were shown to offer greater control over flow and elasticity, critical for applications such as food processing [49].
The YP–Alg–XG bioink mixtures were prepared with a fixed YP concentration of 8% (w/v), while Alg was included at 1% or 2% (w/v), concentrations suitable for effective cross-linking after printing [18,19]. In addition, XG was tested at concentrations of 0.3%, 0.5%, or 1% (w/v) due to its high viscosity and rapid recovery properties, as described previously. (Figure 4, Table 3).
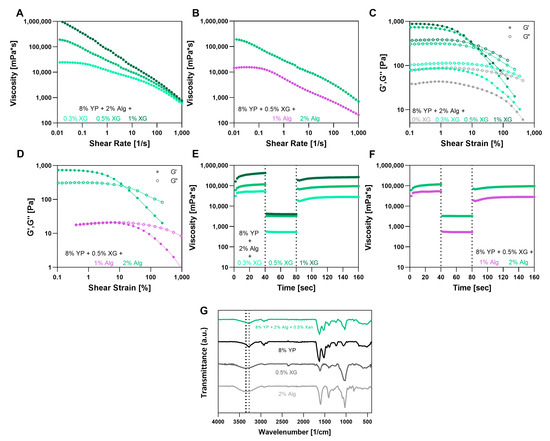
Figure 4.
Alginate (Alg), Xanthan gum (XG) and Yeast protein (YP) triple bioink characterization. (A,B) Viscosity vs. shear rate curves of triple blends at different (A) XG concentrations and (B) Alg concentrations. (C,D) Strain sweep curves of triple blends at different (C) XG concentrations and (D) Alg concentrations. (E,F) Three-cycle thixotropy tests of triple blends at different (E) XG concentrations and (F) Alg concentrations. (G) FTIR spectra of Alg, XG, YP and the triple blend.

Table 3.
Parameters for the triple mixtures of YP–Alg–XG.
All tested bioink formulations exhibited a flow behavior index n below 1, which confirms their shear-thinning nature over a wide range of shear rates. This rheological behavior became more pronounced as the concentration of XG increased, showing a change from n value of 0.86 ± 0.02 without XG to 0.36 ± 0.03 with 1% (w/v) XG (Figure 4A). In contrast, while changes in Alg concentration did not result in a dramatic shift in n values, they did noticeably affect the shear thinning behavior (Figure 4B). In particular, the bioink with 2% (w/v) Alg exhibited higher viscosity values across the entire shear rate range and began to decrease already at low shear rates, indicating an earlier onset of shear thinning compared to the bioink with 1% (w/v) Alg, which showed a more constant viscosity at low shear rate. Notably, only the bioinks containing 8% (w/v) YP, 2% (w/v) Alg, and either 0.5% or 1% (w/v) XG demonstrated a storage modulus (G′) that was higher than the loss modulus (G″). These formulations also exhibited G′ values at least an order of magnitude higher than the other samples, indicating improved mechanical stability and elastic behavior which is crucial for bioinks in extrusion bioprinting (Figure 4C,D).
The thixotropic recovery abilities of the YP–Alg–XG triple mixtures were either comparable to or lower than those of the YP–Alg binary bioinks and were lower than the YP–XG binary bioinks (Figure 4E,F). The highest recovery value, 85 ± 6%, was observed in the mixture comprising 8% (w/v) YP, 2% (w/v) Alg, and 0.5% (w/v) XG. This outcome suggests that interactions and entanglements between the two polysaccharides may increase structural complexity, limiting fully thixotropic recovery. Despite this, all binary and triple mixtures exhibited relatively high thixotropic recovery after shear, supporting the re-establishment of their rheological properties, which is crucial for maintaining shape fidelity in 3D printing [46].
In the FTIR analysis of the triple mixture compared to each individual component (Figure 4G), peaks corresponding to the YP were observed, and no new peaks were detected.
Based on the rheological analysis, we hypothesized that the formulation consisting of 8% (w/v) YP, 2% (w/v) Alg, and 0.5% (w/v) XG has a potential to serve as an edible bioink for 3D printing while leading to desirable post-cross-linking characteristics. This composition provides an optimal balance between flow properties and mechanical stability, exhibiting a pronounced shear-thinning profile with a reduced flow behavior index. In addition, this composition exhibited high G′ values, a dominant elastic behavior (G′ > G″) and sufficient recovery capabilities, which are essential properties for smooth extrusion through the nozzle while maintaining the mechanical stability of the printed filament.
3.5. Mechanical Characterization of Crosslinked Triple Hydrogels
After extrusion and deposition within the support material during the fabrication process, the bioinks undergo cross-linking and are subsequently extracted. As illustrated schematically in Figure 5A, the YP–Alg–XG blend forms an ionically crosslinked network with calcium ions during gelation. Therefore, we aimed to investigate the mechanical properties of the blends at these stages. Alg gels crosslinked ionically with CaCl2 typically experience volume shrinkage during the crosslinking process [55,56]. To evaluate the effect of YP incorporation on this phenomenon, the volumes of the gels relative to the mold volumes were measured after 30 min of incubation in a 0.1 M CaCl2 solution (refer to Section 2.4 in the Methods). As shown in Figure 5B, the binary and triple gels maintained their original dimensions, showing relative volumes of 104 ± 10% and 103 ± 7%, respectively. These slight volume increases above 100% may suggest minimal swelling, likely due to water uptake during crosslinking, which is consistent with the inherent hydrophilicity of the polysaccharide–protein matrix. In contrast, the pure Alg and Alg–XG gels experienced significant shrinkage, resulting in a reduced relative volume of 70 ± 7% and 80 ± 7%, respectively. These results further highlight the advantage of using protein–polysaccharide blends as edible bioinks to enhance dimensional stability during gel formation.
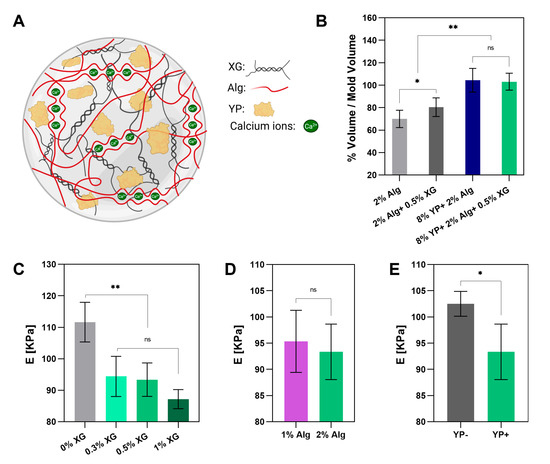
Figure 5.
Mechanical characterization of crosslinked hydrogels. (A) Schematic representation of the YP–Alg–XG hydrogel network crosslinked by calcium ions. (B) Post-crosslinking volume changes in hydrogels. (C–E) Compression modulus of: (C) 8% (w/v) YP + 2% (w/v) Alg with varying XG concentrations, (D) 8% (w/v) YP + 0.5% (w/v) ZG with varying Alg concentrations, (E) 2% (w/v) Alg + 0.5% (w/v) XG with and without 8% (w/v) YP. *, ** and ns refer to the statistically significant differences of p < 0.05, p < 0.01, and not significant, respectively. (Figure 5A created in BioRender. Havazelet, B. (2025) https://BioRender.com/6wq57qp, accessed on 24 October 2025.)
The compression modulus (E) of the triple YP–Alg–XG crosslinked gels was evaluated as a function of XG concentration (Figure 5C), Alg concentration (Figure 5D), and the presence of YP (Figure 5E). The results show that including XG reduces the gels’ compressive modulus; however, no statistically significant differences were observed within the tested range of 0.3–1% (w/v) XG. Similarly, varying the Alg concentration between 1% (w/v) and 2% (w/v) did not significantly affect the modulus. The triple gel containing YP exhibited a lower modulus than the corresponding YP–Alg binary gel despite maintaining the same concentration of each individual component. In contrast, in the tapioca–YP system reported by Kong et al. (2025) [12], the crosslinked bioink exhibited an increase in hardness upon protein addition compared to tapioca alone, demonstrating an opposite trend to that observed in our study. Our findings suggest that both XG and YP contribute to reducing gel stiffness. At the same time, the specific concentrations of XG and Alg, within the tested ranges, have a relatively limited effect on the mechanical properties. These conclusions differ from the rheological results, where higher XG or Alg increased G′ (Table 3). As compression tests used crosslinked gels and rheometer assessed uncross linked mixtures, salt crosslinking may lessen the influence of XG or Alg concentrations.
In compression testing of the crosslinked hydrogels a two-phase stress–strain curves were observed, featuring a lower slope up to ~30–40% strain, followed by a steeper slope (Figure 6A), likely indicating a structural transition occurring under stress. As shown in previous studies [57,58,59], the initial phase may reflect water redistribution and rearrangement of the softer components within the network. As strain increases, the network becomes more compact, and resistance rises due to compression of denser or more rigid domains. To evaluate the durability of the crosslinked hydrogels considering these stress–strain profiles, we conducted a four-cycle compression test (Figure 6C and Figure S3) of the triple cross-linked gel (8% (w/v) YP, 2% (w/v) Alg, 0.5% (w/v) XG), applying four loading–unloading cycles at a rate of 3 mm/min. Each test was performed at a constant maximum strain of either 10%, 20%, 50%, or 70%, applied uniformly across all four cycles. These strain levels were chosen to represent the low-slope (10%, 20%) and high-slope (50%, 70%) regions of the stress–strain curve. The tests conducted at strain levels of up to 50% demonstrated consistent maximum stress across cycles (Figure 6C), highlighting the elastic nature of the hydrogels. At 70% strain, the stress decreased after the first cycle and stabilized over the following three cycles (Figure 6C). This behavior could suggest that an irreversible structural transition may begin to occur under this high strain. These results indicate effective property retention under moderate strain, as further illustrated in Figure 6D, which shows gels before compression and after 50% or 70% deformation.
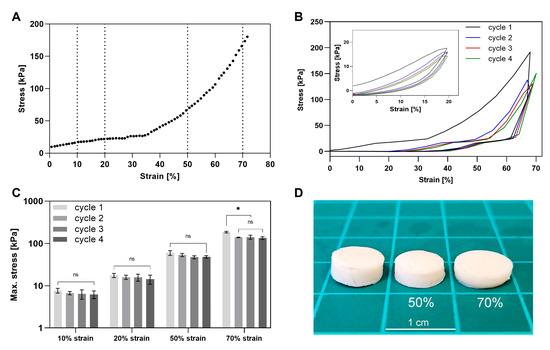
Figure 6.
Cyclic compression test of crosslinked hydrogels. (A) Representative stress–strain curve of a hydrogel under non-cyclic compression up to 70% strain. (B) Stress–strain curves from 4-cycle compression tests at 20% and 70% strain. (C) Maximum stress per cycle during cyclic compression at 10%, 20%, 50%, and 70% strain. (D) Image of gel before compression and after four cycles of 50% or 70% compression. * refer to the statistically significant differences of p < 0.05, and ns to not significant.
3.6. Three-Dimensional Printing of YP–Alg–XG Bioink Blends
After characterizing the rheological and mechanical properties of the triple YP–Alg–XG mixtures, we sought to evaluate their printability using extrusion bioprinting. To enable rapid crosslinking while maintaining the structural integrity of the printed constructs, we employed embedded 3D bioprinting (Figure 7A). In this technique, granular support material serves as an external matrix that stabilizes the deposited filaments during printing and enables rapid crosslinking through various mechanisms [60]. High infill percentages were selected to mimic the dense texture typically required in food or meat-analog printing [20]. In our system, ionic crosslinking was achieved using CaCl2 within CarGrow, a granular support material composed of κ-Carrageenan microgels [34].
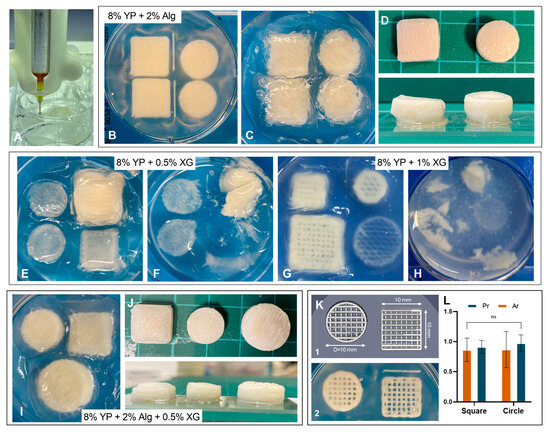
Figure 7.
Embedded 3D printing of edible bioinks. (A) Printing process demonstration within CarGrow. (B–D) 8% (w/v) YP + 2% (w/v) Alg blend: (B,C) images of printed structures after printing, and (D) images of printed objects post-extraction with top and side views. (E–H) 8% (w/v) YP + XG blends: images of (E) 0.5% (w/v) and (G) 1% (w/v) XG within CarGrow after printing. Images of (F) 0.5% (w/v) and (H) 1% (w/v) XG within CarGrow after extraction. (I,J) The triple blends composed of 8% (w/v) YP + 2% (w/v) Alg + 0.5% (w/v) XG (I) within CarGrow support bed and (J) Post-extraction with top and side views. (K) Designed and printed grid structures (square and circular). (L) Ratios of printed-to-designed pore perimeter (Pr) and pore area (Ar) for both geometries.
We printed bulk objects with square and cylindrical geometries within the support material using the following bioinks (Table 1): the binary composition of 8% (w/v) YP + 2% (w/v) Alg, 8% (w/v) YP + 0.5% (w/v) or 1% (w/v) XG and the triple composition of 8% (w/v) YP + 2% (w/v) Alg + 0.5% (w/v) XG. The YP–Alg bioink was successfully printed into the granular bed, forming well-defined and dense structures (Figure 7B,C). However, when attempting to control the infill density of the structures, the results were highly sensitive and inconsistent at both 30% and 50% infill settings, producing overlay dense patterns. Following printing, the structures were successfully extracted due to the rapid ionic crosslinking of the Alg chains (Figure 7D). The resulting constructions were robust, stable, and could be freely handled; however, slight deformation was observed at their lower part. Next, we printed 8% (w/v) YP with either 0.5% (w/v) or 1% (w/v) XG within the granular bed. Since both YP and XG exhibit limited ability to form crosslinked networks in salt, we expected that the printed structures would lack sufficient resolution and could not be extracted post printing. As shown in Figure 7E,G the printed objects exhibited an overall structural geometry similar to those printed with Alg bioink. However, the constructions could not be extracted from the support material due to the absence of gelation at both XG concentrations (Figure 7F,H). These findings, together with the rheological characterization, led to the assumption that the triple YP–Alg–XG blend bioink will offer better performance. In this formulation, Alg provides structural stability through ionic crosslinking, while XG enhances flow properties during extrusion. Therefore, we assessed the printability of the bioink composed of the YP–Alg–XG triple blend at the optimal concentrations identified in Section 3.4: 8% (w/v) YP, 2% (w/v) Alg, and 0.5% (w/v) XG. The printed structures demonstrated high shape fidelity across both geometries and infill densities (6I with 50% infill and Figure 7K with 30% infill). In addition, we could successfully extract the bulk objects printed with 50% infill; the constructions were stable and robust (Figure 7J). These results highlight the potential of the YP–Alg–XG triple blend formulation for fabricating bulk edible structures.
To assess the dimensional fidelity of the printed constructs, grids of the triple blend (8% w/v YP + 2% w/v Alg + 0.5% w/v XG) with 30% infill were analyzed to obtain pore area and perimeter values, which were then compared to the corresponding computer-aided design (CAD) dimensions (Figure 7K). The normalized parameters Ar (pore area) and Pr (pore perimeter) were calculated to quantify the printing accuracy [43]. For the square grid, the average pore area and perimeter were 0.55 ± 0.10 mm2 and 3.32 ± 0.24 mm, respectively, whereas for the circular grid, they were 0.45 ± 0.14 mm2 and 2.92 ± 0.37 mm. The obtained Ar and Pr values ranged between 0.77 ± 0.19 and 0.97 ± 0.14, indicating good overall printing fidelity and limited deviation from the designed constructs (Figure 7L). These values are comparable to those reported by Hendel et al. for pea protein–κ-carrageenan-based printed emulsions, which also demonstrated high shape fidelity [43].
This composition may serve as a standalone material or be combined with other edible components in a layer-by-layer approach to create protein-rich, animal-free food products as demonstrated in previous studies using soy, pea, and other plant-based protein sources [19,20,61].
3.7. Stability Characterization of Crosslinked Hydrogels
An important aspect of food products is their dimensional stability under different conditions. To evaluate gel dimensions under storage conditions, crosslinked hydrogels were incubated in PBS at 4 °C and their relative weight was measured over time (Figure 8A). The Alg–only gel swelled rapidly to 3.5× its initial weight within 24 h, began visibly degrading by day two, and fully dissolved within two weeks. In contrast, the YP–Alg and YP–Alg–XG gels swelled to ~2× their initial weight within the first 50 h and remained stable for at least two weeks, with no significant difference between them. A similar effect was reported in a previous study [27], where the swelling of Agarose, Gellan, and Xanthan gum gels decreased when individually mixed with pea protein. The triple blend printed samples also retained their structure after two days as can be seen at Figure 8B.
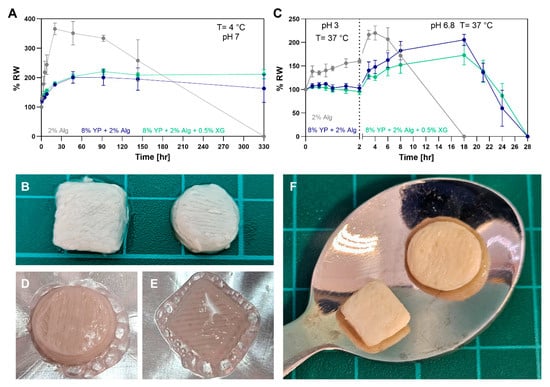
Figure 8.
Stability characterization of hydrogels. (A) Relative weight (RW) over time of Alg, YP–Alg and YP–Alg–XG in PBS (pH 7, 4 °C) for storage. (B) Printed triple blend after two days in PBS (pH 7, 4 °C). (C) Relative weight (RW) over time of Alg, YP–Alg and YP–Alg–XG in simulated digestion: 2 h in PBS simulating gastric fluids (pH 3, 37 °C) followed by PBS simulating intestinal fluids (pH 6.8, 37 °C). (D–F) Printed triple blend (D,E) during and (F) after frying in canola oil at 150 °C for 3 min.
Stability was also tested under simulated pH conditions of the gastrointestinal tract (Figure 8C). Crosslinked hydrogels were first incubated in PBS mimicking the pH conditions of gastric fluids at pH 3 and 37 °C for 2 h, then transferred to pH 6.8 PBS mimicking the pH conditions of intestinal fluids (the buffers content is detailed in Section S4 of the Supplementary Material). As shown in previous studies, Alg gels swell slightly in gastric fluid (pH 3) and more significantly in intestinal fluid (pH 7) [62,63]. This pH-responsive behavior makes them suitable for protecting compounds intended for intestinal release, such as proteins [64], drugs [65], and supplements [62,66]. During the gastric pH phase, the Alg gel swelled by ~1.5×, while the YP–containing gels remained nearly unchanged. Upon transition to intestinal pH conditions, the Alg gel swelled to 200% of its original weight within 2 h and fully dissolved by 18 h. The YP–Alg and YP–Alg–XG gels showed slower swelling, reaching 200% and 150% after 19 h, respectively, and dissolved more gradually, within 28 h. Similarly, freeze-dried RGD-modified Alg scaffolds containing plant proteins (soy and pea protein) showed slower degradation and lower weight loss in PBS at 37 °C compared to protein-free scaffolds [19], supporting our findings that protein incorporation enhances gel stability under physiological conditions.
In contrast to our study, Bodenberger et al. (2016) developed covalently crosslinked YP hydrogels using THPC [10]. These chemically crosslinked networks exhibited swelling ratios of up to twelvefold of the dry mass after ~100 h in PBS at pH 7.4, with swelling decreasing as protein concentration increased due to denser crosslinking. The hydrogels also showed high chemical and thermal stability, maintaining their structure across a broad pH range (3–11) and at temperatures up to 100 °C.
The results reported above indicate that the addition of YP, either alone or in combination with XG, significantly enhances gel stability under both storage and gastrointestinal pH conditions. Gel dissolution occurs when the balance between osmotic swelling pressure and the gel’s elastic resistance is disrupted, a phenomenon described by Flory’s theory [59,60]. In this context, ionic crosslinking relies on physical bond hence offers lower resistance compared to covalent crosslinking. Consequently, when a solvent penetrates these networks, ions diffuse to the solvent along the concentration gradient. The incorporation of YP increases the density of the network and fosters intermolecular interactions, such as hydrogen bonding and entanglements. These enhancements enable the gel network to better withstand disruptive forces in its environment. On the other hand, the denser network may hinder diffusion processes, which are essential for applications such as the controlled release of therapeutic molecules.
As an edible material, we aimed to investigate the response of the printed and cured bioink (YP–Alg–XG) to high temperature. Square and cylindrical structures were printed, extracted from the support material and subsequently fried in canola oil at 150 °C for 5 min (Figure 8D,E). As shown in Figure 8F the resulting objects remained stable and robust with no collapse of the layer-by-layer architecture, probably due to protein denaturation and water loss. These results further highlight the potential of the triple mixture as an edible bioink for fabricating structurally stable, food-grade printed constructs.
Overall, the combined findings highlight the distinct yet complementary roles of each component in determining the performance of the developed bioinks. The binary YP–Alg system exhibited adequate rheological properties for printing but limited shear thinning and structural recovery, which decreased as alginate concentration increased. The constructions from the YP-Alg blend did not fully retain their shape after extraction. In contrast, the YP–XG mixtures showed pronounced shear-thinning behavior and high recovery across all tested concentrations; however, their lack of rapid ionic crosslinking upon contact with the calcium-containing support material limited their suitability for embedded bioprinting. The triple blend formulation YP-Alg-XG integrated the advantages of both systems, yielding inks with balanced flow behavior, rapid post-printing stabilization, and robust structural integrity. In this blend, Alg contributed to ionic crosslinking and shape retention, XG governed shear-thinning and recovery behavior, and YP provided cohesive network formation and nutritional value. Taken together, these results demonstrate that combining a crosslink able polysaccharide with one that improves flow properties offers an effective strategy for designing printable, protein-based soft materials.
4. Conclusions
This work expands the use of yeast protein (YP) into the field of soft material 3D printing, demonstrating its potential as a sustainable and functional component in advanced formulation design. By combining YP with biocompatible polysaccharides, specifically alginate (Alg) and xanthan gum (XG), the research demonstrates how tailored mixtures can create printable formulations with desirable rheological and mechanical properties. The objective was to evaluate how varying compositions of YP, Alg, and XG affect viscosity, shear-thinning behavior, gel strength, swelling capacity, thixotropic recovery, and printability. Binary bioink blends (YP–Alg, YP–XG) and a triple bioink blend (YP–Alg–XG) were characterized and printed using embedded extrusion-based bioprinting.
YP alone remained a liquid without gelation capacity. YP–Alg bioinks showed increased viscosity and shear-thinning, characterized with thixotropic recovery decreased with higher Alg content. YP–XG bioinks formed stronger gel-like network and maintained high recovery after shear and even stronger shear-thinning behavior but exhibited low crosslinking ability after printing. The most favorable properties were observed in the triple bioink blends (8% (w/v) YP + 2% (w/v) Alg + 0.5% (w/v) XG), which achieved high viscosity, elastic modulus, and high recovery abilities. In this triple formulation, Alg provides post-printing structural stability through calcium-induced ionic crosslinking, while XG enhances rheological behavior and shear responsiveness. Crosslinked triple gels resisted shrinkage at crosslinking, retained their structure under compression, and remained stable during storage and under pH conditions simulating digestion. This formulation also enabled the printing of geometrically precise, structurally robust edible constructs, even after frying.
This study proposes a practical method for creating printable, protein-rich materials using YP and polysaccharides. The combination of these three components effectively harnesses their strengths, making it an ideal bioink for developing customized, animal-free food products or soft biomaterials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polysaccharides6040101/s1, Figure S1: Images illustrating preparation process of the cross-linked hydrogels; Figure S2: Strain sweeps and tests of the polysaccharides alone: 0.5% (w/v) XG and 2% (w/v) Alg; Figure S3: Stress–strain curves of hydrogels under cyclic and non-cyclic compression. (A) Representative stress–strain curve of a hydrogel under non-cyclic compression up to 70% strain. (B–E) four-cycle compression curves at (B) 10%, (C) 20%, (D) 50%, and (E) 70% strain.
Author Contributions
Conceptualization, O.P.-E., N.H. and H.B.-P.; Methodology, M.D.-P. and S.L.; Investigation, O.P.-E. and N.H.; Writing—original draft, O.P.-E. and N.H.; Writing—review and editing, M.D.-P., S.L. and H.B.-P.; Supervision, H.B.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
Havazelet Bianco-Peled is the incumbent of the Wolfson Chair in Chemical Engineering, which supported this work. The schematic representation in the graphical abstract was created in BioRender. Peleg, O. (2025) https://BioRender.com/0z9006o, accessed on 14 August 2025.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, J.; Sun, Y.; Meng, D.; Zhou, Z.; Zhang, Y.; Yang, R. Yeast proteins: The novel and sustainable alternative protein in food applications. Trends Food Sci. Technol. 2023, 135, 190–201. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Agboola, J.O.; Øverland, M.; Skrede, A.; Hansen, J.Ø. Yeast as major protein-rich ingredient in aquafeeds: A review of the implications for aquaculture production. Rev. Aquac. 2021, 13, 949–970. [Google Scholar] [CrossRef]
- Cao, X.; Liu, H.; Yang, M.; Mao, K.; Wang, X.; Chen, Z.; Ran, M.; Hao, L. Evaluation of the nutritional quality of yeast protein in comparison to animal and plant proteins using growing rats and INFOGEST model. Food Chem. 2025, 463, 141178. [Google Scholar] [CrossRef]
- Wang, S.; Huang, F.; Zhao, Y.; Ouyang, K.; Xie, H.; Xiong, H.; Zhang, Y.; Chen, Z.; Zhao, Q. Slow-digestive yeast protein concentrate: An investigation of its in vitro digestibility and digestion behavior. Food Res. Int. 2023, 174, 113572. [Google Scholar] [CrossRef]
- Timira, V.; Chen, X.; Zhou, P.; Wu, J.; Wang, T. Potential use of yeast protein in terms of biorefinery, functionality, and sustainability in food industry. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13326. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, F. Plant Protein Heat-Induced Gels: Formation Mechanisms and Regulatory Strategies. Coatings 2023, 13, 1899. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Yang, G.; Kong, H.; Guo, L.; Wei, G. Recent advances in protein hydrogels: From design, structural and functional regulations to healthcare applications. Chem. Eng. J. 2023, 451, 138494. [Google Scholar] [CrossRef]
- Ma, C.; Xia, S.; Song, J.; Hou, Y.; Hao, T.; Shen, S.; Li, K.; Xue, C.; Jiang, X. Yeast protein as a novel protein source: Processing, functional properties, and potential applications in foods. Innov. Food Sci. Emerg. Technol. 2024, 93, 103606. [Google Scholar] [CrossRef]
- Bodenberger, N.; Kubiczek, D.; Paul, P.; Preising, N.; Weber, L.; Bosch, R.; Hausmann, R.; Gottschalk, K.-E.; Rosenau, F. Beyond bread and beer: Whole cell protein extracts from baker’s yeast as a bulk source for 3D cell culture matrices. Appl. Microbiol. Biotechnol. 2017, 101, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, Z.; Munawar, N.; Zan, L.; Zhu, J. 3D edible scaffolds with yeast protein: A novel alternative protein scaffold for the production of high-quality cell-cultured meat. Int. J. Biol. Macromol. 2024, 259, 129134. [Google Scholar] [CrossRef]
- Kong, Y.; Chen, J.; Guo, R.; Huang, Q. Regulation of tapioca starch 3D printability by yeast protein: Rheological, textural evaluation, and machine learning prediction. J. Food Eng. 2025, 387, 112341. [Google Scholar] [CrossRef]
- Xia, S.; Shen, S.; Song, J.; Li, K.; Qin, X.; Jiang, X.; Xue, C.; Xue, Y. Physicochemical and structural properties of meat analogues from yeast and soy protein prepared via high-moisture extrusion. Food Chem. 2023, 402, 134265. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Song, J.; Li, K.; Hao, T.; Ma, C.; Shen, S.; Jiang, X.; Xue, C.; Xue, Y. Yeast protein-based meat analogues: Konjac glucomannan induces the fibrous structure formation by modifying protein structure. Food Hydrocoll. 2023, 142, 108798. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Injectable and 3D Bioprinted Polysaccharide Hydrogels: From Cartilage to Osteochondral Tissue Engineering. Biomacromolecules 2017, 18, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. State-of-the-art of 3D printing technology of alginate-based hydrogels—An emerging technique for industrial applications. Adv. Colloid Interface Sci. 2021, 293, 102436. [Google Scholar] [CrossRef]
- Ianovici, I.; Zagury, Y.; Redenski, I.; Lavon, N.; Levenberg, S. 3D-printable plant protein-enriched scaffolds for cultivated meat development. Biomaterials 2022, 284, 121487. [Google Scholar] [CrossRef]
- Ianovici, I.; Zagury, Y.; Afik, N.; Hendel, M.; Lavon, N.; Levenberg, S. Embedded three-dimensional printing of thick pea-protein-enriched constructs for large, customized structured cell-based meat production. Biofabrication 2024, 16, 045023. [Google Scholar] [CrossRef]
- Patel, J.; Maji, B.; Moorthy, N.S.H.N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef]
- Vaishnav, A.; Choudhary, D.K. (Eds.) Microbial Polymers: Applications and Ecological Perspectives; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. A Guide to Polysaccharide-Based Hydrogel Bioinks for 3D Bioprinting Applications. Int. J. Mol. Sci. 2022, 23, 6564. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Cai, L.; Zeng, Q.; Wang, P. Protein and protein-polysaccharide composites-based 3D printing: The properties, roles and opportunities in future functional foods. Int. J. Biol. Macromol. 2024, 272, 132884. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Arslan, M.; Li, Z.; Cen, S.; Shi, J.; Huang, X.; Xiao, J.; Zou, X. Application of Protein in Extrusion-Based 3D Food Printing: Current Status and Prospectus. Foods 2022, 11, 1902. [Google Scholar] [CrossRef]
- Wollschlaeger, J.O.; Maatz, R.; Albrecht, F.B.; Klatt, A.; Heine, S.; Blaeser, A.; Kluger, P.J. Scaffolds for Cultured Meat on the Basis of Polysaccharide Hydrogels Enriched with Plant-Based Proteins. Gels 2022, 8, 94. [Google Scholar] [CrossRef]
- Khoeini, R.; Nosrati, H.; Akbarzadeh, A.; Eftekhari, A.; Kavetskyy, T.; Khalilov, R.; Ahmadian, E.; Nasibova, A.; Datta, P.; Roshangar, L.; et al. Natural and Synthetic Bioinks for 3D Bioprinting. Adv. NanoBiomed. Res. 2021, 1, 2000097. [Google Scholar] [CrossRef]
- Sun, J.; Yang, X.; Diao, J.; Wang, Y.; Wang, C. Exploration of Pea Protein Isolate–Sodium Alginate Complexes as a Novel Strategy to Substitute Sugar in Plant Cream: Synergistic Interactions Between the Two at the Interface. Foods 2025, 14, 991. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Zuev, Y.F. Interaction-Induced Structural Transformations in Polysaccharide and Protein-Polysaccharide Gels as Functional Basis for Novel Soft-Matter: A Case of Carrageenans. Gels 2022, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Rashid, A.I.; Waghmare, P.R.; Nobes, D.S. Measurement of the flow behavior index of Newtonian and shear-thinning fluids via analysis of the flow velocity characteristics in a mini-channel. SN Appl. Sci. 2020, 2, 1787. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, H.; Li, Z.; Dai, J.; Cong, H.-P.; Yu, S.-H. Highly compressible and environmentally adaptive conductors with high-tortuosity interconnected cellular architecture. Nat. Synth. 2022, 1, 975–986. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Wang, C.; Huang, G. A facile method to fabricate hybrid hydrogels with mechanical toughness using a novel multifunctional cross-linker. RSC Adv. 2017, 7, 35311–35319. [Google Scholar] [CrossRef]
- Hen, N.; Josef, E.; Davidovich-Pinhas, M.; Levenberg, S.; Bianco-Peled, H. On the Relation between the Viscoelastic Properties of Granular Hydrogels and Their Performance as Support Materials in Embedded Bioprinting. ACS Biomater. Sci. Eng. 2024, 10, 6734–6750. [Google Scholar] [CrossRef]
- Machour, M.; Hen, N.; Goldfracht, I.; Safina, D.; Davidovich-Pinhas, M.; Bianco-Peled, H.; Levenberg, S. Print-and-Grow within a Novel Support Material for 3D Bioprinting and Post-Printing Tissue Growth. Adv. Sci. 2022, 9, e2200882. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, E.; Jo, M.; Choi, Y.J. Characterization of yeast protein isolates extracted via high-pressure homogenization and pH shift: A promising protein source enriched with essential amino acids and branched-chain amino acids. J. Food Sci. 2024, 89, 900–912. [Google Scholar] [CrossRef]
- Li, J.; Karboune, S. A comparative study for the isolation and characterization of mannoproteins from Saccharomyces cerevisiae yeast cell wall. Int. J. Biol. Macromol. 2018, 119, 654–661. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Duijsens, D.; Mukherjee, A.; Wilde, P.J. Pea protein extraction method impacts the protein (micro)structural organisation and in vitro digestion kinetics. Food Funct. 2024, 15, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Sibt-E.-Abbas, M.; Butt, M.S.; Riaz, M.N.; Teferra, T.F.; Ul-Haq, I. Amino Acid Profiling and SDS-PAGE Analysis of Protein Isolates Obtained from Nonconventional Sources. J. Food Qual. 2022, 2022, 1926527. [Google Scholar] [CrossRef]
- De Kruif, C.; Tuinier, R. Polysaccharide protein interactions. Food Hydrocoll. 2001, 15, 555–563. [Google Scholar] [CrossRef]
- Belalia, F.; Djelali, N.-E. Rheological properties of sodium alginate solutions. Rev. Roum. Chim. 2014, 59, 135–145. [Google Scholar]
- Brzezińska, M.; Szparaga, G. The Effect of Sodium Alginate Concentration on The Rheological Parameters Of Spinning Solutions. Autex Res. J. 2015, 15, 123–126. [Google Scholar] [CrossRef]
- Hendel, G.; Hen, N.; Levenberg, S.; Bianco-Peled, H. Pea Protein—ĸ-Carrageenan Nanoparticles for Edible Pickering Emulsions. Polysaccharides 2025, 6, 14. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Kong, T. Aqueous polysaccharide blends based on hydroxypropyl guar gum and carboxymethyl cellulose: Synergistic viscosity and thixotropic properties. Colloid Polym. Sci. 2006, 285, 145–151. [Google Scholar] [CrossRef]
- Kamdem, I.E.N.; Saidou, C.; Ngassoum, M.B.; Ndjouenkeu, R. Synergistic interactions in dilute aqueous solutions between alginate and tropical vegetal hydrocolloids. Heliyon 2020, 6, e04348. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, S.; Zhou, Q.; Wang, X.; Hu, J.; Zhou, P. HIPE-gels performance: Role of sodium hyaluronate conformation and concentration in structure and 3D printing. Food Biosci. 2025, 67, 106361. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Peleg-Evron, O.; Davidovich-Pinhas, M.; Bianco-Peled, H. Crosslinking konjac-glucomannan with kappa-carrageenan nanogels: A step toward the design of sacrificial materials. Int. J. Biol. Macromol. 2023, 227, 654–663. [Google Scholar] [CrossRef]
- Cofelice, M.; Messia, M.C.; Marconi, E.; Cuomo, F.; Lopez, F. Effect of the xanthan gum on the rheological properties of alginate hydrogels. Food Hydrocoll. 2023, 142, 108768. [Google Scholar] [CrossRef]
- Xu, J.; Fan, Y.; Liu, H.; Liu, Q.; Zhamsaranova, S.; Kong, B.; Chen, Q. Improvement of rheological properties and 3D printability of pork pastes by the addition of xanthan gum. LWT 2023, 173, 114325. [Google Scholar] [CrossRef]
- Kaczmarczyk, K.; Kruk, J.; Ptaszek, P.; Ptaszek, A. Pressure Drop Method as a Useful Tool for Detecting Rheological Properties of Non-Newtonian Fluids during Flow. Appl. Sci. 2021, 11, 6583. [Google Scholar] [CrossRef]
- Caballero, M.S.; de Monterrey, T.; Valdez-Fragoso, A.; Salinas-López, A.; Chanes, J.W.; Verardo, V.; Mújica-Paz, H. Rheological parameters of xanthan gum/pectin solutions as afunction of temperature and composition. Rev. Mex. Ing. Quimica 2016, 15, 859–868. [Google Scholar] [CrossRef]
- Baniasadi, H.; Kimiaei, E.; Polez, R.T.; Ajdary, R.; Rojas, O.J.; Österberg, M.; Seppälä, J. High-resolution 3D printing of xanthan gum/nanocellulose bio-inks. Int. J. Biol. Macromol. 2022, 209, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Moeinzadeh, S.; Kim, C.; Pan, C.-C.; Weale, G.; Kim, S.; Abrams, G.; James, A.W.; Choo, H.; Chan, C.; et al. Development and systematic characterization of GelMA/alginate/PEGDMA/xanthan gum hydrogel bioink system for extrusion bioprinting. Biomaterials 2023, 293, 121969. [Google Scholar] [CrossRef]
- Wan, L.Q.; Jiang, J.; Arnold, D.E.; Guo, X.E.; Lu, H.H.; Mow, V.C. Calcium Concentration Effects on the Mechanical and Biochemical Properties of Chondrocyte-Alginate Constructs. Cell. Mol. Bioeng. 2008, 1, 93–102. [Google Scholar] [CrossRef]
- Girón-Hernández, J.; Gentile, P.; Benlloch-Tinoco, M. Impact of heterogeneously crosslinked calcium alginate networks on the encapsulation of β-carotene-loaded beads. Carbohydr. Polym. 2021, 271, 118429. [Google Scholar] [CrossRef]
- Buyanov, A.; Gofman, I.; Saprykina, N. High-strength cellulose–polyacrylamide hydrogels: Mechanical behavior and structure depending on the type of cellulose. J. Mech. Behav. Biomed. Mater. 2019, 100, 103385. [Google Scholar] [CrossRef]
- Sartore, L.; Manferdini, C.; Saleh, Y.; Dey, K.; Gabusi, E.; Ramorino, G.; Zini, N.; Almici, C.; Re, F.; Russo, D.; et al. Polysaccharides on gelatin-based hydrogels differently affect chondrogenic differentiation of human mesenchymal stromal cells. Mater. Sci. Eng. C 2021, 126, 112175. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, A.; Christiansen, J.D. Tension–compression asymmetry in the mechanical response of hydrogels. J. Mech. Behav. Biomed. Mater. 2020, 110, 103851. [Google Scholar] [CrossRef]
- McCormack, A.; Highley, C.B.; Leslie, N.R.; Melchels, F.P. 3D Printing in Suspension Baths: Keeping the Promises of Bioprinting Afloat. Trends Biotechnol. 2020, 38, 584–593. [Google Scholar] [CrossRef]
- Barkay-Olami, H.; Zilberman, M. Novel porous soy protein-based blend structures for biomedical applications: Microstructure, mechanical, and physical properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1109–1120. [Google Scholar] [CrossRef]
- Wu, L.; Schroën, K.; Corstens, M. Structural stability and release properties of emulsion-alginate beads under gastrointestinal conditions. Food Hydrocoll. 2024, 150, 109702. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Hogan, S.A.; López-Rubio, A.; Brodkorb, A. Nano- and microstructural evolution of alginate beads in simulated gastrointestinal fluids. Impact of M/G ratio, molecular weight and pH. Carbohydr. Polym. 2019, 223, 115121. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; McClements, D.J. Control of protein digestion under simulated gastrointestinal conditions using biopolymer microgels. Food Res. Int. 2017, 100, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita, R.; Sirbu, I.-O.; Lobiuc, A.; Covasa, M. Sodium Alginate–Starch Capsules for Enhanced Stability of Metformin in Simulated Gastrointestinal Fluids. Biomimetics 2024, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Luo, X.; Zhao, Z.; Yuan, J.; Song, Y.; Liu, C.; Huang, M.; Dong, L.; Xie, H.; Cai, L.; et al. Enhancing viability of Lactobacillus plantarum encapsulated by alginate-gelatin hydrogel beads during gastrointestinal digestion, storage and in the mimic beverage systems. Int. J. Biol. Macromol. 2023, 224, 94–104. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).