Abstract
The synthesis of novel biodegradable polymers as non-viral vectors remains one of the challenging tasks in the field of gene delivery. In this study, the synthesis of the polysaccharide-g-polypeptide copolymers, namely, hyaluronic acid-g-polylysine (HA-g-PLys), using a copper-free strain-promoted azide-alkyne cycloaddition reaction was proposed. For this purpose, hyaluronic acid was modified with dibenzocyclooctyne moieties, and poly-L-lysine with a terminal azido group was obtained using ring-opening polymerization of N-carboxyanhydride of the corresponding protected amino acid, initiated with the amino group azido-PEG3-amine. Two HA-g-PLys samples with different degrees of grafting were synthesized, and the structures of all modified and synthesized polymers were confirmed using 1H NMR and FTIR spectroscopy. The HA-g-PLys samples obtained were able to form nanoparticles in aqueous media due to self-assembly driven by electrostatic interactions. The binding of DNA and model siRNA by copolymers to form polyplexes was analyzed using ethidium bromide, agarose gel electrophoresis, and SybrGreen I assays. The hydrodynamic diameter of polyplexes was ˂300 nm (polydispersity index, PDI ˂ 0.3). The release of a model fluorescently-labeled oligonucleotide in the complex biological medium was significantly higher in the case of HA-g-PLys as compared to that in the case of PLys-based polyplexes. In addition, the cytotoxicity in normal and cancer cells, as well as the ability of HA-g-PLys to facilitate intracellular delivery of anti-GFP siRNA to NIH-3T3/GFP+ cells, were evaluated.
1. Introduction
Gene therapy opens new horizons for treating numerous genetic diseases or preventing certain medical disorders by correcting the underlying genetic problem [1]. Many types of biochemical pathways can be modulated by delivering nucleic acids such as DNA, mRNA, siRNA, or miRNA into cells. Therapeutic gene-based drugs face numerous barriers between administration and delivery to target cells [2]. The main obstacle to the delivery of nucleic acids is their entry into the cell through the cytoplasmic membrane and release from early endosomes. Being negatively charged, nucleic acids are unable to overcome this barrier. Another key obstacle to successful delivery is the low stability of nucleic acids in vivo due to rapid degradation by nucleases. These limitations have been successfully addressed through the use of appropriate delivery systems [3,4].
Systems for gene delivery can be classified into viral and non-viral ones [5,6,7]. Viral delivery systems demonstrate excellent cellular transfection [5,8]. The most commonly used delivery vectors are retroviruses, lentiviruses, adenoviruses, and adeno-associated viruses. Despite their high efficacy, viral vectors also have serious drawbacks, such as limitations in the size of the genetic construct that can be delivered, random recombination (i.e., oncogenic potential), cytotoxicity, and immunogenicity [9]. These disadvantages, as well as the high costs associated with large-scale production, quality control of viral vectors, and special requirements for storage conditions, have increasingly focused attention on the development of non-viral systems.
To date, a wide range of non-viral nucleic acid delivery systems such as liposomes, polymeric nanoparticles, inorganic nanoparticles, and others have been presented in the literature [5,10,11,12,13]. The advantages of non-viral delivery systems, however, often demonstrate lower transfection efficiency compared to viral systems, which is the reason for active development in this field. Gene delivery systems that successfully perform their functions must meet multiple requirements. They must effectively encapsulate and protect genetic constructs, bypass premature elimination mechanisms, achieve cellular internalization, and not be toxic to cells and tissues. The development of delivery systems that meet all these criteria is a challenging task. Among the existing systems, polymeric ones attract the most attention since their properties can be easily adjusted by varying the chemical structure, composition, and chain length.
Interpolyelectrolyte complexes (IPECs) of nucleic acids with polycations, which are also referred to as polyplexes, are of considerable interest for gene delivery [10,11,14]. Thus, a common feature of most polymers designed for nucleic acid delivery is the presence of polycationic fragments. This is necessary both to bind negatively charged nucleic acids and to facilitate interaction with the negatively charged cell membrane. The polycations considered for nucleic acid delivery include synthetic polymers such as polyethylenimine (PEI) [15,16,17], poly(N,N-dimethylethylenediamine methacrylate) and its copolymers [18,19], poly(β-amino esters) [20,21], and cationic polypeptides [22,23,24] such as polylysine (PLys) [25], polyornithine (POrn) [26], and polyarginine (PArg) [27]. Also, they include some natural polymers such as diethylaminoethyl dextran (DEAE) [28], chitosan [29], gelatin [30], and their modified derivatives [31,32].
Unlike many synthetic polymers, cationic polypeptides and natural polymers have positive features, such as biocompatibility and biodegradability. Considering these, polypeptides and polysaccharides are widely used in combination to imitate synthetic viral capsids. For instance, the synthesis of dextran-b-poly(γ-benzyl-L-glutamate) by Cu+-mediated azide-alkyne 1,3-dipolar cycloaddition is reported [33]. The same Cu+-catalyzed click reaction was used by Liu et al. to synthesize chitosan-g-PLys dendrons [34]. Another approach for the synthesis of chitosan-g-PLys was proposed by Yu et al. [35]. In particular, the authors used ring-opening polymerization (ROP) of ε-benzoxycarbonyl L-lysine N-carboxyanhydride (Lys(Z) NCA) initiated by the amino groups of 6-O-triphenylmethyl chitosan. It was shown that the gene transfection ability of chitosan-g-PLys was better than that of chitosan. Other than the mentioned techniques, the conjugation of dextran to polyhistidine (PHis) using the terminal aldehyde group of the polysaccharide and the terminal amino group of PHis to produce dextran-b-PHis was reported by Hwang et al. [36]. The syntheses of dextran-g-PLys copolymers by a reductive amination reaction between PLys·HBr and dextran followed by the study of complexation with DNA was performed by Ferdous et al. [37]. Thus, based on the reported studies it can be noted that the synthesis of polysaccharide-g/b-polypeptide copolymers has been performed using (1) neutral polysaccharide and charged polypeptide, or (2) similarly charged polysaccharide and polypeptide.
It is known that cationic polypeptides, especially PLys, are characterized by excessively strong binding of the nucleic acid. This, in turn, contributes to low release from the polyplex, resulting in low transfection efficacy. In order to improve the transfection efficacy, the PLys structure is often modified to produce Lys-based amphiphilic copolymers [38], pH-sensitive copolymers [39], as well as containing special moieties responsible for interaction with cell surface receptors [40,41,42]. Such modifications favor cell transfection and intracellular gene release. Recently, it was shown that the introduction of negatively charged fragments into polymers [43] or the use as gene delivery systems complexes of PLys with negatively charged polysaccharide heparin [44] facilitates the release of nucleic acids and enhances cell transfection.
Although complexes of HA and PLys can form nanoparticles [45,46] and can be used for gene delivery, the use of their copolymers can provide several advantages. In particular, copolymers form more homogeneous nanocomplexes with DNA/RNA compared to physical complexes. Furthermore, physical mixtures of polymers can dissociate when the pH of the medium or its composition changes, whereas copolymers form more stable nanocomplexes with nucleic acids, which reduces premature release. In addition, the possible release of PLys from physical mixtures may contribute to cell membrane damage, while in copolymers, PLys is fixed on HA, which reduces cytotoxicity. Thus, in this study, we proposed the synthesis of a graft-copolymer containing a weak polyanionic polysaccharide, namely hyaluronic acid (HA), as the main chain and strong polycation, namely PLys, as side chains.
Earlier, Laga et al. reported the conjugation of PLys to HA via oxidation of HA to introduce aldehyde groups followed by reaction with PLys amino groups [47]. This approach is suitable for the preparation of coatings or cross-linked hydrogels but not graft-copolymers. Synthesis of HA-g-PLys faces several challenges. First of all, to graft PLys to HA, the carboxylic groups of HA should be modified or activated, but PLys should contain terminal functionality suitable for the reaction with modified/activated HA. This task can be solved successfully by click reactions such as azide-alkyne 1,3-dipolar cycloaddition [48] or thiol-ene click reaction [49]. Despite the high efficiency of click reactions catalyzed by copper(I) (CuAAC) in the synthesis of graft-copolymers [50,51], the use of metal catalysts may be a problem for further biomedical applications of synthesized copolymers because of the potential toxicity of metal ions chelated by copolymers. The use of thiolene chemistry also has some drawbacks. For example, UV-initiated thiolene reactions (250–365 nm) can damage biopolymer structures. Residues of free thiols (e.g., from cysteamine) can cause oxidative stress in cells, and maleimide groups can react with serum thiols (e.g., albumin). Thus, careful control of residual unreacted groups and purification of the final products is required.
In this study, a copper-free strain-promoted azide-alkyne cycloaddition reaction (SPAAC) was selected as a more predictable method for the synthesis of biomedical copolymers such as HA-g-PLys [52]. Unlike CuAAC, SPAAC does not have such a drawback as the possible complexation of copolymers with Cu ions and, at the same time, proceeds with high reactivity and selectivity under mild reaction conditions [52]. Using SPAAC, here we synthesized and characterized two HA-g-PLys samples differing in the content of grafted PLys. The HA-g-PLys samples obtained were able to form nanoparticles in aqueous media due to self-assembly driven by electrostatic interactions. In addition, the polyplexes of HA-g-PLys and DNA or model siRNA were formed and characterized with regard to their physicochemical characteristics and completeness of nucleic acid binding. The ability of HA-g-PLys to release model nucleic acid and provide intracellular delivery of siRNA was also demonstrated.
2. Materials and Methods
2.1. Chemicals, Solvents, and Supplements
Hyaluronic acid sodium salt (from Streptococcus equi, molecular weight of 1500–1700 kDa), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) hydrochloride (EDC) (≥98%), N-hydroxysuccinimide (NHS) (98%), ε-carboxybenzyl-L-lysine (Lys(Z)) (≥99%), triphosgene (98%), α-pinene (99%), 33% hydrobromide solution in acetic acid, deuterium oxide (D2O, 99.9%), dimethyl sulfoxide-d6 (DMSO-d6, 99.8%), chloroform-d (CDCl3, 99.8%) were purchased from Sigma-Aldrich (Darmstadt, Germany) and used as received. N-hydroxysuccinimide ester of DBCO (DBCO-NHS, >95%), azide-PEG3-amine (N3-PEG3-NH2, >95%) were products of Lumiprobe (Moscow, Russia). Trifluoroacetic acid (≥99.9%) and N-Boc-1,2-diaminoethane (N-Boc-ethylenediamine, EDA-Boc, 98%) were the products of Carl Roth (Karlsruhe, Germany) and Aladdin Scientific (Los Angeles, CA, USA).
Organic solvents, namely 1,4-dioxane, diethyl ether, petroleum ether, dimethyl sulfoxide (DMSO), dimethylformamide (DMF), and ethyl acetate, were purchased from Vecton (St. Petersburg, Russia). Anhydrous solvents purified using standard protocols were used for the synthesis and isolation of Lys(Z) N-carboxyanhydride and graft-copolymers.
Cellulose membranes with molecular weight cut-off (MWCO) of 1000, 10,000, and 25,000 produced by Orange Scientific (Braine-l’Alleud, Belgium) were used for the purification of PLys, HA-g-PLys, and HA, respectively.
Agarose, ethidium bromide, 4× Gel Loading Dye, and 1 kb DNA Ladder were purchased from Eurogen (Moscow, Russia). DNA from salmon testes and DNAse were purchased from Sigma-Aldrich (Darmstadt, Germany). Oligonucleotides—Fam-dT18, dT18 and dA18, as well as anti-GFP siRNA, were ordered from DNA-Synthesis (Moscow, Russia). SYBR Green I 10,000× dye concentrate in DMSO was a product of Thermo Fisher Scientific (Bremen, Germany). Scrambled siRNA (27 bp) for control (sense 5′-GUAAGUGUAAACAACACGACAUCCUUC-3′ and antisense: 3′-CAUUCACAUUUGUUGUGCUGUAGGAAG-5′) was synthesized by GenTerra (Moscow, Russia). GenJect™-40 transfection agent was purchased from MOLECTA (Moscow, Russia).
Salts and alkalis used for the preparation of buffer solutions were products of Vecton (St. Petersburg, Russia). Deionized water was used for the preparation of all solutions. All buffers before use were filtered through the 0.45 µm membrane filters (Millipore, Sigma-Aldrich, Darmstadt, Germany).
Cell lines (human lung epithelial cells (BEAS-2B), human adenocarcinoma cells (A549), human embryonic kidney cells (HEK-293), and mouse fibroblasts (NIH-3T3/GFP+)) were received from the Institute of Cytology of the Russian Academy of Sciences (St. Petersburg, Russia). Cells were cultured using Corning T-75/T-175 vials (Sigma-Aldrich, Darmstadt, Germany) using basal media DMEM (Sigma-Aldrich, Darmstadt, Germany) and LHC-9 (ThermoFisher Scientific, Bremen, Germany). Trypsin, fetal bovine serum (FBS), solutions of penicillin (10,000 U/mL) and streptomycin (10 mg/mL) were products of Biochrom GmbH (Berlin, Germany). Cell experiments were performed in 96-well plates (SPL Lifesciences, Pocheon, Gyeonggi, Republic of Korea).
2.2. Methods
2.2.1. Modification of HA with EDA(Boc)
HA (100 mg, 0.25 mmol carboxyl groups, 1 eq.) was dissolved in 0.1 M MES (pH 5.6) with a concentration of 5 mg/mL under stirring and heating to 50 °C. The solution was allowed to cool to room temperature, and NHS (115 mg, 0.5 mmol, 2 eq.) pre-dissolved in 1 mL of 0.1 M MES (pH 5.6) was added to the reaction mixture under stirring (900 rpm) for 5 min. Then, EDC (57 mg, 0.3 mmol, 1.2 eq.) pre-dissolved in 1 mL of 0.1 M MES (pH 5.6) was added to the reaction mixture, and the system was left for another 30 min to obtain activated carboxyl groups. The pH of the solution was then adjusted accurately to 8.3 with a 1 M solution of NaOH. After that, EDA(Boc) dissolved in 2 mL of 1,4-dioxane was added to the reaction mixture at the EDA(Boc)/-COOH ratios (mol/mol) equal to 1/1 and 3/1. The reaction mixtures were stirred at room temperature for 24 h. The modified HA was purified using dialysis (MWCO 25,000) against a water/methanol mixture (50:50, v/v) to remove unreacted EDA(Boc) for 24 h. Dialysis against water was then performed for 8 h, and to remove electrostatically bound amines against a 1 M solution of NaCl for 8 h. Finally, the dialysis against water for 24 h was performed to remove all salts. The resulting solutions were lyophilized. The degree of modification was determined from 1H NMR spectra by relating the integrals of the proton signals of the acetyl and tert-butyl groups in HA-EDA(Boc). 1H NMR (500 MHz, D2O), δ (ppm): 1.36 (3CH3 of tBu), 1.95 (CH3-CO), 3.07–3.78 (molecular backbone, 10H), 4.38–4.48 (anomeric protons, 2H).
2.2.2. Deprotection of HA-EDA(Boc)
HA covalently modified with EDA(Boc) (100 mg) was dissolved in 15 mL of 4 M HCl solution in anhydrous 1,4-dioxane. The reaction mixture was stirred at 20 °C for 5 h. Dialysis of the HA-EDA samples after deprotection was performed against a water/methanol mixture (50/50, v/v) using dialysis bags with MWCO 1000 for 24 h, water for 8 h, and to regenerate the amino groups, against 0.1 M NaOH solution for 12 h and, finally, against water for 24 h. The resulting solutions were lyophilized. The completeness of the reaction was determined by 1H NMR spectroscopy by disappearance of tert-butyl group signals (1.35–1.36 ppm) in the spectra of the deprotected product (HA-EDA).
2.2.3. Modification of HA-EDA with DBCO-NHS
A solution of DBCO-NHS (20 mg, 0.046 mmol, 2 eq.) in 10 mL of DMF was added to a solution of HA-EDA, containing primary amino groups (70 mg, 0.023 mmol amino groups, 1 eq.) pre-dissolved in 5 mL of 0.1 M PBS (pH 7.4). The reaction mixture was stirred at room temperature for 24 h. After that, the modified product (HA-DBCO) was purified using dialysis against a water/DMF mixture (50/50, v/v) using membrane bags with MWCO 1000 for 24 h and then against water for 24 h. The resulting solutions were lyophilized. The degree of modification of HA-EDA with DBCO was calculated from the 1H NMR spectra by relating the integral signal intensities of the acetyl group protons of HA and aromatic protons of DBCO to each other.
2.2.4. Synthesis of PLys with Terminal Azide Functionality
Polymerization of Lys(Z) NCA (monomer, M) was carried out in freshly distilled ethyl acetate. A 4% solution of monomer in 1,4-dioxane was prepared, after which a solution of N3-PEG3-NH2 as initiator was added to the reaction mixture to achieve a molar ratio of [M]/[I] = 30. Further, the reaction mixture was left under stirring at 25 °C for 48 h. The resulting polymer was precipitated with a three-fold volume of cooled diethyl ether relative to the volume of the reaction mixture and centrifuged (10,000× g, 5 min). After decantation of the diethyl ether, the precipitate was dried in a vacuum desiccator overnight. The yield of N3-PLys(Z) was 86%.
N3-PLys(Z) (150 mg) was dissolved in 1.5 mL of TFA under stirring. After complete dissolution of the polymer, a 33% solution of HBr in acetic acid (0.38 mL, 4-fold excess to the amount of Z groups) was added to the reaction mixture. The reaction mixture was left to stir at room temperature for 2 h. Then, the deblocked polymer was precipitated with a three-fold excess of cold diethyl ether and separated by centrifugation (10,000× g, 5 min). The precipitate was dissolved in water and dialyzed against water for 24 h (MWCO 1000). The resulting solution was lyophilized. The yield of N3-PLys was 76%.
2.2.5. Synthesis of HA-g-PLys by Metal-Free Click Reaction
A solution of N3-PLys (37 mg, 0.020 mmol, 4 eq azide) in 9 mL of 0.1 M acetate buffer solution (pH 4.0) was added to a solution of HA-DBCO (50 mg, 0.005 mmol, 1 eq alkyne) in 10 mL of 0.1 M acetate buffer (pH 4.0). The reaction mixture was stirred at 22 °C for 24 h. The obtained graft-copolymer was purified with dialysis using the membrane bags with MWCO 10,000 against deionized water for 12 h, 1 M NaCl solution for 12 h, and against water for 24 h. The resulting solution was freeze-dried. The grafting degree (GD) was calculated from the 1H NMR spectra of the graft-copolymers obtained. GD values were calculated as the ratio of integral proton intensities of characteristic signals of PLys (-CH2-NH-, 2.91–2.95 ppm) to those of HA (-CH3-CO-, 1.95 ppm) multiplied by the ratio of DP in these copolymers.
2.2.6. Characterization of Polymers
The structure and composition of polymers were analyzed using 1H NMR and FTIR spectroscopy. 1H NMR spectra were recorded using a Bruker 500 MHz Avance III spectrometer (Billerica, MA, USA). FTIR spectra in KBr tablets were recorded using an IRAffinity-1 Fourier transform spectrometer (Shimadzu, Kyoto, Japan).
Size-exclusion chromatography (SEC) was performed using a Styragel Column chromatography column (HMW6E, particle size 15–20 μm, column size 7.8 mm × 300 mm; Waters, Milford, CT, USA) installed into a Shimadzu HPLC system (Kyoto, Japan) consisting of LC-10AD plunger pump, degasser DGU-14A, low-pressure mixer FCV-10AL and refractometric RID-10A detector, controller SCL-10A and equipped with LC Solution GPC software, version 1.25 (Shimadzu, Kyoto, Japan).
2.2.7. Formation of HA-g-PLys/siRNA Polyplexes
HA-g-PLys was dissolved in water at a concentration of 1 mg/mL and exposed to ultrasound for 30 s. The HA-g-PLys/siRNA polyplex was obtained by adding a calculated aliquot of a siRNA stock solution to a copolymer solution with the required concentration to reach the desired N/P values (ratio of moles of amino groups of the polymer to moles of phosphate groups of siRNA). After combining the solutions containing siRNA and copolymer, they were immediately vortexed (1000 rpm/min) and left for equilibrating at 22 °C for 1 h to form stable polyplexes.
2.2.8. Physicochemical Characterization of Polyplexes
For the determination of the physicochemical characteristics of polyplexes, the hydrodynamic diameter (DH), polydispersity indexes (PDI), and zeta-potential were measured for the aqueous dispersions (0.1–0.2 mg/mL) of nanoparticles by dynamic and electrophoretic light scattering (Zetasizer Nano ZS, Malvern, UK). Polyplexes obtained were also analyzed using TEM (Jeol JEM-2100, Kyoto, Japan). Samples were prepared by dropping an aqueous nanoparticle dispersion (0.2 mg/mL) onto carbon/formvar-coated Cu-grids (300 mesh), air-drying, and staining with 2% (w/v) uranyl acetate (30–60 s). Excess stain was blotted, and grids were stored at room temperature for 24 h before imaging. Dry-state nanoparticle diameters were identified from TEM images using ImageJ software, version 1.53v (NIH, Bethesda, MD, USA).
2.2.9. Study of Nucleic Acids Binding Efficacy
(1) Ethidium Bromide Assay for DNA
To study the efficiency of DNA binding by HA-g-PLys, the 96-well flat-bottomed plates were used. The wells were filled with a dispersion of HA-g-PLys nanoparticles in 0.01 M Tris·HCl buffer (pH 7.8). Then, a DNA solution (300 ng/well) and ethidium bromide (4 μL, 0.1 mg/mL) were added to each well to achieve the pre-calculated N/P ratios in the range of 2–10 and mixed by pipetting. The total volume of solution per well was 100 µL. The plate was incubated under shaking at 25 °C for 30 min. After that, the fluorescence intensity was measured at λex/em = 530/590 nm using a fluorescent plate reader Varioskan LUX (Thermo Fisher Scientific, Waltham, MA, USA). The efficacy of binding was evaluated by calculating the relative fluorescence (%) using the following equation:
where F is the fluorescence signal intensity of ethidium bromide in the presence of polyplex, F0 is the fluorescence intensity of ethidium bromide in the absence of DNA, and FDNA is the fluorescence intensity of ethidium bromide in the presence of free DNA.
(2) SybrGreen I Assay for siRNA
The efficiency of siRNA binding to the polymer was analyzed using the SybrGreen I binding assay [53]. With this aim, the freshly prepared solutions of HA-g-PLys@siRNA polyplexes (90 µL), containing 6 pmol siRNA per well at selected N/P ratios, were placed in the wells of a 96-well flat-bottomed plate. The plate was incubated at 22 °C for 30 min, and then 10 µL of 100× SybrGreen I solution was added to each well. After that, the fluorescence intensity was measured at wavelengths λex/em = 497/520 nm using a fluorescent plate reader. The unbound siRNA amount (%) is proportional to relative fluorescence calculated using Equation (1), but for fluorescence measured for free SybrGreen I (Fo) and SybrGreen I in the presence of free siRNA (FsiRNA) or the polyplex (F).
2.2.10. Agarose Gel Electrophoresis Assay
The completeness of HA-g-PLys DNA binding and its stability in the polyplex in the presence of DNAse was assessed using agarose gel electrophoresis. The fresh solutions of HA-g-PLys@DNA at N/P ratios of 0.2–10 were prepared and incubated for 1 h at 25 °C. Next, 4X Gel Loading buffer was added to the particle solutions (15 µL, 1 mg/mL). In the case of the stability study, 1 µL DNAase solution (0.13 units/mL) was added to 15 µL HA-g-PLys@DNA (1 mg/mL). The resulting mixture was incubated at 37 °C for 1 h. Electrophoresis (80 V 15 min, 100 V 45 min) was performed in 1% agarose gel, pre-stained with ethidium bromide (0.5 mg/mL), and using TAE buffer (40 mM Tris-acetate, 1 mM EDTA). Fluorescence imaging was taken at a wavelength of 392 nm. Agarose gel electrophoresis was carried out using a BlueMarine 100 horizontal chamber and an electrophoresis instrument SERVA (Catoosa, OK, USA). Gel images were documented using a GE Healthcare Life Sciences AI600 Imager system (Marlborough, MA, USA).
2.2.11. Release Study of Model siRNA from Polyplexes
In these experiments, FAM-dT18 was used for the release study from the polyplexes with HA-g-PLys. The study was performed for the polyplexes prepared at the N/P ratio = 10 in 0.01 M phosphate saline buffer solution (PBS) (pH 7.4) at 37 °C. At certain time intervals, namely, 1, 2, 6, 10, and 24 h, aliquots of 200 µL were taken and centrifuged using membrane tubes (MWCO 30,000). The filtrate was collected and analyzed for fluorescence signal of free FAM-dT18 (λex/em = 495/517 nm). The amount of free model siRNA was calculated using a calibration plot previously built for solutions with concentrations of 1.0, 0.5, 0.25, 0.125, and 0.0625 pmol/μL.
The percent amount of released FAM-dT18 (Q, %) was calculated using the following equation:
where C is a determined concentration of a solution at a certain time point (µg/mL), V is a solution volume at a certain time point (mL), and Q0 is a loaded amount of oligonucleotide (µg).
2.2.12. Biological Evaluation
A549 and NIH 3T3/GFP+ cells were cultured in basal DMEM medium supplemented with 10% FBS and 1% penicillin-streptomycin. BEAS-2B cells were cultured in LHC-9 basal medium supplemented with 10% FBS and 1% penicillin-streptomycin.
(1) Cytotoxicity
BEAS-2B, A549, and HEK-293 cells were used to evaluate the cytotoxicity of the HA-g-PLys nanoparticles. The CellTiterBlue (CTB) assay, based on the ability of living cells to convert resazurin into fluorescent resorufin, was used for the study. Cells were seeded into wells of a 96-well plate at a concentration of 8000 cells/100 μL of medium. After 24 h of incubation under humidified conditions (5% CO2, 37 °C), the medium was removed, and the dispersions of nanoparticles in culture medium at concentrations of 100, 50, 25, 12.5, 6.25 μg/mL were added to the wells with adhered cells. After incubating the cells with nanoparticles for 24 h, the medium was aspirated, and 100 μL of CTB solution in medium (medium to CTB ratio 10:1) was added to each well. Cells were incubated at 37 °C for 1 h, and the fluorescence (λex = 544 nm, λem = 590 nm) was measured by a Fluoroscan Ascent plate reader(Thermo Fisher Scientific, Waltham, MA, USA). Cells incubated without the tested nanoparticles were used as a negative (blank) control. The viability of cells in the presence of tested nanoparticles was calculated with respect to the control using the following equation:
where As is the optical density of the tested sample, Ac is the optical density of the control, and Ab is the optical density of the medium.
(2) Gene silencing
NIH 3T3/GFP+ cells were used to test the efficiency of GFP gene silencing by anti-GFP siRNA. For this purpose, 5000 cells per well of a 96-well plate were seeded in 100 µL of medium and incubated under humidified conditions (5% CO2, 37 °C) for 24 h. After that, the medium was removed, and 200 µL of a suspension of polyplexes in culture medium containing anti-GFP siRNA and the appropriate amount of polymer from the calculated N/P value was added to each well. Scrambled siRNA was used as a negative control (siControl). As a positive control system, the delivery of anti-GFP siRNA by commercial GenJect-40 transfecting agent was carried out. The concentration of siRNA in all cases was 50 nmol/L. After 72 h after transfection, the fluorescence intensity of the cells was assessed using flow cytometry (NovoCyte Combo Package, ACEA Biosciences, Agilent, Santa Barbara, CA, USA).
2.2.13. Statistics
The results are presented as the mean value ± SD (n = 3 for physicochemical experiments and n = 4 for biological experiments). Statistical significance of biological results was analyzed using a t-test (GraphPad Software, version Prism 10.1.2, La Jolla, CA, USA). p < 0.05 was considered a statistically significant difference.
3. Results and Discussion
3.1. HA-g-PLys Synthesis and Characterization
Since the aim of the study was to produce HA-g-PLys and to evaluate the ability of this copolymer to serve as an siRNA delivery system, the first step was to synthesize the target graft-copolymer using metal-free click chemistry, specifically SPAAC. For this purpose, a dibenzocyclooctyne-containing derivative of hyaluronic acid (HA-DBCO) and PLys containing an azide group at the terminal position (N3-PLys) were initially synthesized.
3.1.1. Modification of Hyaluronic Acid
In order to introduce the DBCO moiety into the HA structure, HA was first modified with N-Boc-ethylenediamine (EDA(Boc)) (Figure 1). For this, the carboxyl groups of HA were pre-activated using the EDC/NHS system. Conjugation of amines to carboxyl groups using the EDC/NHS activation is a widely used method for the efficient formation of amide bonds [54].
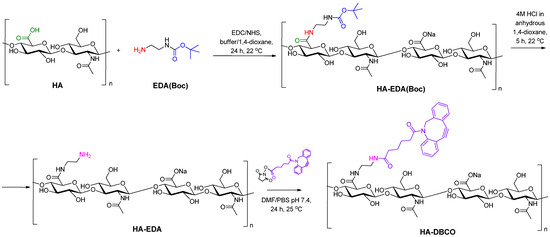
Figure 1.
Scheme of HA modification with Boc-ethylenediamine (EDA(Boc)) and then with dibenzocyclooctyne moiety (DBCO).
Ratios of EDA(Boc) to carboxyl groups of HA were varied during the reaction (Table 1). HA samples covalently modified with EDA(Boc) were characterized by 1H NMR spectroscopy. The degree of modification of HA was calculated by the ratio of the integral intensities of the signals of the acetyl group of the N-acetylglucosamine fragment (δ = 1.95 ppm) and the Boc-group of EDA(Boc) (δ = 1.36 ppm) (Supplementary Materials, Figure S1). Increasing the content of EDA-Boc in the reaction medium led to an increase in the degree of HA modification (substitution degree, SD) (Table 1). The removal of the Boc protecting group then led to the liberation of a primary amino group, enabling further modification (Figure 1). The disappearance of the Boc-group signal in the 1H NMR spectra (δ = 1.36 ppm) indicated the complete deprotection (Supplementary Materials, Figure S2).

Table 1.
The ratios of reagents and modification degrees in the reactions of HA modification with EDA and then DBCO.
Finally, a reaction of HA-EDA with DBCO-NHS ester was performed (Figure 1). The reaction was carried out in a DMF/PBS (pH 7.4) mixture to achieve solubility of all reagents. The success of modification was confirmed using 1H NMR spectroscopy with the appearance of signals characteristic of DBCO, namely aromatic protons at δ = 7.28–7.60 ppm and methylene protons of -CH2-NCO- moiety at 5.13 ppm (Supplementary Materials, Figure S3). Initially, the DBCO-NHS ester to amino groups of HA-EDA ratio was set as 2. In this case, the modification was successful only for HA-EDA containing 7% EDA. At the same time, the efficacy of modification of the HA-EDA sample containing 14% EDA with DBCO was only 52%. A two-fold increase in the initial DBCO-NHS ester/amino groups of HA-EDA ratio allowed for total DBCO conjugation (Table 1). Thus, samples of HA containing 7 and 14% DBCO groups in the HA structure (HA-DBCO-7 and HA-DBCO-14) were obtained and used in further click reaction.
The method of HA functionalization with DBCO proposed in this study has not been previously described. Comparison of our results with previously reported data on HA modification by small functional molecules demonstrates their consistency. Recently, Fu et al. reported the modification of low molecular weight HA with 2-(cyclooct-2-ynyloxy)ethanamine [55]. Despite using an excess of amino-containing cyclooctyne, the authors estimated the degree of substitution of 25%, indicating that approximately every fourth disaccharide unit was substituted. Similarly, Takahashi et al. used a stepwise procedure to modify HA, introducing an azide-containing linker, 4-azidobutanoic acid, instead of the cyclooctyne moiety [56]. Comparison of modification of low molecular weight (80,000) and high molecular weight (800,000) HA revealed similar degrees of substitution, ranging from 10.8 to 12.6%. Han et al. reported modification of HA with amino-PEG4-DBCO, achieving a degree of substitution of 13% [57], which is similar to our results.
3.1.2. Synthesis of Poly(L-Lysine) with Terminal Azido Groups
N3-PLys was synthesized using ring-opening polymerization (ROP) of Lys(Z) NCA and N3-PEG3-NH2 as an initiator and further removal of Z-protective groups. Synthesis of homopolypeptides by ROP with terminal functionality provided by a primary amine-containing functional molecule is well known [33,58,59]. In our case, the reaction was carried out in anhydrous 1,4-dioxane for 48 h using [M]/[I] = 30. The used initiator contained a primary amino group, which launches ROP of NCA under normal (amine) mechanism of polymerization (Figure 2). As a result, the final polypeptide contains an azido group in the terminal position, which is necessary for the SPAAC grafting reaction.
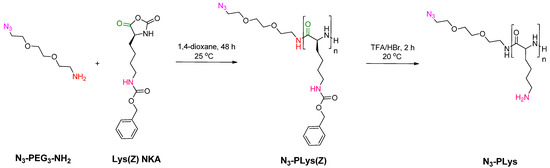
Figure 2.
Scheme of the synthesis of PLys containing terminal azide functionality (N3-PLys) by ROP of Lys(Z) NCA using N3-PEG3-NH2 as initiator.
The structure of the resulting polypeptide was confirmed using 1H NMR spectroscopy (Supplementary Materials, Figure S4). The degree of polymerization (DP) was determined by comparing the average integral intensities of the methylene protons of -O-CH2-C6H5 group (δ = 4.94–4.97 ppm) and -CH2-NH- group (δ = 2.91–2.95 ppm) in PLys(Z) to the integral intensity of methylene protons of -CH2-NH- group in the initiator (δ = 3.34–3.36 ppm). The calculated DP was found to be 29. SEC analysis revealed a number average molecular weight (Mn) of 7150 and dispersity (Đ) of 1.23 for the resulting N3-PLys(Z). The DP value calculated from Mn determined by SEC was 27, which is consistent with the NMR data. The disappearance of the benzyl protecting group signals (4.98 ppm, -O-CH2-C6H5 and 7.2–7.3 ppm, -C6H5) in the 1H NMR spectra of PLys after deblocking indicates complete deprotection of the polypeptide (Supplementary Materials, Figure S5). To confirm the presence of the azide functionality in the resulting polypeptide, the sample was analyzed using FTIR spectroscopy (Figure 3). In the FTIR spectrum, the characteristic bands at 3295 and 3070 cm−1 correspond to the -NH and NH3+ stretching vibrations in the peptide bond and side chains of PLys, respectively. The bands at 2928 and 2867 cm−1 are attributed to the -CH2 stretching vibrations in the side chain of PLys. The absorption bands at 1656 and 1544 cm−1 are characteristic of the amide I and amide II. A band at 2116 cm−1 corresponds to the stretching vibration of the azido group. Thus, the used approach allowed the successful introduction of azide functionality into PLys at the polymerization step.
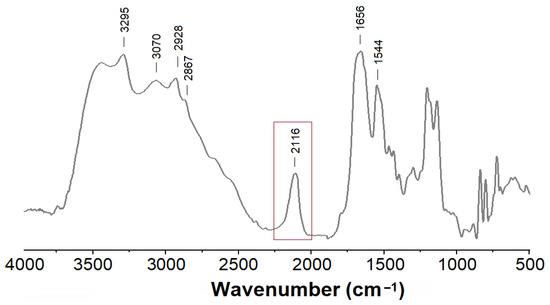
Figure 3.
FTIR spectrum of N3-PLys.
3.1.3. Synthesis of Graft-Copolymers by Metal-Free Click Reaction
In the synthesis of the graft-copolymer, PLys chains containing terminal azido groups were grafted to HA-DBCO via SPAAC (Figure 4). It is known that the efficiency of the click reaction depends on a number of factors and can vary from rather low (~30%) to very high values (75–100%) [60,61,62,63]. In particular, high grafting densities can be achieved by increasing the excess of grafting polymer chains (up to 4), extending the reaction time (up to 3–7 days), and sometimes using elevated temperature (30–40 °C). Low grafting densities are typically achieved with a grafting component excess of 1.2–2.0 and a reaction time of 24–48 h.
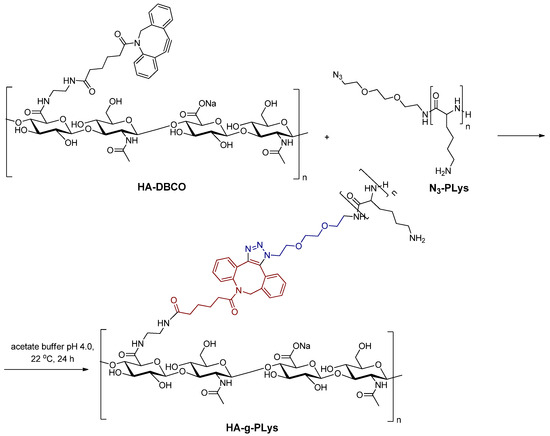
Figure 4.
Scheme of the HA-g-PLys synthesis by SPAAC of HA-DBCO and N3-PLys.
In order to prepare graft-copolymers, HA-DBCO containing 7 and 14% DBCO units was used. Given the relatively low theoretical grafting densities of 7 and 14%, the reaction was carried out for 24 h at room temperature (22 °C) using an excess of N3-PLys. The reaction was carried out in acetate buffer at pH 4.0 (pKa of HA is between 3 and 4 [64]) to avoid the formation of interpolyelectrolyte complexes. The resulting graft-copolymers were purified using dialysis through membrane bags, allowing for the removal of unreacted N3-PLys as described in Section 2.2.5. The structure of the obtained products was confirmed using FTIR and 1H NMR spectroscopy (Figure 5).
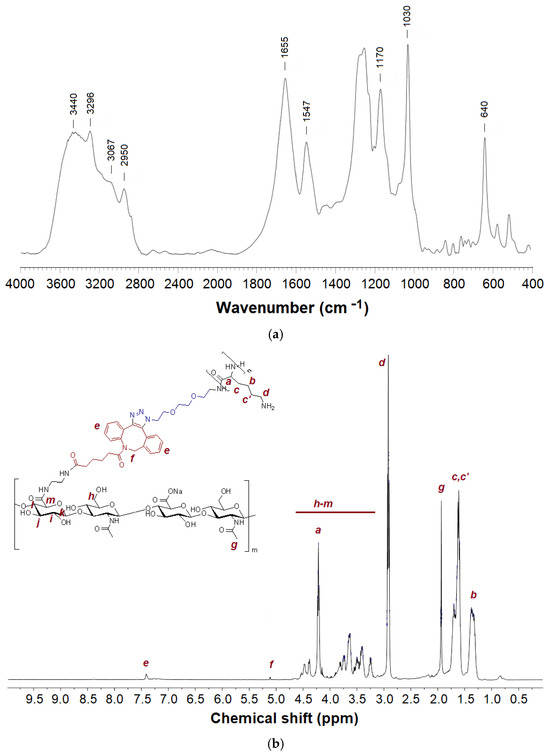
Figure 5.
FTIR (a) and 1H NMR (b) spectra of HA-g-PLys.
In the FTIR spectrum of HA-g-PLys, the signals of both HA and PLys are clearly detected (Figure 5a) [65,66]. Specifically, the characteristic bands at 3440, 3295, and 3067 cm−1 correspond to hydrogen-bonded -OH and -NH groups in HA and PLys, -NH stretching vibrations in the N-acetyl group and peptide bonds of PLys as well as -NH3+ groups in the side chains of PLys, respectively. The signal at 2948 cm−1 can be attributed to the -C-H stretching vibrations in both HA and PLys. The absorption bands at 1655 and 1548 cm−1 are characteristic of the amide I and amide II in PLys. At the same time, the bands at 1170, 1030, and 640 cm−1 belong to asymmetrical stretching vibrations of the -C-O-C- group (O-bridge), stretching vibrations of the -C-O- group, and out-of-plane bending vibrations of the -O-H group in HA, respectively. Furthermore, the disappearance of the band corresponding to the azido group, present in N3-PLys, confirms the success of the click reaction.
Similar to FTIR spectroscopy, analysis of graft-copolymers using 1H NMR spectroscopy also revealed the signals from both the main polysaccharide chain (HA) and the grafted PLys (Figure 5b). The grafting degrees, calculated from the ratio of integral intensities of specific proton signals in HA and PLys, were found to be 2.7% for HA-DBCO-7 and 4.5% for HA-DBCO-14. Thus, the grafting reaction efficiencies were 38% for HA-DBCO-7 and 32% for HA-DBCO-14. Such moderate grafting efficacy values may be explained by two reasons. First, unlike modification with small molecules such as EDA and DBCO, grafting of polypeptides involves steric hindrance and consequently limited availability of the terminal functionality of the polypeptide. Second, the high viscosity of the reaction system due to HA also imposes a limitation on the efficiency of the grafting process [67].
3.2. Formation and Characterization of Empty Nanoparticles and Polyplexes with Model siRNA
Since HA-g-PLys contains both negatively and positively charged polymer chains, the graft-copolymers can form IPECs in aqueous medium due to self-assembly driven mainly by electrostatic interactions of oppositely charged functional groups and, to a lesser extent, hydrophobic interactions of the DBCO moieties. Indeed, direct dissolution of HA-g-PLys in water resulted in the formation of positively charged nanoparticles with relatively large hydrodynamic diameters (DH) (Table 2).

Table 2.
Characteristics of empty nanoparticles and their complexes with model siRNA and DNA (DLS and ELS measurements in water, concentration 0.1 mg/mL; dT-dA (18 bp) was used as a physicochemical model of siRNA).
DNA or a physicochemical model of siRNA was used to evaluate the formation of polyplexes between HA-g-PLys and nucleic acid. Specifically, an oligothymidine-oligoadenine duplex (dT-dA, 18 bp) was used as a siRNA model to form polyplexes and assess their physicochemical characteristics. The polyplexes were prepared in water by direct dissolution of the components under vigorous mixing. In contrast to the empty nanoparticles, the polyplexes exhibited smaller sizes and lower polydispersity indices (Table 2). This suggests that the empty nanoparticles are quite loose, while polyplexes are more compact due to more pronounced electrostatic cross-linking. Furthermore, increasing the content of nucleic acid (model siRNA or DNA) in the polyplex promoted the formation of more compact nanoparticles. As expected, the formation of polyplexes was accompanied by a noticeable reduction in the zeta-potential values, resulting from the partial compensation of the positive charge. These results are in line with previously reported data [68]. Furthermore, the characteristics of the obtained polyplexes meet the requirements for drug delivery systems (DH < 300 nm and PDI < 0.3) [69,70,71]. Comparison of DNA polyplexes produced using HA-g-PLys and a mixture of HA and PLys homopolymers (HA/PLys) showed that larger nanoparticles were formed in the latter case (Table 2).
The morphology of HA-g-PLys-2@dT-dA polyplexes formed at N/P ratios of 10 and 50 was examined by TEM (Figure S6, Supplementary Materials). Both samples showed spherical nanoparticles, with average diameters of 185 ± 75 nm (N/P = 50) and 126 ± 15 nm (N/P = 10). The smaller nanoparticle size observed by TEM compared to DLS likely results from dehydration during sample preparation on the TEM grid.
In addition, DNA binding efficiency was investigated by detecting the decrease in fluorescence intensity of ethidium bromide (EtBr) as a result of binding to the copolymer. When EtBr binds to DNA, the fluorescence intensity of the dye increases due to the intercalation effect. Subsequently, when the copolymer is added to the resulting complex, the latter displaces the bound EtBr, resulting in a decrease in fluorescence intensity. The N/P ratio at which the fluorescence intensity reaches a plateau is optimal for complete DNA binding by this polycation. As can be seen from Figure 6a, the complete binding of DNA by HA-g-PLys-2 occurs at the N/P > 2. This result is also supported by agarose gel electrophoresis, indicating the absence of free DNA in the polyplexes obtained at the N/P > 2 (Figure 6b). In turn, the use of N/P < 1, such as 0.5 or 0.2, indicates incomplete DNA binding, which was confirmed using the presence of a band of free DNA for these samples in agarose gels after electrophoresis (Figure 6b).
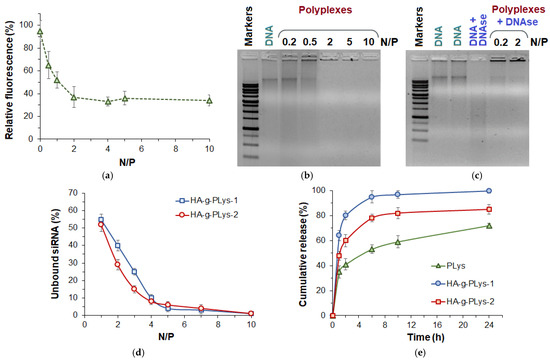
Figure 6.
EtBr assay (a) and agarose gel electrophoresis (b,c) results demonstrating the efficiency of DNA binding by copolymer at various N/P ratios (a,b) and stability in the presence of polyplexes (c). CybrGreen I assay results demonstrating the presence of unbound dT-dA (as physicochemical model of siRNA) at different N/P ratios (d) and cumulative release of Fam-dT18 from polyplexes with HA-g-PLys and PLys (0.01 M PBS/DMEM + FCS = 50/50, 37 °C) (e).
An important property of nucleic acid delivery systems is the ability to protect nucleic acid from the destructive action of enzymes. The ability of HA-g-PLys/DNA polyplexes to stabilize DNA was studied in the presence of DNAase. As can be seen from Figure 6c, free DNA is completely degraded in the presence of DNAse. At the same time, no degradation of DNA in complex with HA-g-PLys was observed even at high DNA loading (Figure 6c).
In the case of siRNA, a SybrGreen I assay was performed to evaluate the efficiency of binding. SybrGreen I dye is known to form complexes with double-stranded nucleic acids, causing a conformational change in the dye that reduces its mobility. This, in turn, results in the release of its energy in the form of fluorescence [72]. Consequently, the fluorescence of the dye increases significantly when bound to free double-stranded nucleic acids (siRNA/DNA). However, nucleic acid molecules complexed with the polymer in the polyplex are not accessible for interaction with the dye. To determine the efficiency of model siRNA complexation, the composition of the polyplexes was varied across a range of N/P ratios from 1 to 10. As shown in Figure 6d, the synthesized HA-g-PLys samples demonstrated complete siRNA binding at N/P ratios of 5 and above.
3.3. Release Study
To investigate the effect of HA in the polyplex on nucleic acid release, Fam-labeled dT18 (Fam-dT18) was used to prepare polyplexes with HA-g-PLys. Additionally, PLys was used as a control polymer for comparison. The release study was performed in a 0.01 M PBS (pH 7.4)/DMEM + FCS mixture (50/50, v/v) at 37 °C using polyplexes obtained at an N/P ratio of 10. As expected, the polyplexes formed from different polymers exhibited distinct release profiles (Figure 6e). In particular, the slowest release was observed for PLys as carrier, with less than 70% release within 24 h. In contrast, the use of HA-g-PLys as a carrier resulted in faster nucleic acid release. Notably, the fastest oligonucleotide release was observed for HA-g-PLys-1, which contains the lowest amount of PLys, achieving 90–100% release within 24 h. The observed trends are attributed to the presence of HA in the polyplex, which acts as a competing weak polyanion, reducing the electrostatic binding of the nucleic acid to the polycation.
For comparison, Gonzalez-Ferreiro et al. reported oligonucleotide release of 15–55% within 24 h from alginate/PLys microparticles, depending on their preparation method [73]. Jia et al. studied siRNA release from light-sensitive PEGylated amphiphilic polypeptide polyplexes in PBS solution [74]. They observed strong retention of siRNA by the amphiphilic cationic polypeptide in the absence of light (only 5% release within 6 h), with enhanced release upon light exposure. Similarly, Osipova et al. reported limited siRNA release (less than 10% over 5 days) from amphiphilic cationic polypeptides containing both lysine and glutamic acid units in PBS and DMEM media [75]. However, incubation of polyplexes in DMEM medium containing serum facilitated siRNA release due to competitive displacement by negatively charged bovine serum albumin (~60% after 3 days). In contrast, polyplexes with PLys as the delivery system released no more than 10% under the same conditions. Compared to the amphiphilic polypeptides reported by Osipova et al., the faster release observed with HA-g-PLys-based polyplexes may be attributed to the lower content of hydrophobic components, which provide additional retention through multiple hydrophobic interactions in amphiphilic polypeptides [75].
3.4. Biological Evaluation
3.4.1. Cytotoxicity
The cytotoxicity of HA-g-PLys was evaluated in human lung epithelial cells (BEAS-2B), human lung adenocarcinoma cells (A549), and human embryonic kidney cells (HEK-293) after 24 h of co-incubation (Figure 7). The number of viable cells was determined using the CellTiter-Blue (CTB) assay, which measures the ability of live and metabolically active cells to convert resazurin to resorufin. For comparison, PLys was also tested under the same conditions as a positive control. Cell viability in the tested samples was normalized to cells incubated under the same conditions without the addition of polymers (negative control).
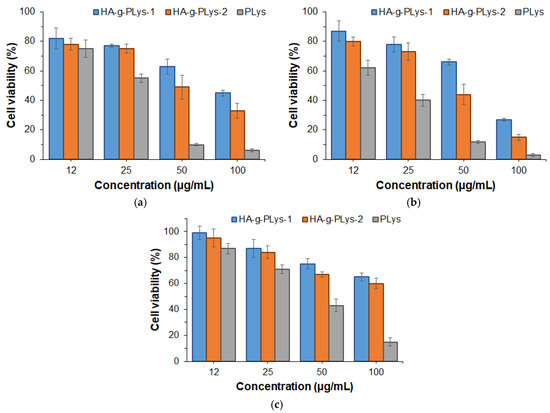
Figure 7.
Viability of BEAS-2B (a), A549 (b), and HEK-293 (c) cells in the presence of HA-g-PLys and PLys (CTB assay, 24 h). Viability data are presented as percentages normalized to the blank control.
From the data presented in Figure 7, it is evident that PLys exhibits significant toxicity toward all cell lines. Specifically, viability of BEAS-2B and HEK-293 normal cells above 75% was observed at a PLys concentration of ≤12 and 25 µg/mL, respectively. At the same concentrations of PLys, the viability of A549 cancer cells was lower than that of BEAS-2B, with pronounced cytotoxicity observed across the entire concentration range. In turn, HA-g-PLys demonstrated reduced cytotoxicity due to the higher biocompatibility of HA. Notably, like PLys, HA-g-PLys demonstrated less cytotoxicity in normal cells (BEAS-2B and HEK-293) and greater cytotoxicity in cancer cells (A549). These results are consistent with previously published data [40,76,77].
3.4.2. Gene Silencing
In order to evaluate the ability of HA-g-PLys for gene delivery, the efficiency of GFP gene silencing in mouse fibroblast cells (NIH-3T3/GFP+) was examined. NIH-3T3/GFP+ cells expressed GFP protein due to the integration of the GFP gene into their genome. The delivery of anti-GFP siRNA into the cells, which induces mRNA interference, is expected to suppress GFP synthesis.
Unlike lipids, for which practically used N/P ratios are rather low, polymers generally require N/P ratios ranging from 20 to 300 [78]. Low N/P ratios often result in physically unstable complexes. For instance, siRNA-containing polyplexes obtained at N/P = 5 in 150 mM NaCl solution exhibit rapid aggregation [79]. Both the efficiency of nucleic acid encapsulation and the colloidal stability of the polyplexes improve with increasing N/P ratio [79,80]. In turn, the cytotoxicity of polyplexes tends to increase at high N/P ratios due to excess cationic charge, which can disrupt cell membranes and induce apoptosis [81,82]. However, this effect can be reduced by using grafted polymers with anionic or natural shielding polymers [83,84], and N/P = 20–50 can provide an acceptable balance between siRNA delivery and cytotoxicity of copolymers [85,86]. Considering this, the HA-g-PLys-2@siRNA polyplexes prepared at N/P ratios of 25 and 50 were used in the GFP gene silencing study.
Gene silencing efficiency was investigated by flow cytometry after 72 h incubation of NIH-3T3/GFP+ cells with HA-g-PLys-2@anti-GFP siRNA polyplex. The efficiency of GFP gene silencing was quantified by measuring the reduction in GFP fluorescence. Transfection using a GenJect-40 commercial agent served as the positive control. Untreated cells were used as a negative control to normalize transfection results across other systems. In addition, siControl, representing the polyplex of HA-g-PLys-2 with scrambled siRNA (N/P = 50), was included for comparison.
The results shown in Figure 8 clearly demonstrate that the N/P ratio significantly influences gene silencing efficacy. Specifically, gene silencing efficiency increased with increasing N/P ratio, which is consistent with previous reports [87,88]. Moreover, at N/P = 25, transfection was very low (~25%) and comparable to that achieved using PLys as a delivery system. A three-fold decrease in the GFP production was detected when N/P was 50. In this case, gene silencing reached 65%, which was comparable to the results obtained using a commercially available transfecting agent (~68%). In contrast, transfection efficiency using PLys as a delivery system was significantly lower, not exceeding 15% at the same N/P ratio.
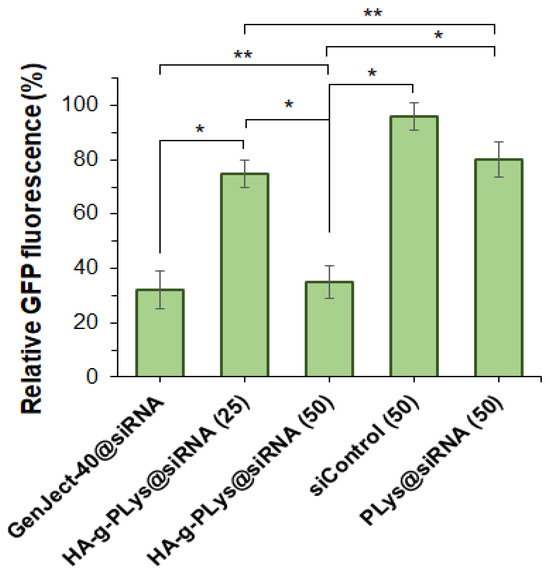
Figure 8.
Silencing of GFP gene in NIH-3T3/GFP+ cells after treatment with polyplexes containing anti-GFP siRNA (72 h). * Values in the brackets are N/P ratios. ** Complex of siRNA with GenJect-40 was prepared according to manufacturer’s recommendations on optimal composition (GenJect-40/siRNA = 6, wt.). Concentration of siRNA in all cases was 50 nM. * p ˂ 0.001; ** p ≥ 0.05.
4. Conclusions
In this study, two graft-copolymers of hyaluronic acid and poly-L-lysine with different grafting degrees were synthesized using SPAAC click chemistry. HA was first modified with DBCO linkers (7% and 14% substitution, confirmed using 1H NMR and FTIR), while azide-terminated PLys was prepared via ROP using azido-PEG3-amine as initiator. The resulting HA-g-PLys copolymers had grafting degrees of 2.7% (HA-DBCO-7) and 4.5% (HA-DBCO-14). These copolymers self-assembled into loosely packed nanoparticles (365–540 nm) via electrostatic interactions between HA and PLys and hydrophobic interactions among DBCO moieties. HA-g-PLys effectively condensed DNA (N/P > 2) and siRNA (N/P > 5) into compact polyplexes (160–260 nm). Compared to free PLys, the copolymers showed higher cell viability and a more pronounced oligonucleotide release. The use of HA-g-PLys for intercellular siRNA delivery resulted in GFP gene silencing efficiency of up to 65% (N/P = 50), matching commercial transfection agents and outperforming PLys alone. Thus, the combination of strong polycation and weak polyanion can provide good transfection due to efficient cell penetration and facilitated intracellular release.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polysaccharides6030060/s1, Figure S1: 1H NMR spectrum of HA-EDA-Boc (D2O, 25 °C).; Figure S2: 1H NMR spectrum of HA-EDA (D2O, 25 °C); Figure S3: 1H NMR spectrum of HA-DBCO (D2O, 25 °C); Figure S4: 1H NMR spectrum of N3-PLys (DMSO-d6, 25 °C); Figure S5: 1H NMR spectrum of PLys (D2O, 25 °C); Figure S6: TEM images of HA-g-PLys-2@dT-dA prepared at N/P = 50 (a) and N/P = 10 (b).
Author Contributions
Conceptualization, V.K.-V. and E.K.-V.; methodology, V.K.-V., P.T., A.D. and T.T.; formal analysis, V.K.-V., P.T. and E.K.-V.; investigation, P.T., N.G. and A.D.; resources, V.K.-V., T.T. and E.K.-V.; data curation, V.K.-V., P.T. and E.K.-V.; writing—original draft preparation, V.K.-V. and E.K.-V.; writing—review and editing, E.K.-V. and T.T.; visualization, V.K.-V. and E.K.-V.; supervision, V.K.-V. and E.K.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data are available within the article and its Supplementary Materials.
Acknowledgments
The research was performed with the use of the equipment of the Research Park of SPbU (Chemical Analysis and Materials Research Centre, Magnetic Resonance Research Center, and Centre for Molecular and Cell Technologies).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HA | Hyaluronic acid |
| PLys | Poly(L-lysine) |
| siRNA | Small interfering RNA |
| DBCO | Dibenzocyclooctyne |
| EDA | Ethylenediamine |
| NCA | N-carboxyanhydride |
| ROP | Ring-opening polymerization |
| NHS | N-hydroxysuccinimide |
| CuAAC | Cu-catalyzed azide-alkyne cycloaddition |
| SPAAC | Strain-promoted azide-alkyne cycloaddition |
| SEC | Size-exclusion chromatography |
| FTIR | Fourier transform infrared spectroscopy |
| PDI | Polydispersity index |
| dT-dA | Oligothymidine-oligoadenine duplex |
| GFP | Green fluorescent protein |
| PBS | Phosphate-buffered saline |
References
- Bulaklak, K.; Gersbach, C.A. The once and future gene therapy. Nat. Commun. 2020, 11, 5820. [Google Scholar] [CrossRef] [PubMed]
- Bolhassani, A. Improvements in chemical carriers of proteins and peptides. Cell Biol. Int. 2019, 43, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.K.; Gowrav, M.P.; Gangadharappa, H.V. Materials for Gene Delivery Systems. In Interaction of Nanomaterials with Living Cells; Springer Nature Singapore: Singapore, 2023; pp. 411–437. [Google Scholar]
- Sung, Y.; Kim, S. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef]
- Taghdiri, M.; Mussolino, C. Viral and Non-Viral Systems to Deliver Gene Therapeutics to Clinical Targets. Int. J. Mol. Sci. 2024, 25, 7333. [Google Scholar] [CrossRef]
- Gerhardt, A.; Voigt, E.; Archer, M.; Reed, S.; Larson, E.; Van Hoeven, N.; Kramer, R.; Fox, C.; Casper, C.; Yan, Y.; et al. Non-viral vectors for RNA delivery. J. Control Release 2022, 342, 241–279. [Google Scholar]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging non-viral vectors for gene delivery. J. Nanobiotechnology 2023, 21, 272. [Google Scholar] [CrossRef]
- De Haan, P.; Van Diemen, F.R.; Toscano, M.G. Viral gene delivery vectors: The next generation medicines for immune-related diseases. Hum. Vaccin. Immunother. 2021, 17, 14–21. [Google Scholar] [CrossRef]
- Kotterman, M.A.; Chalberg, T.W.; Schaffer, D.V. Viral Vectors for Gene Therapy: Translational and Clinical Outlook. Annu. Rev. Biomed. Eng. 2015, 17, 63–89. [Google Scholar] [CrossRef]
- Cai, X.; Dou, R.; Guo, C.; Tang, J.; Li, X.; Chen, J.; Zhang, J. Cationic Polymers as Transfection Reagents for Nucleic Acid Delivery. Pharmaceutics 2023, 15, 1502. [Google Scholar] [CrossRef]
- Pietersz, G.; Tang, C.-K.; Apostolopoulos, V. Structure and Design of Polycationic Carriers For Gene Delivery. Mini-Rev. Med. Chem. 2006, 6, 1285–1298. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, B.; Jiang, H.; Wang, B.; Ma, B. Cationic lipids and polymers mediated vectors for delivery of siRNA. J. Control Release 2007, 123, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther.-Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef]
- Valente, J.F.A.; Pereira, P.; Sousa, A.; Queiroz, J.A.; Sousa, F. Effect of Plasmid DNA Size on Chitosan or Polyethyleneimine Polyplexes Formulation. Polymers 2021, 13, 793. [Google Scholar] [CrossRef]
- Khan, M. Polymers as Efficient Non-Viral Gene Delivery Vectors: The Role of the Chemical and Physical Architecture of Macromolecules. Polymers 2024, 16, 2629. [Google Scholar] [CrossRef]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in gene therapy: Current status and clinical applications. J. Control Release 2023, 362, 667–691. [Google Scholar] [CrossRef] [PubMed]
- Kichler, A.; Leborgne, C.; Coeytaux, E.; Danos, O. Polyethylenimine-mediated gene delivery: A mechanistic study. J. Gene Med. 2001, 3, 135–144. [Google Scholar] [CrossRef]
- Agarwal, S.; Zhang, Y.; Maji, S.; Greiner, A. PDMAEMA based gene delivery materials. Mater. Today 2012, 15, 388–393. [Google Scholar] [CrossRef]
- Ritt, N.; Ayaou, A.; Zentel, R. RAFT Synthesis of Reactive Multifunctional Triblock-Copolymers for Polyplex Formation. Macromol. Chem. Phys. 2021, 222, 2100122. [Google Scholar] [CrossRef]
- Kasza, K.; Elsherbeny, A.; Moloney, C.; Hardie, K.R.; Cámara, M.; Alexander, C.; Gurnani, P. Hybrid Poly(β-amino ester) Triblock Copolymers Utilizing a RAFT Polymerization Grafting-From Methodology. Macromol. Chem. Phys. 2023, 224, 2300262. [Google Scholar] [CrossRef]
- Karlsson, J.; Rhodes, K.R.; Green, J.J.; Tzeng, S.Y. Poly(beta-amino ester)s as gene delivery vehicles: Challenges and opportunities. Expert Opin. Drug Deliv. 2020, 17, 1395–1410. [Google Scholar] [CrossRef]
- Aydinlioglu, E.; Abdelghani, M.; Le Fer, G.; van Hest, J.C.M.; Sandre, O.; Lecommandoux, S. Robust Polyion Complex Vesicles (PICsomes) Based on PEO-b-poly(amino acid) Copolymers Combining Electrostatic and Hydrophobic Interactions: Formation, siRNA Loading and Intracellular Delivery. Macromol. Chem. Phys. 2023, 224, 2200306. [Google Scholar] [CrossRef]
- Kumar, D.; Sahu, B.; Banerjee, S. Amino Acid-Derived Smart and Functional Polymers for Biomedical Applications: Current Status and Future Perspectives. Macromol. Chem. Phys. 2023, 224, 2300207. [Google Scholar] [CrossRef]
- Stepanova, M.; Nikiforov, A.; Tennikova, T.; Korzhikova-Vlakh, E. Polypeptide-Based Systems: From Synthesis to Application in Drug Delivery. Pharmaceutics 2023, 15, 2641. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, Z.; Shen, Y. Effects of chirality on gene delivery efficiency of polylysine. Chin. J. Polym. Sci. 2016, 34, 94–103. [Google Scholar] [CrossRef]
- Conejos-Sánchez, I.; Gallon, E.; Niño-Pariente, A.; Smith, J.A.; De la Fuente, A.G.; Di Canio, L.; Pluchino, S.; Franklin, R.J.M.; Vicent, M.J. Polyornithine-based polyplexes to boost effective gene silencing in CNS disorders. Nanoscale 2020, 12, 6285–6299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-T.; Yu, M.; Niu, Y.-J.; Liu, W.-Z.; Pang, W.-H.; Ding, J.; Wang, J.-C. Polyarginine-Mediated siRNA Delivery: A Mechanistic Study of Intracellular Trafficking of PCL-R15/siRNA Nanoplexes. Mol. Pharm. 2020, 17, 1685–1696. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J. Studies of DEAE-dextran-mediated gene transfer. Biotechnol. Appl. Biochem. 1997, 25, 47–51. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Madkhali, O.; Mekhail, G.; Wettig, S.D. Modified gelatin nanoparticles for gene delivery. Int. J. Pharm. 2019, 554, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Schatz, C.; Louguet, S.; Le Meins, J.; Lecommandoux, S. Polysaccharide-block-polypeptide Copolymer Vesicles: Towards Synthetic Viral Capsids. Angew. Chem. Int. Ed. 2009, 48, 2572–2575. [Google Scholar] [CrossRef]
- Liu, T.; Chen, S.; Zhang, S.; Wu, X.; Wu, P.; Miao, B.; Cai, X. Transferrin-functionalized chitosan-graft-poly(L-lysine) dendrons as a high-efficiency gene delivery carrier for nasopharyngeal carcinoma therapy. J. Mater. Chem. B 2018, 6, 4314–4325. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, X.; Lu, T.; Sun, J.; Tian, H.; Hu, J.; Wang, Y.; Zhang, P.; Jing, X. Poly(L-lysine)-Graft-Chitosan Copolymers: Synthesis, Characterization, and Gene Transfection Effect. Biomacromolecules 2007, 8, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Choi, C.W.; Kim, H.W.; Kim, D.H.; Kwak, T.W.; Lee, H.M.; Kim, C.H.; Chung, C.W.; Jeong, Y.I.; Kang, D.H. Dextran-b-poly(L-histidine) copolymer nanoparticles for pH-responsive drug delivery to tumor cells. Int. J. Nanomed. 2013, 2013, 3197. [Google Scholar]
- Ferdous, A. Poly(L-lysine)-graft-dextran copolymer: Amazing effects on triplex stabilization under physiological pH and ionic conditions (in vitro). Nucleic Acids Res. 1998, 26, 3949–3954. [Google Scholar] [CrossRef]
- Pilipenko, I.; Korovkina, O.; Gubina, N.; Ekimova, V.; Ishutinova, A.; Korzhikova-Vlakh, E.; Tennikova, T.; Korzhikov-Vlakh, V. Random Copolymers of Lysine and Isoleucine for Efficient mRNA Delivery. Int. J. Mol. Sci. 2022, 23, 5363. [Google Scholar] [CrossRef]
- Korovkina, O.; Polyakov, D.; Korzhikov-Vlakh, V.; Korzhikova-Vlakh, E. Stimuli-Responsive Polypeptide Nanoparticles for Enhanced DNA Delivery. Molecules 2022, 27, 8495. [Google Scholar] [CrossRef]
- Dzhuzha, A.; Gandalipov, E.; Korzhikov-Vlakh, V.; Katernyuk, E.; Zakharova, N.; Silonov, S.; Tennikova, T.; Korzhikova-Vlakh, E. Amphiphilic Polypeptides Obtained by Post-Polymerization Modification of Poly-l-Lysine as Systems for Combined Delivery of Paclitaxel and siRNA. Pharmaceutics 2023, 15, 1308. [Google Scholar] [CrossRef]
- Yi, A.; Sim, D.; Lee, Y.-J.; Sarangthem, V.; Park, R.-W. Development of elastin-like polypeptide for targeted specific gene delivery in vivo. J. Nanobiotechnology 2020, 18, 15. [Google Scholar] [CrossRef]
- Bravo-Anaya, L.M.; Garbay, B.; Nando-Rodríguez, J.L.E.; Carvajal Ramos, F.; Ibarboure, E.; Bathany, K.; Xia, Y.; Rosselgong, J.; Joucla, G.; Garanger, E.; et al. Nucleic acids complexation with cationic elastin-like polypeptides: Stoichiometry and stability of nano-assemblies. J. Colloid Interface Sci. 2019, 557, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Osipova, O.; Zakharova, N.; Pyankov, I.; Egorova, A.; Kislova, A.; Lavrentieva, A.; Kiselev, A.; Tennikova, T.; Korzhikova-vlakh, E. Amphiphilic pH-sensitive polypeptides for siRNA delivery. J. Drug Deliv. Sci. Technol. 2022, 69, 103135. [Google Scholar] [CrossRef]
- Korzhikov-Vlakh, V.; Katernuk, I.; Pilipenko, I.; Lavrentieva, A.; Guryanov, I.; Sharoyko, V.; Manshina, A.A.; Tennikova, T.B. Photosensitive Poly-l-lysine/Heparin Interpolyelectrolyte Complexes for Delivery of Genetic Drugs. Polymers 2020, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Casey-Power, S.; Vardar, C.; Ryan, R.; Behl, G.; McLoughlin, P.; Byrne, M.E.; Fitzhenry, L. NAD+-associated-hyaluronic acid and poly(L-lysine) polyelectrolyte complexes: An evaluation of their potential for ocular drug delivery. Eur. J. Pharm. Biopharm. 2023, 192, 62–78. [Google Scholar] [CrossRef]
- Pan, W.; Yin, D.-X.; Jing, H.-R.; Chang, H.-J.; Wen, H.; Liang, D.-H. Core-Corona Structure Formed by Hyaluronic Acid and Poly(L-lysine) via Kinetic Path. Chin. J. Polym. Sci. 2019, 37, 36–42. [Google Scholar] [CrossRef]
- Laga, R.; Carlisle, R.; Tangney, M.; Ulbrich, K.; Seymour, L.W. Polymer coatings for delivery of nucleic acid therapeutics. J. Control Release 2012, 161, 537–553. [Google Scholar] [CrossRef]
- Korovkina, O.M.; Korzhikov-Vlakh, V.A.; Polyakov, D.S.; Solomakha, O.A.; Dzhuzha, A.Y.; Tennikova, T.B.; Korzhikova-Vlakh, E.G. Polysaccharide-g-Polypeptide Copolymers: Synthesis by Metal-Free Click Reaction and Evaluation as Gene Delivery Systems. Macromol. Chem. Phys. 2025, 226, 2400501. [Google Scholar] [CrossRef]
- García-Astrain, C.; Chen, C.; Burón, M.; Palomares, T.; Eceiza, A.; Fruk, L.; Corcuera, M.Á.; Gabilondo, N. Biocompatible Hydrogel Nanocomposite with Covalently Embedded Silver Nanoparticles. Biomacromolecules 2015, 16, 1301–1310. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Demirci, G.; Tasdelen, M.A. Synthesis and characterization of graft copolymers by photoinduced CuAAC click chemistry. Eur. Polym. J. 2015, 66, 282–289. [Google Scholar] [CrossRef]
- Degirmenci, A.; Sanyal, R.; Sanyal, A. Metal-Free Click-Chemistry: A Powerful Tool for Fabricating Hydrogels for Biomedical Applications. Bioconjug. Chem. 2024, 35, 433–452. [Google Scholar] [CrossRef]
- Leggate, J.; Allain, R.; Isaac, L.; Blais, B.W. Microplate fluorescence assay for the quantification of double stranded DNA using SYBR Green I dye. Biotechnol. Lett. 2006, 28, 1587–1594. [Google Scholar] [CrossRef]
- Fischer, M.J.E. Amine Coupling Through EDC/NHS: A Practical Approach. Methods Mol. Biol. 2010, 627, 55–73. [Google Scholar] [PubMed]
- Fu, S.; Dong, H.; Deng, X.; Zhuo, R.; Zhong, Z. Injectable hyaluronic acid/poly(ethylene glycol) hydrogels crosslinked via strain-promoted azide-alkyne cycloaddition click reaction. Carbohydr. Polym. 2017, 169, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Suzuki, Y.; Suhara, T.; Omichi, K.; Shimizu, A.; Hasegawa, K.; Kokudo, N.; Ohta, S.; Ito, T. In Situ Cross-Linkable Hydrogel of Hyaluronan Produced via Copper-Free Click Chemistry. Biomacromolecules 2013, 14, 3581–3588. [Google Scholar] [CrossRef]
- Han, S.-S.; Yoon, H.Y.; Yhee, J.Y.; Cho, M.O.; Shim, H.-E.; Jeong, J.-E.; Lee, D.-E.; Kim, K.; Guim, H.; Lee, J.H.; et al. In situ cross-linkable hyaluronic acid hydrogels using copper free click chemistry for cartilage tissue engineering. Polym. Chem. 2018, 9, 20–27. [Google Scholar] [CrossRef]
- Vlakh, E.G.; Grachova, E.V.; Zhukovsky, D.D.; Hubina, A.V.; Mikhailova, A.S.; Shakirova, J.R.; Sharoyko, V.V.; Tunik, S.P.; Tennikova, T.B. Self-assemble nanoparticles based on polypeptides containing C-terminal luminescent Pt-cysteine complex. Sci. Rep. 2017, 7, 41991. [Google Scholar] [CrossRef]
- Kar, M.; Malvi, B.; Das, A.; Panneri, S.; Gupta, S. Sen Synthesis and characterization of poly-l-lysine grafted SBA-15 using NCA polymerization and click chemistry. J. Mater. Chem. 2011, 21, 6690. [Google Scholar] [CrossRef]
- Obhi, N.K.; Peda, D.M.; Kynaston, E.L.; Seferos, D.S. Exploring the Graft-To Synthesis of All-Conjugated Comb Copolymers Using Azide–Alkyne Click Chemistry. Macromolecules 2018, 51, 2969–2978. [Google Scholar] [CrossRef]
- Savaş, B.; Öztürk, T.; Meyvacı, E.; Hazer, B. Synthesis and characterization of comb-type graft copolymers by redox polymerization and “click” chemistry method. SN Appl. Sci. 2020, 2, 181. [Google Scholar] [CrossRef]
- Savaş, B.; Öztürk, T. Poly(4-vinylbenzyl-g-β-butyrolactone) graft copolymer synthesis and characterization using ring-opening polymerization, free-radical polymerization, and “click” chemistry techniques. J. Chem. Sci. 2024, 136, 65. [Google Scholar] [CrossRef]
- Öztürk, T.; Asan, N. Synthesis and Characterization of Poly(vinyl chloride-graft-ethylene glycol) Graft Copolymers by “Click” Chemistry. Hacet. J. Biol. Chem. 2018, 1, 35–42. [Google Scholar]
- Zheng, X.; Wang, B.; Tang, X.; Mao, B.; Zhang, Q.; Zhang, T.; Zhao, J.; Cui, S.; Chen, W. Absorption, metabolism, and functions of hyaluronic acid and its therapeutic prospects in combination with microorganisms: A review. Carbohydr. Polym. 2023, 299, 120153. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.C.; Pereira, H.C.B.; da Silva, M.d.L.C.; Vasconcelos, A.F.D.; Celligoi, M.A.P.C. Improvement Production of Hyaluronic Acid by Streptococcus zooepidemicus in Sugarcane Molasses. Appl. Biochem. Biotechnol. 2017, 182, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, M.; Shoham, G. FTIR spectra of solid poly-l-lysine in the stretching NH mode range. Biophys. Chem. 2007, 125, 166–171. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Cheraghi, R.; Alipour, M.; Nazari, M.; Hosseinkhani, S. Optimization of conditions for gene delivery system based on PEI. Nanomed. J. 2017, 4, 8–16. [Google Scholar]
- Ferrari, R.; Sponchioni, M.; Morbidelli, M.; Moscatelli, D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: The checkpoints on the road from the synthesis to clinical translation. Nanoscale 2018, 10, 22701–22719. [Google Scholar] [CrossRef]
- Williams, J. Nanoparticle drug delivery system for intravenous delivery of topoisomerase inhibitors. J. Control Release 2003, 91, 167–172. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Zipper, H. Investigations on DNA intercalation and surface binding by SYBR Green I, its structure determination and methodological implications. Nucleic Acids Res. 2004, 32, e103. [Google Scholar] [CrossRef] [PubMed]
- González Ferreiro, M.; Tillman, L.; Hardee, G.; Bodmeier, R. Characterization of alginate/poly-l-lysine particles as antisense oligonucleotide carriers. Int. J. Pharm. 2002, 239, 47–59. [Google Scholar] [CrossRef]
- Jia, J.; Yang, J.; Qian, L.; Zhou, B.; Tang, X.; Liu, S.; Wu, L.; Chen, J.; Kuang, Y. Controlled siRNA Release of Nanopolyplex for Effective Targeted Anticancer Therapy in Animal Model. Int. J. Nanomed. 2024, 19, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Osipova, O.; Sharoyko, V.; Zashikhina, N.; Zakharova, N.; Tennikova, T.; Urtti, A.; Korzhikova-Vlakh, E. Amphiphilic polypeptides for VEGF siRNA delivery into retinal epithelial cells. Pharmaceutics 2020, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Salma-Ancane, K.; Sceglovs, A.; Tracuma, E.; Wychowaniec, J.K.; Aunina, K.; Ramata-Stunda, A.; Nikolajeva, V.; Loca, D. Effect of crosslinking strategy on the biological, antibacterial and physicochemical performance of hyaluronic acid and ɛ-polylysine based hydrogels. Int. J. Biol. Macromol. 2022, 208, 995–1008. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, X.; Liu, Z.; Meng, R.; Chen, X.; Guo, N. Antimicrobial, antioxidant, and antitumor activity of epsilon-poly-L-lysine and citral, alone or in combination. Food Nutr. Res. 2016, 60, 31891. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Tapeinos, C.; Torrieri, G.; Känkänen, V.; El-Sayed, N.; Python, A.; Hirvonen, J.T.; Santos, H.A. Non-viral nanoparticles for RNA interference: Principles of design and practical guidelines. Adv. Drug Deliv. Rev. 2021, 174, 576–612. [Google Scholar] [CrossRef]
- Alameh, M.; Lavertu, M.; Tran-Khanh, N.; Chang, C.-Y.; Lesage, F.; Bail, M.; Darras, V.; Chevrier, A.; Buschmann, M.D. siRNA Delivery with Chitosan: Influence of Chitosan Molecular Weight, Degree of Deacetylation, and Amine to Phosphate Ratio on in Vitro Silencing Efficiency, Hemocompatibility, Biodistribution, and in Vivo Efficacy. Biomacromolecules 2018, 19, 112–131. [Google Scholar] [CrossRef]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef]
- Lechanteur, A.; Sanna, V.; Duchemin, A.; Evrard, B.; Mottet, D.; Piel, G. Cationic Liposomes Carrying siRNA: Impact of Lipid Composition on Physicochemical Properties, Cytotoxicity and Endosomal Escape. Nanomaterials 2018, 8, 270. [Google Scholar] [CrossRef]
- Hanafy, M.S.; Dao, H.M.; Xu, H.; Koleng, J.J.; Sakran, W.; Cui, Z. Effect of the amount of cationic lipid used to complex siRNA on the cytotoxicity and proinflammatory activity of siRNA-solid lipid nanoparticles. Int. J. Pharm. X 2023, 6, 100197. [Google Scholar] [CrossRef]
- Choi, K.Y.; Saravanakumar, G.; Park, J.H.; Park, K. Hyaluronic acid-based nanocarriers for intracellular targeting: Interfacial interactions with proteins in cancer. Colloids Surf. B Biointerfaces 2012, 99, 82–94. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, S.; Hu, H.; Wu, G.; Feng, M.; Zhang, W.; Luo, X. Poly(ethylene glycol)-block-polyethylenimine copolymers as carriers for gene delivery: Effects of PEG molecular weight and PEGylation degree. J. Biomed. Mater. Res. Part A 2008, 84A, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.K.; Kai, D.; Tu, G.X.E.; Deen, G.R.; Too, H.P.; Loh, X.J. Enhanced transfection of a macromolecular lignin-based DNA complex with low cellular toxicity. Biosci. Rep. 2018, 38, BSR20181021. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shi, H.; Chu, X.; Zhou, X.; Sun, P. A rapid and efficient polyethylenimine-based transfection method to prepare lentiviral or retroviral vectors: Useful for making iPS cells and transduction of primary cells. Biotechnol. Lett. 2016, 38, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Wang, C.; Huang, Y.; Shi, Q.; Fernandes, J.C.; Dai, K.; Tang, G.; Zhang, X. Polyethylenimine600-beta-cyclodextrin: A promising nanopolymer for nonviral gene delivery of primary mesenchymal stem cells. Int. J. Nanomed. 2013, 2013, 1935. [Google Scholar] [CrossRef]
- Lehner, R.; Wang, X.; Hunziker, P. Plasmid linearization changes shape and efficiency of transfection complexes. Eur. J. Nanomed. 2013, 5, 28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).