Feruloylated Arabinoxylans from Nixtamalized Maize Bran By-Product as a Baking Ingredient: Physicochemical, Nutritional, and Functional Properties
Abstract
1. Introduction
2. Materials and Methods
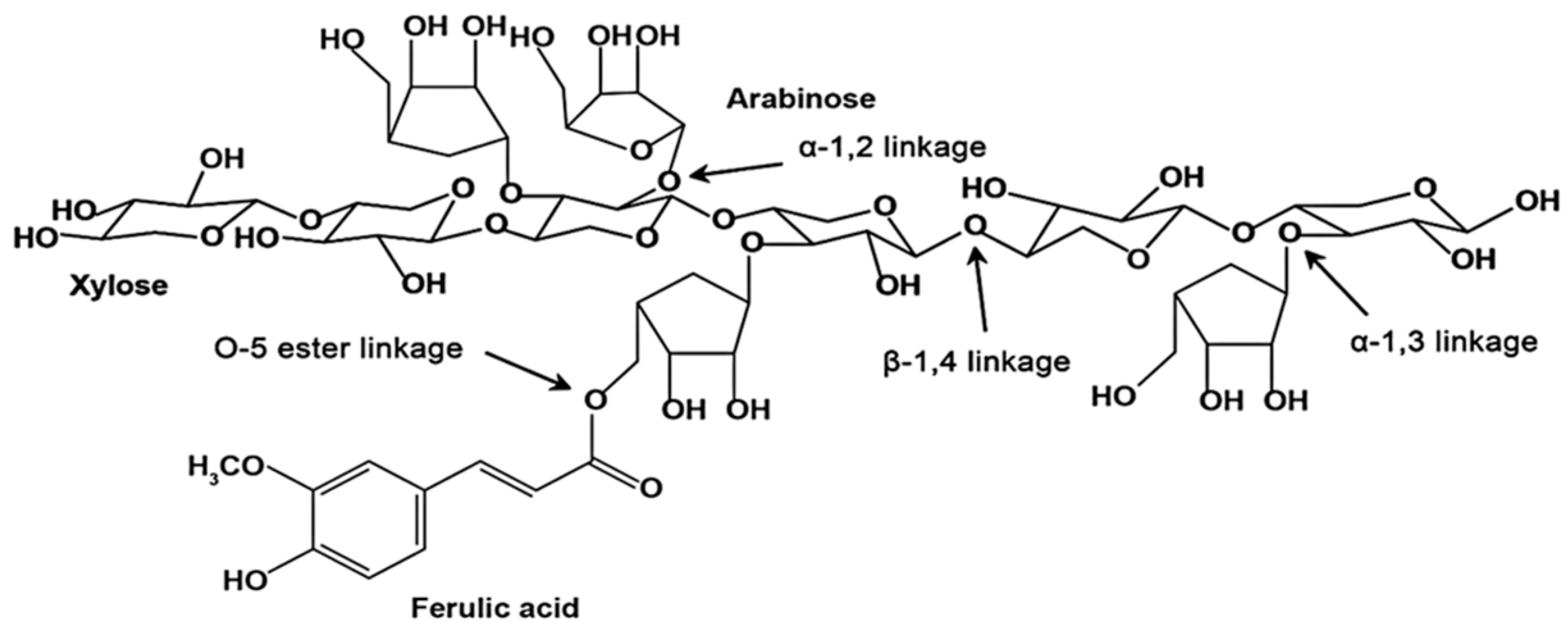
2.1. Extraction and Characterization of Arabinoxylans
2.2. Treatments
2.3. Preparation of White Bread with Added FAXs
2.4. Texture Profile Analysis (TPA)
2.5. Color Evaluation
2.6. Proximate Composition
2.7. Total Phenols and Antioxidant Capacity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Texture Profile Analysis (TPA)
3.2. Color Evaluation
3.3. Proximate Composition
3.4. Total Phenols and Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serna-Saldivar, S.O. Understanding the functionality and manufacturing of nixtamalized maize products. J. Cereal Sci. 2021, 99, 103205. [Google Scholar] [CrossRef]
- Ramírez-Araujo, H.; Gaytán-Martínez, H.M.; Reyes-Vega, M.L. Alternative technologies to the traditional nixtamalization process: Review. Trends Food Sci. Tech. 2019, 85, 34–43. [Google Scholar] [CrossRef]
- Rosentrater, K.A. A review of corn masa processing residues: Generation, properties, and potential utilization. Waste Manag. 2006, 26, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.J.; Inglett, G.E.; Liu, S.X. Utilisation of corn (Zea mays) bran and corn fiber in the production of food components. J. Sci. Food Agric. 2009, 90, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Malunga, L.N.; Beta, T. Isolation and identification of feruloylated arabinoxylan mono- and oligosaccharides from undigested and digested maize and wheat. Heliyon 2016, 2, e00106. [Google Scholar] [CrossRef] [PubMed]
- Saeed, F.; Hussain, M.; Arshad, M.S.; Afzaal, M.; Munir, H.; Imran, M.; Tufail, T.; Anjum, F.M. Functional and nutraceutical properties of maize bran cell wall non-starch polysaccharides. Int. J. Food Prop. 2020, 24, 233–248. [Google Scholar] [CrossRef]
- Zhang, Z.; Smith, C.; Li, W. Extraction and modification technology of arabinoxylans from cereal by-products: A critical review. Food Res. Int. 2014, 65, 423–436. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Rascón-Chu, A.; Márquez-Escalante, J.A.; Micard, V.; Ponce de León, N.; Gardea, A. Maize bran gum: Extraction, characterization and functional properties. Carbohyd. Polym. 2006, 69, 280–285. [Google Scholar] [CrossRef]
- Aguedo, M.; Fougnies, C.; Dermience, M.; Richel, A. Extraction by three processes of arabinoxylans from wheat bran and characterization of the fractions obtained. Carbohyd Polym. 2014, 105, 317–324. [Google Scholar] [CrossRef]
- Lin, S.; Agger, J.W.; Wilkens, C.; Meyer, A.S. Feruloylated Arabinoxylan and Oligosaccharides: Chemistry, Nutritional Functions, and Options for Enzymatic Modification. Annu. Rev. Food Sci. Technol. 2021, 12, 331–354. [Google Scholar] [CrossRef]
- Tse, T.; Schendel, R.R. Cereal Grain Arabinoxylans—Processing Effects and Structural Changes during Food and Beverage Fermentations. Fermentation 2023, 9, 914. [Google Scholar] [CrossRef]
- Huang, M.; Bai, J.; Buccato, D.G.; Zhang, J.; He, Y.; Zhu, Y.; Yang, Z.; Xiao, X.; Daglia, M. Cereal-derived water-unextractable arabinoxylans: Structure feature, effects on baking products and human health. Foods 2024, 13, 2369. [Google Scholar] [CrossRef]
- Mexican Ministry of Economy, Learn More About the Baking Industry in Mexico [Secretaría de Economía. Conoce Más Sobre la Industria Panificadora en México]. 2017. Available online: https://www.gob.mx/se/articulos/conoce-mas-sobre-la-industria-panificadora-en-mexico (accessed on 30 August 2024).
- Mexican Official Standard NOM-F-159-S-1979; White Bread. [Norma Oficial Mexicana NOM-F-159-S-1979; Pan blanco de caja]. Available online: https://dof.gob.mx/nota_detalle.php?codigo=4829330&fecha=06/08/1979&print=true (accessed on 30 August 2024).
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of bread dough with added fiber polysaccharides and phenolic antioxidants: A review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, A.; Buksa, K. Properties and Functionality of Cereal Non-Starch Polysaccharides in Breadmaking. Appl. Sci. 2023, 13, 2282. [Google Scholar] [CrossRef]
- Zannini, E.; Bravo Núñez, Á.; Sahin, A.W.; Arendt, E.K. Arabinoxylans as Functional Food Ingredients: A Review. Foods 2022, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Saeed, F.; Pasha, I.; Anjum, F.M.; Sultan, M.T. Arabinoxylans and Arabinogalactans: A Comprehensive Treatise. Crit. Rev. Food Sci. Nutr. 2011, 51, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Pietiäinen, S.; Moldin, A.; Ström, A.; Malmberg, C.; Langton, M. Effect of physicochemical properties, pre-processing, and extraction on the functionality of wheat bran arabinoxylans in breadmaking—A review. Food Chem. 2022, 383, 132584. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Baez-Gonzalez, J.G.; Carvajal-Millan, E.; Muy-Rangel, D.; Urias-Orona, V.; Martinez-Lopez, A.L.; Marquez-Escalante, J.A.; Heredia, J.B.; Beta, T.; Niño-Medina, G. Alkali-extracted feruloylated arabinoxylans from nixtamalized maize bran byproduct: A synonymous with soluble antioxidant dietary fiber. Waste Biomass Valori. 2020, 11, 403–409. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Gutiérrez-Soto, G.; Urías-Orona, V.; Hernández-Luna, C.E. Effect of laccase from Trametes maxima CU1 on physicochemical quality of bread. Cogent Food Agric. 2017, 3, 132876. [Google Scholar] [CrossRef]
- Commission Internationale De L’ecleirage. Cie 15: Technical Report: Colorimetry, 3rd ed.; CIE Publications: Vienna, Austria, 2004; p. 7. [Google Scholar]
- ColorHexa: Color Encyclopedia: Information and Conversion. 2024. Computer Software. Available online: www.colorhexa.com (accessed on 14 September 2024).
- Onishi, M.; Inoue, M.; Araki, T.; Iwabuchi, H.; Sagara, Y. Characteristic coloring curve for white bread during baking. Biosci. Biotechnol. Biochem. 2011, 75, 255–260. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemist International (AOAC). Methods of Analysis of AOAC International, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Niño-Medina, G.; Muy-Rangel, D.; Urías-Orona, V. Chickpea (Cicer arietinum) and soybean (Glycine max) hulls: Byproducts with potential use as a source of high value-added food products. Waste Biomass. Valor. 2017, 8, 1199–1203. [Google Scholar] [CrossRef]
- López-Contreras, J.J.; Zavala-García, F.; Urías-Orona, V.; Martínez-Ávila, G.C.G.; Rojas, R.; Niño-Medina, G. Chromatic, phenolic and antioxidant properties of Sorghum bicolor genotypes. Not. Bot. Horti Agrobo. 2015, 43, 366–370. [Google Scholar] [CrossRef]
- Minitab, version 17.0; Statistical Software, Computer Software; Minitab: State College, PA, USA, 2010. Available online: www.minitab.com (accessed on 20 August 2024).
- Szczesniak, A.S. Texture is a sensory property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef]
- Morales-Ortega, A.; Niño-Medina, G.; Carvajal-Millán, E.; Gardea-Béjar, A.; Torres-Chávez, P.; López-Franco, Y.; Rascón-Chu, A.; Lizardi-Mendoza, E. Los arabinoxilanos ferulados de cereales. una revisión de sus características fisicoquímicas y capacidad gelificante. [Ferulated arabinoxylans from cereals. A review of their physico-chemical characteristics]. Rev. Fitotec. Mex. 2013, 36, 439–446. [Google Scholar]
- Buksa, K.; Nowotna, A.; Ziobro, R.; Gambus, H. Rye flour enriched with arabinoxylans in rye bread making. Food Sci. Technol. Int. 2015, 21, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Soto, F.A.; Serna-Saldívar, S.E.; Welti-Chanes, J. Effect of arabinoxylans and laccase on batter rheology and quality of yeast-leavened gluten free breads. J. Cereal Sci. 2017, 73, 10–17. [Google Scholar] [CrossRef]
- Biliaderis, C.G.; Izydorczyk, M.S.; Rattan, O. Effect of arabinoxylans on bread-making quality of wheat flours. Food Chem. 1995, 53, 165–171. [Google Scholar] [CrossRef]
- Buksa, K.; Nowotna, A.; Ziobro, R. Application of cross-linked and hydrolyzed arabinoxylans in baking of model rye bread. Food Chem. 2016, 192, 991–996. [Google Scholar] [CrossRef]
- Michalska, A.; Amigop-Benavent, M.; Zielinski, H.; del Castillo, M.D. Effect of bread making on formation of Maillard reaction products contributing to the overall antioxidant activity of rye bread. J. Cereal Sci. 2008, 48, 123–132. [Google Scholar] [CrossRef]
- Brainard, D.H. Color Appearance and Color Difference Specification. In The Science of Color, 2nd ed.; Shevell, S.K., Ed.; Elsevier: Oxford, UK, 2003; pp. 191–216. [Google Scholar]
- Mokrzycki, W.S.; Tatol, M. Color difference ΔE—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Karma, I.G.M. Determination and Measurement of Color Dissimilarity. Int. J. Eng. Emerg. Technol. 2020, 5, 67–71. [Google Scholar] [CrossRef]
- Zhang, L.; van Boven, A.; Mulder, J.; Grandia, J.; Chen, X.D.; Boom, R.M.; Schutyser, M.A.I. Arabinoxylans-enriched fractions: From dry fractionation of wheat bran to the investigation on bread baking performance. J. Cereal Sci. 2019, 87, 1–8. [Google Scholar] [CrossRef]
- Han, S.; Wang, Y.; Zhang, Y.; Lan, H.; Li, X.; Wan, J.; Yue, C.; Feng, J.; Luo, D.; Bai, Z. Effects of water-unextractable arabinoxylan from wheat processing wastewater on the quality characteristics of multigrain bread. LWT—Food Sci. Technol. 2024, 210, 116867. [Google Scholar] [CrossRef]
- Bender, D.; Regner, M.; D’Amico, S.; Jäger, H.; Tömösközi, S.; Schoenlechner, R. Effect of Differently Extracted Arabinoxylan on Gluten-Free Sourdough-Bread Properties. J. Food Qual. 2018, 2018, 5719681. [Google Scholar] [CrossRef]
- Jiang, D.; Chiaro, C.; Maddali, P.; Prabhu, K.S.; Peterson, D.G. Identification of hydroxycinnamic acid−maillard reaction products in low-moisture baking model systems. J. Agric. Food Chem. 2009, 57, 9932–9943. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Balandrano, D.D.; Báez-González, J.G.; Carvajal-Millán, E.; Méndez-Zamora, G.; Urías-Orona, V.; Amaya-Guerra, C.A.; Niño-Medina, G. Feruloylated Arabinoxylans from Nixtamalized Maize Bran Byproduct: A Functional Ingredient in Frankfurter Sausages. Molecules 2019, 24, 2056. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Katsantonis, D.; Kleisiaris, F. Evaluation of quality attributes, nutraceutical components and antioxidant potential of wheat bread substituted with rice bran. J. Cereal Sci. 2015, 65, 74–80. [Google Scholar] [CrossRef]
- Koegelenberg, D.; Chimphango, A.F.A. Effects of wheat-bran arabinoxylan as partial flour replacer on bread properties. Food Chem. 2017, 221, 1606–1613. [Google Scholar] [CrossRef]
- Snelders, J.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Impact of wheat bran derived arabinoxylanoligosaccharides and associated ferulic acid on dough and bread properties. J. Agr. Food Chem. 2014, 62, 7190–7199. [Google Scholar] [CrossRef]
- Zhang, D.; Rudjito, R.C.; Pietiäinen, S.; Chang, S.C.; Idström, A.; Evenäs, L.; Vilaplana, F.; Jiménez-Quero, A. Arabinoxylan supplemented bread: From extraction of fibers to effect of baking, digestion, and fermentation. Food Chem. 2023, 413, 135660. [Google Scholar] [CrossRef]
- Marcato, D.C.; Spagnol, C.M.; Salgado, H.R.N.; Isaac, V.L.B.; Correa, M.A. New and potential properties, characteristics, and analytical methods of ferulic acid: A review. Braz. J. Pharm. Sci. 2022, 58, e18747. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Antioxidant Properties, Metabolism, Application and Mechanism of Ferulic Acid in Medicine, Food, Cosmetics, Livestock and Poultry. Antioxidants 2024, 13, 853. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; He, Y.; Lu, F. The structure-antioxidant activity relationship of dehydrodiferulates. Food Chem. 2018, 269, 480–485. [Google Scholar] [CrossRef] [PubMed]
| FAXs Extraction Time | Composition (%) | Dietary Fiber (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Xyl | Ara | Gal | Glc | Pro | Ara/Xyl | Soluble | Insoluble | |
| 2 h | 33.43 | 27.73 | 2.52 | 4.51 | 1.00 | 0.82 | 86.56 | ND |
| 4 h | 29.69 | 25.89 | 2.50 | 1.98 | 0.86 | 0.87 | 89.95 | ND |
| 6 h | 30.28 | 26.64 | 3.12 | 1.26 | 0.62 | 0.87 | 86.14 | ND |
| FAXs Extraction Time | Total Phenols (mg/g) | Ferulic Acid Monomers (mg/g) | Ferulic Acid Oligomers (mg/g) |
|---|---|---|---|
| 2 h | 9.01 | 1.943 | 0.289 |
| 4 h | 7.16 | 1.263 | 0.231 |
| 6 h | 6.48 | 0.368 | 0.107 |
| Treatment Nomenclature | Treatment Description |
|---|---|
| TC (control) | Bread + 0.00% FAXs |
| T1 | Bread + 0.15% FAXs 2 h |
| T2 | Bread + 0.30% FAXs 2 h |
| T3 | Bread + 0.15% FAXs 4 h |
| T4 | Bread + 0.30% FAXs 4 h |
| T5 | Bread + 0.15% FAXs 6 h |
| T6 | Bread + 0.30% FAXs 6 h |
| Ingredient | Quantity |
|---|---|
| Flour | 100 g |
| Water | 62 mL |
| Salt | 3 g |
| Sugar | 4 g |
| Yeast | 3 g |
| FAXs | 0.15/0.30 g |
| Treatments | Parameter | ||||
|---|---|---|---|---|---|
| Hardness (N) | Springiness | Cohesiveness | Chewiness (N) | Resilience | |
| TC | 64.43 ± 7.60 a | 0.92 ± 0.012 b | 0.60 ± 0.015 c | 36.00 ± 4.63 a | 0.20 ± 0.018 c |
| T1 | 40.67 ± 1.56 bc | 0.93 ± 0.001 ab | 0.66 ± 0.005 ab | 25.30 ± 0.76 bc | 0.32 ± 0.006 ab |
| T2 | 40.20 ± 2.39 bc | 0.94 ± 0.001 a | 0.67 ± 0.025 ab | 25.54 ± 2.00 bc | 0.30 ± 0.018 ab |
| T3 | 51.03 ± 0.07 b | 0.93 ± 0.001 ab | 0.67 ± 0.036 ab | 32.33 ± 1.82 ab | 0.31 ± 0.030 ab |
| T4 | 45.45 b ± 6.90 bc | 0.93 ± 0.009 ab | 0.64 ± 0.019 bc | 27.24 ± 4.33 bc | 0.28 ± 0.016 b |
| T5 | 34.32 ± 1.19 c | 0.93 ± 0.005 ab | 0.66 ± 0.010 ab | 21.43 ± 1.05 c | 0.31 ± 0.012 ab |
| T6 | 39.73 b ± 3.31 bc | 0.94 ± 0.004 a | 0.70 ± 0.002 a | 26.38 ± 2.29 bc | 0.34 ± 0.010 a |
| Treatments | Parameter | ||||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h* | ΔE | Color View | |
| TC | 53.22 ± 0.64 bc | 12.59 ± 0.17 a | 26.04 ± 0.05 a | 28.97 ± 0.15 a | 64.03 ± 0.34 c | ----- |  |
| T1 | 56.16 ± 2.21 ab | 12.18 ± 0.18 ab | 26.61 ± 0.43 a | 29.14 ± 0.17 a | 65.52 ± 0.24 bc | 3.04 ± 2.19 bc |  |
| T2 | 53.57 ± 1.69 bc | 12.45 ± 0.41 ab | 27.00 ± 0.06 a | 29.68 ± 0.11 a | 65.29 ± 0.54 bc | 2.07 ± 0.68 cd |  |
| T3 | 53.99 ± 1.73 bc | 9.86 ± 0.37 c | 21.82 ± 0.03 c | 23.60 ± 0.75 b | 65.01 ± 0.32 bc | 5.30 ± 0.41 ab |  |
| T4 | 50.49 ± 0.18 c | 12.69 ± 0.28 a | 24.93 ± 0.36 b | 28.21 ± 0.68 a | 62.01 ± 1.08 d | 2.96 ± 0.76 bcd |  |
| T5 | 58.30 ± 2.21 a | 12.16 ± 0.38 ab | 26.84 ± 0.50 a | 29.28 ± 0.77 a | 66.47 ± 1.09 ab | 5.20 ± 1.46 ab |  |
| T6 | 59.40 ± 0.54 a | 11.58 ± 0.20 b | 26.60 ± 0.60 a | 29.48 ± 0.61 a | 67.44 ± 0.52 a | 6.32 ± 0.32 a |  |
| Treatments | Component (%) | aw | |||||
|---|---|---|---|---|---|---|---|
| Moi | Pro | Fat | Cfb | Ash | Car | ||
| TC | 33.45 ± 0.32 a | 14.02 ± 0.68 a | 1.00 ± 0.01 b | 1.11 ± 0.002 b | 4.58 ± 0.03 a | 45.80 ± 0.40 b | 0.92 ± 0.011 a |
| T1 | 32.49 ± 0.11 a | 13.73 ± 0.44 a | 1.11 ± 0.16 b | 1.16 ± 0.005 a | 4.60 ± 0.13 a | 46.88 ± 0.26 b | 0.89 ± 0.005 ab |
| T2 | 32.62 ± 0.10 a | 14.21 ± 0.07 a | 0.68 ± 0.01 c | 1.15 ± 0.016 a | 4.58 ± 0.15 a | 46.73 ± 0.15 b | 0.89 ± 0.012 ab |
| T3 | 29.31 ± 0.35 c | 14.14 ± 0.84 a | 1.14 ± 0.01 b | 1.15 ± 0.006 ab | 4.50 ± 0.01 a | 49.73 ± 0.52 a | 0.88 ± 0.028 b |
| T4 | 31.09 ± 0.24 b | 14.55 ± 0.05 a | 1.69 ± 0.01 a | 1.15 ± 0.004 a | 4.73 ± 0.15 a | 46.76 ± 0.33 b | 0.89 ± 0.010 ab |
| T5 | 30.49 ± 0.35 b | 13.82 ± 0.27 a | 1.44 ± 0.06 b | 1.15 ± 0.008 ab | 4.42 ± 0.10 a | 48.66 ± 0.44 a | 0.90 ± 0.003 ab |
| T6 | 33.43 ± 0.33 a | 14.01 ± 0.21 a | 0.88 ± 0.02 bc | 1.16 ± 0.011 a | 4.51 ± 0.01 a | 45.99 ± 0.51 b | 0.89 ± 0.006 ab |
| Treatments | Functional Property | |||
|---|---|---|---|---|
| Total Phenols * | DPPH ** | ABTS ** | FRAP ** | |
| TC | 1.24 ± 0.10 c | 9.36 ± 0.20 c | 8.86 ± 0.20 b | 3.05 ± 0.13 b |
| T1 | 1.98 ± 0.11 a | 16.56 ± 0.20 ab | 17.57 ± 0.43 a | 4.65 ± 0.17 a |
| T2 | 1.93 ± 0.10 a | 16.34 ± 0.67 ab | 17.57 ± 0.94 a | 4.24 ± 0.45 a |
| T3 | 1.68 ± 0.11 ab | 17.01 ± 0.49 ab | 17.19 ± 0.09 a | 4.09 ± 0.71 ab |
| T4 | 1.95 ± 0.12 a | 17.51 ± 0.60 a | 16.68 ± 0.77 a | 5.07 ± 0.34 a |
| T5 | 1.75 ± 0.17 ab | 16.03 ± 0.53 b | 16.43 ± 0.73 a | 4.82 ± 0.16 a |
| T6 | 1.57 ± 0.06 b | 16.89 ± 0.35 ab | 17.64 ± 0.20 a | 5.07 ± 0.31 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Balandrano, D.D.; Báez-González, J.G.; Carvajal-Millán, E.; Urías-Orona, V.; Méndez-Zamora, G.; Niño-Medina, G. Feruloylated Arabinoxylans from Nixtamalized Maize Bran By-Product as a Baking Ingredient: Physicochemical, Nutritional, and Functional Properties. Polysaccharides 2025, 6, 59. https://doi.org/10.3390/polysaccharides6030059
Herrera-Balandrano DD, Báez-González JG, Carvajal-Millán E, Urías-Orona V, Méndez-Zamora G, Niño-Medina G. Feruloylated Arabinoxylans from Nixtamalized Maize Bran By-Product as a Baking Ingredient: Physicochemical, Nutritional, and Functional Properties. Polysaccharides. 2025; 6(3):59. https://doi.org/10.3390/polysaccharides6030059
Chicago/Turabian StyleHerrera-Balandrano, Daniela D., Juan G. Báez-González, Elizabeth Carvajal-Millán, Vania Urías-Orona, Gerardo Méndez-Zamora, and Guillermo Niño-Medina. 2025. "Feruloylated Arabinoxylans from Nixtamalized Maize Bran By-Product as a Baking Ingredient: Physicochemical, Nutritional, and Functional Properties" Polysaccharides 6, no. 3: 59. https://doi.org/10.3390/polysaccharides6030059
APA StyleHerrera-Balandrano, D. D., Báez-González, J. G., Carvajal-Millán, E., Urías-Orona, V., Méndez-Zamora, G., & Niño-Medina, G. (2025). Feruloylated Arabinoxylans from Nixtamalized Maize Bran By-Product as a Baking Ingredient: Physicochemical, Nutritional, and Functional Properties. Polysaccharides, 6(3), 59. https://doi.org/10.3390/polysaccharides6030059









