Role of Chitosan Characteristics on the Properties of Curcumin-Loaded Carriers and Their Potential Application in Ophthalmologic Infection Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Materials for the Formation of the Capsules
2.1.2. Materials for the Implementation of Microbiological Studies
- Cell culture
- Viruses
- Reference substances
2.2. Methods and Procedures
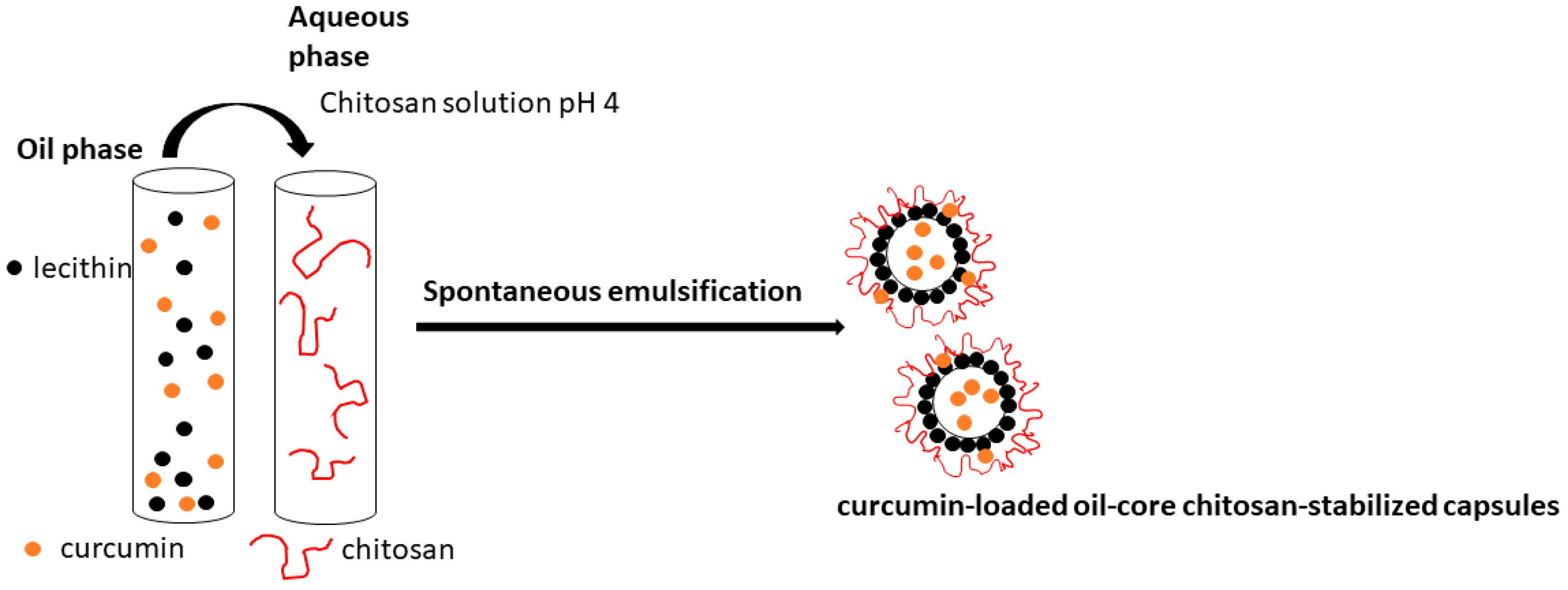
2.2.1. Preparation of the Curcumin-Loaded Capsules
2.2.2. Preparation of Simulated Ocular Fluids
- Simulated tear fluid (STF)
- Simulated aqueous humor (SAH)
- Simulated vitreous humor (SVH)
2.2.3. Evaluation of the Loaded Amount of Curcumin
2.2.4. Stability of the Capsules in Simulated Ocular Fluids
2.2.5. Characterization of the Charge and Size of the Capsules
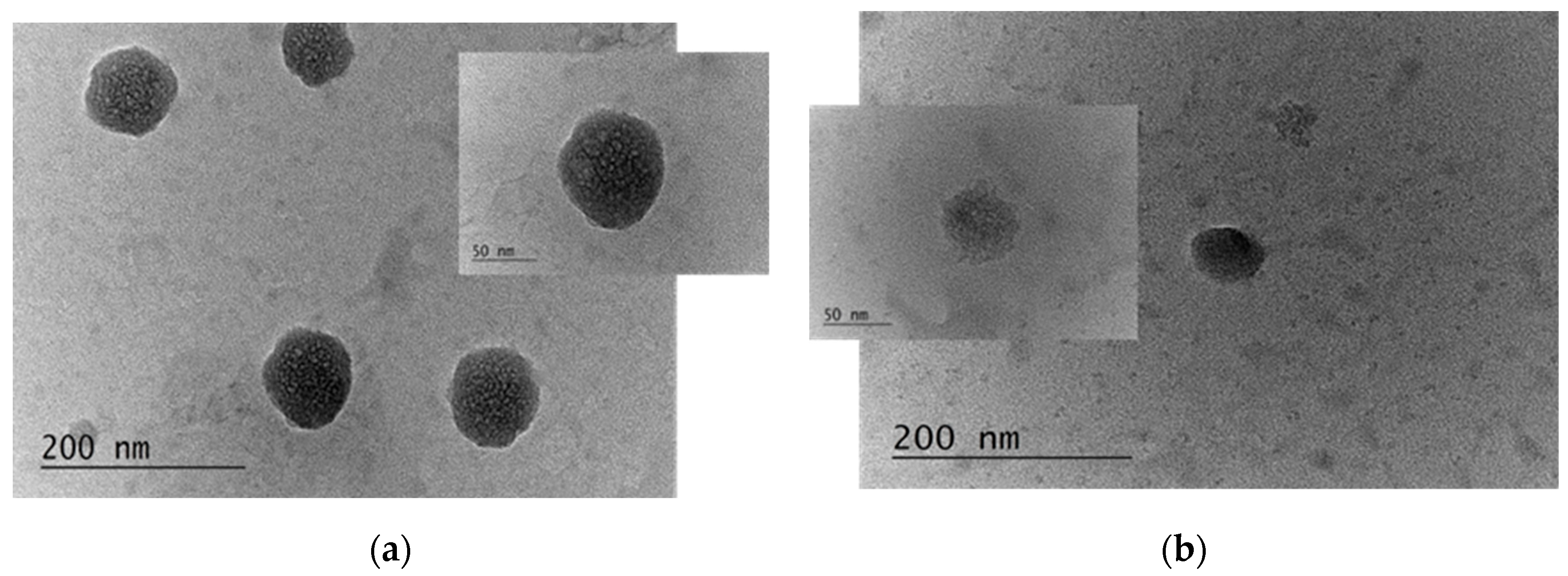
2.2.6. Visualization of the Produced Capsules
2.3. Microbiology Studies
2.3.1. Determination of Cytotoxicity and Phototoxicity
2.3.2. Determination of Infectious Viral Titers
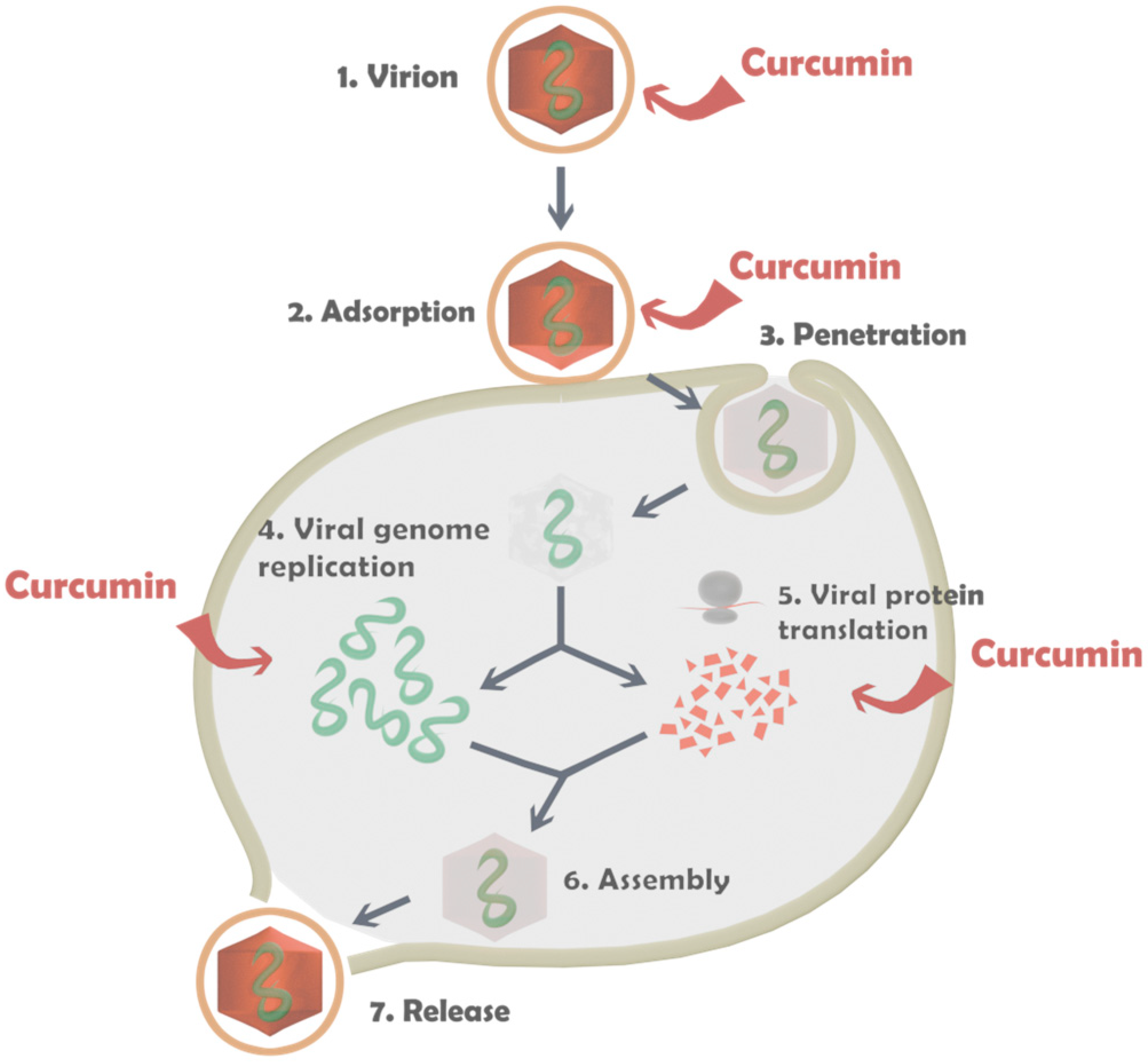
2.3.3. Antiviral Activity Assay
2.3.4. Determination of the Effect on Extracellular Virions
2.3.5. Effect on Viral Adsorption
2.3.6. Statistical Analysis
3. Results
3.1. Characterization of the Properties and Stability of the Produced Capsules
3.2. Analysis of the Microbiology Studies
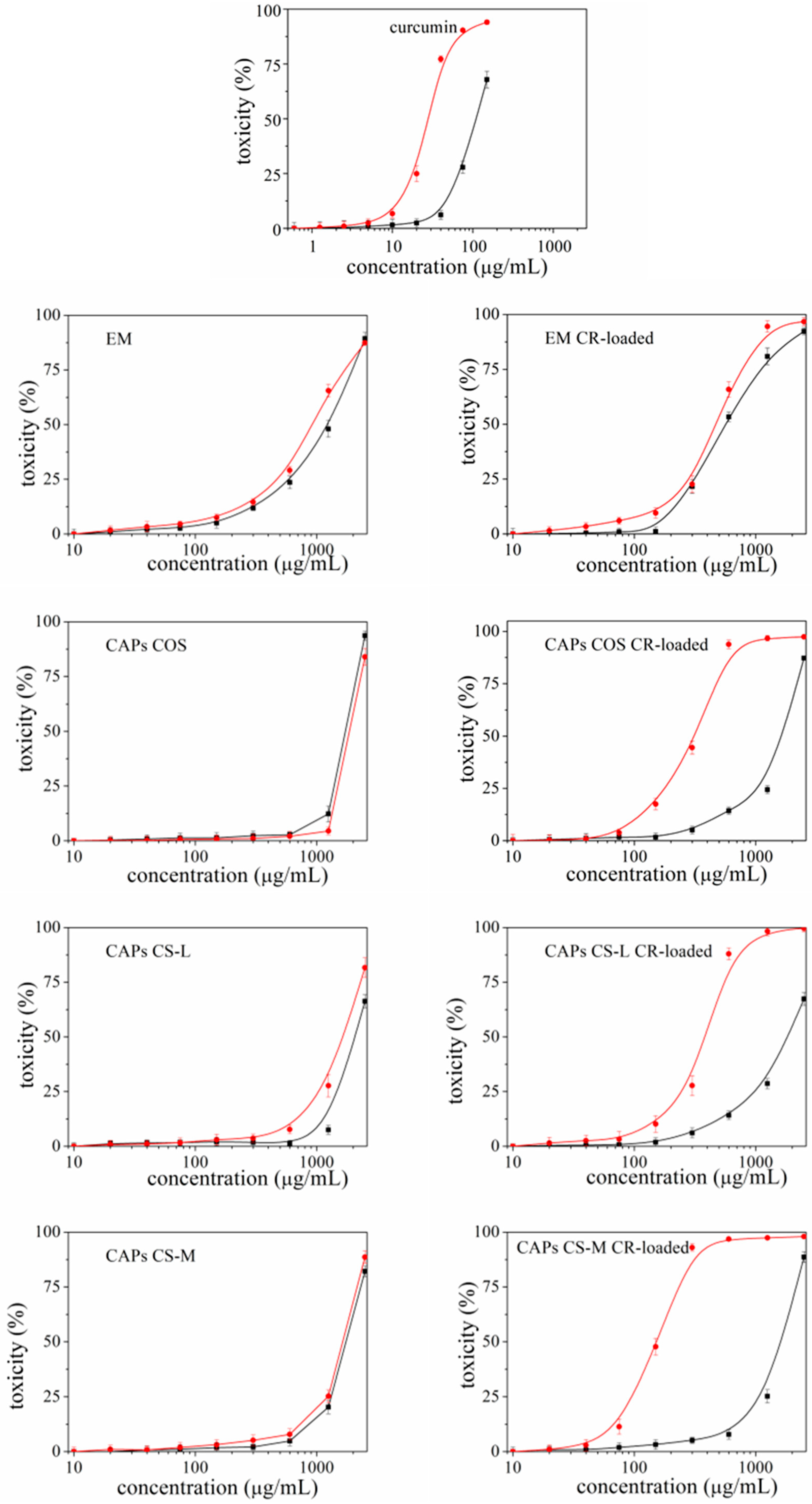
3.2.1. Cytotoxicity and Phototoxicity of the Carriers
3.2.2. Antiviral Activity of the Capsules
4. Discussion
- (1)
- (2)
- The activity of the carriers can be increased several times by incorporating CR [43,44,45]. For example, in the present study, the microbiological experiments showed that the CR-loaded emulsion and capsules produced from CS-L have a few times higher activity compared to the unloaded ones. The experiments demonstrate that changing even one form of the components of the structural composition may not lead to the desired effect and the system may not be fully productive. In the present study, the variation in the molecular weight of the chitosan results in different activity of the structures. The capsules containing CS-L showed the highest activity of all the tested samples, while the registered activity for the capsules with CS-H was almost three times lower.
- (3)
- CR is known to possess some phototoxicity. An ability for which it is being studied (but not yet recognized as an official therapeutic) as a photosensitizer most often in the treatment of cancers [46,47,48]. However, when it is not a matter of antitumor activity, the manifestation of this phototoxicity can often turn out to be negative, especially if the aim is to apply CR externally on the skin or eyes—areas that have direct contact with sunlight. The present study proved that, depending on the type of carriers used, it is possible to reduce the unwanted phototoxicity of the compound. The most effective in this regard was EM-CR-loaded, which reduced the manifestation of CR phototoxicity more than three times.
5. Conclusions
- (1)
- The size of the unloaded capsules depends on the characteristics of the chitosan used for the stabilization of the structures. The size of the capsules formed with chitosan with a higher molecular weight is larger compared to capsules produced with the low-molecular polymer samples (at a similar degree of acetylation of the polymer). The thickness of the chitosan layer on the unloaded carriers is significantly greater compared to the film formed on the CR-loaded ones. However, a correlation between the size of unloaded and CR-loaded capsules did not register, and it was supposed that the size of the capsules almost does not depend on the presence of CR in the core.
- (2)
- The registered ζ-potential from the dispersion of capsules stabilized with chitosan indicates the achievement of a positive charge of the structures after the chitosan adsorption but the ζ-potential of the capsules almost does not depend on the physicochemical characteristics of the polymer. The increase in the positive charge is registered for the CR-loaded capsules.
- (3)
- The encapsulation efficiency of CR into the carriers is evaluated at above 94% and almost does not depend on the characteristics of chitosan.
- (4)
- The aggregation is registered in all samples of chitosan-stabilized capsules (unloaded and CR-loaded) when the carriers are redispersed in artificial ocular fluids (STF, SAH, and SVH) and the released amount of CR is in the range of 0.8 to 1% (or ca. 1.5 µg/mL). It is assumed that there is a correlation between the stability of the capsules and the released amount of CR. The capsules stabilized by CAPs COS CR-loaded are more stable because they released the lowest amount of CR.
- (5)
- The characterization of the emulsions indicates that the size of the CR-loaded droplets is larger compared to the unloaded ones. Moreover, the electrokinetic charge of the unloaded droplets is highly negative but the incorporation of the CR results in a slight decrease in the charge because of the deposition of the positively charged CR molecules on the droplet surface, not only in the core of the structures. The encapsulation efficiency of CR in emulsion droplets is slightly higher compared to the chitosan-stabilized capsules. The emulsions are shown significant stability when the carriers are redispersed in artificial ocular fluids and the released amount of CR is ca. 0.61% (or ca. 0.93 µg/mL).
- (6)
- The registered cyto- and phototoxicity of carriers containing CR is significantly reduced compared to the solution of pure CR. Moreover, the activity of the unloaded carriers can be increased several times by incorporating the compound. The experimental results demonstrate that the variation in the properties of even one component of the structural composition can provoke different activities in the carriers.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrillo, F.; Petrillo, A.; Sasso, F.P.; Schettino, A.; Maione, A.; Galdiero, M. Viral Infection and Antiviral Treatments in Ocular Pathologies. Microorganisms 2022, 10, 2224. [Google Scholar] [CrossRef] [PubMed]
- Clare, G.; Kempen, J.H.; Pavésio, C. Infectious eye disease in the 21st century—An overview. Eye 2024, 38, 2014–2027. [Google Scholar] [CrossRef] [PubMed]
- Vinod, K.; Sidoti, P.A. Glaucoma care during the coronavirus disease 2019 pandemic. Curr. Opin. Ophthalmol. 2021, 32, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Van Der Hoek, L.; Pyrc, K.; Jebbink, M.F.; Vermeulen-Oost, W.; Berkhout, R.J.M.; Wolthers, K.C. Identification of a New Human Coronavirus. Nat. Med. 2004, 10, 368–373. [Google Scholar] [CrossRef]
- Sen, M.; Honavar, S.G.; Sharma, N.; Sachdev, M.S. COVID-19 and Eye: A Review of Ophthalmic Manifestations of COVID-19. Indian J. Ophthalmol. 2021, 69, 488–509. [Google Scholar] [CrossRef]
- Bagga, B.; Kate, A.; Joseph, J.; Dave, V.P. Herpes simplex infection of the eye: An introduction. Community Eye Health 2020, 33, 68–70. [Google Scholar]
- Aldaas, K.; Challa, P.; Weber, D.J.; Fleischman, D. Infections and glaucoma. Surv. Ophthalmol. 2022, 67, 637–658. [Google Scholar] [CrossRef]
- Singh, R.B.; Ichhpujani, P.; Thakur, S.; Jindal, S. Promising therapeutic drug delivery systems for glaucoma: A comprehensive review. Ther. Adv. Ophthalmol. 2020, 12, 2515841420905740. [Google Scholar] [CrossRef]
- Pater, K.D.; Silva, L.B.; Park, Y.; Shakouri, T.; Keskin-Erdogan, Z.; Sawadkar, P.; Cho, K.J.; Knowles, J.C.; Chau, D.Y.S.; Kim, H.-W. Recent advances in drug delivery systems for glaucoma treatment. Mater. Today Nano 2022, 18, 100178. [Google Scholar]
- Cvenkel, B.; Kolko, M. Devices and treatment address low adherence in glaucoma patients: A narrative review. J. Clin. Med. 2023, 12, 151. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorca, V.; Cicero, A.F.G. Clinical applications of astaxantin in the treatment of ocular diseases: Emerging insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Guo, H.; Kern, T.S.; Huang, K.; Zheng, L. Curcumin inhibits neuronal and vascular degeneration in retina after ischemia and reperfusion injury. PLoS ONE 2011, 6, e23194. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, A.; Hashemi, F. Protective effects of curcumin on ischemic reperfusion of rat retina. Comp. Clin. Pathol. 2019, 28, 89–95. [Google Scholar] [CrossRef]
- Kevin, T.T.M.; Nur Idanis, A.S.; Anastasha, B.; Mohd Faris, M.R.; Faizah, O.; Taty Anna, K. Curcumin minimises histopathological and immunological progression in the ankle joints of collagen-induced arthritis rats. Med. Health 2020, 15, 26–36. [Google Scholar]
- Kamal, D.A.M.; Salamt, N.; Yusuf, A.N.M.; Kashim, M.; Mokhtar, M.H. Potential health benefits of curcumin on female reproductive disorders: A review. Nutrients 2021, 13, 3126. [Google Scholar] [CrossRef]
- Buccarello, L.; Dragotto, J.; Hassanzadeh, K.; Maccarone, R.; Corbo, M.; Feligioni, M. Retinal ganglion cell loss in an ex vivo mouse model of optic nerve cut is prevented by curcumin treatment. Cell Death Discov. 2021, 7, 394. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Alonso, M.J.; Sanchez, A. The potential of chitosan in ocular drug delivery. JPP 2003, 55, 1451–1463. [Google Scholar] [CrossRef]
- Sable, A.A.; Kunwar, A.; Barik, A. Alginate and chitosan-based delivery systems for improving the bioavailability and therapeutic efficacy of curcumin. Pharmaceutics 2024, 16, 423. [Google Scholar] [CrossRef]
- Dmour, I. Absorption enhancement strategies in chitosan-based nanosystems and hydrogels intended for ocular delivery: Latest advances for optimization of drug permeation. Carbohydr. Polym. 2024, 343, 122486. [Google Scholar] [CrossRef]
- Haider, A.; Khan, S.; Iqbal, D.N.; Shrahili, M.; Haider, S.; Mohammad, K.; Mohammad, A.; Rizwan, M.; Kanwal, Q.; Mustafa, G. Advances in chitosan-based drug delivery systems: A comprehensive review for therapeutic applications. Eur. Polym. J. 2024, 210, 112983. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Calvo, P.; Remufifin-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Development of positively charged colloidal drug carriers: Chitosan-coated polyester nanocapsules and submicron-emulsions. Colloid Polym. Sci. 1997, 275, 46–53. [Google Scholar] [CrossRef]
- Kurniawansyah, I.S.; Rusdiana, T.; Sopyan, I.; Ramoka, H.; Wahab, H.A.; Subarnas, A. In situ ophthalmic gel forming of poloxamer 407 and hydroxypropyl methylcellulose mixtures for sustained ocular delivery of chloramphenicol: Optimization study by factorial design. Heliyon 2020, 11, e05365. [Google Scholar]
- Hayashi, R.; Hayashi, S.; Arai, K.; Yoshida, S.; Chikuda, M.; Machida, S. Evaluating the Biostability of Yellow and Clear Intraocular Lenses with a System Simulating Natural Intraocular Environment. Transl. Vis. Sci. Technol. 2016, 5, 11. [Google Scholar] [CrossRef]
- Kummer, M.P.; Abbott, J.J.; Dinser, S.; Nelson, B.J. Artificial vitreous humor for in vitro experiments. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 6407–6410. [Google Scholar]
- Nong, H.V.; Hung, L.X.; Thang, P.N.; Chinh, V.D.; Vu, L.V.; Dung, P.T.; Trung, T.V.; Nga, P.T. Fabrication and vibration characterization of curcumin extracted from turmeric (Curcuma longa) rhizomes of northern Vietnam. SpringerPlus 2016, 5, 1147. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determination in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Cytopathic Effect Inhibition Assay. Creative Diagnostics. 2025. Available online: https://antiviral.creative-diagnostics.com/cc50-ic50-assay.html (accessed on 15 January 2025).
- Rabenau, H.F.; Schwebke, I.; Blümel, J. Guideline for testing chemical disinfectants regarding their virucidal activity within the field of human medicine. Bundesgesundheitsbl 2020, 63, 645–655. [Google Scholar] [CrossRef]
- Lopes, N.; Ray, S.; Espada, S.F.; Bomfim, W.A.; Ray, B.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol. 2017, 102, 605–612. [Google Scholar] [CrossRef]
- Milkova, V. Electrosteric stabilization of oil/water emulsions by adsorption of chitosan oligosaccharides—An electrokinetic study. Carbohydr. Polym. 2021, 265, 118072. [Google Scholar] [CrossRef]
- Bernabé-Pineda, M.; Ramírez-Silva, M.; Romero-Romo, M.; Gonzalez Vergara, E.; Rojas-Hernandez, A. Determination of acidity constants of curcumin in aqueous solution and apparent rate constant its decomposition. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2024, 60, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F.; Bikádi, Z.; Simonyi, M. Circular dichroism spectroscopic studies reveal pH dependent binding of curcumin in the minor groove of natural and synthetic nucleic acids. Org. Biomol. Chem. 2004, 2, 2902–2910. [Google Scholar] [CrossRef] [PubMed]
- Jen, M.; Lee, S.; Lee, G.; Lee, D.; Pang, Y. Intramolecular Charge Transfer of Curcumin and Solvation Dynamics of DMSO Probed by Time-Resolved Raman Spectroscopy. Int. J. Mol. Sci. 2022, 23, 1727. [Google Scholar] [CrossRef]
- Md Saari, N.H.; Chua, L.S.; Hasham, R.; Yuliati, L. Curcumin-Loaded Nanoemulsion for Better Cellular Permeation. Sci. Pharm. 2020, 88, 44. [Google Scholar] [CrossRef]
- Derman, S.; Uzunoglu, D.; Acar, T.; Arasoglu, T.O.; Ucak, S.; Ozalp, V.; Mansuroglu, B. Antioxidant Activity and Hemocompatibility Study of Quercetin Loaded Plga Nanoparticles. Iran. J. Pharm. Res. 2020, 19, 424–435. [Google Scholar]
- Vaiss, D.P.; Rodrigues, J.L.; Yurgel, V.C.; Guedes, F.C.; Mendonça da Matta, L.L.; Barros, P.A.B.; Vaz, G.R.; dos Santos, R.N.; Matte, B.F.; Kupski, L.; et al. Curcumin and quercetin co-encapsulated in nanoemulsions for nasal administration: A promising therapeutic and prophylactic treatment for viral respiratory infections. Eur. J. Pharm. Sci. 2024, 197, 106766. [Google Scholar] [CrossRef]
- Ma, Z.; Haddadi, A.; Molavi, O.; Lavasanifar, A. Micelles of poly (ethylene oxide) b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J. Biomed. Mater. Res. 2008, 86, 300–310. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Ravi Kumar, M.N.V. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- do Bonfim, C.M.; Monteleoni, L.F.; Calmon, M.d.F.; Cândido, N.M.; Provazzi, P.J.S.; Lino, V.d.S.; Rabachini, T.; Sichero, L.; Villa, L.L.; Quintana, S.M.; et al. Antiviral activity of curcumin nanoemulsion associated with photodynamic therapy in vulvar cell lines transducing different variants of HPV-16, Artificial Cells. Nanomed. Biotechnol. 2020, 48, 515–524. [Google Scholar]
- Yang, K.C.; Lin, J.C.; Tsai, H.H.; Hsu, C.Y.; Shih, V.; Hu, C.M.J. Nanotechnology advances in pathogen- and host-targeted antiviral delivery: Multipronged therapeutic intervention for pandemic control. Drug Deliv. Transl. Res. 2021, 11, 1420–1437. [Google Scholar] [CrossRef]
- Khizar, S.; Alrushaid, N.; Khan, F.A.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Elaissari, A. Nanocarriers based novel and effective drug delivery system. Int. J. Pharm. 2023, 632, 122570. [Google Scholar] [CrossRef] [PubMed]
- Allard, E.; Passirani, C.; Benoit, J.P. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials 2009, 30, 2302–2318. [Google Scholar] [CrossRef] [PubMed]
- Trigo Gutierrez, J.K.; Zanatta, G.C.; Ortega, A.L.M.; Balastegui, M.I.C.; Sanita, P.V.; Pavarina, A.C.; Barbugli, P.A.; de Oliveira Mima, E.G. Encapsulation of curcumin in polymeric nanoparticles for antimicrobial Photodynamic Therapy. PLoS ONE 2017, 12, e0187418. [Google Scholar] [CrossRef]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.A.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A.; et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine 2015, 11, 195–206. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer. Pharmaceutics 2021, 13, 1562. [Google Scholar] [CrossRef]
- Buggiani, G.; Troiano, M.; Rossi, R.; Lotti, T. Photodynamic therapy: Off-label and alternative use in dermatological practice. Photodiagnosis Photodyn Ther. 2008, 5, 134–138. [Google Scholar] [CrossRef]
- Skevaki, C.L.; Galani, I.E.; Pararas, M.V.; Giannopoulou, K.P.; Tsakris, A. Treatment of Viral Conjunctivitis with Antiviral Drugs. Drugs 2011, 71, 331–347. [Google Scholar] [CrossRef]
- Hoffman, J. Overview of antiviral medications used in ophthalmology. Community Eye Health 2020, 33, 85–88. [Google Scholar]
- MacCormack, M.A. Photodynamic therapy in dermatology: An update on applications and outcomes. Semin. Cutan. Med. Surg. 2008, 27, 52–62. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, Y.; Luo, X.; Lei, W.; Xie, L. Antiviral and virucidal effects of curcumin on transmissible gastroenteritis virus in vitro. J. Gen. Virol. 2020, 101, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017, 142, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Milkova, V.; Vilhelmova-Ilieva, N.; Gyurova, A.; Kamburova, K.; Dimitrov, I.; Tsvetanova, E.; Georgieva, A.; Mileva, M. Remdesivir-loaded Nanoliposomes Stabilized by Chitosan/Hyaluronic Acid Film with a Potential Application in the Treatment of Coronavirus Infection. Neurol. Int. 2023, 15, 1320–1338. [Google Scholar] [CrossRef] [PubMed]
- Milkova, V.; Kamburova, K.; Martinov, P.; Vilhelmova-Ilieva, N.; Rashev, V. Chitosan-Based Nanocarriers for Delivery of Remdesivir. Sci. Pharm. 2023, 91, 37. [Google Scholar] [CrossRef]
| Samples | Size * [nm] | Polydispersity Index | ζ-Potential [mV] | EE% |
|---|---|---|---|---|
| PDI | ||||
| EM | 145.5 ± 5.5 | 0.12 | −55.4 ± 1.3 | - |
| EM CR-loaded | 192.7 ± 4.8 | 0.17 | −47.7 ± 3.0 | 98.6 |
| CAPs CS-L | 204.4 ± 3.9 | 0.22 | 68.6 ± 0.9 | - |
| CAPs CS-L CR-loaded | 207 ± 3.6 | 0.18 | 89.8 ± 7.0 | 98.0 |
| CAPs CS-M | 312.5 ± 0.2 | 0.34 | 70.7 ± 1.2 | - |
| CAPs CS-M CR-loaded | 280.3 ± 8.9 | 0.40 | 106.7 ± 5.9 | 94.6 |
| CAPs COS | 269.4 ± 3.5 | 0.11 | 58.7 ± 0.8 | - |
| CAPs COS CR-loaded | 233.4 ± 8.2 | 0.25 | 86.3 ± 2.1 | 96.4 |
| Sample | Stability in STF | Stability in SAH | Stability in SVH |
|---|---|---|---|
| Release Amount CR in [%] and [μg/mL] | Release Amount CR in [%] and [μg/mL] | Release Amount CR in [%] and [μg/mL] | |
| EM CR-loaded | 0.56 (0.86) | 0.68 (1.04) | 0.58 (0.88) |
| CAPs CS-L CR-loaded | 0.80 (1.52) | 0.96 (1.46) | 0.70 (1.06) |
| CAPs CS-M CR-loaded | 0.93 (1.37) | 0.78 (1.16) | 0.63 (0.93) |
| CAPs COS CR-loaded | 0.73 (1.09) | 0.66 (0.99) | 0.53 (0.8) |
| Sample | Mean CC50 ± SD (µg/mL) | PIF * | |
|---|---|---|---|
| −Irr *** | +Irr **** | ||
| CR | 110.49 ± 5.999 | 27.88 ± 0.581 | 3.96 |
| CAPs COS | 1725.17 ± 22.486 | 1862.36 ± 45.271 | 0.93 |
| CAPs COS CR-loaded | 1657.98 ± 25.087 | 323.11 ± 12.393 | 5.13 |
| CAPs CS-L | 2496.61 ± 53.461 | 2012.59 ± 58.426 | 1.24 |
| CAPs CS-L CR-loaded | 2366.59 ± 84.875 | 506.93 ± 14.170 | 4.67 |
| EM | 1280.38 ± 88.110 | 916.04 ± 41.45 | 1.4 |
| EM CR-loaded | 557.62 ± 28.65 | 466.10 ± 15.272 | 1.2 |
| CAPs CS-M | 1745.38 ± 58.63 | 1638.61 ± 53.27 | 1.07 |
| CAPs CS-M CR-loaded | 1638.59 ± 47.577 | 154.97 ± 8.558 | 10.57 |
| Chlorpromazine ** | 16.98 ± 0.316 | 2.59 ± 0.166 | 6.56 |
| Sample | 48 h | 120 h | ||
|---|---|---|---|---|
| CC50 Mean ± SD (μg/mL) | MTC (μg/mL) | CC50 Mean ± SD (μg/mL) | MTC (μg/mL) | |
| CR | 105.2 ± 6.3 *** | 10.0 | 94.4 ± 6.2 *** | 10.0 |
| CAPs COS | 1780.5 ± 12.3 *** | 1000.0 | 1548.2 ± 12.8 | 1000.0 |
| CAPs COS CR-loaded | 1721.7 ± 13.2 *** | 1000.0 | 1597.2 ± 14.9 ** | 1000.0 |
| EM | 1350.3 ± 10.6 *** | 1000.0 | 1123.8 ± 12.1 *** | 1000.0 |
| EM CR-loaded | 611.4 ± 12.7 *** | 320.0 | 520.8 ± 9.2 *** | 320.0 |
| CAPs CS-L | ˃2500.0 *** | 1000.0 | 2320.6 ± 16.7 *** | 1000.0 |
| CAPs CS-L CR-loaded | 2403.8 ± 15.6 *** | 1000.0 | 1800.0 ± 14.3 *** | 1000.0 |
| CAPs CS-M | 1708.6 ± 13.7 *** | 1000.0 | 1640.9 ± 13.3 *** | 1000.0 |
| CAPs CS-M CR-loaded | 1658 ± 11.4 *** | 1000.0 | 1448 ± 10.8 *** | 1000.0 |
| ACV | 182.4 ± 6.8 | 100.0 | nd | nd |
| REM | nd | nd | 1543.6 ± 5.4 | 640.0 |
| Sample | HSV-1 (Victoria Strain) | HCoV-OC43 | ||
|---|---|---|---|---|
| IC50 Mean ± SD (μg/mL) | SI | IC50 Mean ± SD (μg/mL) | SI | |
| CR | 20.6 ± 2.3 *** | 5.1 | 8.2 ± 2.2 *** | 11.5 |
| CAPs COS | - | - | - | - |
| CAPs COS CR-loaded | 389.7 ± 4.8 *** | 4.4 | 295.4 ± 2.9 *** | 5.4 |
| EM | - | - | - | - |
| EM CR-loaded | 124.7 ± 4.2 *** | 4.9 | 25.8 ± 3.2 | 20.8 |
| CAPs CS-L | - | - | - | - |
| CAPs CS-L CR-loaded | 245.4 ± 5.7 *** | 9.8 | 84.2 ± 5.1 *** | 21.4 |
| CAPs CS-M | - | - | - | - |
| CAPs CS-M CR-loaded | 364.7 ± 6.1 *** | 4.7 | 238.1 ± 4.8 *** | 6.9 |
| ACV | 1.6 ± 0.3 | 114.0 | nd | nd |
| REM | nd | nd | 28.7 | 53.8 |
| Sample | Δlg | ||||
|---|---|---|---|---|---|
| 15 min | 30 min | 45 min | 60 min | 120 min | |
| CR | 1.5 | 2.0 | 3.25 | 3.25 | 3.25 |
| CAPs COS | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 |
| CAPs COS CR-loaded | 0.25 | 0.5 | 0.75 | 0.75 | 0.75 |
| EM | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| EM CR-loaded | 0.25 | 0.5 | 0.75 | 0.75 | 0.75 |
| CAPs CS-L | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 |
| CAPs CS-L CR-loaded | 0.5 | 0.5 | 0.5 | 0.75 | 0.75 |
| CAPs CS-M | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| CAPs CS-M CR-loaded | 0.5 | 0.5 | 0.75 | 0.75 | 0.75 |
| 70% etanol | 6.25 | 6.25 | 6.25 | 6.25 | 6.0 |
| Sample | Δlg | ||||
|---|---|---|---|---|---|
| 15 min | 30 min | 45 min | 60 min | 120 min | |
| CR | 1.25 | 1.75 | 2.5 | 3.0 | 3.5 |
| CAPs COS | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 |
| CAPs COS CR-loaded | 0.5 | 0.5 | 0.5 | 0.75 | 0.75 |
| EM | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 |
| EM CR-loaded | 0.5 | 0.5 | 0.5 | 0.5 | 0.75 |
| CAPs CS-L | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 |
| CAPs CS-L CR-loaded | 0.5 | 0.75 | 0.75 | 0.75 | 0.75 |
| CAPs CS-M | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 |
| CAPs CS-M CR-loaded | 0.5 | 0.75 | 0.75 | 0.75 | 0.75 |
| 70% etanol | 5.5 | 5.25 | 5.25 | 5.25 | 5.0 |
| Sample | Δlg | |||
|---|---|---|---|---|
| 15 min | 30 min | 45 min | 60 min | |
| CR | 1.5 | 1.5 | 2.0 | 2.0 |
| CAPs COS | 0.5 | 0.5 | 0.5 | 0.5 |
| CAPs COS CR-loaded | 0.5 | 0.5 | 1.5 | 1.5 |
| EM | 0.5 | 0.5 | 0.5 | 0.5 |
| EM CR-loaded | 0.5 | 0.5 | 1.0 | 1.5 |
| CAPs CS-L | 0.5 | 0.5 | 0.5 | 0.5 |
| CAPs CS-L CR-loaded | 0.5 | 0.5 | 0.75 | 1.0 |
| CAPs CS-M | 0.5 | 0.5 | 0.5 | 0.5 |
| CAPs CS-M CR-loaded | 0.25 | 0.25 | 0.75 | 0.75 |
| Sample | Δlg | ||||
|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 90 min | 120 min | |
| CR | 1.5 | 1.5 | 2.0 | 2.25 | 2.25 |
| CAPs COS | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| CAPs COS CR-loaded | 0.5 | 0.5 | 1.25 | 1.5 | 1.5 |
| EM | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| EM CR-loaded | 0.5 | 0.5 | 1.0 | 1.5 | 1.5 |
| CAPs CS-L | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| CAPs CS-L CR-loaded | 0.5 | 0.5 | 1.0 | 1.25 | 1.25 |
| CAPs CS-M | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| CAPs CS-M CR-loaded | 0.5 | 0.5 | 0.75 | 1.0 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milkova, V.; Martinov, P.; Vilhelmova-Ilieva, N.; Iliev, I. Role of Chitosan Characteristics on the Properties of Curcumin-Loaded Carriers and Their Potential Application in Ophthalmologic Infection Therapy. Polysaccharides 2025, 6, 22. https://doi.org/10.3390/polysaccharides6010022
Milkova V, Martinov P, Vilhelmova-Ilieva N, Iliev I. Role of Chitosan Characteristics on the Properties of Curcumin-Loaded Carriers and Their Potential Application in Ophthalmologic Infection Therapy. Polysaccharides. 2025; 6(1):22. https://doi.org/10.3390/polysaccharides6010022
Chicago/Turabian StyleMilkova, Viktoria, Petar Martinov, Neli Vilhelmova-Ilieva, and Ivan Iliev. 2025. "Role of Chitosan Characteristics on the Properties of Curcumin-Loaded Carriers and Their Potential Application in Ophthalmologic Infection Therapy" Polysaccharides 6, no. 1: 22. https://doi.org/10.3390/polysaccharides6010022
APA StyleMilkova, V., Martinov, P., Vilhelmova-Ilieva, N., & Iliev, I. (2025). Role of Chitosan Characteristics on the Properties of Curcumin-Loaded Carriers and Their Potential Application in Ophthalmologic Infection Therapy. Polysaccharides, 6(1), 22. https://doi.org/10.3390/polysaccharides6010022






