Metal–Phenolic Network-Loaded Sodium Alginate-Based Antibacterial and Antioxidant Films Incorporated with Geranium Essential Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
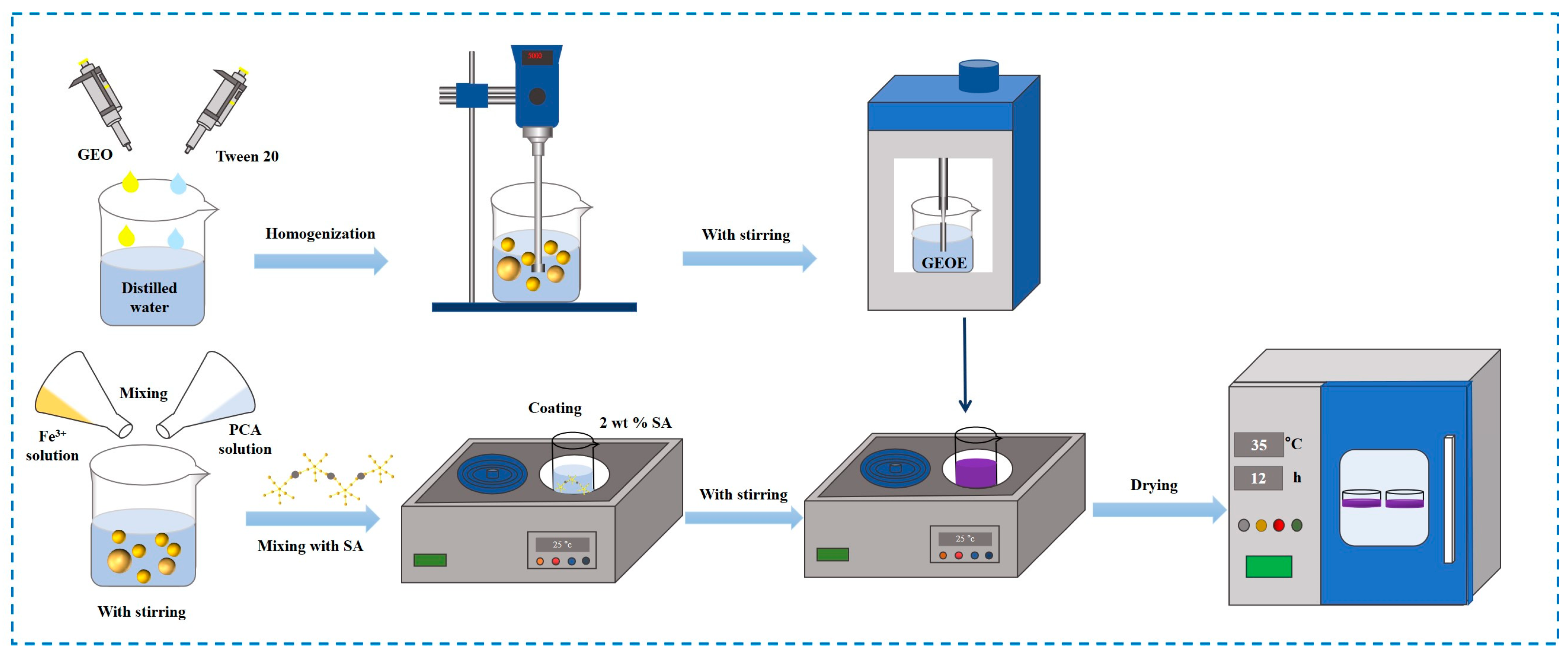
2.2. Fabrication of Geranium Essential Oil Emulsion
2.3. Film Preparation
2.4. Characterization of the Film
2.4.1. Thickness and Water Content
2.4.2. The Distribution of Geranium Essential Oil
2.4.3. Scanning Electron Microscopy
2.4.4. Fourier Transform Infrared
2.4.5. X-Ray Diffraction
2.4.6. Color
2.4.7. Ultraviolet–Visible Analysis
2.4.8. Thermal Gravimetric Analysis
2.4.9. Mechanical Properties
2.5. Functional Properties of the Films
2.5.1. Antioxidant Activity
2.5.2. Antibacterial Activity
2.5.3. Hemolysis Properties
2.6. Statistical Analysis
3. Results and Discussion
3.1. Thickness and Water Content
3.2. The Distribution of GEO
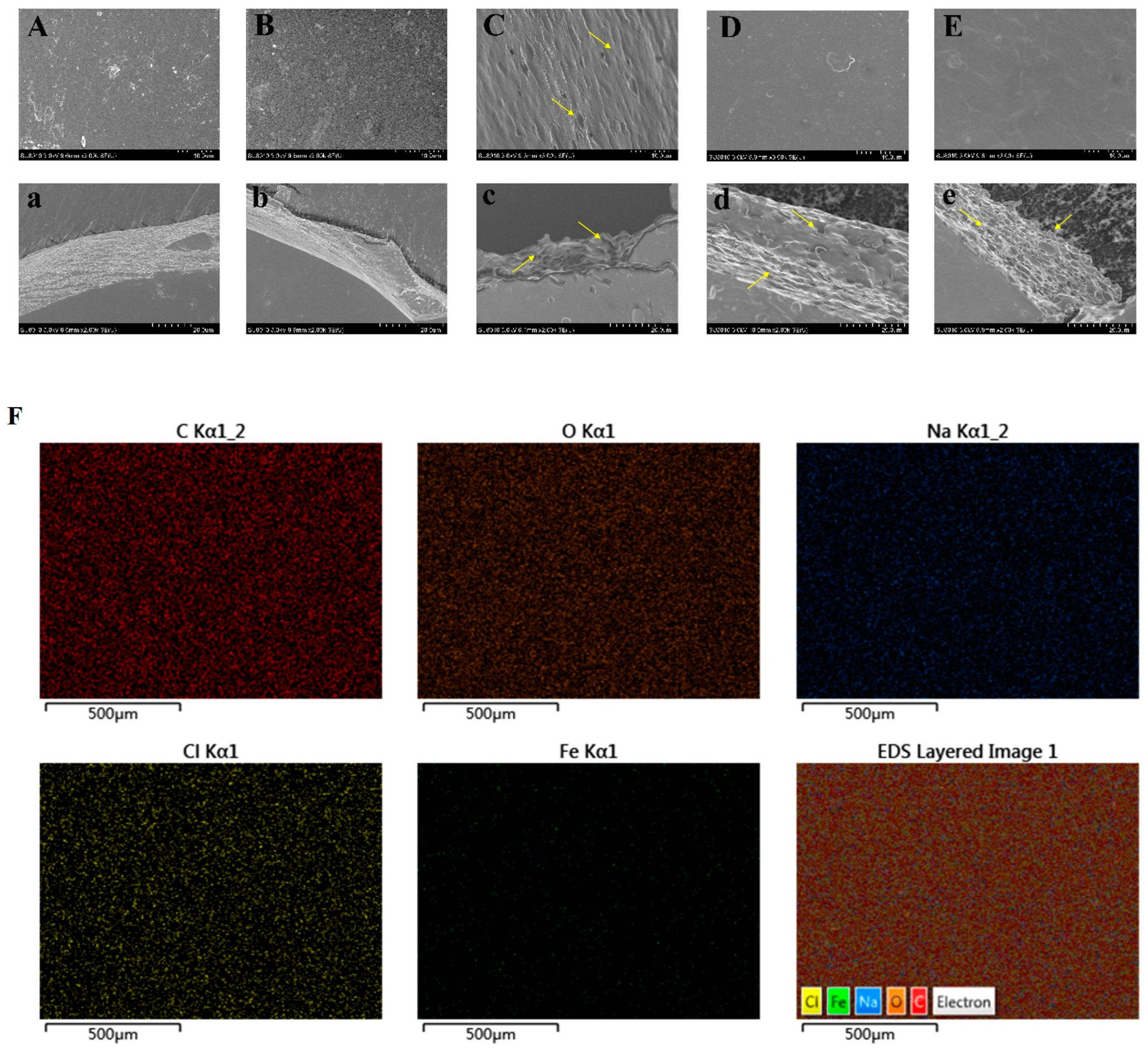
3.3. SEM
3.4. FTIR
3.5. XRD
3.6. Color
3.7. UV
3.8. TGA
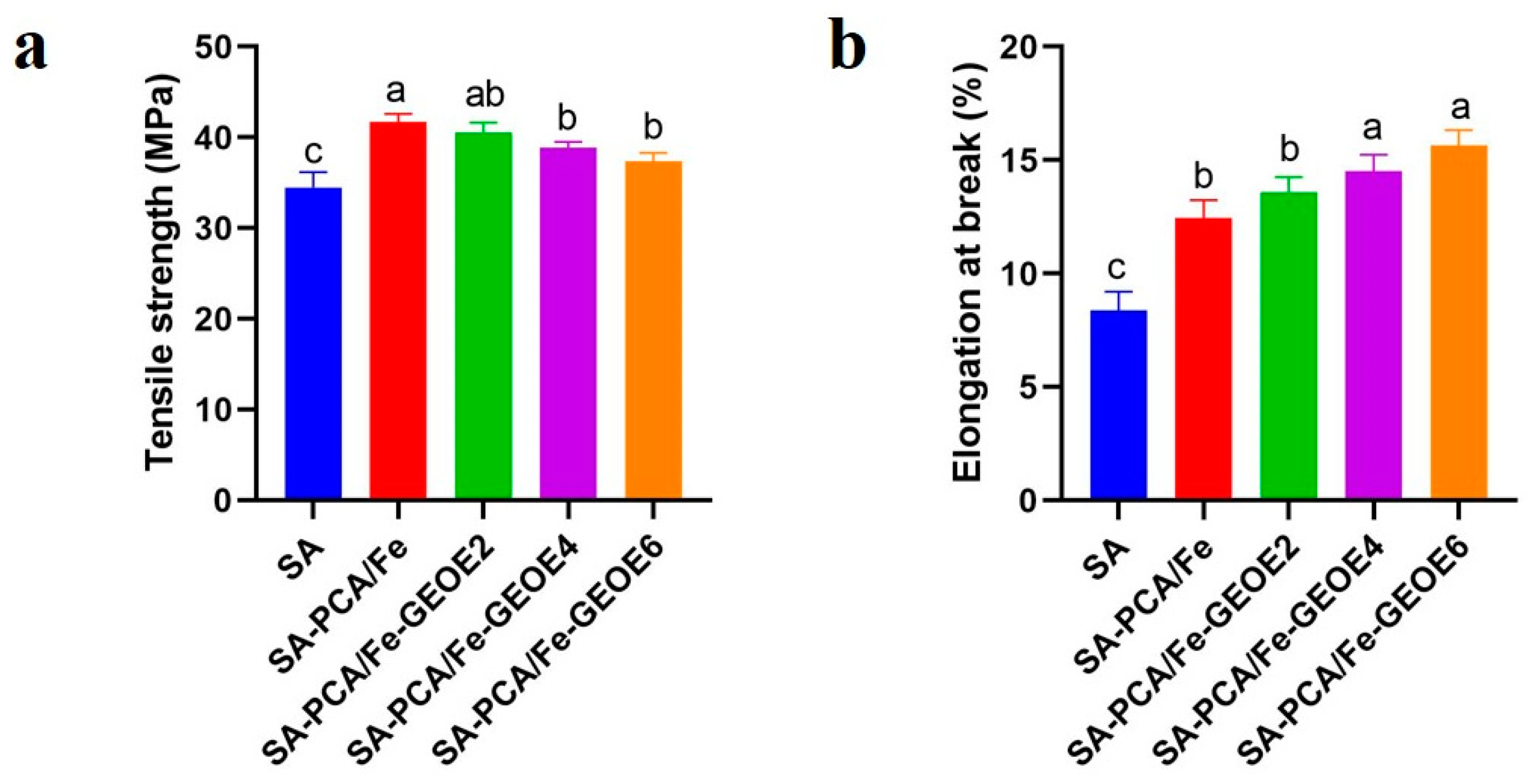
3.9. Mechanical Properties
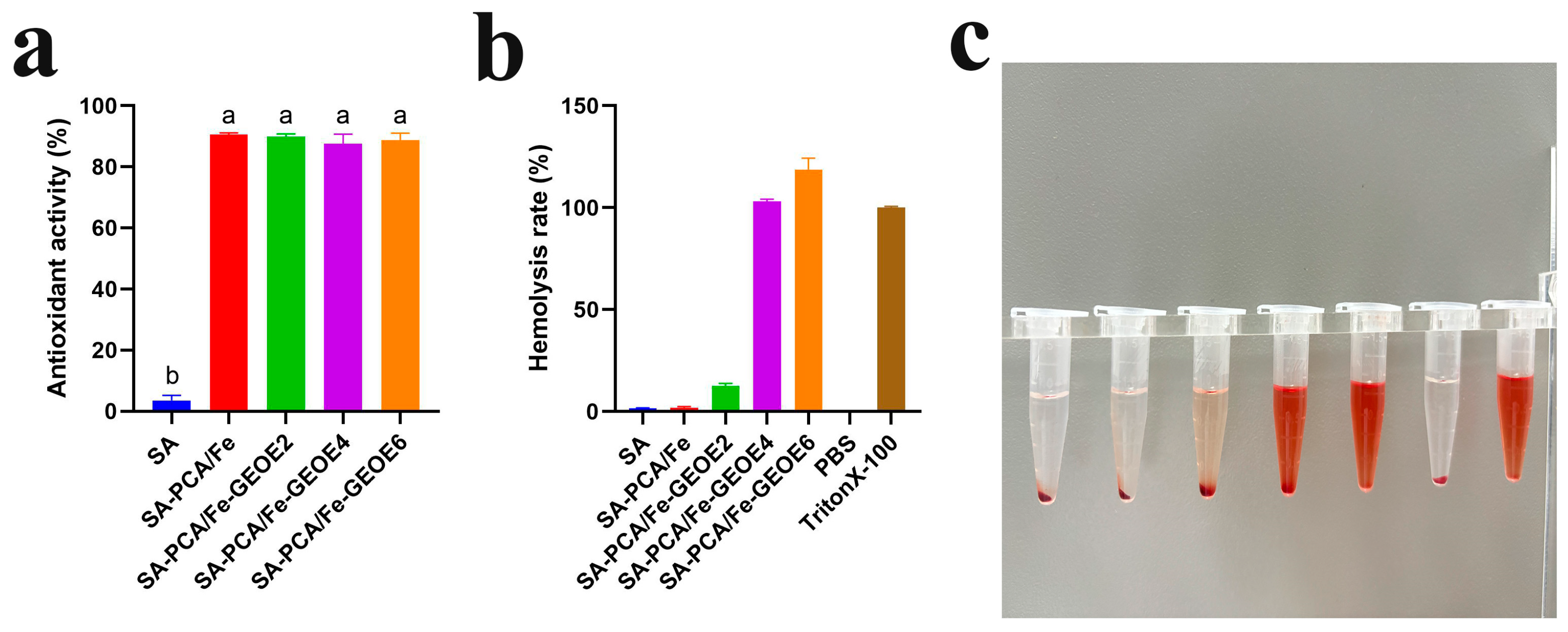
3.10. Antioxidant Activity
3.11. Antibacterial Activity
3.12. Hemolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, M.-Y.; Zhou, Q.; Zhang, J.; Li, T.; Cheng, J.; Liu, Q.; Xu, W.-R.; Zhang, Y.-C. Antioxidant and antibacterial properties of essential oils-loaded β-cyclodextrin-epichlorohydrin oligomer and chitosan composite films. Colloids Surf. B Biointerfaces 2022, 215, 112504. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, Y.Y.; Zhu, Z.; Xu, B.; Meng, L.H.; Yang, T.; Zhang, L.; Qian, J.Y.; Liu, F.S. Preparation and characterization of peach gum/chitosan polyelectrolyte composite films with dual cross-linking networks for antibacterial packaging. Int. J. Biol. Macromol. 2024, 261, 129754. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wu, Z.; Shen, M.; Rong, L.; Liu, W.; Xiao, W.; Xie, J. Improve properties of sweet potato starch film using dual effects: Combination Mesona chinensis Benth polysaccharide and sodium carbonate. LWT-Food Sci. Technol. 2021, 140, 110679. [Google Scholar] [CrossRef]
- Barbosa-Nuñez, J.A.; Espinosa-Andrews, H.; Cardona, A.A.V.; Haro-González, J.N. Polymer-based encapsulation in food products: A comprehensive review of applications and advancements. J. Future Foods 2025, 5, 36–49. [Google Scholar] [CrossRef]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Natural polymers in bio-degradable/edible film: A review on environmental concerns, cold plasma technology and nanotechnology application on food packaging—A recent trends. Food Chem. Adv. 2022, 1, 100135. [Google Scholar] [CrossRef]
- Yan, P.; Lan, W.; Xie, J. Modification on sodium alginate for food preservation: A review. Trends Food Sci. Technol. 2024, 143, 104217. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, K.; Bai, B. A critical review of sodium alginate-based composites in water treatment. Carbohydr. Polym. 2024, 331, 121850. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.F.; Ren, Y.M.; Chen, S.G.; Liu, D.H.; Ye, X.Q.; Tian, J.H. Development and characterization of pH responsive sodium alginate hydrogel containing metal-phenolic network for anthocyanin delivery. Carbohydr. Polym. 2023, 320, 121234. [Google Scholar] [CrossRef] [PubMed]
- Yun, G.; Richardson, J.J.; Biviano, M.; Caruso, F. Tuning the Mechanical Behavior of Metal–Phenolic Networks through Building Block Composition. ACS Appl. Mater. Inter. 2019, 11, 6404–6410. [Google Scholar] [CrossRef]
- Mao, S.; Ren, Y.; Wei, C.; Chen, S.; Ye, X.; Jinhu, T. Development of novel EGCG/Fe loaded sodium alginate-based packaging films with antibacterial and slow-release properties. Food Hydrocoll. 2023, 145, 109032. [Google Scholar] [CrossRef]
- Fan, G.; Cottet, J.; Rodriguez-Otero, M.R.; Wasuwanich, P.; Furst, A.L. Metal–Phenolic Networks as Versatile Coating Materials for Biomedical Applications. ACS Appl. Bio Mater. 2022, 5, 4687–4695. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miao, Y.; Yang, L.; Zhao, Y.; Wu, K.; Lu, Z.; Hu, Z.; Guo, J. Recent Advances in the Development and Antimicrobial Applications of Metal–Phenolic Networks. Adv. Sci. 2022, 9, 2202684. [Google Scholar] [CrossRef]
- Yun, G.; Kang, D.G.; Rheem, H.B.; Lee, H.; Han, S.Y.; Park, J.; Cho, W.K.; Han, S.M.; Choi, I.S. Reversed Anionic Hofmeister Effect in Metal–Phenolic-Based Film Formation. Langmuir 2020, 36, 15552–15557. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Okpara, E.S.; Adedara, I.A.; Guo, X.; Klos, M.L.; Farombi, E.O.; Han, S. Molecular mechanisms associated with the chemoprotective role of protocatechuic acid and its potential benefits in the amelioration of doxorubicin-induced cardiotoxicity: A review. Toxicol. Rep. 2022, 9, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Salarbashi, D.; Tajik, S.; Shojaee-Aliabadi, S.; Ghasemlou, M.; Moayyed, H.; Khaksar, R.; Noghabi, M.S. Development of new active packaging film made from a soluble soybean polysaccharide incorporated Zataria multiflora Boiss and Mentha pulegium essential oils. Food Chem. 2014, 146, 614–622. [Google Scholar] [CrossRef]
- Cheng, J.; Velez, F.J.; Singh, P.; Cui, L. Fabrication; characterization, and application of pea protein-based edible film enhanced by oregano essential oil (OEO) micro- or nano-emulsion. Curr. Res. Food Sci. 2024, 8, 100705. [Google Scholar] [CrossRef]
- Meerasri, J.; Sukatta, U.; Rugthaworn, P.; Klinsukhon, K.; Khacharat, L.; Sakayaroj, S.; Chollakup, R.; Sothornvit, R. Synergistic effects of thyme and oregano essential oil combinations for enhanced functional properties of sericin/pectin film. Int. J. Biol. Macromol. 2024, 263, 130288. [Google Scholar] [CrossRef]
- Abedi, E.; Sayadi, M.; Oliyaei, N. Fabrication and characterization of emulsion-based edible film containing cinnamon essential oil using chia seed mucilage. Int. J. Biol. Macromol. 2024, 266, 131173. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; Cháfer, M.; Hernández, M.; Chiralt, A.; González-Martínez, C. Antimicrobial activity of polysaccharide films containing essential oils. Food Control. 2011, 22, 1302–1310. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Ji, X.; Shen, M.; Chen, X.; Qi, X.; Li, Y.; Xie, J. Characterization of gallic acid-Chinese yam starch biodegradable film incorporated with chitosan for potential use in pork preservation. Food Res. Int. 2023, 164, 112331. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Jin, T.Z.; Chen, W.; He, Q.; Zou, Z.; Zhao, H.; Ye, X.; Guo, M. Preparation and characterization of gellan gum-chitosan polyelectrolyte complex films with the incorporation of thyme essential oil nanoemulsion. Food Hydrocoll. 2021, 114, 106570. [Google Scholar] [CrossRef]
- He, Q.; Zhang, L.; Song, L.; Zhang, X.; Liu, D.; Hu, Y.; Guo, M. Inactivation of Staphylococcus aureus using ultrasound in combination with thyme essential oil nanoemulsions and its synergistic mechanism. LWT-Food Sci. Technol. 2021, 147, 111574. [Google Scholar] [CrossRef]
- Rong, L.; Shen, M.; Wen, H.; Ren, Y.; Xiao, W.; Xie, J. Preparation and characterization of hyacinth bean starch film incorporated with TiO2 nanoparticles and Mesona chinensis Benth polysaccharide. Int. J. Biol. Macromol. 2021, 190, 151–158. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Lu, L.J.; Xu, J.; Ning, H.Y.; Lu, L.X. Development of chitosan-based antibacterial and antioxidant bioactive film incorporated with carvacrol-loaded modified halloysite nanotube. Food Hydrocoll. 2023, 145, 109102. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.Z.; He, W.; Du, T.; Wang, S.C.; Hu, P.Y.; Pan, B.; Jin, J.J.; Liu, L.Z.; Wang, J.L. A tailored slow-release film with synergistic antibacterial and antioxidant activities for ultra-persistent preservation of perishable products. Food Chem. 2024, 430, 136993. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.D.; Zhu, J.; Wu, L.X.; Qiao, Z.R.; Li, L.; Yan, J.K. Preparation, characterization, rheological and antioxidant properties of ferulic acid-grafted curdlan conjugates. Food Chem. 2019, 300, 125221. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.M.; Yu, D.D.; Wu, J.X.; Mao, S.F.; Chen, P.; Chen, S.G.; Gao, Q.; Ye, X.Q.; Tian, J.H. Preparation and physicochemical properties characterization of hesperetin-grafted pectin conjugate. Int. J. Biol. Macromol. 2023, 243, 124887. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Sadeghi, K.; Seo, J. Recent advances in poly (vinyl alcohol)/natural polymer based films for food packaging applications: A review. Food Packag. Shelf Life 2022, 33, 100904. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, M.; McClements, D.J.; Liu, F.; Cheng, C.; Xiong, J.; Zhu, M.; Chen, S. Investigation of a novel smart and active packaging materials: Nanoparticle-filled carrageenan-based composite films. Carbohydr. Polym. 2023, 301, 120331. [Google Scholar] [CrossRef]
- Zou, Z.; Ismail, B.B.; Zhang, X.; Yang, Z.; Liu, D.; Guo, M. Improving barrier and antibacterial properties of chitosan composite films by incorporating lignin nanoparticles and acylated soy protein isolate nanogel. Food Hydrocoll. 2023, 134, 108091. [Google Scholar] [CrossRef]
- Li, J.; Shen, M.; Xiao, W.; Li, Y.; Pan, W.; Xie, J. Regulating the physicochemical and structural properties of different starches by complexation with tea polyphenols. Food Hydrocoll. 2023, 142, 108836. [Google Scholar] [CrossRef]
- Amalraj, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Preparation, characterization and antimicrobial activity of polyvinyl alcohol/gum arabic/chitosan composite films incorporated with black pepper essential oil and ginger essential oil. Int. J. Biol. Macromol. 2020, 151, 366–375. [Google Scholar] [CrossRef]
- Kim, H.-J.; Roy, S.; Rhim, J.-W. Gelatin/agar-based color-indicator film integrated with Clitoria ternatea flower anthocyanin and zinc oxide nanoparticles for monitoring freshness of shrimp. Food Hydrocoll. 2022, 124, 107294. [Google Scholar] [CrossRef]
- Ge, X.Z.; Hu, Y.Y.; Shen, H.S.; Liang, W.; Sun, Z.Z.; Zhang, X.Y.; Li, W.H. Pheophorbide-a as a Light-Triggered Liposomal Switch: For the Controlled Release of Alpinia galanga (A. galanga) Essential Oil and Its Stability, Antioxidant, and Antibacterial Activity Assessment. J. Agric. Food Chem. 2023, 71, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Ying, R.; Wang, W.; Guo, Y.; He, Y.; Mo, X.; Xue, C.; Mao, X. A Macroporous Hydrogel Dressing with Enhanced Antibacterial and Anti-Inflammatory Capabilities for Accelerated Wound Healing. Adv. Funct. Mater. 2020, 30, 2000644. [Google Scholar] [CrossRef]


| Samples | Thickness (μm) | Water Content (%) | S. aureus Inhibition Zone (mm) | E. coli Inhibition Zone (mm) |
|---|---|---|---|---|
| SA | 31.60 ± 3.51 d | 14.58 ± 0.38 a | - | - |
| SA-PCA/Fe | 38.20 ± 0.84 c | 11.99 ± 1.39 a | 8.33 ± 0.15 b | 6.93 ± 0.15 b |
| SA-PCA/Fe-GEOE2 | 40.20 ± 0.84 c | 7.28 ± 2.17 b | 9.10 ± 0.20 b | 7.67 ± 0.15 b |
| SA-PCA/Fe-GEOE4 | 44.60 ± 2.30 b | 8.94 ± 0.81 b | 9.53 ± 0.15 a | 8.83 ± 0.35 a |
| SA-PCA/Fe-GEOE6 | 49.60 ± 1.52 a | 7.75 ± 1.52 b | 10.13 ± 0.31 a | 9.50 ± 0.26 a |
| Samples | L* | a* | b* |
|---|---|---|---|
| SA | 36.34 ± 5.08 a | 0.33 ± 0.12 a | −0.28 ± 0.06 a |
| SA-PCA/Fe | 15.42 ± 2.81 b | 0.27 ± 0.05 a | −1.64 ± 0.19 b |
| SA-PCA/Fe-GEOE2 | 15.44 ± 1.44 b | 0.18 ± 0.09 b | −1.67 ± 0.10 b |
| SA-PCA/Fe-GEOE4 | 15.53 ± 0.51 b | 0.31 ± 0.03 a | −1.68 ± 0.07 b |
| SA-PCA/Fe-GEOE6 | 16.51 ± 1.19 b | 0.29 ± 0.01 a | −1.46 ± 0.04 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Mao, S.; Ye, X.; Tian, J. Metal–Phenolic Network-Loaded Sodium Alginate-Based Antibacterial and Antioxidant Films Incorporated with Geranium Essential Oil. Polysaccharides 2025, 6, 15. https://doi.org/10.3390/polysaccharides6010015
Ren Y, Mao S, Ye X, Tian J. Metal–Phenolic Network-Loaded Sodium Alginate-Based Antibacterial and Antioxidant Films Incorporated with Geranium Essential Oil. Polysaccharides. 2025; 6(1):15. https://doi.org/10.3390/polysaccharides6010015
Chicago/Turabian StyleRen, Yanming, Shuifang Mao, Xingqian Ye, and Jinhu Tian. 2025. "Metal–Phenolic Network-Loaded Sodium Alginate-Based Antibacterial and Antioxidant Films Incorporated with Geranium Essential Oil" Polysaccharides 6, no. 1: 15. https://doi.org/10.3390/polysaccharides6010015
APA StyleRen, Y., Mao, S., Ye, X., & Tian, J. (2025). Metal–Phenolic Network-Loaded Sodium Alginate-Based Antibacterial and Antioxidant Films Incorporated with Geranium Essential Oil. Polysaccharides, 6(1), 15. https://doi.org/10.3390/polysaccharides6010015






