Auricularia Auricula Polysaccharide-Mediated Green Synthesis of Highly Stable Au NPs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
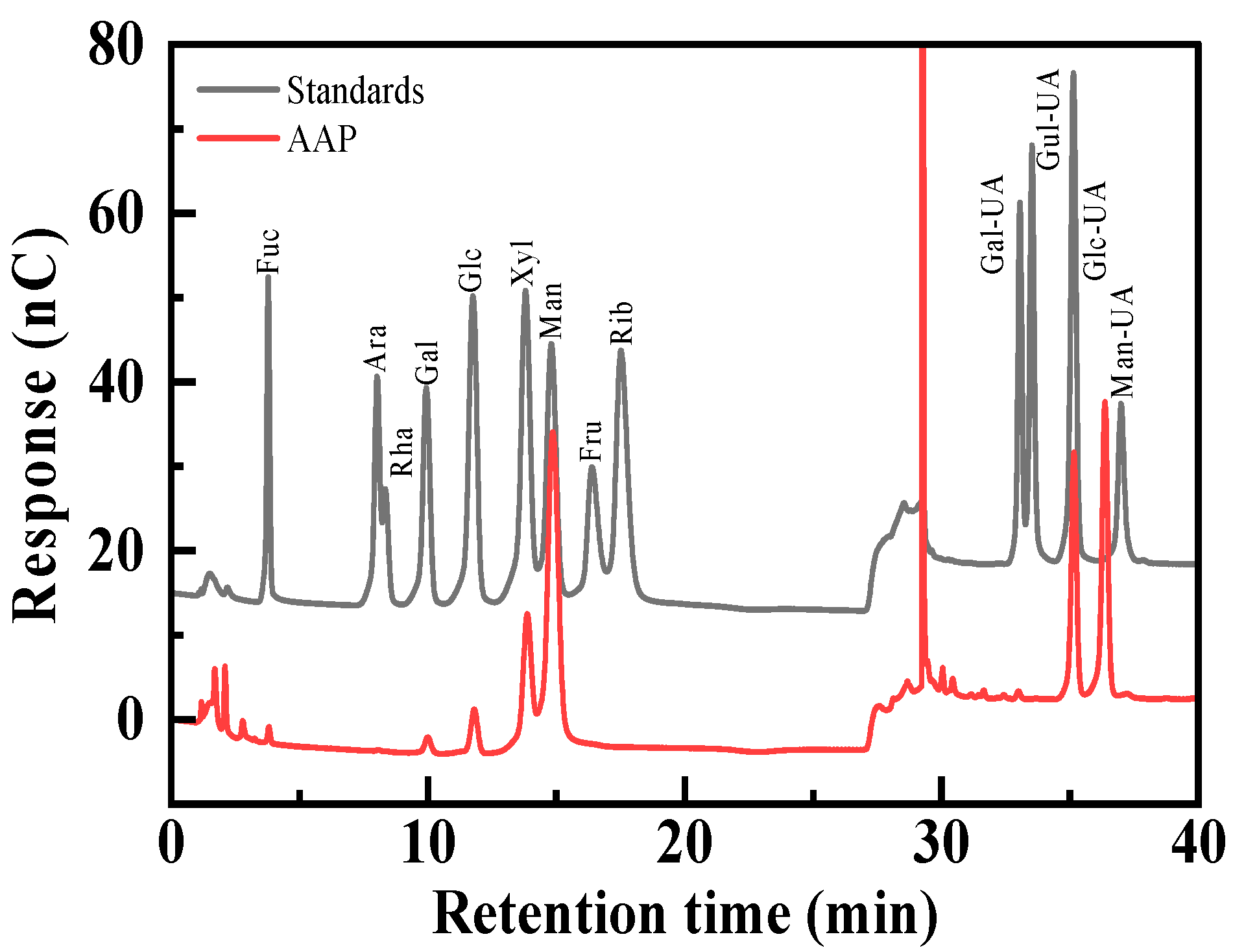
2.2. Preliminary Characterization of AAP
2.3. Synthesis of AAP-Au NPs
2.4. Characterization of AAP-Au NPs
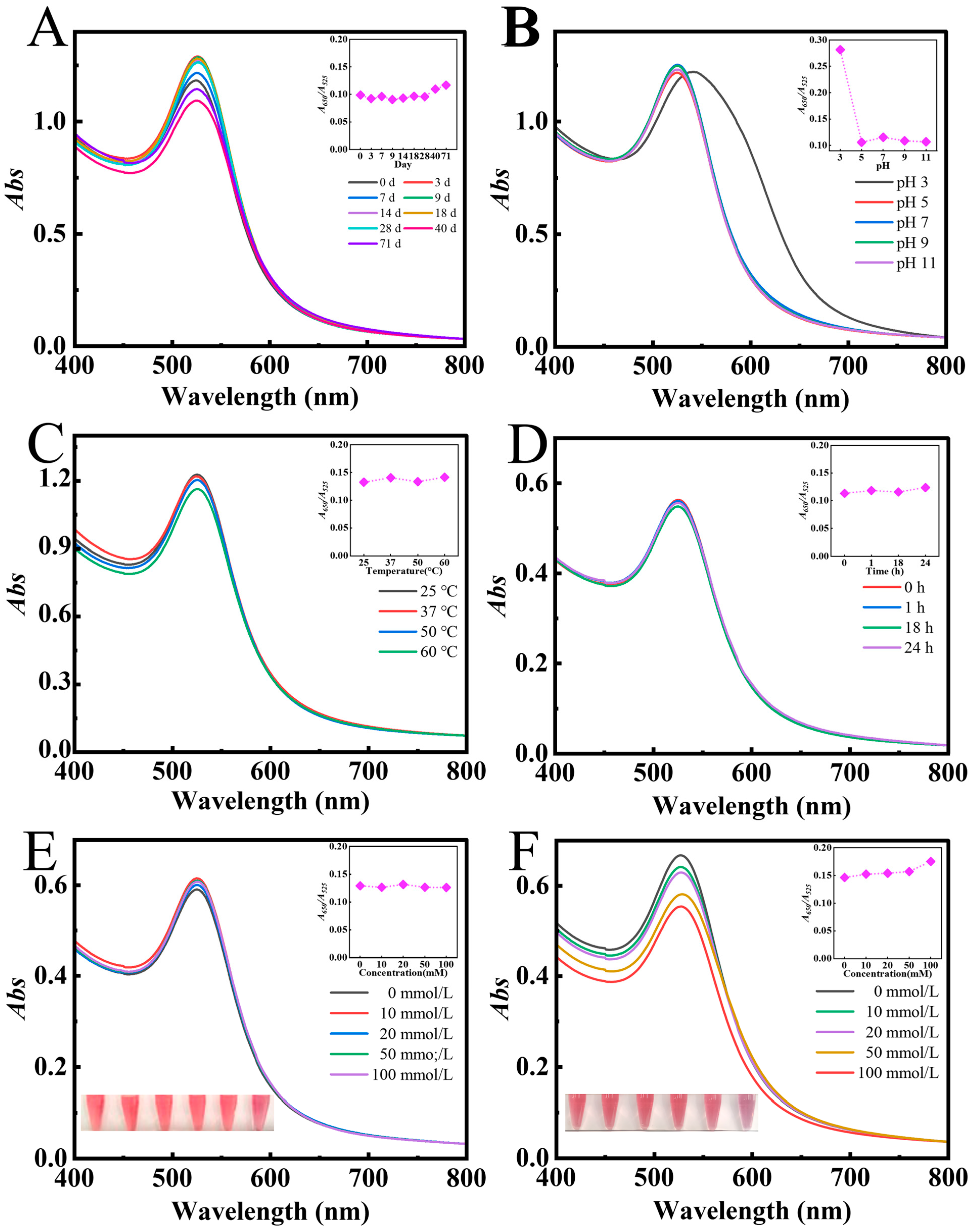
2.5. The Stability Analysis of AAP-Au NPs
2.6. The Antioxidant Properties of AAP-Au NPs
3. Results and Discussion
3.1. Optimization of Conditions for the Green Synthesis of AAP-Au NPs
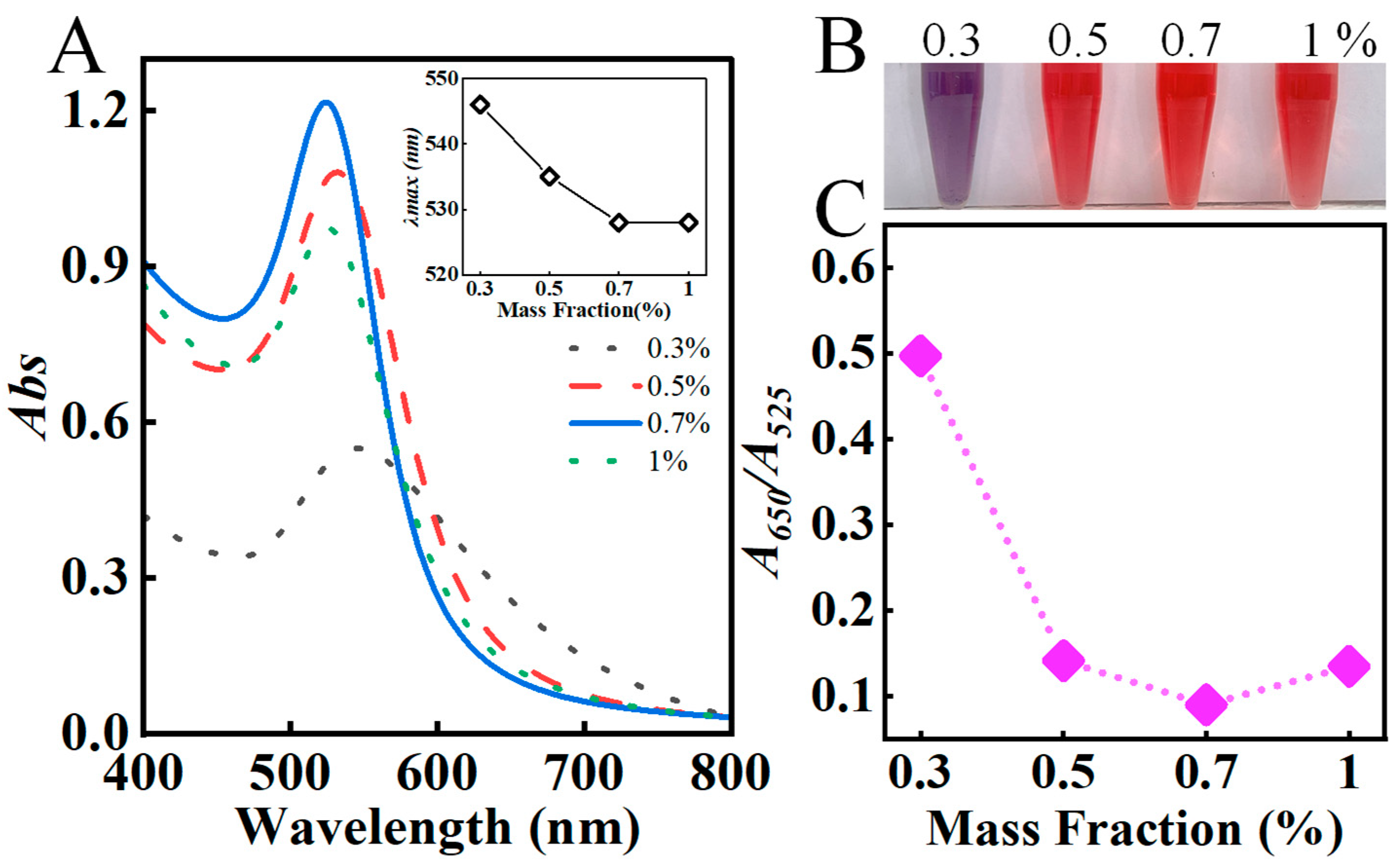
3.1.1. Effect of Various Mass Fractions of AAP
3.1.2. Effect of pH
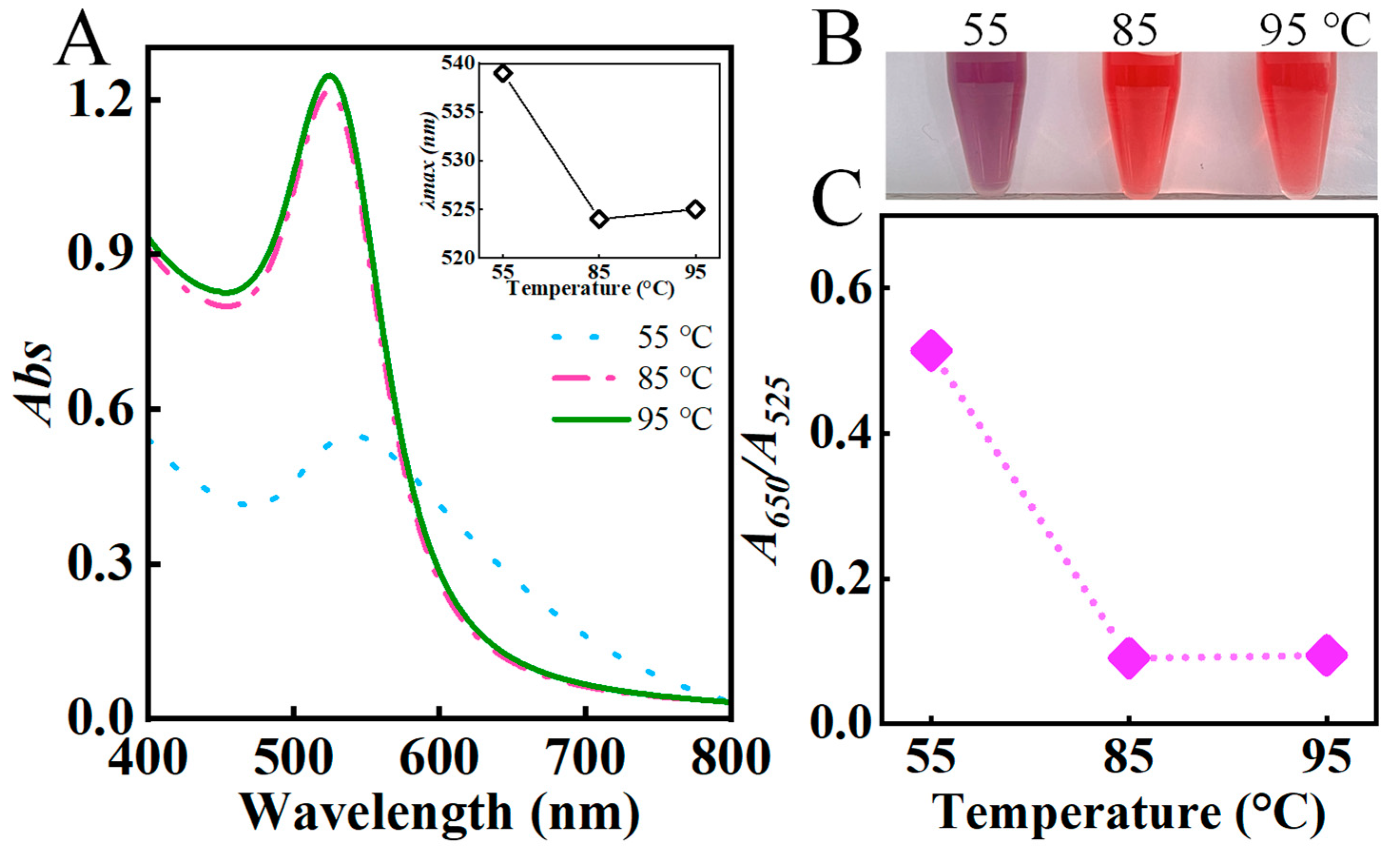
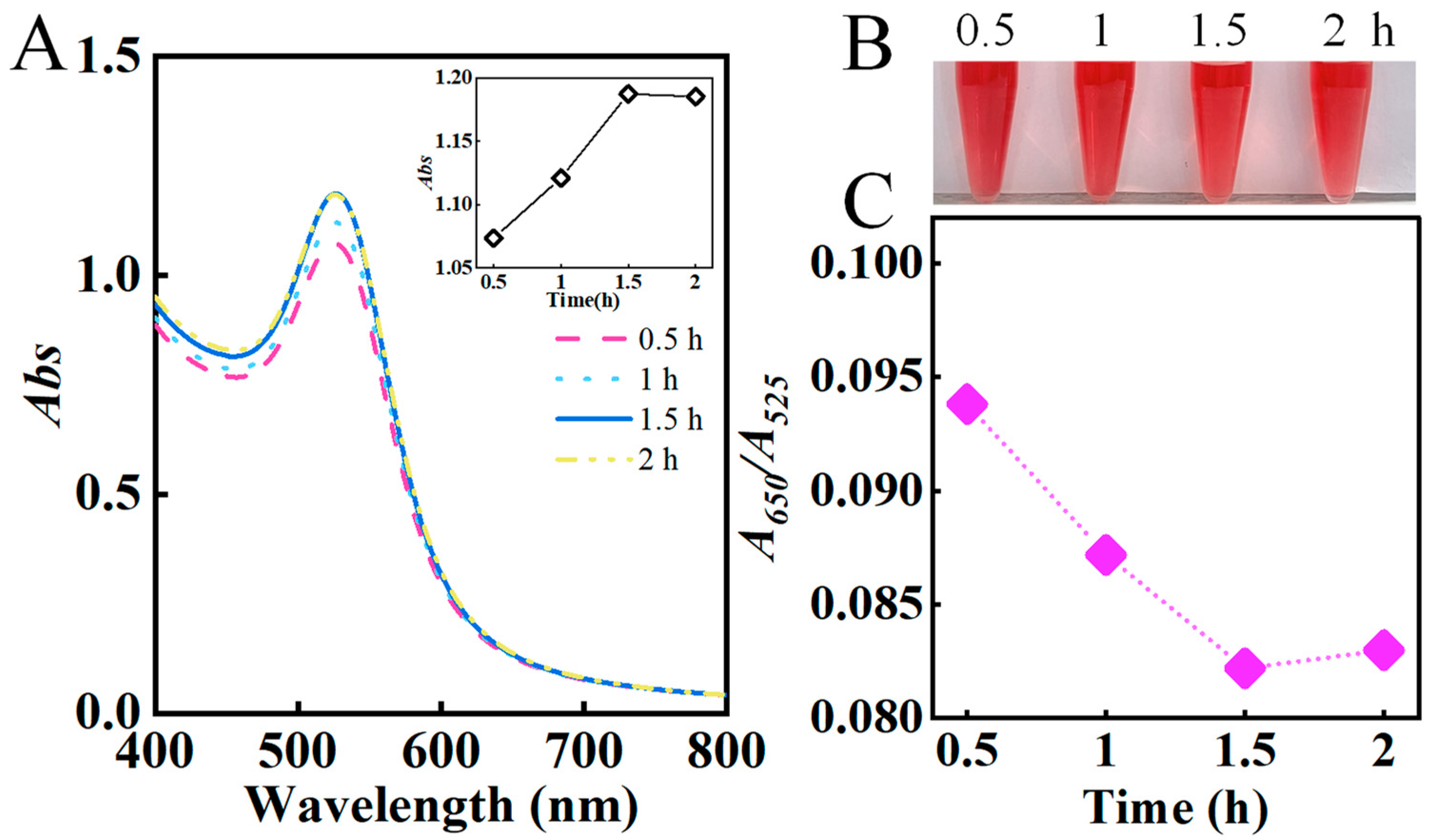
3.1.3. Effect of Reaction Temperature and Time
3.1.4. Effect of Concentrations of Gold Precursor
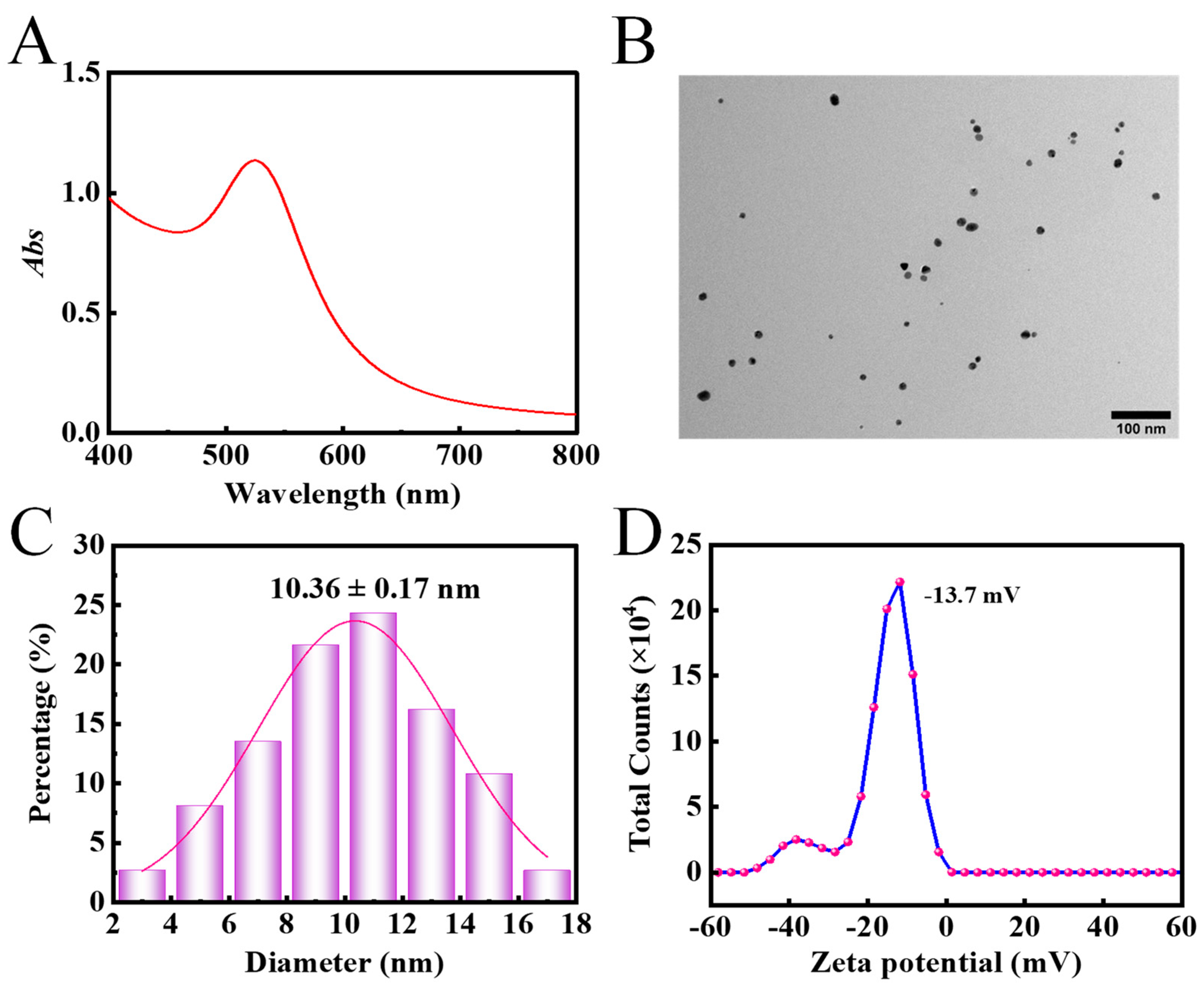
3.2. The Structural and Morphological Characterisation of AAP-Au NPs
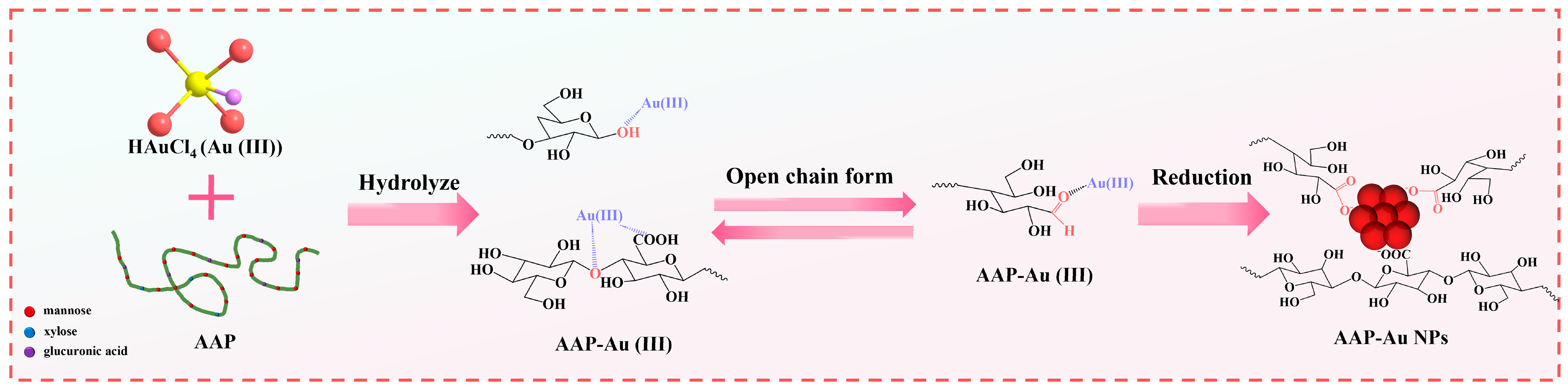
3.3. Synthesis Mechanism of AAP-Au NPs
3.4. The Stability of AAP-Au NPs
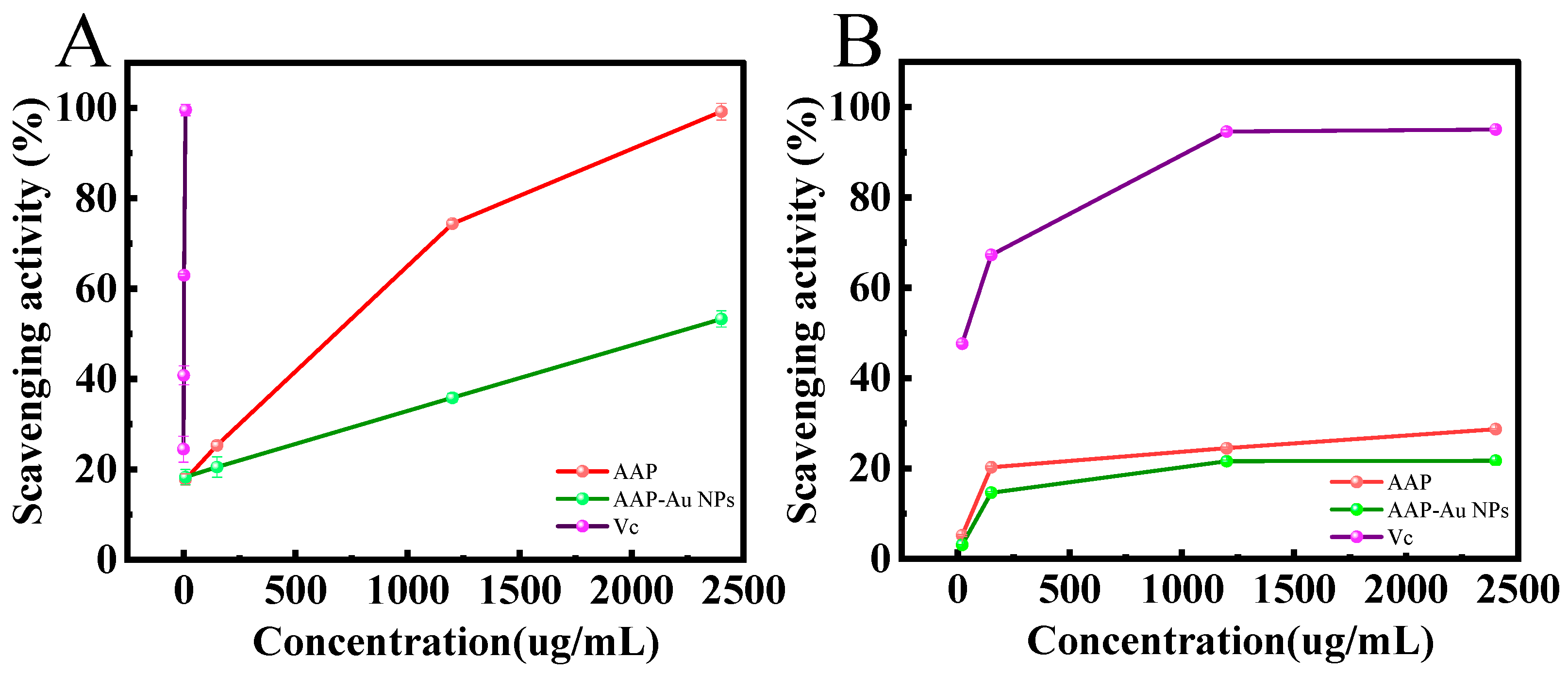
3.5. Scavenging Activity of AAP-Au NPs on ABTS and DPPH Free Radicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Shao, S.; Yue, H.; Wang, X.; Zhang, W.; Chen, F.; Zheng, L.; Xing, J.; Qin, Y. High stability Au NPs: From design to application in nanomedicine. Int. J. Nanomed. 2021, 16, 6067–6094. [Google Scholar] [CrossRef] [PubMed]
- Nasaruddin, R.R.; Chen, T.; Yao, Q.; Zang, S.; Xie, J. Toward greener synthesis of gold nanomaterials: From biological to biomimetic synthesis. Coord. Chem. Rev. 2021, 426, 213540. [Google Scholar] [CrossRef]
- Xu, L.; Xu, M.; Sun, X.; Feliu, N.; Feng, L.; Parak, W.J.; Liu, S. Quantitative comparison of gold nanoparticle delivery via the enhanced permeation and retention (EPR) effect and mesenchymal stem cell (MSC)-based targeting. ACS Nano 2023, 17, 2039–2052. [Google Scholar] [CrossRef] [PubMed]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Reddy, B.; Rachamalla, S.S.; Sistla, R. Natural polysaccharide functionalized gold nanoparticles as biocompatible drug delivery carrier. Int. J. Biol. Macromol. 2015, 80, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Roy, S.; Sanpui, P.; Jaiswal, A. Plasmonic gold nanorattle impregnated chitosan nanocarrier for stimulus responsive theranostics. ACS Appl. Biol. Mater. 2019, 2, 4812–4825. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Hou, G.; Wang, Y.; Wang, J.; Sun, T.; Ji, L.; Suo, A.; Yao, Y. A dual-targeted hyaluronic acid-gold nanorod platform with triple-stimuli responsiveness for photodynamic/photothermal therapy of breast cancer. Acta Biomater. 2019, 83, 400–413. [Google Scholar] [CrossRef]
- Khan, M.S.; Vishakante, G.D.; Siddaramaiah, H. Gold nanoparticles: A paradigm shift in biomedical applications. Adv. Colloid Interface Sci. 2013, 199–200, 44–58. [Google Scholar] [CrossRef]
- Mzwd, E.; Ahmed, N.M.; Suradi, N.; Alsaee, S.K.; Altowyan, A.S.; Almessiere, M.A.; Omar, A.F. Green synthesis of gold nanoparticles in Gum Arabic using pulsed laser ablation for CT imaging. Sci. Rep. 2022, 12, 10549. [Google Scholar] [CrossRef]
- Iranpour, P.; Ajamian, M.; Safavi, A.; Iranpoor, N.; Abbaspour, A.; Javanmardi, S. Synthesis of highly stable and biocompatible gold nanoparticles for use as a new X-ray contrast agent. J. Mater. Sci. Mater. Med. 2018, 29, 48. [Google Scholar] [CrossRef]
- He, J.; Unser, S.; Bruzas, I.; Cary, R.; Shi, Z.; Mehra, R.; Aron, K.; Sagle, L. The facile removal of CTAB from the surface of gold nanorods. Colloids Surf. B 2018, 163, 140–145. [Google Scholar] [CrossRef]
- Gevaerd, A.; Caetano, F.R.; Oliveira, P.R.; Zarbin, A.J.G.; Bergamini, M.F.; Marcolino-Junior, L.H. Thiol-capped gold nanoparticles: Influence of capping amount on electrochemical behavior and potential application as voltammetric sensor for diltiazem. Sens. Actuators B: Chem. 2015, 220, 673–678. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiang, Y.; Sheng, R.; Tomas, H.; Rodrigues, J.; Gu, Z.; Zhang, H.; Gong, Q.; Luo, K. Polysaccharide-based nanomedicines for cancer immunotherapy: A review. Bioact. Mater. 2021, 6, 3358–3382. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Duan, B.; Xu, X.; Zhang, L. Progress in rigid polysaccharide-based nanocomposites with therapeutic functions. J. Mater. Chem. B 2017, 5, 5690–5713. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, Y.; Wu, Y.; Dai, F.; Yuan, M.; Wang, F.; Bai, Y.; Deng, H. Natural polysaccharides based self-assembled nanoparticles for biomedical applications–A review. Int. J. Biol. Macromol. 2021, 192, 1240–1255. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Oh, J. Marine polysaccharide-based nanomaterials as a novel source of nanobiotechnological applications. Int. J. Biol. Macromol. 2016, 82, 315–327. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Wang, G. Review on marine carbohydrate-based gold nanoparticles represented by alginate and chitosan for biomedical application. Carbohydr. Polym. 2020, 244, 116311. [Google Scholar] [CrossRef]
- Ahmed, H.B. Recruitment of various biological macromolecules in fabrication of gold nanoparticles: Overview for preparation and applications. Int. J. Biol. Macromol. 2019, 140, 265–277. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Meng, F.; Su, C.; Li, J. Polysaccharide-based gold nanomaterials: Synthesis mechanism, polysaccharide structure-effect, and anticancer activity. Carbohydr. Polym. 2023, 321, 121284. [Google Scholar] [CrossRef]
- Lyu, F.; Xu, X.; Zhang, L. Natural polysaccharides with different conformations: Extraction, structure and anti-tumor activity. J. Mater. Chem. B 2020, 8, 9652–9667. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, R.; Yang, W.; Liu, H.; Yang, H.; Zhao, Q. Carboxymethylated hyperbranched polysaccharide: Synthesis, solution properties, and fabrication of hydrogel. Carbohydr. Polym. 2015, 128, 179–187. [Google Scholar] [CrossRef]
- Tomofuji, Y.; Matsuo, K.; Terao, K. Kinetics of denaturation and renaturation processes of double-stranded helical polysaccharide, xanthan in aqueous sodium chloride. Carbohydr. Polym. 2022, 275, 118681. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhang, H.; Zong, X.; Li, S.; Wang, J.; Wang, Y.; Jin, M. Polysaccharides from Auricularia auricula: Preparation, structural features and biological activities. Carbohydr. Polym. 2020, 247, 116750. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Zhou, R.; You, S.; Wu, S.; Wang, Q.; Cui, S.W. Gelation mechanism of polysaccharides from Auricularia auricula-judae. Food Hydrocoll. 2018, 76, 35–41. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Jiang, K. Antioxidant activity in vitro and in vivo of the polysaccharides from different varieties of Auricularia auricula. Food Funct. 2016, 7, 3868–3879. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jia, Y.; Xu, N.; Tang, L.; Chang, Y. Auricularia auricula-judae (Bull.) polysaccharides improve obesity in mice by regulating gut microbiota and TLR4/JNK signaling pathway. Int. J. Biol. Macromol. 2023, 250, 126172. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Li, J.; Sun, L.; Zhong, L.; Ma, Q. An in vitro comparison of the antioxidant activities of chitosan and green synthesized gold nanoparticles. Carbohydr. Polym. 2019, 211, 161–172. [Google Scholar] [CrossRef]
- Jia, X.; Yao, Y.; Yu, G.; Qu, L.; Li, T.; Li, Z.; Xu, C. Synthesis of gold-silver nanoalloys under microwave-assisted irradiation by deposition of silver on gold nanoclusters/triple helix glucan and antifungal activity. Carbohydr. Polym. 2020, 238, 116169. [Google Scholar] [CrossRef]
- Snitka, V.; Naumenko, D.O.; Ramanauskaite, L.; Kravchenko, S.A.; Snopok, B.A. Generation of diversiform gold nanostructures inspired by honey’s components: Growth mechanism, characterization, and shape separation by the centrifugation-assisted sedimentation. J. Colloid Interface Sci. 2012, 386, 99–106. [Google Scholar] [CrossRef]
- Suganya, K.S.; Govindaraju, K.; Kumar, V.G.; Karthick, V.; Parthasarathy, K. Pectin mediated gold nanoparticles induces apoptosis in mammary adenocarcinoma cell lines. Int. J. Biol. Macromol. 2016, 93, 1030–1040. [Google Scholar] [CrossRef]
- Cisneros, J.S.; Chain, C.Y.; Rivas Aiello, M.B.; Parisi, J.; Castrogiovanni, D.C.; Bosio, G.N.; Martire, D.O.; Vela, M.E. Pectin-coated plasmonic nanoparticles for photodynamic therapy: Inspecting the role of serum proteins. ACS Omega 2021, 6, 12567–12576. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Noruzi, M.; Zare, D.; Khoshnevisan, K.; Davoodi, D. Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. Spectrochim. Acta A 2011, 79, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhai, M.; Peng, J.; Xu, L.; Li, J.; Wei, G. Synthesis, size control and fluorescence studies of gold nanoparticles in carboxymethylated chitosan aqueous solutions. J. Colloid Interface Sci. 2007, 316, 398–404. [Google Scholar] [CrossRef]
- Laksee, S.; Puthong, S.; Teerawatananond, T.; Palaga, T.; Muangsin, N. Highly efficient and facile fabrication of monodispersed Au nanoparticles using pullulan and their application as anticancer drug carriers. Carbohydr. Polym. 2017, 178, 178–191. [Google Scholar] [CrossRef]
- Pienpinijtham, P.; Thammacharoen, C.; Ekgasit, S. Green synthesis of size controllable and uniform gold nanospheres using alkaline degradation intermediates of soluble starch as reducing agent and stabilizer. Macromol. Res. 2012, 20, 1281–1288. [Google Scholar] [CrossRef]
- Chen, X.; Xue, Z.; Ji, J.; Wang, D.; Shi, G.; Zhao, L.; Feng, S. Hedysarum polysaccharides mediated green synthesis of gold nanoparticles and study of its characteristic, analytical merit, catalytic activity. Mater. Res. Bull. 2021, 133, 111070. [Google Scholar] [CrossRef]
- Kumar, S.; Yoon, S.H.; Kim, G.H. Bridging the nanogap electrodes with gold nanoparticles using dielectrophoresis technique. Curr. Appl. Phys. 2009, 9, 101–103. [Google Scholar] [CrossRef]
- Muangnapoh, T.; Sano, N.; Yusa, S.I.; Viriya-Empikul, N.; Charinpanitkul, T. Facile strategy for stability control of gold nanoparticles synthesized by aqueous reduction method. Curr. Appl. Phys. 2010, 10, 708–714. [Google Scholar] [CrossRef]
- Mironov, I.V.; Makotchenko, E.V. The Hydrolysis of AuCl4− and the stability of aquachlorohydroxocomplexes of gold(III) in aqueous solution. J. Solut. Chem. 2009, 38, 725–737. [Google Scholar] [CrossRef]
- Sun, L.; Pu, S.; Li, J.; Cai, J.; Zhou, B.; Ren, G.; Ma, Q.; Zhong, L. Size controllable one step synthesis of gold nanoparticles using carboxymethyl chitosan. Int. J. Biol. Macromol. 2019, 122, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Pestov, A.; Nazirov, A.; Modin, E.; Mironenko, A.; Bratskaya, S. Mechanism of Au(III) reduction by chitosan: Comprehensive study with 13C and 1H NMR analysis of chitosan degradation products. Carbohydr. Polym. 2015, 117, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.R.; Heo, J.H. Simultaneous stabilization and functionalization of gold nanoparticles via biomolecule conjugation: Progress and perspectives. ACS Appl. Mater. Inter. 2021, 13, 42311–423286. [Google Scholar] [CrossRef] [PubMed]
- Wiącek, A.; Chibowski, E. Zeta potential, effective diameter and multimodal size distribution in oil/water emulsion. Colloid Surf. A 1999, 159, 253–261. [Google Scholar] [CrossRef]
- Wiącek, A.E.; Chibowski, E.; Wilk, K. Studies of oil-in-water emulsion stability in the presence of new dicephalic saccharide-derived surfactants. Colloid Surf. B 2002, 25, 243–256. [Google Scholar] [CrossRef]
- Demirbas, A.; Büyükbezirci, K.; Celik, C.; Kislakci, E.; Karaagac, Z.; Gokturk, E.; Kati, A.; Cimen, B.; Yilmaz, V.; Ocsoy, I. Synthesis of long-term stable gold nanoparticles benefiting from red raspberry (Rubus idaeus), strawberry (Fragaria ananassa), and blackberry (Rubus fruticosus) extracts–gold ion complexation and investigation of reaction conditions. ACS Omega 2019, 4, 18637–18644. [Google Scholar] [CrossRef]
- Koo, S.H.; Lee, J.S.; Kim, G.H.; Lee, H.G. Preparation, characteristics, and stability of glutathione-loaded nanoparticles. J. Agric. Food Chem. 2011, 59, 11264–11269. [Google Scholar] [CrossRef]


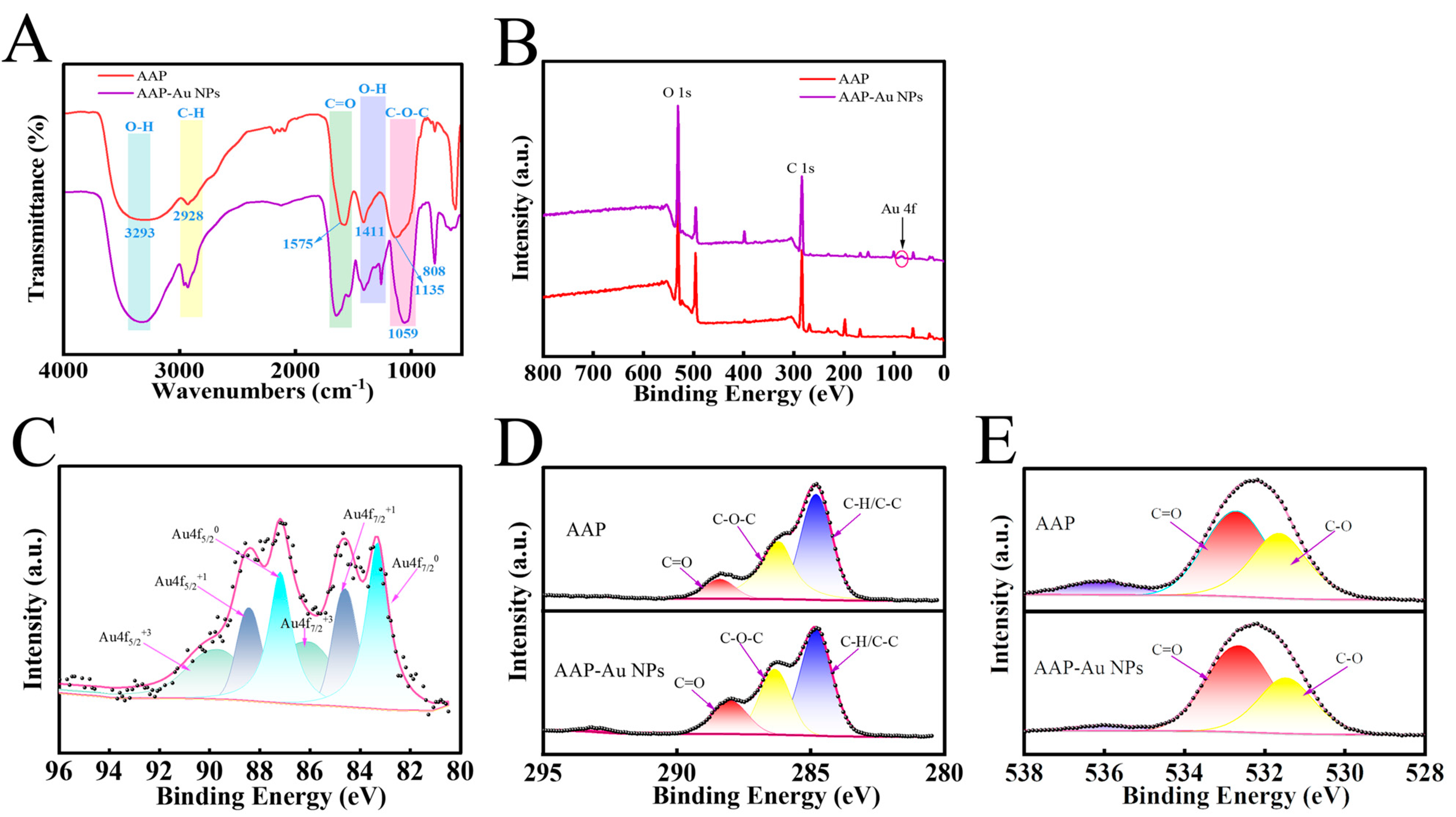
| AAP | AAP-Au NPs | |||
|---|---|---|---|---|
| Peak Position (BE) | Percentage (%) | Peak Position (BE) | Percentage (%) | |
| C=O | 288.39 | 9.44 | 288.02 | 18.67 |
| C-O-C/C-OH | 286.16 | 38.64 | 286.33 | 30.8 |
| C-H/C-C | 284.8 | 38.64 | 284.8 | 50.53 |
| AAP | AAP-Au NPs | |||
|---|---|---|---|---|
| Peak Position (BE) | Percentage (%) | Peak Position (BE) | Percentage (%) | |
| C=O | 532.71 | 57.71 | 532.61 | 63.18 |
| C-O | 531.64 | 42.29 | 531.48 | 36.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Gu, L.; Ye, Y.; Zhang, M. Auricularia Auricula Polysaccharide-Mediated Green Synthesis of Highly Stable Au NPs. Polysaccharides 2024, 5, 643-655. https://doi.org/10.3390/polysaccharides5040041
Liu H, Gu L, Ye Y, Zhang M. Auricularia Auricula Polysaccharide-Mediated Green Synthesis of Highly Stable Au NPs. Polysaccharides. 2024; 5(4):643-655. https://doi.org/10.3390/polysaccharides5040041
Chicago/Turabian StyleLiu, Haoqiang, Liyu Gu, Yuanzhen Ye, and Minwei Zhang. 2024. "Auricularia Auricula Polysaccharide-Mediated Green Synthesis of Highly Stable Au NPs" Polysaccharides 5, no. 4: 643-655. https://doi.org/10.3390/polysaccharides5040041
APA StyleLiu, H., Gu, L., Ye, Y., & Zhang, M. (2024). Auricularia Auricula Polysaccharide-Mediated Green Synthesis of Highly Stable Au NPs. Polysaccharides, 5(4), 643-655. https://doi.org/10.3390/polysaccharides5040041







