Abstract
Beta-glucan (β-glucan), a naturally occurring complex polysaccharide, has drawn attention for its diverse health benefits, including immune system modulation. β-glucan was extracted from two fungi, Monascus purpureus (Mp) and Monascus kaoliang (Mk), cultured in Saccharina japonica via submerged fermentation. The yield, solubility, total sugar, reducing sugar, protein content, Fourier Transform Infrared Spectroscopy (FTIR), X-ray Diffraction (XRD), Thermogravimetric analysis (TGA), Scanning Electron Microscopy (SEM), in vitro free radical scavenging activity, and cytotoxicity were analyzed. A significant yield of β-glucans, with the contents of 51.30 ± 1.54% in Mp and 44.24 ± 1.18% in Mk was observed on a dry weight basis. Water solubility slightly varied, measuring 36.25 ± 1.14% in Mp and 31.25 ± 0.94% in Mk. Total sugar and reducing sugar content in Mp and Mk derived β-glucans were 114.75 ± 2.54 mg/g and 100.25 ± 1.86 mg/g, 7.38 ± 0.78 mg/g, and 8.39 ± 0.46 mg/g, respectively. FTIR spectra resembled the standard, and TGA confirmed heat stability. XRD patterns indicated that the extracted β-glucans, including the standard one, showed the most prominent diffraction peaks in the lower 2θ range, suggesting similar crystalline phases; however, they differed in crystallinity and degree of amorphous content. SEM images displayed characteristic rough and fibrous shapes and surfaces for extracted β-glucans but it was uniform and of a regular shape in the standard sample. The isolated β-glucans exhibited in vitro free radical scavenging and no cytotoxicity was observed in the MTS assay. Therefore, utilizing S. japonica as a substrate in the fermentation process by Monascus spp. presents a unique opportunity in the production and utilization of β-glucans.
1. Introduction
Several bioactive polysaccharides have been identified in fungi [1,2] with glucans emerging as the predominant forms located within the cell wall. The cell wall of fungal cells comprises four primary components: glucan, chitin, mannan, and mannoprotein. These carbohydrates are crucial for preserving the shape and viability of fungal cells when faced with osmotic challenges, and they act as the interface between the cell and its environment. Glucans are typically arranged as triple helical polymers, contributing to the rigidity of the cell wall [3]. Numerous glucans have been documented from diverse origins through the application of various methods. Due to their remarkable capacity to boost and activate the immune systems in both humans and animals these glucans are recognized as standard biological response modifiers [4]. Glucans are polysaccharides consisting of glucose molecules linked through α or β (1 → 3), (1 → 4), and (1 → 6) glycoside bonds. Structurally complex, insoluble glucose homopolymer glucans are found in the cell walls of fungi, as well as in algae and bacteria [5]. While their primary molecular structure is generally uniform, the bonding type, molecular mass, and molecular configuration may vary, contingent upon the microbial source. Generally, between 65% and 90% of the cell wall glucan is β-glucan, but other glucans have been identified in various fungal cell walls. The β-glucan serves as the basic structural component to which other cell wall constituents are covalently attached [6].
Recent investigations have revealed that β-glucan derived from the cell wall of Saccharomyces cerevisiae demonstrates antioxidative activity, particularly in terms of scavenging free radicals [7]. β-glucans have proven effective in the treatment of various conditions such as diabetes, hypercholesterolemia, microbial infections, cancer, and skin care [8,9,10,11]. The active component, β-glucan, has been noted for its phagocytic and candidacidal activities. Given the health benefits associated with β-D-glucan, numerous isolation and purification processes for this polysaccharide have been developed [2,4,7]. However, many of these methods involve hot alkali, acids, or a combination of both, potentially leading to significant degradation of glucose chains, low yields, and limited purity [11]. Consequently, alternative processes incorporating hot water and enzyme treatment have been explored [7,12]. This paper provides a summary of recent research on the isolation process, structure, and technological functionality of fungal β-glucans, underscoring their relevance to human health. Both soluble and insoluble β-glucans have potential applications in pharmaceutical and chemical industries and cosmetics [13]. β-glucans from various sources have a wide range of potential applications in food production, serving as a thickening agent, fat substitute, and emulsifier [14].
It has been well-established that fungi are a prime source of β-glucan, showing potential in both quantitative and qualitative aspects for industrial β-glucan production. However, there is no previous report available on β-glucan production using Monascus spp. In some prior research, the authors demonstrated the suitability of Monascus spp. for pigment and lovastatin production using Saccharina japonica as a fermentation substrate [15,16]. The substrate plays a vital role in the production of secondary metabolites, including glucan, pigments, and lovastatin, via microbial fermentation. For industrial β-glucan production, the cost-effectiveness, availability, and price of substrate are critical factors. Low-cost marine biomass, such as seaweeds, offers a promising substrate for microbial conversion and β-glucans production, as it does not compete with agricultural crops. Seaweeds are rich in carbohydrates such as alginate, phlorotanin, fucoxanthin, fucosterol, fucoidan, laminarin, glucan, cellulose, and mannitol [17,18]. Among these, the brown seaweed Saccharina japonica stands out due to its low cost and extensive cultivation in East Asian countries [19]. Using S. japonica as a substrate provides a renewable and economical alternative for natural β-glucan production. Additionally, there is no report on the utilization of S. japonica as a fermentation substrate for β-glucan production with Monascus spp. Therefore, the present study aimed to produce β-glucan using S. japonica as a fermentation substrate by M. purpureus and M. kaoliang. The methodology and resources for the extraction of β-glucan outlined in this paper provide an alternative production strategy for industrial applications, including the food, pharmaceutical, and cosmeticeutical sectors. The study also involved the characterization of the isolated β-glucan based on various physicochemical and biofunctional parameters, including production yield and solubility, total sugar, reducing sugar and protein content, Fourier Transform Infrared Spectroscopy (FTIR), Thermogravimetric analysis (TGA), X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM), in vitro free radical scavenging activity, and cytotoxicity.
2. Materials and Methods
2.1. Collection and Preparation of Microorganisms and Substrate
Strains of M. purpureus KCCM 60168 and M. kaoliang KCCM 60154 were obtained from the Korean Culture Centre of Microorganisms (KCCM). Cultivation of these red molds took place on potato dextrose agar (PDA) and yeast extract agar (YEA) at 30 °C for 12 days to prepare the inoculum for submerged fermentation of S. japonica. S. japonica cultivated in the Gijang area of Busan at the adult sporophyte stage and sundried for local consumption was purchased from a local market in Busan, Republic of Korea, for use in the study. The sample was dried again in a hot air oven for 12 h at 50 °C and ground to a size of 0.2 mm using a sterile blender. Upon confirming the purity and optimal growth of the cultures, they were employed in the study, while the remaining plates were aseptically stored at 4 °C for future use. Sub-culturing occurred at 2-week intervals to sustain viability and retain the desired characteristics.
2.2. Submerged Fermentation
The inoculum for M. purpureus and M. kaoliang was prepared following the method [15] with some modifications. Briefly, 10 mL of distilled water was added to a fully-grown agar plate, scraped aseptically, and left for 5 min to obtain a homogeneous spore suspension. A mixture of 1 g of finely ground S. japonica powder in 50 mL of distilled water was placed in a 100 mL conical flask. External nitrogen (peptone) was added at 0.05 g, and the mixture was autoclaved for 15 min at 121 °C. The insolubilized (pellet) seaweed was discarded when the temperature of the autoclaved flask reached 75 °C. After cooling, externally filtrated glucose of 1 g was added, and 3 mL of fungal inoculum was added to the substrate. The mixture was then incubated at 30 °C for 15 days and the fungal mycelium was separated from the liquid medium, homogenized using a blender, and freeze-dried at −48 °C.
2.3. β-Glucan Extraction from Monascus spp.
The dried red mold (0.5 g) was initially dissolved in distilled water and homogenized using a homogenizer (Model: HG-15D, Dhaihan Scientific, Seoul, Republic of Korea) at 10,000 rpm for 5 min. Subsequently, it was boiled for 2 h while stirring to extract water-soluble polysaccharides. The mixture was then cooled and filtered to remove solid residue. The filtrate was mixed with 25 mL of 1 N NaOH and boiled for another 5 h to extract alkali-soluble β-glucan. The mixture was cooled and neutralized to pH 7.0 using acetic acid. Ethanol was gradually added to reach a final concentration of 70% ethanol in the solution to precipitate the β-glucan. The solution was then discarded, and the β-glucan pellet was collected. The β-glucan pellet was washed with ethanol to remove any remaining impurities. Finally, the β-glucan underwent lyophilization using a freeze-drier at −48 °C for further analysis.
2.4. Assay for Determination of β-Glucan Content
The freeze-dried glucan (20 mg) was placed in a 15 mL Corning tube, and 0.4 mL of 2 M KOH was introduced. The mixture was stirred at 130 rpm for 30 min in an ice-cold water bath. Following this, 1.6 mL of 1.2 M sodium acetate buffer (pH 3.8) was added, and the solution was thoroughly mixed. Subsequently, 40 µL of GlucazymeTM (a suspension containing exo-1,3-β glucanase, endo-1,3-β-glucanase, β-glucosidase, and chitinase) was incorporated into the mixture. The blend was then allowed to sit in an ice water bath for 2 min before transferring the Corning tube to a water bath set at 40 °C for 16 h. Following this incubation, it was subjected to a water bath at 80 °C for 2 h. Next, 10 mL of water was added to each tube and thoroughly mixed. The tubes underwent centrifugation at 12,000× g for 15 min, and the resulting supernatant was utilized for glucan analysis. For glucan analysis, a 25 µL sample was withdrawn into a cuvette, and 1 mL of GOPOD reagent (freeze-dried enzymes containing glucose oxidase, peroxidase, and 4-aminoantipyrine) was combined with GOPOD reagent buffer (following the Megazyme yeast β-glucan assay kit protocol) and added to the tube. The mixture underwent incubation at 40 °C for 20 min and then at 80 °C for 10 min. Absorbance was measured at 510 nm against a blank (25 µL sodium acetate buffer, 200 mM, pH 5.0).
β-Glucan (% w/w) = ΔE × F × 12.04/0.1 × 100/W × 1/1000 × 162/180 = ΔE × F/W × 10.836
ΔE = Absorbance was measured relative to the reagent blank
F = The conversion from absorbance to µg was determined by dividing the absorbance of the standard (150 µg of D-glucose) by the GOPOD absorbance of this 150 µg.
12.04/0.1 = Volume correction (0.1 mL taken from 12.04 mL).
100/W = Factor used to express β-glucan as a percentage of the sample weight.
1/1000 = Conversion from µg to mg.
W = The sample weight analyzed in mg.
162/180 = Factor for converting free D-glucose to anhydro-D-glucose, as found in β-glucan.
2.5. Determination of Water Solubility
To assess the water solubility of β-glucan, 10 mg of the sample was dissolved in 1 mL of distilled water, and the resulting suspension was stirred at 25 °C for 24 h. Subsequently, the sample underwent centrifugation at 16,000× g for 15 min, and the supernatant was combined with three volumes of ethanol to induce precipitation of the solubilized glucan. The resulting precipitate was collected through centrifugation for 20 min, followed by vacuum drying at 40 °C and subsequent weighing. The solubility was measured using the following formula:
2.6. Total Sugar, Reducing Sugar, and Total Protein Analysis of β-Glucan
To determine total sugar, the phenol-sulfuric acid method was employed. In a 1 mL cuvette, 100 µL of the sample was taken, and then 100 µL of phenol was added, followed by a 5-min incubation. Subsequently, 500 µL of H2SO4 was added and vortexed. The absorbance was measured at 510 nm, with glucose used as the standard. Reducing sugar was measured using DNS solution. In 100 µL of sample, 1 mL of DNS was added and then boiled for 10 min, and the absorbance was measured at 570 nm against the reagent blank. The total protein content of the sample was measured using Bradford solution. Initially, 100 µL of the sample was taken in a cuvette, and then 1 mL of Bradford solution was added and measured at 595 nm against the reagent blank.
2.7. FTIR Analysis of β-Glucan
The freeze-dried β-glucan sample of 10 mg was utilized for FTIR analysis in the frequency range of 4000–500 cm−1 using Spectrum X (Perkin Elmer, Waltham, MA, USA) following the method of Ul Ashraf et al. [20]. The glucan (fraction 1 and fraction 2 individually) was placed on the surface of the attenuated total reflection crystal, and pressure was exerted to remove air from the powder particles. The sample’s spectrum was obtained by averaging 128 scans at four different resolutions.
2.8. Thermogravimetric Analysis (TGA) of β-Glucan
The TGA of isolated and standard β-glucan (S. cereviacese, Sigma-Aldrich, St. Louis, MO, USA) was performed following the method [21] with slight modifications. The thermal behavior was evaluated using thermogravimetric (TG) analysis on a thermogravimetric analyzer (Pyris 1 TGA, Perkin Elmer Life and Analytical Sciences, Branford, CT, USA) under nitrogen (25 mL/min). Precisely 10 mg of sample was placed in open alumina crucibles and subjected to heating in the range of 50 °C to 800 °C at a heating rate of 10 °C/min. The thermo curve was generated by plotting percent weight loss against temperature to predict the thermal behaviors of the polysaccharides.
2.9. X-ray Diffraction (XRD) Analysis
The crystallinity of native and modified polysaccharides was evaluated using X-ray diffraction (XRD) on a Brüker D8-Advance diffractometer (Bruker Corporation, Rheinstetten, Germany) following the methods of Liu et al. [22]. The scattering angle of 2θ was measured from 10° to 80° with a step size of 0.05 and an exposure time of 1 s at each step. The obtained data were analyzed using the software HighScore Plus (version 3.0.4; Almelo, The Netherlands).
2.10. Scanning Electron Microscopic (SEM) Analysis of β-Glucan
The external morphology of β-glucan sample was observed under SEM following the method of Jahromi et al. [23] with modifications. The sample was freeze-dried and gold-coated. The coated sample was viewed under SEM using the voltage of 10 kV.
2.11. Radical Scavenging Activity of β-Glucan
2.11.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
The DPPH free radical scavenging activity of the extracted β-glucan was assessed following the method [4] with slight modifications. In brief, 50 µL of the sample (10 mg/mL) was blended with 100 µL of a freshly prepared 0.20 mM DPPH solution. The mixture was kept in incubation at room temperature for 30 min in the dark. Following incubation, the absorbance was measured at 517 nm using a microplate spectrophotometer (Infinite M200 NanoQuant, Tecan, Zurich, Switzerland). DPPH was purchased from Sigma-Aldrich, St. Louis, MO, USA. The scavenging activity was determined using the following equation:
where, As represents the absorbance of the sample in the presence of DPPH chemical, A0 is the absorbance of the pigment with ethanol, and Ac is the absorbance of DPPH chemical without the sample.
2.11.2. Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP assay of the β-glucan sample was used to determine the Fe3+ scavenging activity. One mL of 2,4,6-tripyridyltriazine (TPTZ) solution (10 mM, in 40 mM HCl), 1 mL of 20 mM FeCl3, and 10 mL of 0.3 M acetate buffer (pH 3.6) were mixed to prepare the FRAP reagent. Sample solutions of 150 μL (10 mg/mL) were added to 850 µL of the FRAP reagent, followed by incubation at 37 °C for 20 min. Then, absorbance was measured at 593 nm.
2.11.3. Hydroxyl Radical Scavenging Activity
The hydroxyl radical scavenging activity was assessed using a method previously outlined [24], incorporating certain modifications. To determine hydroxyl radical scavenging activity, 200 µL of FeSO4 (1.5 mM), 200 µL of H2O2, 100 µL of sodium salicylate (20 mM), and 200 µL (10 mg/mL) of the sample were mixed together. Then the reaction mixture was incubated at 37 °C and absorbance was measured at 510 nm. The scavenging activity was assessed through the application of the subsequent formula:
where, Acontrol is the reagent without sample and Asample is the reagent with glucan sample.
2.12. Cytotoxicity of β-Glucan by MTS Assay
The cytotoxicity of β-glucan was performed following the previous method by MTS assay [25]. To assess the cytotoxicity of glucan on Caco-2 cells, the Caco-2 cell line (KCLB 30037) was purchased from the Korean Cell Line Bank (KCLB) and cultivated at 37 °C under 95% air and 5% CO2 (both v/v) in Dulbecco’s Eagle Medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% (w/v) Fetal Bovine Serum (FBS) and 1% (w/v) streptomycin-penicillin (100 μg/mL and 100 IU/mL, respectively). In this experiment, samples at a concentration of 10 µg/mL were used. Cell viability was evaluated using the CellTiter 96® AQueous non-radioactive cell proliferation assay kit (Promega, Walldorf, Germany). Cells (6.2 × 103 cells/well) were seeded into a 96-well plate with a clear flat bottom, incubated for 24 h, and then treated with extracts at various concentrations, followed by an additional 24 h of incubation. Subsequently, 20 μL of MTS/PMS solution (35 μL PMS in 700 μL MTS, prepared just before use) was added to each well (containing 100 μL of medium), and the mixture was incubated for 2 h. The MTS assay is based on the conversion of a yellow tetrazolium salt (MTS) in the presence of phenazine methosulfonate (PMS) to a soluble purple formazan, formed by the actions of dehydrogenases in metabolically active cells. The formazan level was measured by absorbance at 490 nm using a microplate spectrophotometer (Infinite M200 NanoQuant, Tecan, Zurich, Switzerland); the absorbance is directly proportional to the number of living cells.
2.13. Statistical Analysis
Values are presented as means ± standard deviation of triplicates. Statistical analysis was performed by one-way analysis of variance (ANOVA) using SPSS software (version 25.0, SPSS Inc., Chicago, IL, USA). Significance differences among the means were determined using Duncan’s Multiple Range Test and p ≤ 0.05 was regarded as significant.
3. Results and Discussion
3.1. Production Yield and Water Solubility of β-Glucan
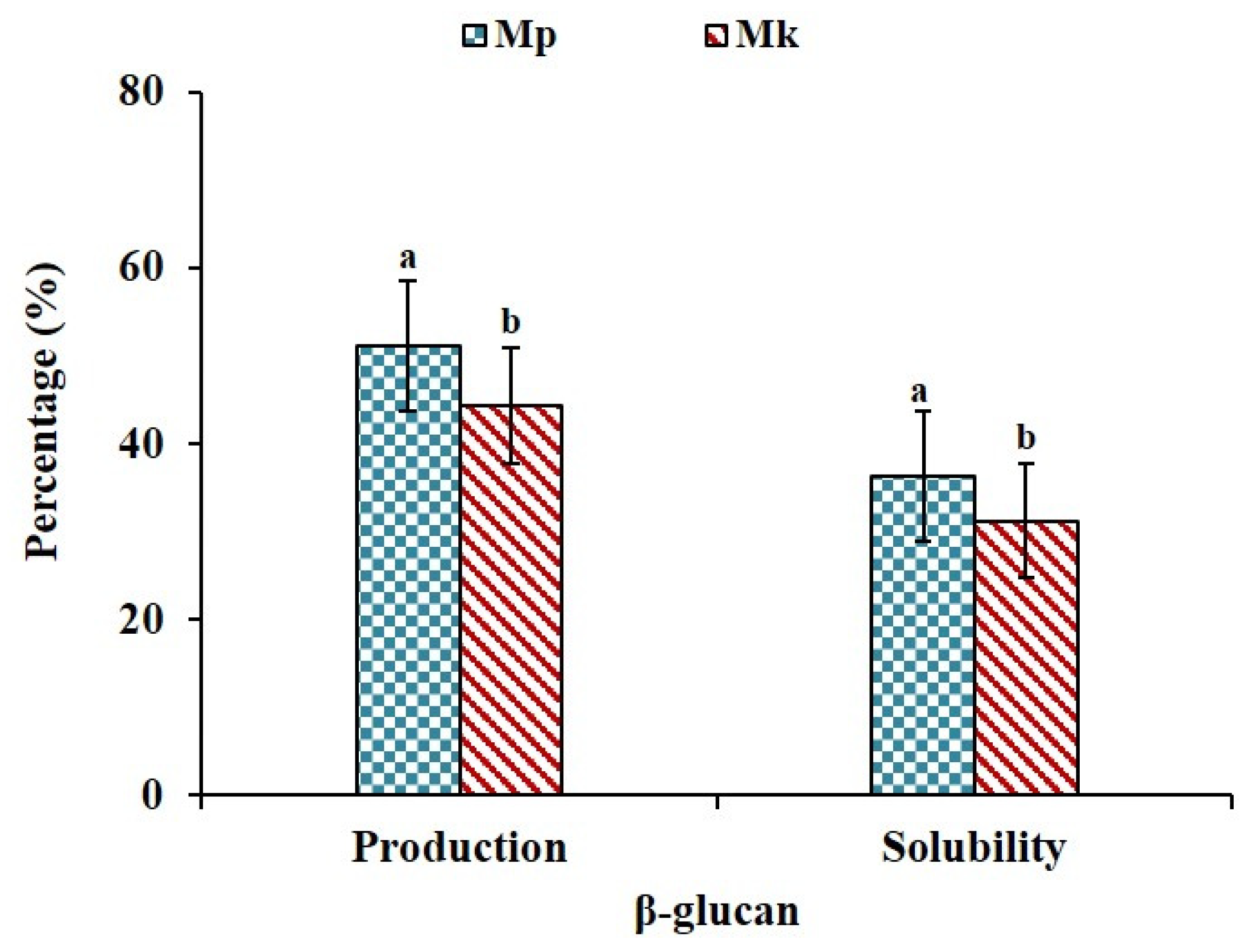
The production yield and water solubility of β-glucan are presented in Figure 1. The production yield of β-glucan in Mp was approximately 51.30%, whereas the production yield in Mk was slightly lower at 44.24% on a dry weight basis. β-glucan obtained from Mp showed slightly higher water solubility at 36.25% compared to the β-glucan recovered from Mk, which exhibited a solubility of 31.25%. The difference in β-glucan production and water solubility between Mp and Mk is quite noticeable. There is an increasing trend in β-glucan production and water solubility in Mp compared to Mk. Very few studies have reported the production of β-glucan using Monascus spp. A recent report by Jayasekara et al. [26] stated that the β-glucan content in Xylaria sp. BCC 1067 was 50.54% of dry mycelium; however, they could extract 11.58% of total β-glucan. The solubility of β-glucan in water depends on molecular weight as well. An increase in the molecular weight of oat β-glucan from 1.13 × 105 to 9.04 × 105 g/mol and from 1.65 × 105 to 8.51 × 105 g/mol results in a notable reduction in its water solubility, with it decreasing from 72.8% to 68.2% and from 72.3% to 67.2%, respectively [25].
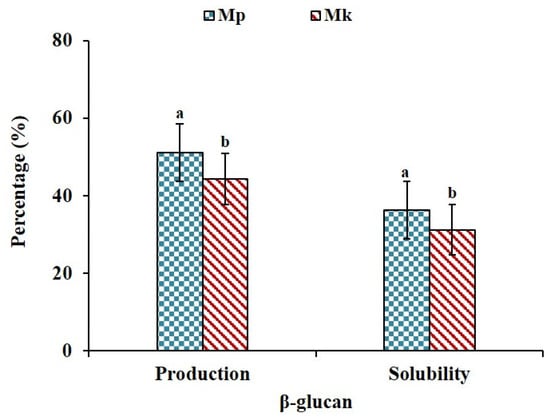
Figure 1.
Production yield and solubility of β-glucan produced by Monascus purpures (Mp) and Monascus kaoliang (Mk) under submerged fermentation in Saccharina japonica. Different superscript small letters on each parameter indicate significant differences (p ≤ 0.05).
The nutritional requirements of fungi are crucial for their growth and industrial fermentation processes. Typically, fungal growth substrates consist of carbon and nitrogen sources, along with a small number of elements specific to fungi. Malt extract and potato extract are commonly used media for fungal growth, providing the essential nutrients for fungal sporulation and growth. Choosing more effective fermentation media can enhance fungal yield and boost the production of primary and secondary metabolites [26]. Moreover, various sugars have an impact on fungal growth and their saccharide levels. The utilization of saccharides by fungi for energy and as a carbon source depends on sugar availability, cultural conditions, and the strain’s adaptation to the substrate. In glycolysis, the initial step involves the phosphorylation of glucose to glucose-6-phosphate, which is subsequently converted to fructose-6-phosphate through an isomerization reaction. Notably, the substitution of glucose with fructose in the sugar supply leads to a direct conversion to fructose-6-phosphate.
In addition to sugars and carbon sources, the mycelial formation of Monascus spp. can be influenced by efficient nitrogen sources, including peptone. Nevertheless, the selection of carbon and nitrogen sources in the growth media depends on the strain cultivation process [27]. Therefore, from an industrial perspective, the principle optimization strategies, incorporating selected components such as peptone, have been established as the basis for tailoring media to enhance Monascus spp. metabolite production. In the current study, S. japonica was employed as a submerged fermentation medium for M. purpureus and M. kaoliang, demonstrating β-glucan production comparable to the reference value. Consequently, it can be inferred that submerged fermentation of S. japonica is a viable approach for β-glucan production.
Solubility is a critical factor for any compound, determining its suitability for industrial applications in the food, pharmaceutical, and cosmetic industries. The water solubility of β-glucan plays a key role in supporting bioavailability, functional activity, stability, emulsifying properties, facilitating absorption in the body, and contributing to various health benefits. Notably, β-glucan is recognized for its ability to reduce blood cholesterol levels and enhance glycemic control. The water solubility of β-glucan is indispensable for its integration into food products, including baked goods, beverages, and soups, without compromising textures or sensory properties. The solubility of glucan and its derivatives is contingent upon the degree of substitution. Moreover, the solubility of these derivatives is influenced by the nature of the introduced anionic group in the molecule [28]. The enhancement of antitumor activities primarily results from water solubility and the introduction of sulfate groups [16]. Moreover, research by Sun et al. [29] suggests that water-soluble β-glucan may offer greater benefits to human health compared to its water-insoluble counterpart.
3.2. Total Sugar, Reducing Sugar, and Total Protein Content of β-Glucan
Table 1 presents the total sugar, reducing sugar, and total protein content of the extracted β-glucan. The highest total sugar content of β-glucan obtained from Mp was found to be 114.75 ± 2.54 mg/g, followed by 100.25 ± 1.86 mg/g in β-glucan obtained from Mk. Conversely, the reducing sugar content of β-glucan obtained from Mk was 8.39 ± 0.46 mg/g, slightly higher than the reducing sugar content of β-glucan obtained from Mp, which was 7.38 ± 0.78 mg/g. The protein content of β-glucan extracted from Mp and Mk was 0.67 ± 0.11% and 0.71 ± 0.08%, respectively, showing no significant variation.

Table 1.
The total sugar, reducing sugar, and total protein content of β-glucan isolated from Monascus purpureus (Mp) and Monascus kaoliang (Mk).
The total sugar content of β-glucan is a scientifically important characteristic as it determines the functional and nutritional properties, influencing the feasibility of industrial applications. The physicochemical and functional properties, such as viscosity, solubility, and the gel-forming ability of β-glucan are determined by both total and reducing sugar content. These properties play a crucial role in the various applications of β-glucan in the food, pharmaceutical, and cosmetic industries. The protein content of β-glucan extracted from beet was reported as 1.5 ± 0.0%, which was comparatively higher than the present value [22].
3.3. FTIR Analysis of β-Glucan
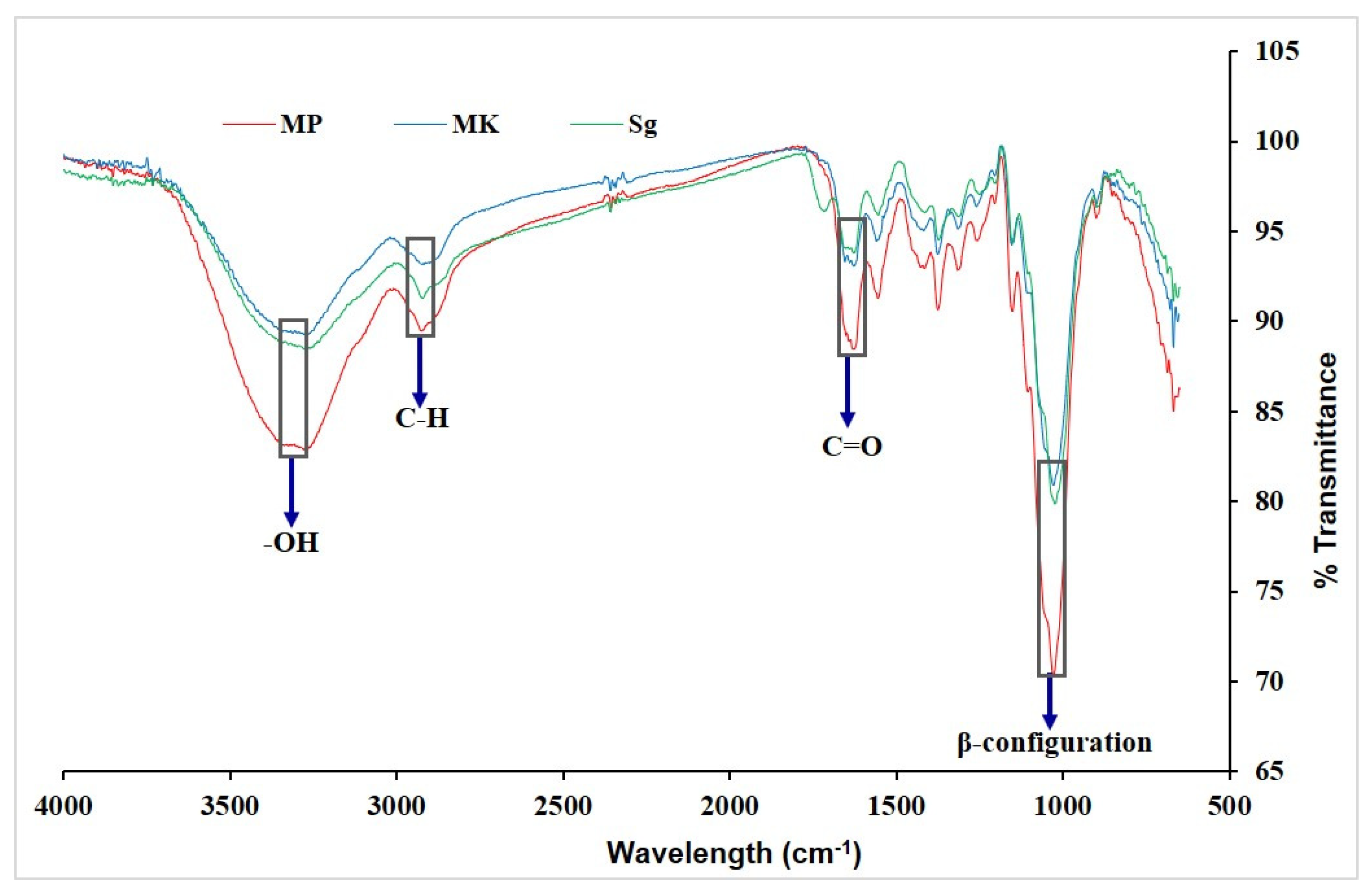
The FTIR spectroscopy process deals with the absorption of infrared radiation by the sample molecules. It was performed to identify the functional groups of the β-glucan molecule. This technique is responsive to the position and anomeric configuration of glycoside linkages in β-glucans. Figure 2 shows the FTIR spectrum of β-glucan isolated from M. purpureus, M. kaoliang, and standard β-glucan. Both extracted and standard β-glucan exhibited infrared absorption peaked within the ranges of 3000–3500 cm−1, 2800–3000 cm−1, 1700–1600 cm−1, and 890 cm−1, indicative of –OH (hydroxyl group), C–H (alkyl C–H stretches), C=O (carbonyl group), and the β-configuration of the glucan linkage, respectively [17,30].
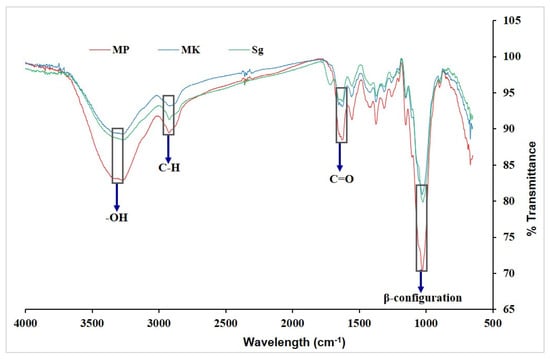
Figure 2.
FTIR spectrum of β-glucan. Mp: β-glucan isolated from Monascus purpureus; Mk: β-glucan isolated from Monascus kaoliang; Sg: Standard β-glucan.
The spectra showed a wide and intense peak in the range of 3249 to 3316 cm−1, attributed to the stretching vibration of hydroxyl groups (OH), while a shift in the carbonyl group (CO) was observed from 1634 to 1638 cm−1 [31]. The spectrums for the extracted and standard β-glucan were found to coincide. The typical absorption peak of β-glucan was found at 891.91 cm−1. Additionally, there were peaks at 2917.77, 1373.06, 1150.33, and 1023.05 cm−1, which indicate β-(1-3)-linkages [32]. FTIR is a suitable approach for analyzing β-glucan in crude high molecular weight extracted from various raw materials [31].
3.4. Thermogravimetric Analysis (TGA) of β-Glucan
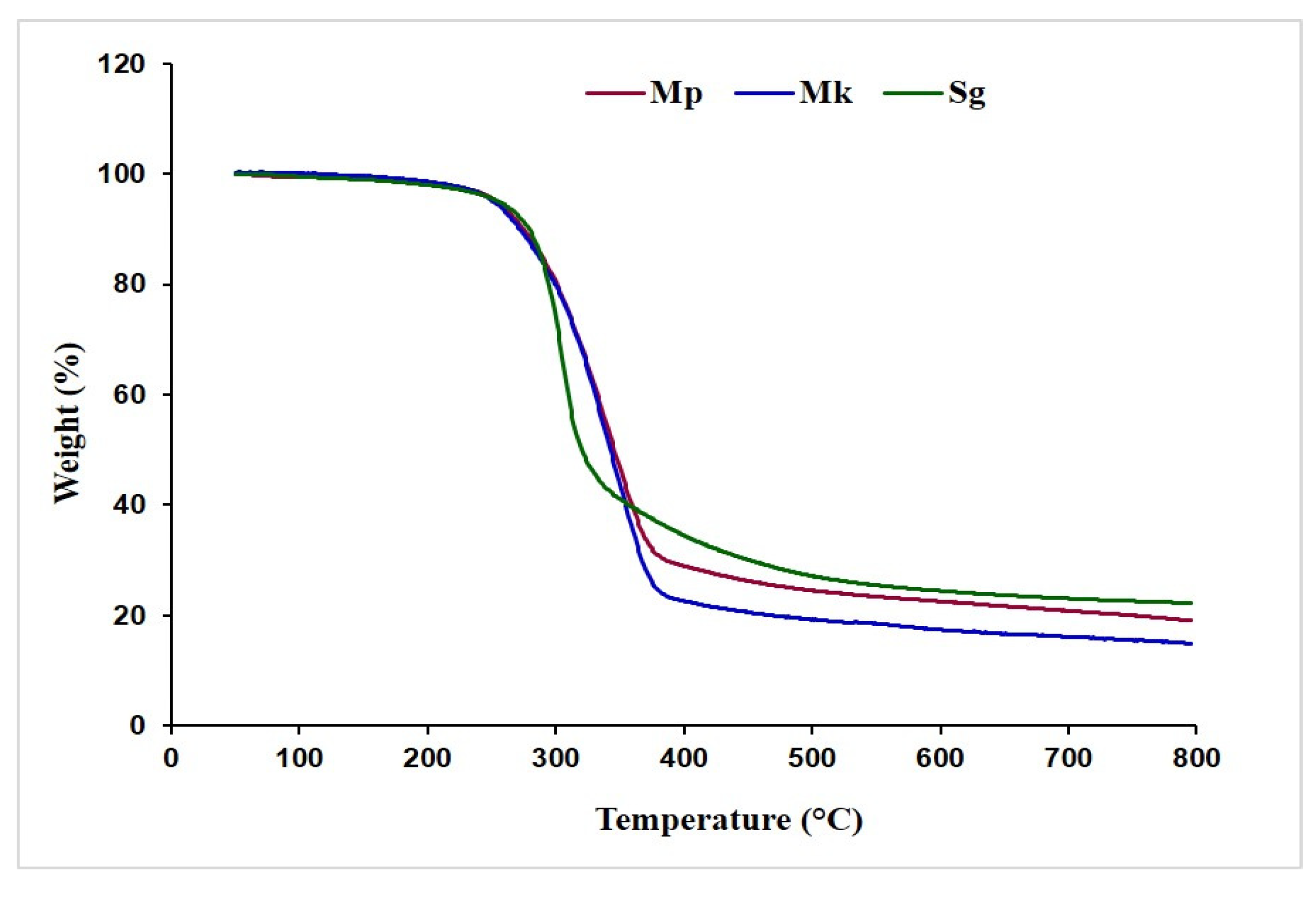
Figure 3 illustrates the comparative thermogravimetric changes in the behavior of β-glucan isolated from M. purpureus and M. kaoliang alongside the standard sample. At 125 °C, the samples exhibited a slight weight reduction, with the weight ranging from approximately 98.25% to 99.01% of the initial mass. This weight loss is likely due to the evaporation of moisture content. All the samples demonstrated high stability until reaching 224.0 °C, where the obtained mass remained around 97.23%. As the temperature exceeded 240 °C, there was a notable decline in the mass of all β-glucan samples, and at 380 °C, the masses of β-glucan samples Mp, Mk, and Sg decreased to 28.44%, 24.50%, and 33.63%, respectively. After a further temperature increase, weight reduction occurred at a slower rate, resulting in final residue masses of 22.21%, 15.17%, and 22.22% for Mp, Mk, and Sg, respectively. Sg demonstrated the highest stability in TGA treatment, followed by Mp and Mk.
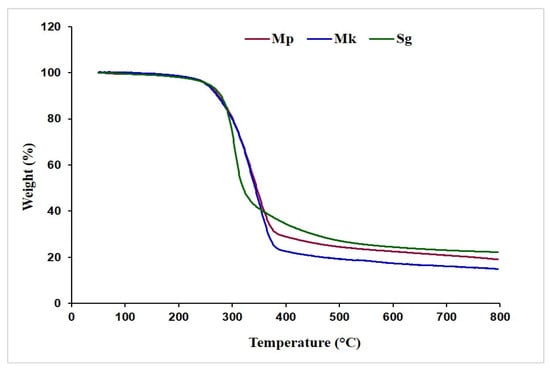
Figure 3.
Thermogravimetric curve of β-glucan. Mp: β-glucan isolated from Monascus purpureus; Mk: β-glucan isolated from Monascus kaoliang; Sg: Standard β-glucan.
Thermogravimetric analysis of β-glucan was applied to examine its thermal stability and decomposition characteristics across a broad temperature range. This analytical technique assesses alterations in mass in response to temperature variations, enabling the observation of weight loss, thermal degradation, decomposition, and other thermal transitions of β-glucan. The thermogravimetric analysis provides a visual representation of these processes, offering insights into the thermal behavior of β-glucan. A similar pattern of weight degradation was reported [33], which showed that in the initial phase, the weight loss observed in β-glucan was attributed to the elimination of free and hydrated water within the sample. The subsequent weight loss, occurring in the temperature range of 227 °C to 340 °C and accounting for 40% of the total weight loss, corresponds to the primary thermal decomposition of β-glucan. TGA is typically capable of discerning material composition when there are notable variations in the thermal behaviors of the components [33]. Therefore, TGA is a very fruitful tool for understanding the temperature-dependent pattern of extracted β-glucan, providing thermal characteristics for potential applications in various fields.
3.5. X-ray Diffraction (XRD) Analysis of β-Glucan
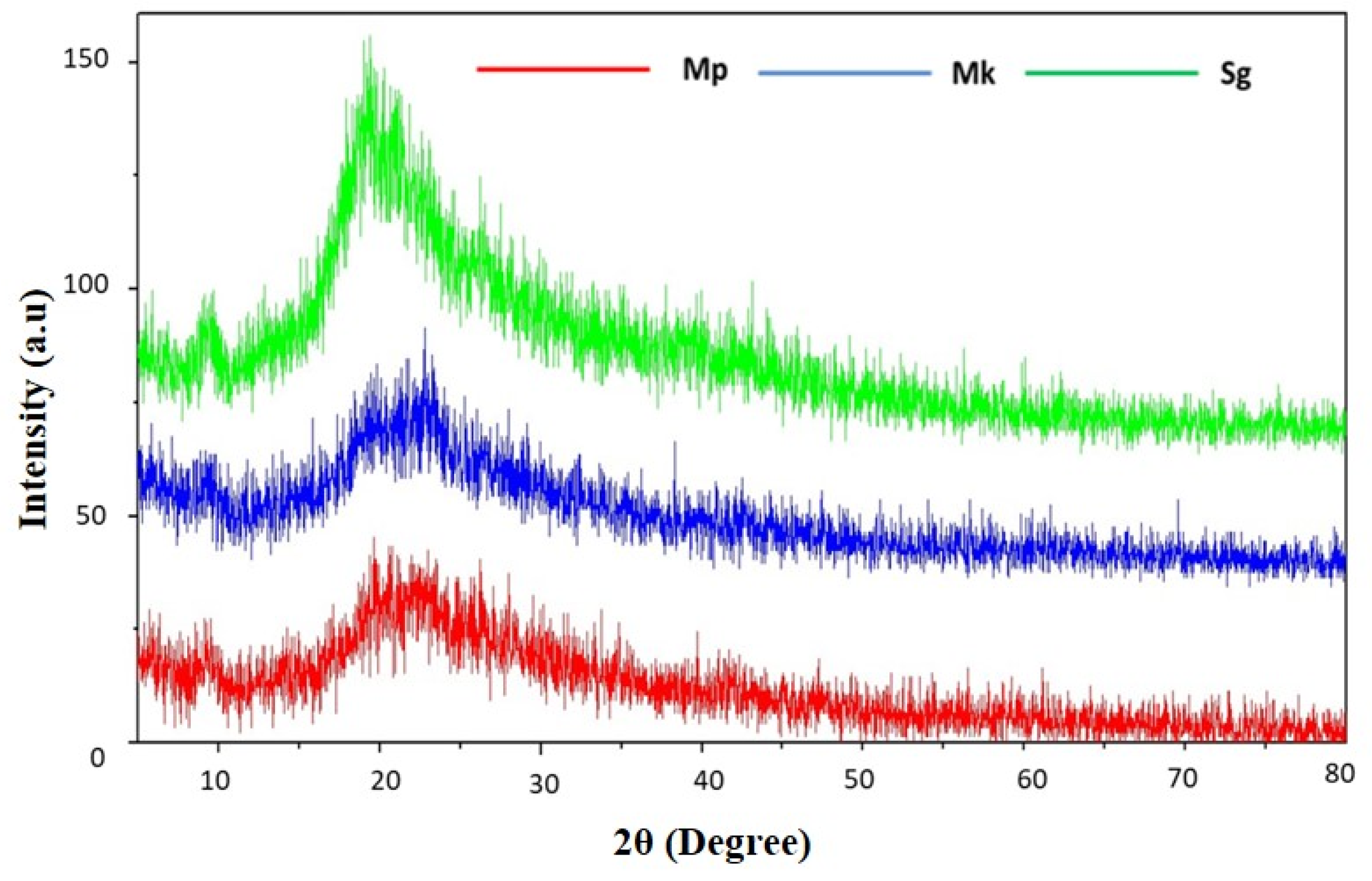
X-ray diffraction stands as an established method for examining crystal lattice arrangements, providing valuable insights into the degree of crystallinity in a sample. The XRD pattern of extracted and standard β-glucan is presented in Figure 4. There is a similar diffraction pattern between the extracted and standard β-glucan, indicating the purity of the extracted one. The XRD profile of β-glucan commonly exhibits a broad peak at approximately 2θ°, arising from X-ray scattering by the helical arrangement within the beta-glucan molecule; the peak’s intensity may vary based on the beta-glucan source and preparation method. The diffraction peak was observed at 2–10° and 20°, which are characteristic peaks of β-glucan, and this finding is in accord with previous reports [4,34]. XRD is a suitable method for determining the crystal structure of a material by measuring how X-rays are scattered by its atoms. The scattered X-rays are then detected and employed to calculate the crystal structure. β-Glucan comprises a diverse array of sugars, such as glucose, fructose, and sucrose, forming a complex molecule. The helical configuration of the β-glucan molecule allows for the utilization of its XRD pattern to ascertain the dimensions of the helix.
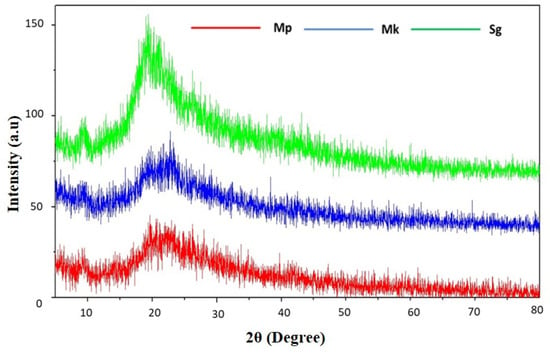
Figure 4.
X-ray diffraction (XRD) pattern of β-glucan. Mp: β-glucan isolated from Monascus purpureus; Mk: β-glucan isolated from Monascus kaoliang; Sg: Standard β-glucan.
3.6. Scanning Electron Microscopic View of β-Glucan
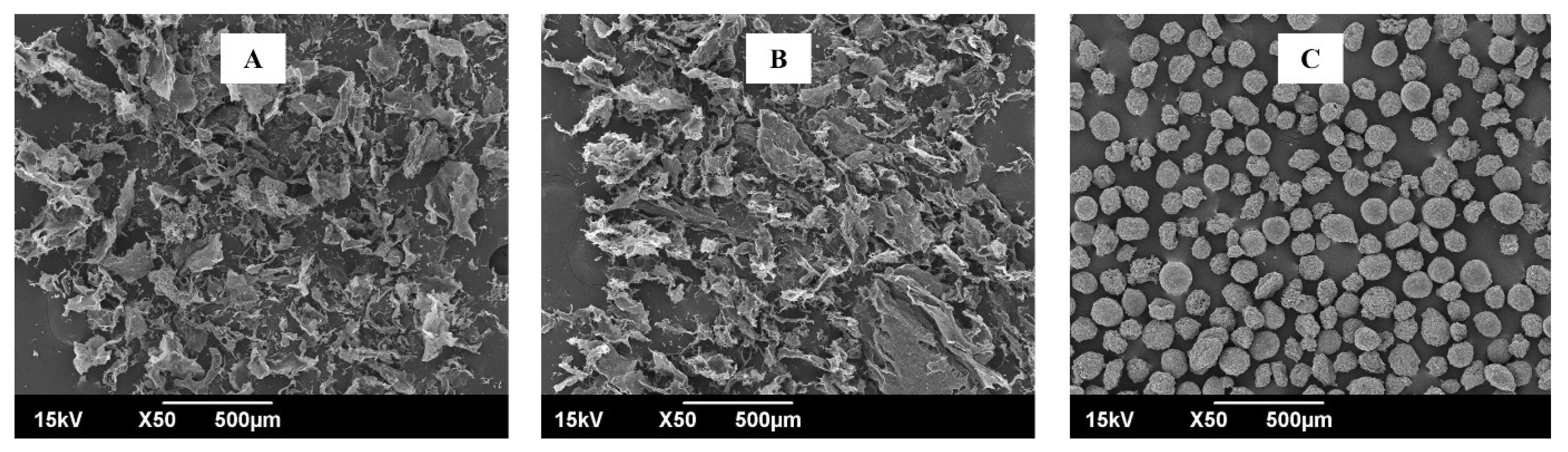
The microscopic morphology of standard β-glucan, β-glucan extracted from M. purpureus, and β-glucan extracted from M. kaoliang were evaluated by SEM (Figure 5). The standard β-glucan particles exhibited uniformity with spherical shapes; however, the extracted β-glucan formed irregular aggregates that coalesced into masses comprising spherical to elongated particles. Similar uniform and spherical standard β-glucan and the β-glucan extracted from Saccharomyces cerevisiae with irregular geometric shapes with sharp edges, along with aggregated particles that coalesce to form large masses, were reported [35,36]. The extracted beta-glucan displayed a porous and spongy appearance, devoid of any discernible traces of the cell wall structure. Nevertheless, disparities in the size distribution and shape of the particles were observed. A comprehensive examination of the samples indicated that the extracted β-glucans exhibited an irregular and wide particle size distribution. The extracted sample featured a few clusters with rounded structures and small, loosely dispersed particles showcasing geometric shapes. In contrast, the commercial β-glucans displayed larger, rounder, and more homogeneous clusters. In brief, SEM images displayed characteristic rough and fibrous shapes and surfaces for extracted β-glucans but they were a uniform and regular shape in the standard sample. The variations in structure among the samples likely resulted from the employed extraction method, particularly in the drying and milling stages. A microstructure with comparable characteristics to those obtained in this study for β-glucans extracted using an alkaline aqueous method was demonstrated [37].
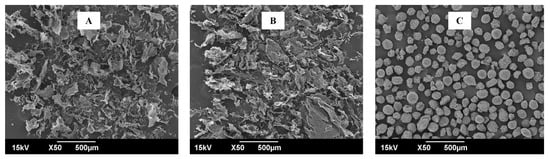
Figure 5.
Scanning electron microscopic view of β-glucan. (A) β-glucan isolated from Monascus purpureus; (B) β-glucan isolated from Monascus kaoliang; (C) Standard β-glucan.
3.7. Radical Scavenging Activity of β-Glucan
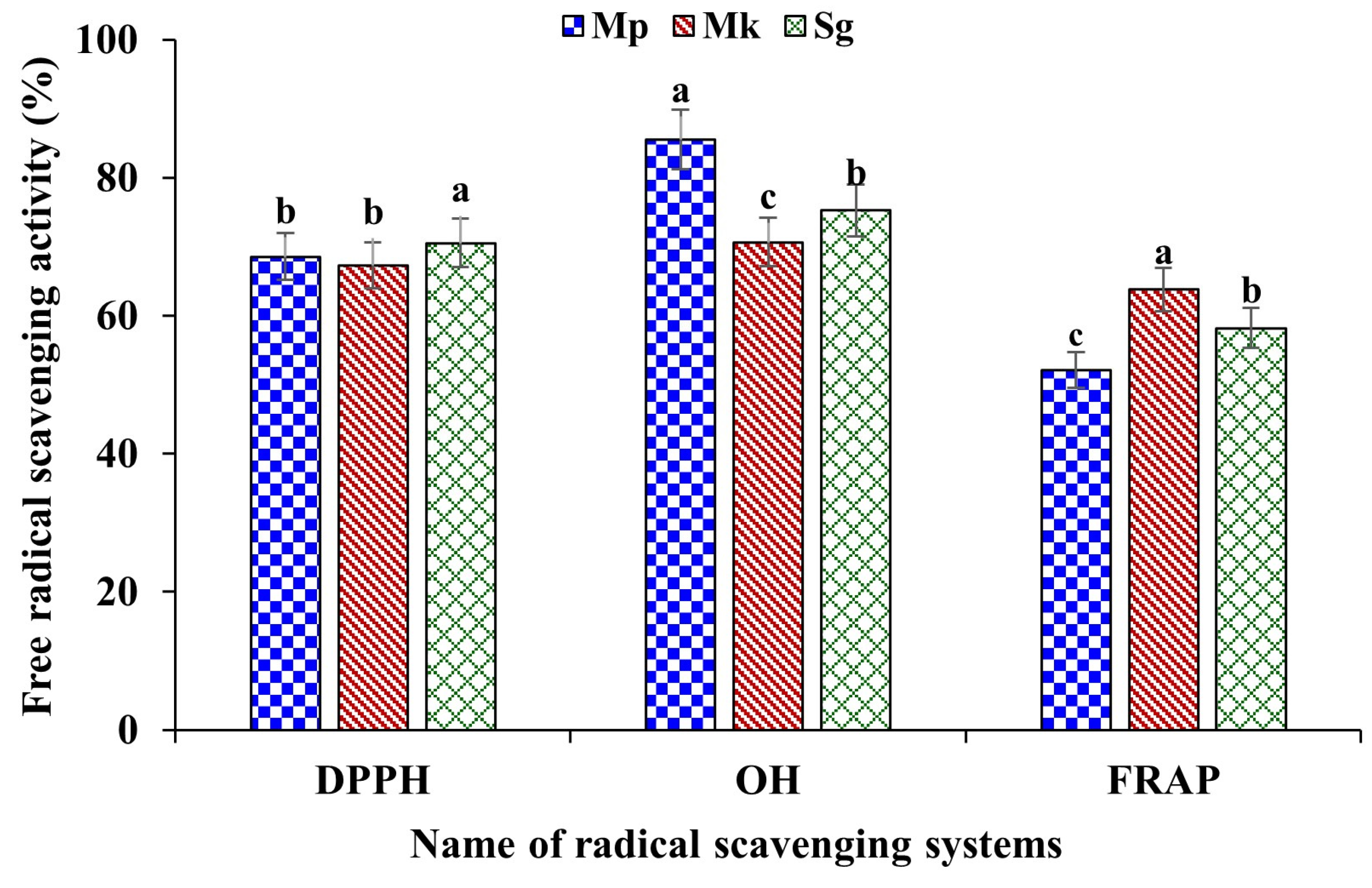
The in vitro free radical scavenging activities, namely DPPH, OH, and FRAP of the extracted β-glucan, are presented in Figure 6. The β-glucan extracted from M. purpureus, M. kaoliang, and standard β-glucan showed strong free radical scavenging activity, with variations between 67.31% and 70.54% of DPPD free radical scavenging activity. In relation to this, standard β-glucan showed significantly higher (p ≤ 0.05) activity compared to extracted samples. The OH radical scavenging activity of β-glucan extracted from M. purpureus exhibited higher activity (85.52%) than β-glucan extracted from M. kaoliang and standard β-glucan, whereas the FRAP activity of β-glucan extracted from M. kaoliang showed higher activity of 63.77% compared to β-glucan extracted from M. purpureus (52.10%) and standard β-glucan (58.20%). Free radicals are unstable, highly reactive molecules that cause cellular damage and contribute to various physical problems such as cellular damage, mutation, oxidative stress, inflammation, and neurological effects. DPPH, a stable free radical, finds extensive application in antioxidant assays to gauge the effectiveness of compounds in mitigating oxidative stress. This assay is instrumental in assessing the capacity of substances, including natural compounds or extracts, to counteract oxidative damage by neutralizing free radicals. It is a frequently utilized method in antioxidant research to quantify the radical scavenging activity of diverse compounds. The DPPH activity of barley β-glucan and found considerable radical scavenging activity [4]. Hydroxyl radical scavenging activity is the capacity of a substance to eliminate hydroxyl free radicals from any system. Evaluating the scavenging activity against hydroxyl radicals is a prevalent method in antioxidant research. This approach enables the assessment of natural compounds or extracts, gauging their potential to neutralize these radicals and provide protection against oxidative damage. The hydroxyl radical scavenging activity of β-glucan varied depending on the source of β-glucan. For example, β-glucan extracted from barley exhibited stronger hydroxyl radical scavenging activity compared to β-glucan extracted from black yeast or oats [38]. Although the mechanisms by which β-glucan scavenges hydroxyl radicals are not yet clarified, the different structures of β-glucan, which may be associated with the source and extraction method of obtaining β-glucan, may influence antioxidant activity.
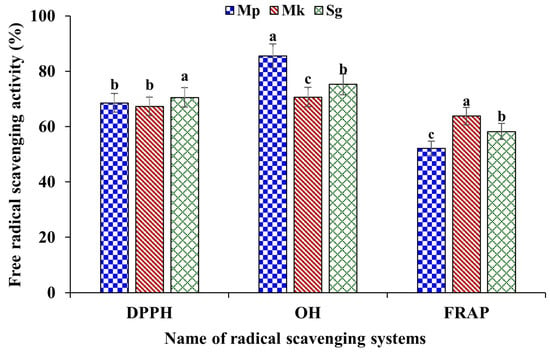
Figure 6.
The in vitro free radical scavenging activity of β-glucan. Mp: β-glucan isolated from Monascus purpureus; Mk: β-glucan isolated from Monascus kaoliang and Sg: Standard β-glucan. Different small letter on each column bar for individual property indicates significant difference (p ≤ 0.05).
The FRAP method has found extensive application in assessing the antioxidant capacity of food and health products. The fundamental concept behind the FRAP method for gauging total antioxidant capacity lies in the ability of antioxidants to convert Fe3+ to Fe2+ under acidic conditions. The cleavage of glycoside bonds results in an augmented presence of reducing sugars, thereby elevating the reducibility of degraded polysaccharides. The in vitro FRAP activity of β-glucan extracted from barley was found to be strong, and the activity was found to increase with increasing the fermentation period [39]. β-glucan possesses the ability to neutralize free radicals due to its distinctive molecular configuration, thus working as a potent antioxidant. Many research reports are proving that the free radical scavenging activity of β-glucan is effective in relieving oxidative stress and inflammation in the body. β-glucan exerts positive effects on human health, including reducing serum cholesterol, prebiotic and anticancer activities, and having antioxidant properties. Although the specific mechanisms through which β-glucan scavenges hydroxyl radicals remain unclear, it is noteworthy that the diverse structures of β-glucan associated with the source and extraction method could impact antioxidant activity [38]. The mechanisms of antioxidant properties of β-glucans are not well-known; however, previous studies showed that the anomeric hydrogen molecules could be related to this [40]. Studies have indicated that the efficacy of β-glucan in scavenging free radicals is predominantly dependent on its molecular and structural characteristics [40].
3.8. Cytotoxicity of Glucan by MTS Assay
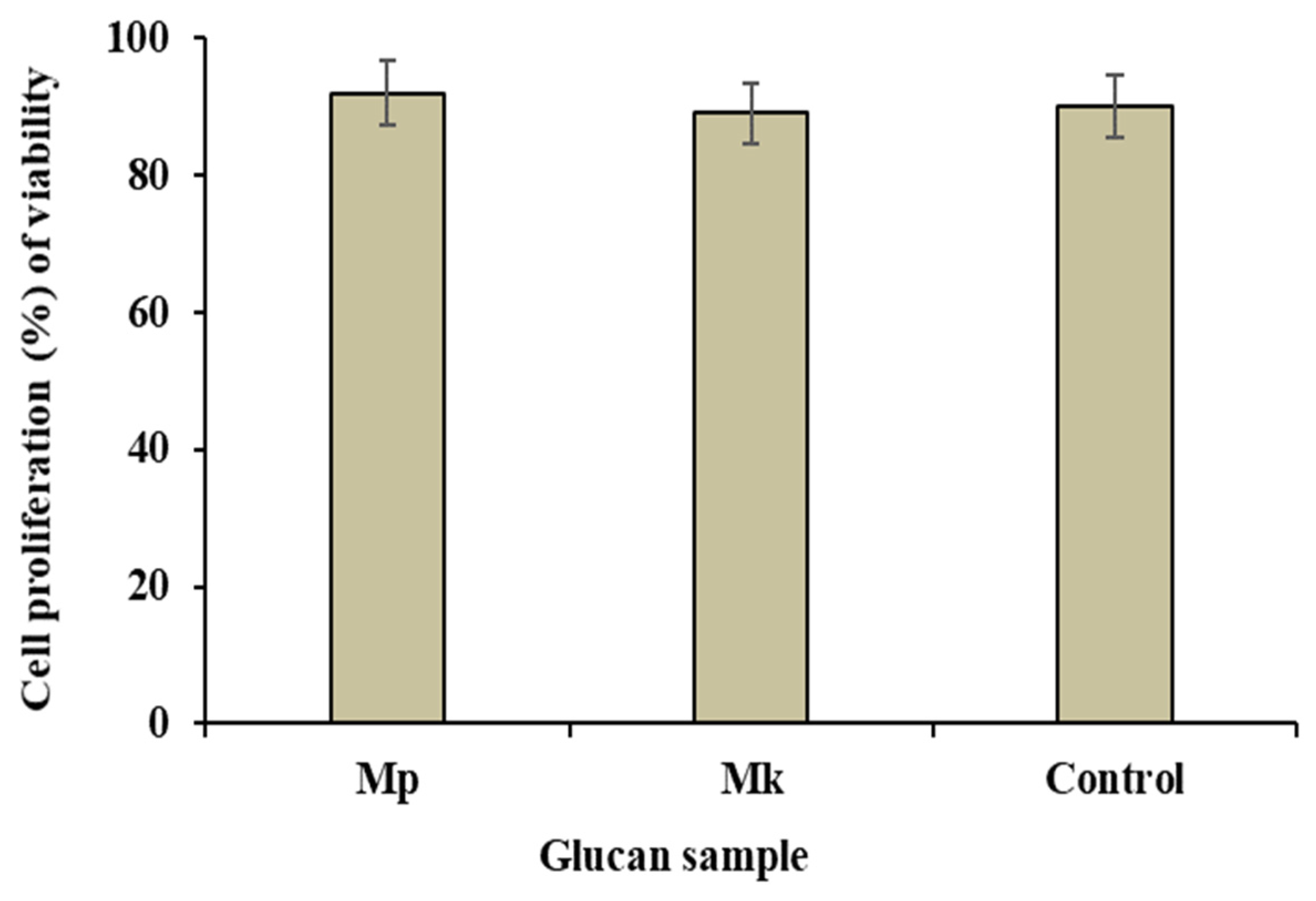
The cytotoxic study employing the MTS assay assesses the effects of a substance on cell viability. The cytotoxicity of β-glucan samples extracted from M. purpureus and M. kaoliang to Caco-2 cells (% of cell viabilities) was determined and compared with standard β-glucan (Figure 7). The cell viability was reduced by β-glucan extracted from M. purpureus and M. kaoliang to 92.10% and 89.24%, respectively, whereas the value was 90.68 in standard β-glucan. Therefore, it can be concluded that the extracted β-glucan did not exhibit any cytotoxic effects. Cytotoxicity assays are commonly employed in the food and pharmaceutical industries, providing valuable insights into the potential toxic effects of compounds. The human intestinal Caco-2 cell line is extensively used for in vitro toxicology studies [25]. This cell line was utilized to assess new oral drugs for permeability and toxicity, highlighting that those substances highly toxic to intestinal epithelial cells may not be considered viable drugs [41]. In a separate study, Fernandes et al. [42] found that a crude fungal extract of Alternaria alternate exhibited moderate toxicity to HeLa cells. The water extracts of fermented and unfermented Houttuynia cordata were moderately toxic to human HL-60 and Molt-4 cell lines [43]. The MTS assay is efficient due to its sensitivity, simplicity, and ability to provide reliable results, making it an accepted method for toxicity studies in various scientific investigations. In the present study, our findings indicate that both β-glucans were not cytotoxic. Consequently, these β-glucans may serve as potential candidates for functional foods and pharmaceuticals.
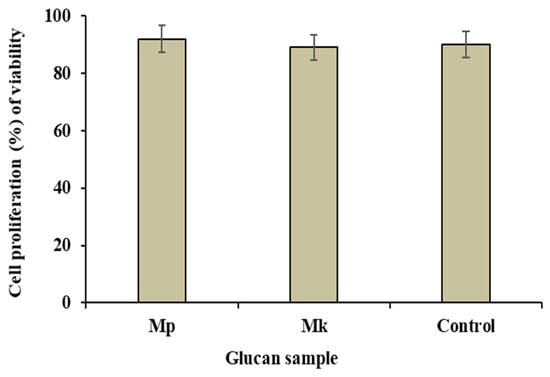
Figure 7.
The cell proliferation, represented as the percentage of viability, of Caco-2 cells after a 24-h incubation with β-glucan. Mp: β-glucan isolated from Monascus purpureus; Mk: β-glucan isolated from Monascus kaoliang; Control: Standard β-glucan. The assessment was conducted using the MTS technique, a non-radioactive cell proliferation assay.
4. Conclusions
The isolation and characterization of β-glucan extracted from Monascus spp. utilizing S. japonica as a submerged fermented substrate provide valuable insights into the structural and functional characteristics of the compounds. While the yield of β-glucan was appreciable, its solubility in water was found to be limited. The total sugar content was determined to be 114.75 ± 2.54 mg/g and 110.25 ± 1.86 mg/g, respectively, in the extracted β-glucan from M. purpureus and M. kaoliang. This determination is crucial for assessing the functional and nutritional properties, including the feasibility of industrial applications. The FTIR spectra confirm the identity and purity of the extracted β-glucan compared to the standard. TGA analysis demonstrated the feasibility of applying the extracted β-glucan in thermal-based manufacturing. SEM views revealed similarities between the extracted β-glucan and previously reported ones. Moreover, the extracted β-glucan exhibited in vitro free radical scavenging activity and showed no toxicity in the MTS assay. Therefore, using S. japonica as a substrate in the fermentation process by Monascus spp. could play a unique role in β-glucan production and applications. In the food industry, β-glucan enhances nutritional value and improves product quality, while in the pharmaceutical sector, it offers potential health benefits and can be used in novel treatment supplements. Additionally, its skin-friendly properties make it suitable for various skincare applications. Further research on the in vivo biological activities and health benefits associated with β-glucans may open new avenues for the development of novel functional compounds with diverse applications in the field of biotechnology and beyond.
Author Contributions
S.S.: Methodology, Investigation, Writing—Original draft, Formal analysis, Visualization, Conceptualization. W.J.J.: Formal analysis, Project administration, Writing—review and editing, Validation, Investigation. M.H.: Writing—Original draft, Investigation, Formal analysis. I.-S.K.: Conceptualization, Supervision, Project administration, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The research did not receive any external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no competing interests among them.
References
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.; Van Griensven, L.J. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Olsen, L.M.; Carbonero, E.R.; Baggio, C.H.; Freitas, C.S.; Marcon, R.; Santos, A.R.; Gorin, P.A.; Iacomini, M. Anti-inflammatory and analgesic properties in a rodent model of a (1→ 3), (1→ 6)-linked β-glucan isolated from Pleurotus pulmonarius. Eur. J. Pharmacol. 2008, 597, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Lowman, D.W.; West, L.J.; Bearden, D.W.; Wempe, M.F.; Power, T.D.; Ensley, H.E.; Haynes, K.; Williams, D.L.; Kruppa, M.D. New insights into the structure of (1→ 3, 1→ 6)-β-D-glucan side chains in the Candida glabrata cell wall. PLoS ONE 2011, 6, e27614. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.P.; Zhou, H.M.; Zhu, K.R.; Li, Q. Effect of thermal processing on the molecular, structural, and antioxidant characteristics of highland barley β-glucan. Carbohydr. Polym. 2021, 271, 118416. [Google Scholar] [CrossRef]
- Iorio, E.; Torosantucci, A.; Bromuro, C.; Chiani, P.; Ferretti, A.; Giannini, M.; Cassone, A.; Podo, F. Candida albicans cell wall comprises a branched β-d-(1→ 6)-glucan with β-d-(1→ 3)-side chains. Carbohydr. Res. 2008, 343, 1050–1061. [Google Scholar] [CrossRef]
- Bowman, S.M.; Free, S.J. The structure and synthesis of the fungal cell wall. Bioessays 2006, 28, 799–808. [Google Scholar] [CrossRef]
- Jaehrig, S.C.; Rohn, S.; Kroh, L.W.; Wildenauer, F.X.; Lisdat, F.; Fleischer, L.G.; Kurz, T. Antioxidative activity of (1→ 3), (1→ 6)-β-d-glucan from Saccharomyces cerevisiae grown on different media. LWT-Food Sci. Technol. 2008, 41, 868–877. [Google Scholar] [CrossRef]
- Sousa, P.; Tavares-Valente, D.; Amorim, M.; Azevedo-Silva, J.; Pintado, M.; Fernandes, J. β-Glucan extracts as high-value multifunctional ingredients for skin health: A review. Carbohydr. Polym. 2023, 322, 121329. [Google Scholar] [CrossRef] [PubMed]
- Chaichian, S.; Moazzami, B.; Sadoughi, F.; Haddad Kashani, H.; Zaroudi, M.; Asemi, Z. Functional activities of beta-glucans in the prevention or treatment of cervical cancer. J. Ovarian Res. 2020, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Sivieri, K.; de Oliveira, S.M.; de Souza Marquez, A.; Pérez-Jiménez, J.; Diniz, S.N. Insights on β-glucan as a prebiotic coadjuvant in the treatment of diabetes mellitus: A review. Food Hydrocoll. Health 2022, 2, 100056. [Google Scholar] [CrossRef]
- Wolever, T.M.; Rahn, M.; Dioum, E.; Spruill, S.E.; Ezatagha, A.; Campbell, J.E.; Jenkins, A.L.; Chu, Y. An oat β-glucan beverage reduces LDL cholesterol and cardiovascular disease risk in men and women with borderline high cholesterol: A double-blind, randomized, controlled clinical trial. J. Nutr. 2021, 151, 2655–2666. [Google Scholar] [CrossRef]
- Magnani, M.; Calliari, C.M.; de Macedo Jr, F.C.; Mori, M.P.; de Syllos Cólus, I.M.; Castro-Gomez, R.J. Optimized methodology for extraction of (1→ 3)(1→ 6)-β-D-glucan from Saccharomyces cerevisiae and in vitro evaluation of the cytotoxicity and genotoxicity of the corresponding carboxymethyl derivative. Carbohydr. Polym. 2009, 78, 658–665. [Google Scholar] [CrossRef]
- Zechner-Krpan, V.; Petravić-Tominac, V.; Gospodarić, I.; Sajli, L.; Đaković, S.; Filipović-Grčić, J. Characterization of ß-Glucans isolated from Brewer’s yeast and dried by different methods. Food Technol. Biotechnol. 2010, 48, 189–197. [Google Scholar]
- Tâm, T.M.; Duy, N.Q.; Minh, N.P.; Dao, D.T.A. Optimization of Βeta-Glucan extraction from waste brewer’s yeast Saccharomyces cerevisiae using autolysis, enzyme, ultrasonic and combined enzyme-ultrasonic treatment. Am. J. Res. Commun. 2013, 1, 149–158. [Google Scholar]
- Suraiya, S.; Siddique, M.P.; Lee, J.M.; Kim, E.Y.; Kim, J.M.; Kong, I.S. Enhancement and characterization of natural pigments produced by Monascus spp. using Saccharina japonica as fermentation substrate. J. Appl. Phycol. 2018, 30, 729–742. [Google Scholar] [CrossRef]
- Suraiya, S.; Kim, J.H.; Tak, J.Y.; Siddique, M.P.; Young, C.J.; Kim, J.K.; Kong, I.S. Influences of fermentation parameters on lovastatin production by Monascus purpureus using Saccharina japonica as solid fermented substrate. LWT 2018, 92, 1–9. [Google Scholar] [CrossRef]
- Jang, H.; Lee, J.; Park, Y.K.; Lee, J.Y. Exploring the health benefits and concerns of brown seaweed consumption: A comprehensive review of bioactive compounds in brown seaweed and its potential therapeutic effects. J. Agric. Food Res. 2024, 17, 101215. [Google Scholar] [CrossRef]
- Shibasaki, S.; Ueda, M. Utilization of macroalgae for the production of bioactive compounds and bioprocesses using microbial biotechnology. Microorganisms 2023, 11, 1499. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, P.; Li, S.; Cheng, M.; Duan, D. Isolation and characterization of glutathione S-transferase genes and their transcripts in Saccharina japonica (Laminariales, Phaeophyceae) during development and under abiotic stress. BMC Plant Biol. 2023, 23, 436. [Google Scholar] [CrossRef]
- Ul Ashraf, Z.; Shah, A.; Gani, A.; Gani, A.; Masoodi, F.A.; Noor, N. Nanoreduction as a technology to exploit β-Glucan from cereal and fungal sources for enhancing its nutraceutical potential. Carbohydr. Polym. 2021, 258, 117664. [Google Scholar] [CrossRef]
- Haq, M.; Park, S.K.; Kim, M.J.; Cho, Y.J.; Chun, B.S. Modifications of Atlantic salmon by-product oil for obtaining different ω-3 polyunsaturated fatty acids concentrates: An approach to comparative analysis. J. Food Drug Anal. 2018, 26, 545–556. [Google Scholar] [CrossRef]
- Liu, N.; Couto, R.; Seifried, B.; Moquin, P.; Delgado, L.; Temelli, F. Characterization of oat beta-glucan and coenzyme Q10-loaded beta-glucan powders generated by the pressurized gas-expanded liquid (PGX) technology. Food Res. Int. 2018, 106, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, F.M.; Liang, J.B.; Ho, Y.W.; Mohamad, R.; Goh, Y.M.; Shokryazdan, P. Lovastatin production by Aspergillus terreus using agro-biomass as substrate in solid state fermentation. BioMed Res. Int. 2012, 2012, 196264. [Google Scholar]
- Suraiya, S.; Lee, J.M.; Cho, H.J.; Jang, W.J.; Kim, D.G.; Kim, Y.O.; Kong, I.S. Monascus spp. fermented brown seaweeds extracts enhance bio-functional activities. Food Biosci. 2018, 21, 90–99. [Google Scholar] [CrossRef]
- Kim, H.J.; White, P.J. Impact of the molecular weight, viscosity, and solubility of β-glucan on in vitro oat starch digestibility. J. Agric. Food Chem. 2013, 61, 3270–3277. [Google Scholar] [CrossRef] [PubMed]
- Jayasekara, L.C.B.; Poonsawad, A.; Watchaputi, K.; Wattanachaisaereekul, S.; Soontorngun, N. Media optimization of antimicrobial activity production and beta-glucan content of endophytic fungi Xylaria sp. BCC 1067. Biotechnol. Rep. 2022, 35, e00742. [Google Scholar] [CrossRef]
- Wattanachaisaereekul, S.; Tachaleat, A.; Punya, J.; Haritakun, R.; Boonlarppradab, C.; Cheevadhanarak, S. Assessing medium constituents for optimal heterologous production of anhydromevalonolactone in recombinant Aspergillus oryzae. AMB Express 2014, 4, 52. [Google Scholar] [CrossRef]
- Šandula, J.; Kogan, G.; Kačuráková, M.; Machová, E. Microbial (1→ 3)-β-d-glucans, their preparation, physico-chemical characterization and immunomodulatory activity. Carbohydr. Polym. 1999, 38, 247–253. [Google Scholar] [CrossRef]
- Sun, T.; Li, J.; Qin, Y.; Xie, J.; Xue, B.; Li, X.; Gan, J.; Bian, X.; Shao, Z. Rheological and functional properties of oat β-glucan with different molecular weight. J. Mol. Struct. 2020, 1209, 127944. [Google Scholar] [CrossRef]
- Gonzaga, M.L.C.; Menezes, T.M.; de Souza, J.R.R.; Ricardo, N.M.; Soares, S.D.A. Structural characterization of β glucans isolated from Agaricus blazei Murill using NMR and FTIR spectroscopy. Bioact. Carbohydr. Diet. Fibre 2013, 2, 152–156. [Google Scholar] [CrossRef]
- Khan, A.A.; Gani, A.; Masoodi, F.A.; Amin, F.; Wani, I.A.; Khanday, F.A.; Gani, A. Structural, thermal, functional, antioxidant & antimicrobial properties of β-d-glucan extracted from baker’s yeast (Saccharomyces cereviseae)—Effect of γ-irradiation. Carbohydr. Polym. 2016, 140, 442–450. [Google Scholar]
- Zlatković, D.; Jakovljević, D.; Zeković, Đ.; Vrvić, M. A glucan from active dry baker’s yeast (Saccharomyces cerevisiae): A chemical and enzymatic investigation of the structure. J. Serbian Chem. Soc. 2003, 68, 805–809. [Google Scholar] [CrossRef]
- Eyigor, A.; Bahadori, F.; Yenigun, V.B.; Eroglu, M.S. Beta-Glucan based temperature responsive hydrogels for 5-ASA delivery. Carbohydr. Polym. 2018, 201, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Novák, M.; Synytsya, A.; Gedeon, O.; Slepička, P.; Procházka, V.; Synytsya, A.; Blahovec, J.; Hejlová, A.; Čopíková, J. Yeast β (1-3),(1-6)-d-glucan films: Preparation and characterization of some structural and physical properties. Carbohydr. Polym. 2012, 87, 2496–2504. [Google Scholar] [CrossRef]
- Jameel, F.A.R.; Yassein, S.N. Characterization of β-glucan extracted from Saccharomyces cereviseae and Candida albicans. Plant Arch. 2021, 21, 1722–1727. [Google Scholar] [CrossRef]
- Bacha, U.; Nasir, M.; Iqbal, S.; Anjum, A.A. Nutraceutical, anti-inflammatory, and immune modulatory effects of β-glucan isolated from yeast. BioMed Res. Int. 2017, 2017, 8972678. [Google Scholar] [CrossRef] [PubMed]
- Limberger-Bayer, V.M.; de Francisco, A.; Chan, A.; Oro, T.; Ogliari, P.J.; Barreto, P.L. Barley β-glucans extraction and partial characterization. Food Chem. 2014, 154, 84–89. [Google Scholar] [CrossRef]
- Kofuji, K.; Aoki, A.; Tsubaki, K.; Konishi, M.; Isobe, T.; Murata, Y. Antioxidant activity of β-glucan. Int. Sch. Res. Not. 2012, 2012, 125864. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Tan, C.; Zhao, Y.; Zhu, Y.; Bai, J.; Xiao, X.; Zhang, L.; Teng, D.; Tian, J.; et al. Effects of L. plantarum dy-1 fermentation time on the characteristic structure and antioxidant activity of barley β-glucan in vitro. Curr. Res. Food Sci. 2022, 5, 125–130. [Google Scholar] [CrossRef]
- Ahmad, M.; Gani, A.; Shah, A.; Gani, A.; Masoodi, F.A. Germination and microwave processing of barley (Hordeum vulgare L) changes the structural and physicochemical properties of β-d-glucan & enhances its antioxidant potential. Carbohydr. Polym. 2016, 153, 696–702. [Google Scholar]
- Fernandes, M.D.R.V.; Pfenning, L.H.; Costa-Neto, C.M.D.; Heinrich, T.A.; Alencar, S.M.D.; Lima, M.A.D.; Ikegaki, M. Biological activities of the fermentation extract of the endophytic fungus Alternaria alternata isolated from Coffea arabica L. Braz. J. Pharm. Sci. 2009, 45, 677–685. [Google Scholar] [CrossRef]
- Fernandes, M.B.; Gonçalves, J.E.; Scotti, M.T.; de Oliveira, A.A.; Tavares, L.C.; Storpirtis, S. Caco-2 cells cytotoxicity of nifuroxazide derivatives with potential activity against Methicillin-resistant Staphylococcus aureus (MRSA). Toxicol. Vitr. 2012, 26, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Banjerdpongchai, R.; Kongtawelert, P. Ethanolic extract of fermented Thunb induces human leukemic HL-60 and Molt-4 cell apoptosis via oxidative stress and a mitochondrial pathway. Asian Pac. J. Cancer Prev. 2011, 12, 2871–2874. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).