Abstract
In recent decades, cellulose (Cel) and its modified forms have emerged as a new class of versatile adsorbents for removing dyes from aqueous solutions. This work reports the immobilization of macromolecules obtained from reactions between ethylenediamine (N) and ethylene sulfide (S) in three molar proportions (1:1, 1:2, and 1:4) on the surface of chlorinated cellulose (Cl-Cel), aiming to increase the adsorption capacity of dyes. The materials obtained (NS-Cel, N2S-Cel, and N4S-Cel) were characterized by elemental analysis, which demonstrated immobilization of macromolecules with a ratio of 12 ethylene sulfides to 1 ethylenediamine in the materials NS-Cel and N4S-Cel and a ratio of 10 ethylene sulfides to 1 ethylenediamine in NS-Cel. Intense C-H stretching bands of CH2 groups at 2900 cm−1 in the FT-IR spectra suggest a large amount of the functional group, corroborating the 13C NMR spectra, which presented a signal at 33 ppm referring to methylene carbons. The materials obtained had excellent performance in removing the dyes studied, with the adsorption capacity of the Remazol yellow GR dye being approximately 24 times greater than the raw material (87.70 ± 2.63 mg g−1) for the best-hyperbranched cellulose N4S-Cel and 3.60 ± 0.18 mg g−1 for Cel, and about ten times higher for the dye Remazol red RB (57.84 ± 1.73 mg g−1) for N4S-Cel compared to previously published work for Cel.
1. Introduction
Textile industries discharge large amounts of toxic dyes into water bodies, which can cause various damages to human health and aquatic life [1,2,3,4]. Synthetic dyes have complex structures that make them stable and non-biodegradable, complicating the treatment of wastewater containing this class of organic compounds [3,4,5,6,7]. Due to their toxicity and teratogenic, mutagenic, and carcinogenic effects on aquatic organisms and humans, it is urgent to develop techniques for treating wastewater containing dyed effluents. Therefore, developing new adsorbents with a high adsorption capacity is highly relevant to addressing the challenge of environmental pollution caused by dyes [8,9,10,11].
Cellulose is a biopolymer considered an almost inexhaustible source of raw material and is a promising natural material that researchers have extensively explored. Cellulose becomes even more attractive when modifications are made to its structure to improve its existing properties or to add new potentialities to this material [12,13,14,15,16]. Among the modifications, halogenation is an intermediate route, in which a halogen atom is incorporated into cellulose through a nucleophilic substitution reaction, preferably occurring at the hydroxyl group on carbon 6. This process increases the reactivity of cellulose due to the easier exchange of the halogen compared to the hydroxyl group, allowing for the incorporation of molecules of interest that enhance this renewable raw material [17,18,19].
Hyperbranched macromolecules and dendrimers are examples of molecules of interest. These are gaining prominence due to their architecture, which consists of a multifunctional central core from which numerous branches extend, resembling the branches and roots growing from the trunk of a tree. This structure forms multiple branches that result in versatility in their applications [20,21,22,23,24]. These macromolecules have high density, low viscosity, and many functional groups, enabling various technological applications in developing new materials. They can also have environmental applications, such as metal [25] and dye [21] removal from an aqueous medium. Hyperbranched polymers and dendrimers offer a good opportunity; however, they are a significant challenge in materials science without giving preference to any group. Although not necessarily trivial, synthesizing hyperbranched polymers is relatively more feasible than synthesizing dendrimers; however, the complete characterization of the highly complex and irregular structure and the establishment of a clear structure–property relationship still need to be completed because a hyperbranched polymer consists of many different isomeric macromolecules and has high polydispersity, unlike dendrimers, which have an exact molar mass structure and perfect branching [21,24].
Previous efforts demonstrated that cellulose incorporated with multifunctional hyperbranched groups exhibited excellent pollutant adsorption capacity, and many reports explored the functionalization of cellulose through hyperbranching with large quantities of nitrogen-containing moieties. Yu et al. 2019 [26] developed an amino-terminated hyperbranched polyamide functionalized cellulose with excellent adsorption performance for orange II and Cu(II) ions. Hyperbranched polyethylenimine was explored in this field [27,28]. The paper published by Liu et al. 2024 [8] functionalized loofah cellulose with polyethylenimine and provided an efficient active bright red dye adsorbent. Other potential hyperbranched functionalizations include amino-polyol [29] and aliphatic hyperbranched polyester [30]. However, to date, there have been no reports on functionalizing cellulose through hyperbranching with most sulfur-containing groups.
Therefore, this work proposes the development of new materials with hyperbranched molecular structures through the modification of cellulose with macromolecules formed from reactions between ethylenediamine and ethylene sulfide at different molar ratios. The obtained materials were applied in the removal of two azo dyes from aqueous solutions, which are used in the textile industry, such as Remazol yellow GR and Remazol red RB. The influence of experimental conditions on adsorption was evaluated including pH, time, temperature, and initial dye concentrations.
2. Materials and Methods
2.1. Chemicals
The reagents were cellulose (Aldrich, São Paulo, Brazil), thionyl chloride 99% (Aldrich, São Paulo, Brazil), N, N-dimethylformamide (DMF) 99.8% (Vetec, Duque de Caxias, Brazil), dimethylacetamide (DMA) 99.8% (Vetec, Duque de Caxias, Brazil), ammonium hydroxide 28.0–30.0% (Impex), ethylenediamine 99% (Vetec, Duque de Caxias, Brazil), ethylene sulfide 98% (Vetec, Duque de Caxias, Brazil), acetone 99.5% (Vetec, Duque de Caxias, Brazil), hydrochloric acid 36.5–38% (Impex, Diadema, Brazil), sodium hydroxide 99% (Impex, Diadema, Brazil), and potassium nitrate 99% (Impex, Diadema, Brazil). The dyes were commercial samples and were used as received. Remazol yellow GR dye and Remazol red RB dye were supplied by Dystar Company (Singapore). Deionized water was used in the preparations. All reagents were of analytical grade and used without prior purification.
2.2. Cellulose Chlorination
The details of the synthesis procedure for chlorinated cellulose, which in this work is used as an intermediate step in obtaining new cellulosic derivatives, have been described in previous studies [17,18,31]. In summary, 10.0 g of cellulose was suspended in 200.0 mL of DMF. Then, 35.0 mL of thionyl chloride was added slowly at 353 K, under mechanical stirring, which was maintained for 4 h. Finally, the mixture was washed with an aqueous NH4OH solution to neutralize the pH, followed by washing with deionized water and acetone. The final material obtained after filtration and drying was chlorinated cellulose (Cl-Cel).
2.3. Synthesis of Hyperbranched Cellulose
The synthesis of hyperbranched cellulose derivatives was carried out in two stages. In the first stage, three reactions between ethylenediamine and ethylene sulfide in different molar ratios were developed using 50.0 mL of DMA as the solvent at 328 K for 5 h. For each reaction, 5.0 mL of ethylenediamine was mixed with 4.5 mL, 8.9 mL, and 17.8 mL of ethylene sulfide to correspond to 1:1, 1:2, and 1:4 molar ratios for ethylenediamine to ethylene sulfide, respectively.
In the next stage, 5.0 g of Cl-Cel was added to the reaction mixtures from the first stage without removing any reagents from the system. The systems were maintained under reflux and constant stirring for 4 h at 328 K. Subsequently, the materials were filtered, washed with water, and dried under a vacuum at room temperature. The new materials were designated NS-Cel, N2S-Cel, and N4S-Cel, corresponding to the 1:1, 1:2, and 1:4 molar ratios of ethylenediamine to ethylene sulfide, respectively.
2.4. Point of Zero Charge (pHPZC)
The point of zero charge (pHPZC) of the matrices (NS-Cel, N2S-Cel, and N4S-Cel) was determined using the solid addition method [32]. In a typical procedure, samples of 20.0 mL of 0.1 mol L−1 KNO3 solutions were pH-adjusted between 2.0 and 12.0 with 0.1 mol L−1 HCl and/or NaOH and were placed in twelve Erlenmeyer flasks. The initial pH (pHi) was duly recorded, and the solutions were put into contact with 20.0 mg of each material for 24 h under constant stirring. Subsequently, the solutions were filtered, and the final pH (pHf) was measured using a pH meter Tecnal (Piracicaba-SP) model TEC-3MP. The difference between the initial and final pH was calculated (ΔpH = pHi − pHf) and plotted on a graph of ΔpH as a function of pHi. The value of pHi, where ΔpH is 0, is called the material’s point of zero charge (pHpzc).
2.5. Characterizations
The chlorine, sulfur, and nitrogen contents in the precursor and in the chemically modified biopolymer were determined through elemental analysis on a Perkin Elmer (São Paulo, Brazil), model 2400, elemental analyzer. The Fourier transform infrared spectra (FT-IR) were recorded on a spectrophotometer Bomem (Germany) MB-series. The samples were dispersed in KBr pellets (1% sample), and each spectrum was recorded in the 4000 to 400 cm−1 with 4 cm−1 resolution and 36 scans at room temperature. Solid-state 13C NMR spectra of the samples were obtained on a Bruker AC 300 spectrometer (Germany). For each run, approximately one gram of each solid sample was compacted into a 7 mm zirconium oxide rotor. The measurements were obtained at 75.47 MHz frequency with a magic angle spinning of 4 kHz. In order to increase the signal-to-noise ratio, the CP/MAS technique was used, with a pulse repetition of 3 s and contact times of 50 ms. The X-ray diffraction patterns were obtained on a Shimadzu (Japan) XRD-6000 diffractometer (40 kV, 30 mA), using CuKα radiation (λ = 0.154 nm) as the X-ray source. Patterns were acquired at a scan rate of 2° min−1 in the 2θ = 3–50° range, at a 0.02° step size and a time per step of 0.60 s. The thermogravimetric curves were obtained using a DuPont (U.S.A.) 9900 apparatus operating under a nitrogen atmosphere at a flow rate of 1.67 mL s−1, in the temperature range of 273–1200 K, and at a heating rate of 1.67 K s−1.
2.6. Adsorption
This study used two anionic dyes containing the azo group as a chromophore. The Remazol yellow GR dye has a vinyl sulfone group in its molecular structure, which acts as a reactive group available for interaction with textile fibers [33,34]. The Remazol red RB dye is a bifunctional dye that binds to the textile fiber through monochlorotriazine and vinyl sulfone groups [35,36]. Concentration measurements of the dye solutions in the adsorption tests were performed in triplicate using a Varian (U.S.A.) Cary 300 spectrophotometer at the wavelengths of maximum absorption, 414 nm and 518 nm for Remazol yellow GR and Remazol red RB, respectively. A calibration curve with a linear range between 5.0 and 55.0 mg L−1 was used.
2.6.1. Adsorption Kinetics
The adsorption kinetics of the dyes (Remazol yellow GR and Remazol red RB) on the materials (NS-Cel, N2S-Cel, N4S-Cel, or Cel) were carried out as follows: A total of 20.0 mg of the materials were added to 20.0 mL of 300.0 mg L−1 dye solution and reacted for a time range of 20–300 min under mechanical stirring at 150 rpm and 298 K. The samples were centrifuged, and the UV–visible spectrophotometer model determined equilibrium dye concentrations: Varian Cary 300 [37,38].
The quantity of the dye fixed in the adsorbate, qe (mg g−1), was determined by Equation (1),
C0 and Ce are the initial and equilibrium concentrations in solution (mg·L−1), m is the mass of the material in g, and V is the volume of the solution used in mL.
The experimental results were adjusted to the pseudo-first- [39] and pseudo-second-order [40] adsorption kinetic models as described by Equations (S1) and (S2).
2.6.2. Influence of pH on Adsorption
The effect of pH on adsorption was performed by using dye solutions at a 300.0 mg L−1 initial concentration and a pH of 2.0 to 12.0, which was adjusted with 0.1 mol L−1 NaOH and/or HCl solutions [10,31].
In this procedure, samples containing 20.0 mL of dye solutions were put into contact with 20.0 mg of the new materials (NS-Cel, N2S-Cel, and N4S-Cel) and pristine cellulose in separate processes under agitation at 298 K until saturation of the material occurred. The initial dye concentrations and the dye concentrations after adsorption were measured using the Varian (U.S.A.) Cary 300 spectrophotometer, as described earlier. Equation (1) calculated the amount of dye removed [15,37].
2.6.3. Adsorption Isotherms
Samples containing 20.0 mg of the materials (NS-Cel, N2S-Cel, N4S-Cel, or Cel) were added to 20.0 mL of dye solutions in the 100.0–1000.0 mg L−1 concentration range. These systems were evaluated at three different temperatures (298 K, 308 K, and 318 K) under optimized pH and time conditions with constant stirring. After the adsorption equilibrium time, centrifugation was performed, and aliquots were taken from the supernatant solutions for absorbance readings. The amount adsorbed onto the material was calculated using Equation (1). The linearized forms of the Langmuir [41], Freundlich [42], and Temkin [43] isotherms models were applied to fit the experimental data. The relevant parameters for each isotherm model were obtained from Equations (S3)–(S6).
3. Results and Discussion
3.1. Characterization
The results obtained from elemental analysis characterization are presented in Table 1.

Table 1.
Percentages of chlorine, nitrogen, and sulfur in Cl-Cel, NS-Cel, N2S-Cel, and N4S-Cel.
The presence of 17.58% chlorine in the Cl-Cel material confirmed the success of the chlorination step of cellulose, which is crucial for subsequent reactions. The nearly complete substitution of chlorine in Cl-Cel by the new immobilized molecular structure in each reaction is evidenced by the significant decrease in the percentage of chlorine in the final materials compared to Cl-Cel. This decrease indicated the success of the proposed reaction.
The percentages of sulfur and nitrogen obtained in the final materials further reinforce the success of cellulose modification, as these elements are not present in the raw material. Additionally, the percentages of sulfur and nitrogen incorporated increased with the relative proportion of ethylenediamine and ethylene sulfide. For nitrogen, amounts increased from 1.79 ± 0.05% in NS-Cel to 2.22 ± 0.09% in N2S-Cel and 2.68 ± 0.04% in N4S-Cel. For sulfur, the values changed from 23.92 ± 0.05% in NS-Cel to 26.80 ± 0.05% in N2S-Cel and 37.89 ± 0.03% in N4S-Cel, reinforcing the indication of a higher degree of chlorine atom substitution.
The ratios between the numbers of moles of sulfur and nitrogen, nS/nN, calculated from their respective percentages, resulted in values around 5 in the modification with a molar ratio of 1:2 (N2S-Cel), indicating approximately five sulfur atoms for every nitrogen atom. For reactions conducted with 1:1 (NS) and 1:4 (N4S) molar ratios, the nS/nN ratio was around 6, indicating approximately six sulfur atoms for every nitrogen atom.
The proposed modifications involved the formation of macromolecules with a highly branched structure (Figure 1a,b), where subsequent reactions involving ethylene sulfide molecules to an ethylenediamine molecule occurred until a limit was reached, probably due to steric hindrance.
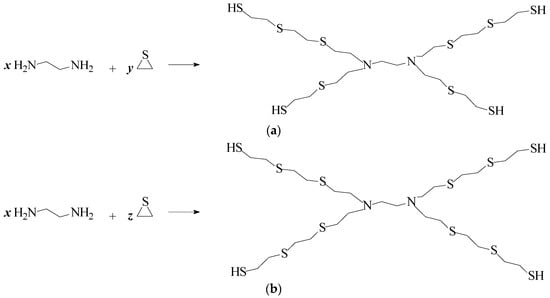
Figure 1.
Macromolecule formation for (a) N2S (x = 1 and y = 2) and (b) NS or N4S (x = 1 and z = 1 or 4, respectively).
The macromolecules are then incorporated onto the cellulose surface by replacing the chlorine atoms present, as shown in the proposed reaction scheme depicted in Figure 2a,b. For the N2S-Cel material, where the ratio is approximately five sulfur atoms for each nitrogen atom, a proportion of approximately ten ethylene sulfide molecules to one ethylenediamine molecule was observed. In contrast, for NS-Cel and N4S-Cel materials, the ratio is approximately twelve ethylene sulfide molecules to one ethylenediamine molecule.
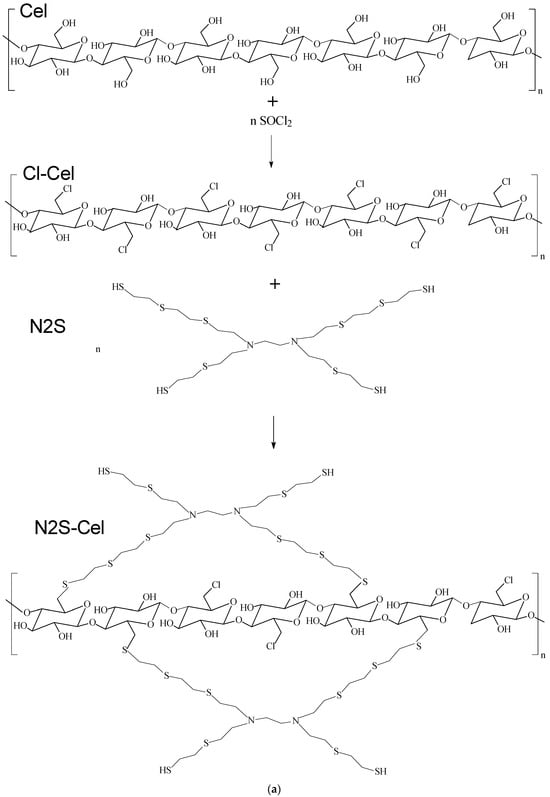
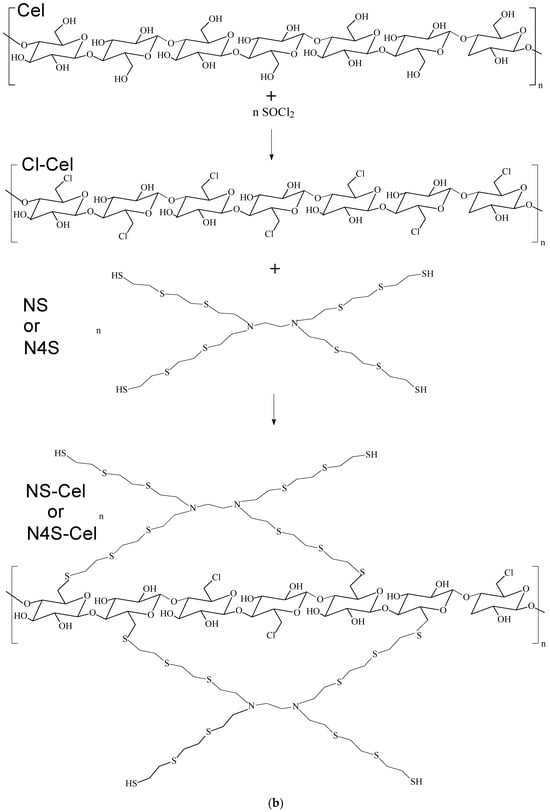
Figure 2.
Reaction scheme for immobilization of macromolecule on cellulose for (a) N2S-Cel and (b) NS-Cel or N4S-Cel.
According to the proposed reaction schemes shown in Figure 2a,b, the structure of cellulose acts as a core from which branches originate from the new ligands. Two or more cellulose segments at different ends of the new macromolecule can be linked, forming a hyperbranched polymer structure [21,44,45].
The FT-IR spectra are given in Figure 3. The bands related to specific functional groups of the raw material can be observed in Figure 3a. These include OH groups appearing in the region between 3400 and 3300 cm−1 with stretching vibrations of these groups present in both the ring and the side chain ν(-CH-OH) and ν(-CH2-OH). The band at 1639 cm−1 corresponds to these groups’ bending vibration δ(O-H), also characterized by bands between 1500 and 1200 cm−1. The band at approximately 2900 cm−1 corresponds to the methylene groups’ stretching, primarily attributed to CH groups, given that the ratio of CH to CH2 groups was 5:1, respectively, in the unmodified cellulose structure. Bands present below 1000 cm−1 were attributed to alcoholic groups present in the biopolymer structure [18].
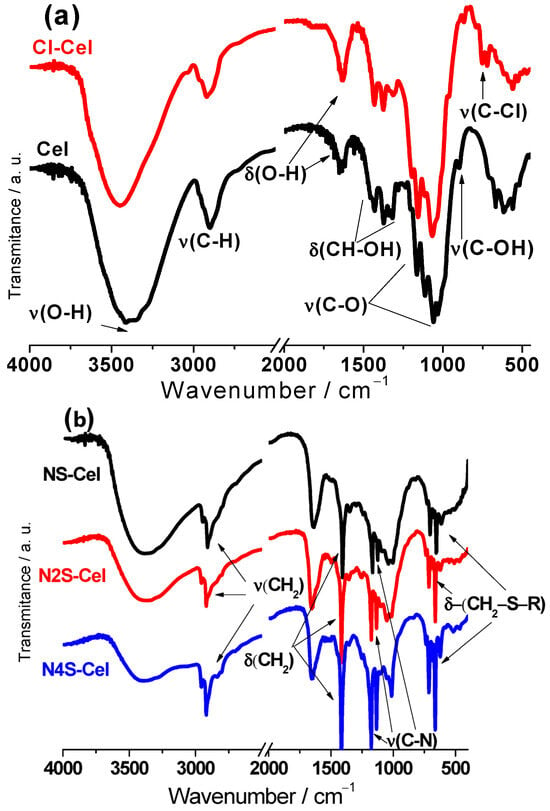
Figure 3.
FT-IR spectra of (a) Cel and Cl-Cel and (b) NS-Cel, N2S-Cel, and N4S-Cel.
The changes confirming the success of the cellulose chlorination step can be observed by comparing the spectra of chlorinated cellulose (Figure 3a, Cl-Cel) with that of raw cellulose (Figure 3a, Cel). The bands at 753 and 709 cm−1 in the Cl-Cel spectrum can be attributed to the stretching vibrations of carbon–chlorine bonds ν(C-Cl), confirming the effectiveness of chlorination. The reaction with thionyl chloride causes a decrease in the intensity of bands between 1500 and 1200 cm−1, which can be attributed to the substitution of hydroxyl at carbon 6 with chlorine, once again confirming the success of the reaction. Another significant change occurred with the C-OH stretching, which displaced from 896 cm−1 in pristine cellulose to 868 cm−1 in the chlorinated sample [31].
The structure of the new agent formed in the reaction between ethylenediamine and ethylene sulfide consists solely of CH2 groups as methylene groups. Therefore, the spectra of the three final materials obtained (Figure 3b) showed a different shape for the absorption band at 2900 cm−1 compared to that observed in the spectrum of unmodified cellulose due to the higher contribution of CH2 groups in these cases. Additionally, the large number of CH2 groups in the branches was indicated by the intense absorption band related to CH2 deformation at 1425 cm−1. Other alterations in the spectra included the band at 1180 cm−1 corresponding to C-N stretching, and bands appearing below 800 cm−1 attributed to CH2-S-R angular deformation, where the group R corresponds to carbon (band at 720 cm−1), indicating the formation of subsequent ethylene sulfide chains, or hydrogen (band at 660 cm−1), representing the end of the chain with SH groups [18].
The results obtained from solid-state 13C NMR are presented in Figure 4a, where the chemical shift signals attributed to the six carbons of the monomeric structure of the starting material can be observed (Figure 4a). The carbon with the largest chemical shift was carbon 1 (C1) at 104 ppm, which is bonded with two oxygen atoms. Next, a shift at 88 ppm (C4c of crystalline cellulose) and 83 ppm (C4a of amorphous cellulose) is attributed to C4, which is bonded to only one oxygen and is responsible for the 1,4′-β-glycosidic bonds. The chemical shift near 74 ppm was related to carbons 2, 3, and 5, which have equivalent chemical environments, meaning they are all secondary carbons bonded to hydroxyls and -CH groups. The carbon with the slightest chemical shift was C6, at 65 ppm (C6c of crystalline cellulose) and 63 ppm (C6a of amorphous cellulose), due to being a primary carbon bonded to hydroxyl and the only -CH2 group present in cellulose [19,46].
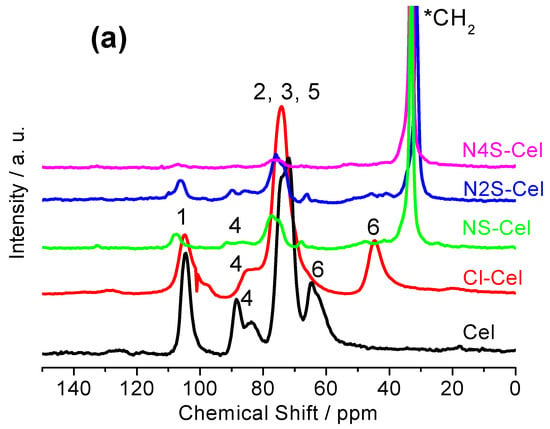
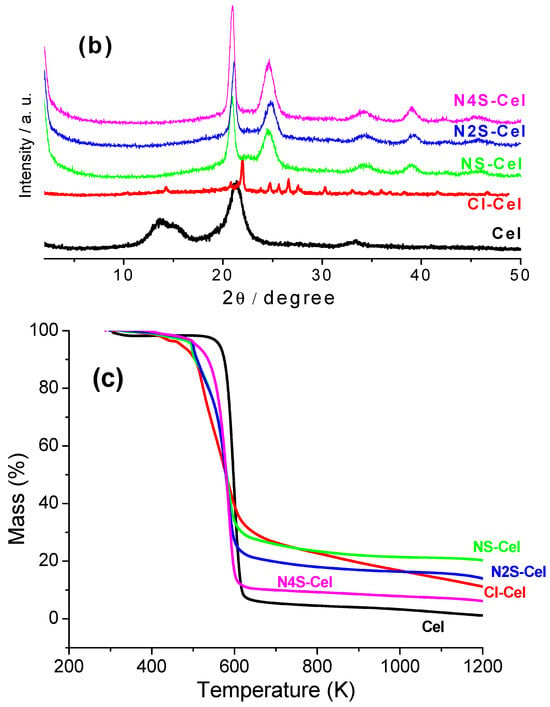
Figure 4.
(a) Solid-state 13C NMR spectra; (b) X-ray diffractograms; and (c) TG curves of Cel, Cl-Cel, NS-Cel, N2S-Cel, and N4S-Cel. *CH2 represents the signal corresponding to the newly incorporated methylene carbons.
The spectrum for Cl-Cel (Figure 4a) showed a significant change in chemical shifts compared to the spectrum of the raw material. The peak of carbon 1 (C1) shifted from 104 to 103 ppm, and C4, which initially had two signals at 88 and 83 ppm, showed a single signal at 83 ppm after modification, with a broad shoulder due to the depolymerization of the amorphous part. No significant changes were observed for C2, C3, and C5 compared to pristine cellulose, presenting only a better-formed peak. At C6, the hydroxyl group was replaced by chlorine, causing the chemical shift to vary from 65 and 63 ppm to 44 ppm due to the depolymerization of the amorphous part, thus confirming the immobilization of chlorine, forming chlorinated cellulose with a degree of substitution close to 1.0, due to the significant shift of C6 [17,18,19].
In the NMR spectra of the final materials (Figure 4a, NS-Cel, N2S-Cel, and N4S-Cel), the presence of a peak with a chemical shift around 31 ppm can be attributed to the CH2 groups from the anchored molecule (*CH2), as these carbons are close to sulfur and/or nitrogen atoms, leading them to a region of lower chemical shift compared to the other carbons in cellulose. The large amount of CH2 groups from the bulky group anchored to the cellulose surface caused the high intensity of the signal at 31 ppm, reducing the signals of the characteristic carbons of the cellulose monomer due to the significant scale difference. This effect was more evident in the N4S-Cel spectrum, where the higher intensity of the signal at 31 ppm is noticeable due to the greater number of incorporated macromolecules, corroborating with the elemental analysis results that showed higher percentages of nitrogen and sulfur for this material [18,31].
Figure 4b shows the XRD patterns of the raw material and the materials obtained subsequently. This technique is essential for verifying changes in crystallinity after the modifications.
Substituting hydroxyl groups in Cel with chlorine to obtain Cl-Cel favors forming hydrogen bonds with characteristics different from those formed in the starting material, leading to different crystallinity. This phenomenon has been widely observed in several studies that utilize the same modification route [19].
Incorporating the synthesized macromolecules into the biopolymer to form the final materials (Figure 4b, NS-Cel, N2S-Cel, and N4S-Cel) resulted in the acquisition of new crystallinity patterns. These patterns were similar due to the number of centers capable of forming new interactions, such as hydrogen bonds, which enable this different organization compared to the raw material. This phenomenon is not commonly observed in most materials reported in the literature, which tend to become amorphous due to the disorder introduced by the incorporation of modifying agents [6,18,31,47,48]. For example, Silva Filho et al. 2013 [18] obtained a cellulosic derivative by incorporating the same nucleophiles used in the present work, in which a simple change in the synthesis process was performed. The ethylenediamine molecule was first incorporated, and in a subsequent step, ethylene sulfide formed an amorphous material.
Figure 4c presents the thermogravimetry results. For pristine cellulose, Figure 4c, the curve showed a single decomposition event between 563 and 647 K, corresponding to a total mass loss of 92%. However, 2% of the mass was released up to 343 K, corresponding to physiosorbed water, and above 647 K, 6% mass loss was related to the decomposition of the organic structure of the polymer chain [49].
The decomposition occurred in three events for chlorinated cellulose (Figure 4c, Cl-Cel). However, the first event should not be considered for thermal stability as it only involved the loss of physiosorbed water, occurring between 386 and 430 K. The second decomposition event occurred between 438 and 534 K, with a mass loss of 23%, and was assigned to the release of HCl and the condensation of hydroxyl groups of carbons 2 and 3, resulting in the release of water. The third event corresponded to the decomposition of the organic support and occurred above 521 K [49,50].
The final materials (Figure 4c, NS-Cel, N2S-Cel, and N4S-Cel) exhibited higher thermal stability than the starting material, a phenomenon observed in various materials reported in the literature [20,34]. This behavior can be explained by the protection provided by the molecules anchored on the cellulose surface, where the modifying agents degrade first, followed by the degradation of the cellulose structure itself, leading to the complete degradation of the final materials at higher temperatures. Additionally, the disruption of bonds to achieve the proposed chemical modifications alters the stability of the materials. Therefore, the observed order of thermal stability was Cel < N4S-Cel < Cl-Cel < N2S-Cel < N2S-Cel.
Through the study of the point of zero charge (pHpzc) of the adsorbent matrices, Figure 5, the pHpzc values were 6.39, 6.58, 6.59, and 6.77 for Cel, NS-Cel, N2S-Cel, and N4S-Cel, respectively. Therefore, the pHpzc value of the synthesized material was slightly higher than that of the raw material due to the presence of amino and thiol groups in NS-Cel, N2S-Cel, and N4S-Cel, which interfere with the charge balance of the raw material, consequently increasing the pHpzc. The region corresponding to pH values lower than their respective pHpzc values indicates that the materials have a positively charged surface, meaning these are the pH values at which the materials retain protons. On the other hand, the region above the pHpzc values represents the pH values at which the materials release protons into the medium, indicating that the surfaces are negatively charged [51,52].
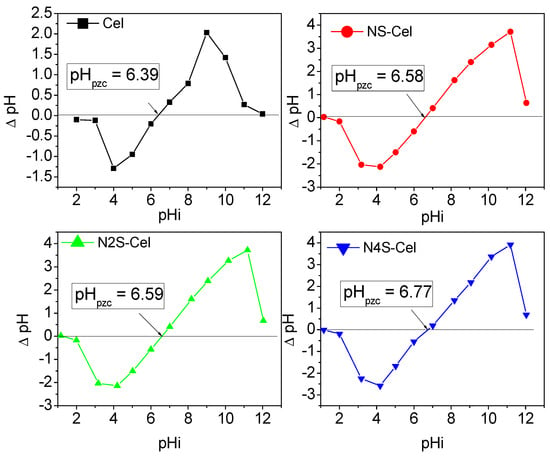
Figure 5.
Point of zero charge of Cel (−■−), NS-Cel (−●−), N2S-Cel (−▲−), and N4S-Cel (−▲−).
3.2. Adsorption
The influence of contact time on the adsorption of Remazol yellow GR and Remazol red RB dyes at 298 K is given in Figure 6.
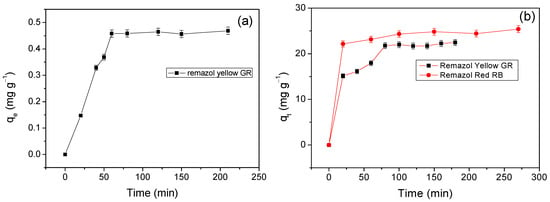
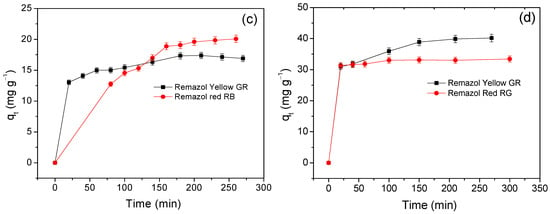
Figure 6.
Effect of contact time on dye adsorption on (a) Cel, (b) NS-Cel, (c) N2S-Cel, and (d) N4S-Cel.
The results in Figure 6a show that after 60 min of contact between pristine cellulose and Remazol yellow GR dye solution, the adsorbed amount became nearly constant, indicating that the contact time was sufficient to establish adsorption equilibrium on the surface of this material. The modified materials NS-Cel, N2S-Cel, and N4S-Cel reached adsorption equilibrium for the Remazol yellow GR dye at 80, 150, and 160 min, respectively, as shown in Figure 6b–d. Although longer contact times were required to reach equilibrium using the modified materials, the adsorption capacity increased compared to pristine cellulose for Remazol yellow GR dye.
Silva et al. 2015 [46] reported that raw cellulose interacted with Remazol red RB dye, reaching adsorption equilibrium after 200 min. In contrast, the NS-Cel, N2S-Cel, and N4S-Cel materials required contact times around 100, 150, and 100 min, respectively (Figure 6b–d). In this case, the modified materials reached equilibrium in a shorter time interval compared to pristine cellulose. Modified cellulose samples also exhibited a higher adsorption capacity, and the values were 25.38 ± 0.76, 20.08 ± 0.60, and 33.44 ± 1.00 mg g−1 for NS-Cel, N2S-Cel, and N4S-Cel, respectively.
Table 2 shows the parameters obtained from the pseudo-first-order [42] and pseudo-second-order models [43]. The highest values of the linear correlation coefficient (R2 ≥ 0.981) were obtained using the pseudo-second-order model in all evaluated cases. Additionally, minor differences between the experimental amount of adsorbed material per gram of adsorbent, qe,exp, and the calculated values, qe,cal, through a pseudo-second-order kinetic model, were observed. Therefore, the pseudo-second-order model was the most suitable for describing the kinetic behavior of dye adsorption in all studied materials. Similar results were also observed in other studies [19,53,54,55,56,57].

Table 2.
Parameters of adsorption kinetic models for Remazol yellow GR or Remazol red RB on Cel, NS-Cel, N2S-Cel, or N4S-Cel.
Figure 7 shows the pH’s influence on the dyes’ adsorption capacity (Remazol yellow GR or Remazol red RB).
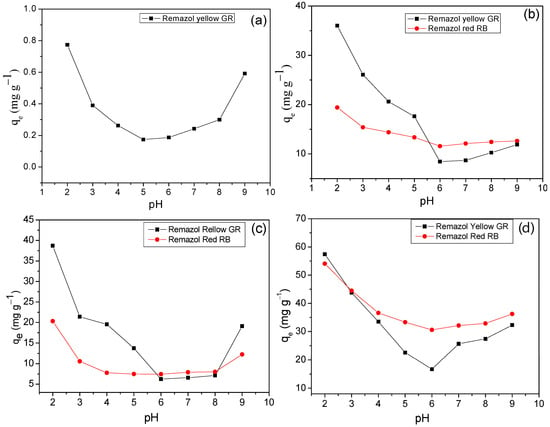
Figure 7.
Effect of pH on dye adsorption on (a) Cel, (b) NS-Cel, (c) N2S-Cel, and (d) N4S-Cel.
The graphs in Figure 7 indicated that all studied materials, Cel (Figure 7a), NS-Cel (Figure 7b), N2S-Cel (Figure 7c), and N4S-Cel (Figure 7d), exhibited higher dye adsorption capacity at pH 2.0, which was subsequently used in further studies. Based on these results, interactions between the dyes and the modified biopolymers N2S-Cel (Figure 8a) and NS-Cel or N4S-Cel (Figure 8b) were proposed under optimal pH conditions for the adsorption, where the adsorbent material surface is protonated due to the acidic pH of the medium (consistent with the results from the point of zero charge, pH < pHpzc), favoring electrostatic interactions and possibly hydrogen bonding between the dyes and the adsorbents. However, van der Waals forces may also play a role in adsorption for dye adsorption involving the interaction between organic chains of the adsorbent and the dyes [58].
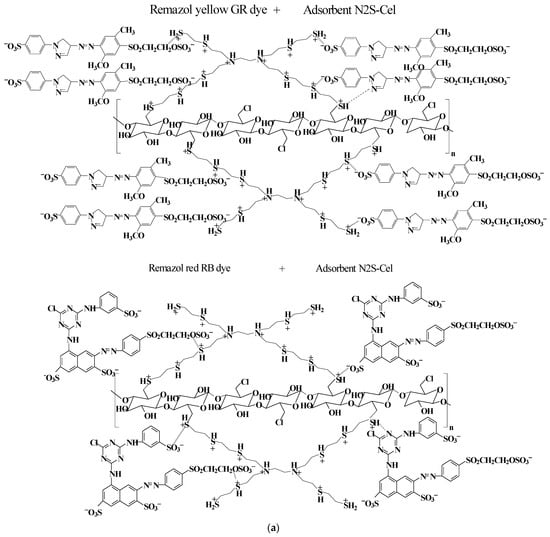
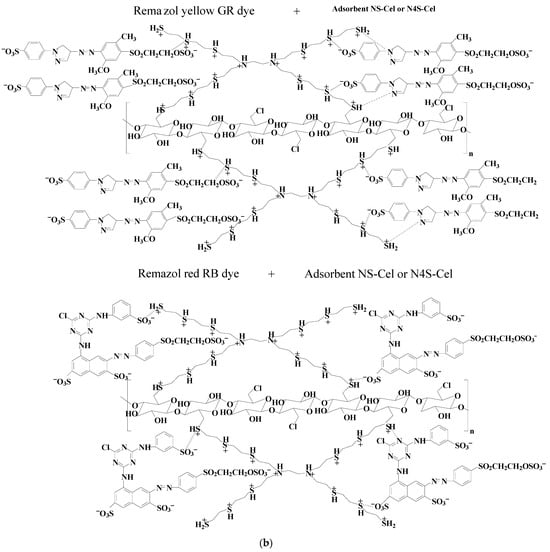
Figure 8.
Scheme of interaction between anionic dye with N2S-Cel (a) or NS-Cel and N4S-Cel (b).
It is essential to highlight that there was a reduction in the dye adsorption capacity on the adsorbent matrices up to pH 8.0, as observed in Figure 8, because an increase in pH decreases the protonation of the matrix surface due to the low concentration of H+, thereby reducing electrostatic attractions between the matrix and the dye. Additionally, competition between the dye and OH− ions for the adsorbent matrix surface is also possible. On the other hand, at pH 9.0, dye adsorption was relatively high, which can be attributed to the transformation of the sulfatethylsulfonic group, -SO2CH2CH2OSO3Na, of the dye into a vinylsulfonic group -SO2CH=CH2. Once the vinylsulfonic group is formed, it can interact covalently with the surface of the modified cellulose, thereby favoring dye adsorption, as reported in similar studies [19,31].
Considering the difference in sizes between the dye structures, the molecules presented distinct preferential sites for interaction with the material, where the larger volume of the Remazol red RB dye structure can hinder its penetration beyond the surface of the branched structure of the adsorbent, unlike the Remazol yellow GR dye structure, which possibly can enter the cavities of the branched structure more quickly, due to the smaller size of the dye molecule, as shown in Figure 8.
Figure 9 shows the adsorption isotherms obtained at pH 2.0 at temperatures of 298 K, 308 K, and 318 K.
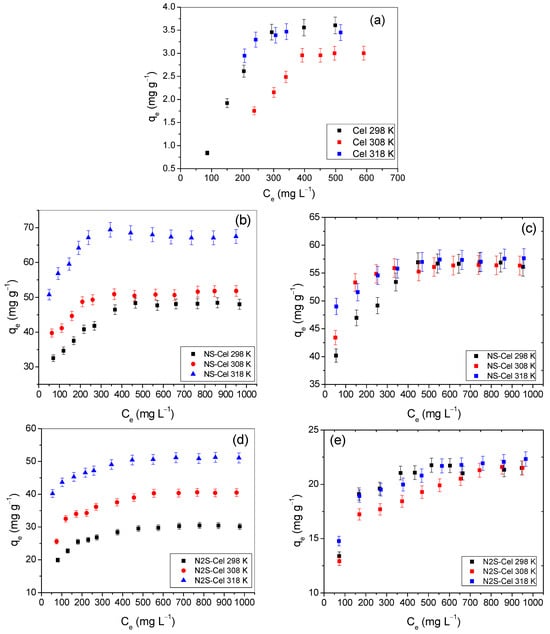
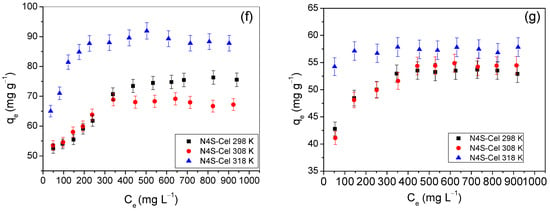
Figure 9.
Adsorption isotherms of Remazol yellow GR on (a) Cel, (b) NS-Cel, (d) N2S-Cel, and (f) N4S-Cel or Remazol red RB on (c) NS-Cel, (e) N2S-Cel, and (g) N4S-Cel at 298 K (-■-), 308 K (-●-), and 318 K (-▲-).
The adsorption of Remazol yellow GR dye on the pristine cellulose showed no significant influence of temperature. Although the curve for 308 K was slightly lower than the others, the values for the maximum adsorption capacities in each isotherm were close to the margin of error of the others (3.60 ± 0.18, 3.45 ± 0.17, and 3.00 ± 0.15 mg g−1 for the isotherms at 298 K, 308 K, and 318 K, respectively, Figure 9a). A similar result was reported by Silva et al. 2015 [46], which showed the adsorption of Remazol red RB dye using raw cellulose as the adsorbent.
When analyzing the adsorption isotherms of Remazol yellow GR dye using NS-Cel and N4S-Cel materials as adsorbents (Figure 9b,f), it is evident that temperature only influenced the adsorption process at 318 K. At 298 K and 308 K, the isotherms showed maximum adsorption capacities within the margin of error of each other. For N2S-Cel (Figure 9d), the isotherms indicated an increase in adsorption capacity with rising temperature, as the error margins did not overlap. In contrast, all isotherms related to the adsorption of Remazol red RB dye (Figure 9c,e,g) were temperature-independent in the dye removal process.
The material modified with a molar ratio of ethylenediamine to ethylene sulfide of 1:4 (N4S-Cel) showed the best results for both dyes, as the adsorption capacities for N4S-Cel, NS-Cel, and N2S-Cel in the removal of Remazol yellow GR dye were 87.70 ± 2.63, 67.10 ± 2.01, and 50.44 ± 1.51 mg g−1, respectively, at pH 2. The respective adsorption capacities for Remazol red RB dye were 57.84 ± 1.73, 57.64 ± 1.73, and 21.92 ± 0.66 mg g−1, respectively.
According to Table 3, the maximum adsorption capacity of the best-hyperbranched cellulose in the present study was lower compared to carboxymethyl cellulose in sodium alginate microgel [59] and cellulose modified with polyethyleneimine [60]. The value was approximately equal to those observed for activated carbon derived from coconut shells [61] and biochar from rice husks [62]. However, the materials presented a higher adsorption capacity compared to cationic cellulose [63] and composite nanofiber membranes containing cuprammonium cellulose [64].

Table 3.
Comparison of adsorption capacity of dyes in other modified cellulose and activated carbon.
In addition, the materials achieved a higher adsorption capacity for Remazol yellow GR dye, which may be related to the size of the dye molecules (Figure 10) since the larger volume of the Remazol red RB dye molecule compared to Remazol yellow GR dye could make it difficult to penetrate the spaces between the branches of the adsorbent materials.
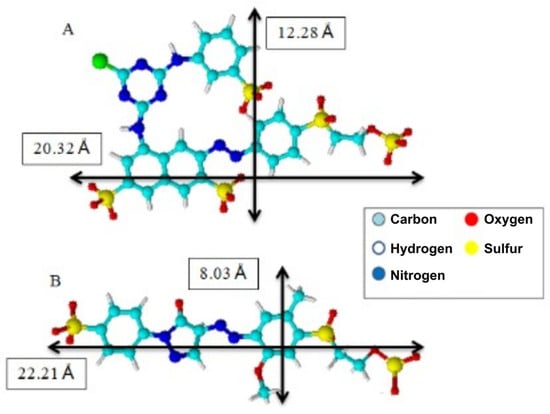
Figure 10.
A schematic illustration of the molecular structures of the dyes Remazol red RB (A) and Remazol yellow GR (B) and their dimensions obtained using Chem Sketch software 12.0.
The experimental results obtained (Figure 9) were fitted with the Langmuir [41], Freundlich [42], and Temkin [43] models using the linearized equations of each model and are presented in Table 4 and Table 5. The results showed that the correlation coefficients calculated by the Langmuir equation were higher (R2 ≥ 0.997) than those obtained by the other two models when considering the data obtained using the modified materials as adsorbents for the dyes. Additionally, the values of RL calculated from Equation (S4) showed that adsorption was favorable (0 < RL < 1). Therefore, the Langmuir model, which assumes monolayer adsorption, is the most suitable for describing the adsorption behavior of dyes by hyperbranched biopolymers [21,65].

Table 4.
Equilibrium isotherm model parameters for the adsorption of Remazol yellow GR on N2S-Cel or N4S-Cel.

Table 5.
Equilibrium isotherm model parameters for the adsorption of Remazol red RB on N2S-Cel or N4S-Cel.
In contrast, when analyzing the data using pristine cellulose as the adsorbent for Remazol yellow GR dye, the experimental data were best fitted to the Temkin model at temperatures of 298 K and 308 K. However, at 318 K, the data fit the Langmuir model. The Temkin model is a modification proposed for the Langmuir model, providing a closer approximation to the adsorption process behavior but calculating the involved parameters differently.
4. Conclusions
Synthesis of the hyperbranched adsorbent matrices for removing anionic dyes in aqueous media was confirmed by the characterizations. Elemental analyses revealed a ratio of 10 ethylene sulfides to 1 ethylenediamine in N2S-Cel and 12 ethylene sulfides to 1 ethylenediamine in NS-Cel and N4S-Cel. FT-IR showed a strong absorption band around 2900 cm⁻¹, indicating a high quantity of CH2 groups, corroborated by 13C NMR results. The new structures exhibited distinct crystallinity profiles due to intermolecular interactions among functional groups. These materials showed promising results in dye removal, particularly N4S-Cel, which had an adsorption capacity of 87.70 ± 2.63 mg g⁻¹ for Remazol yellow GR, significantly higher than the raw material. The adsorption processes were well described by the pseudo-second-order kinetics and Langmuir models. The adsorption sites were attributed to the presence of numerous nitrogen and sulfur functional groups in the hyperbranched cellulose biopolymer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polysaccharides5030025/s1.
Author Contributions
Conceptualization, L.C.B.L., L.d.S.S. and F.J.L.F.; methodology, L.C.B.L., F.J.L.F. and F.d.C.S.; software, L.C.B.L. and L.d.S.S.; validation, L.C.B.L., L.d.S.S., M.G.d.F., J.A.O. and E.C.d.S.F.; formal analysis, L.C.B.L. and F.J.L.F.; writing—original draft preparation, L.C.B.L., L.d.S.S. and E.C.d.S.F.; writing—review and editing, L.C.B.L., L.d.S.S. and E.C.d.S.F.; supervision, J.A.O. and E.C.d.S.F.; project administration, E.C.d.S.F.; funding acquisition, E.C.d.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Federal University of Piauí (UFPI) and the Graduate Program in Chemistry (PPGQ/CCN/UFPI).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Acknowledgments
The authors thank CAPES, CNPq, and FAPEPI for financial support and the UFPI and IFMA for providing work development conditions.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of the data, in the writing of the manuscript, or in the decision to publish the results.
References
- Rahul; Jindal, R. Efficient Removal of Toxic Dyes Malachite Green and Fuchsin Acid from Aqueous Solutions Using Pullulan/CMC Hydrogel. Polymer 2024, 307, 127203. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Lima, E.C.; Pinto, I.S.; Amavisca, C.V.; Royer, B.; Pinto, R.B.; Alencar, W.S.; Pereira, S.F.P. Application of Cupuassu Shell as Biosorbent for the Removal of Textile Dyes from Aqueous Solution. J. Environ. Manag. 2011, 92, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.A.R.; Sousa, K.S.; Cavalcanti, G.R.S.; França, D.B.; Queiroga, L.N.F.; Santos, I.M.G.; Fonseca, M.G.; Jaber, M. Green Biosorbents Based on Chitosan-Montmorillonite Beads for Anionic Dye Removal. J. Environ. Chem. Eng. 2017, 5, 3309–3318. [Google Scholar] [CrossRef]
- Fabryanty, R.; Valencia, C.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Kurniawan, A.; Ju, Y.H.; Ismadji, S. Removal of Crystal Violet Dye by Adsorption Using Bentonite—Alginate Composite. J. Environ. Chem. Eng. 2017, 5, 5677–5687. [Google Scholar] [CrossRef]
- Cai, W.; Wang, M.; Yang, G.Q.; Li, J. High-Performance Nanofiltration Membranes with a Polyamide-Polyester Composite Layer and a Polydopamine Surface Layer for Desalination and Dye Pollutant Removal. Polymer 2023, 268, 125720. [Google Scholar] [CrossRef]
- Silva, L.d.S.; Carvalho, J.D.O.; Bezerra, R.D.D.S.; Silva, M.S.D.; Ferreira, F.J.L.; Osajima, J.A.; Da Silva Filho, E.C. Potential of Cellulose Functionalized with Carboxylic Acid as Biosorbent for the Removal of Cationic Dyes in Aqueous Solution. Molecules 2018, 23, 743. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Wang, A. Adsorption of Dyes onto Palygorskite and Its Composites: A Review. J. Environ. Chem. Eng. 2016, 4, 1274–1294. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, N. Preparation of Polyamine Loofah Cellulose and Its Adsorption for Reactive Brilliant Red K–2BP. Polymer 2024, 292, 126630. [Google Scholar] [CrossRef]
- Xu, R.; Mao, J.; Peng, N.; Luo, X.; Chang, C. Chitin/Clay Microspheres with Hierarchical Architecture for Highly Efficient Removal of Organic Dyes. Carbohydr. Polym. 2018, 188, 143–150. [Google Scholar] [CrossRef]
- Silva, L.S.; Ferreira, F.J.L.; Silva, M.S.; Citó, A.M.G.L.; Meneguin, A.B.; Sábio, R.M.; Barud, H.S.; Bezerra, R.D.S.; Osajima, J.A.; Silva Filho, E.C. Potential of Amino-Functionalized Cellulose as an Alternative Sorbent Intended to Remove Anionic Dyes from Aqueous Solutions. Int. J. Biol. Macromol. 2018, 116, 1282–1295. [Google Scholar] [CrossRef]
- Jiang, F.; Dinh, D.M.; Hsieh, Y. Lo Adsorption and Desorption of Cationic Malachite Green Dye on Cellulose Nanofibril Aerogels. Carbohydr. Polym. 2017, 173, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Wu, X.; Shi, K.; Zhao, Y.; Huang, J.; Zhou, W.; Cai, M.; Guo, L. Surface Plasma Modification of Cellulose Acetate Fiber Filter for the Adsorption of Typical Components in Smoke Components. RSC Adv. 2024, 14, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, R.D.S.; Morais, A.I.S.; Osajima, J.A.; Nunes, L.C.C.; Silva Filho, E.C. Development of New Phosphated Cellulose for Application as an Efficient Biomaterial for the Incorporation/Release of Amitriptyline. Int. J. Biol. Macromol. 2016, 86, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.Q.; Strømme, M.; Lindh, J. Preparation of Porous 2,3-Dialdehyde Cellulose Beads Crosslinked with Chitosan and Their Application in Adsorption of Congo Red Dye. Carbohydr. Polym. 2018, 181, 200–207. [Google Scholar] [CrossRef] [PubMed]
- De Castro Silva, F.; Da Silva, M.M.F.; Lima, L.C.B.; Osajima, J.A.; Da Silva Filho, E.C. Integrating Chloroethyl Phosphate with Biopolymer Cellulose and Assessing Their Potential for Absorbing Brilliant Green Dye. J. Environ. Chem. Eng. 2016, 4, 3348–3356. [Google Scholar] [CrossRef]
- Xie, K.; Zhao, W.; He, X. Adsorption Properties of Nano-Cellulose Hybrid Containing Polyhedral Oligomeric Silsesquioxane and Removal of Reactive Dyes from Aqueous Solution. Carbohydr. Polym. 2011, 83, 1516–1520. [Google Scholar] [CrossRef]
- Musyoka, S.M.; Ngila, J.C.; Moodley, B.; Petrik, L.; Kindness, A. Synthesis, Characterization, and Adsorption Kinetic Studies of Ethylenediamine Modified Cellulose for Removal of Cd and Pb. Anal. Lett. 2011, 44, 1925–1936. [Google Scholar] [CrossRef]
- Silva Filho, E.C.; Lima, L.C.B.; Silva, F.C.; Sousa, K.S.; Fonseca, M.G.; Santana, S.A.A. Immobilization of Ethylene Sulfide in Aminated Cellulose for Removal of the Divalent Cations. Carbohydr. Polym. 2013, 92, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.S.; Silva, M.S.; Ferreira, F.J.L.; Lima, L.C.B.; Bezerra, R.D.S.; Citó, A.M.G.L.; Osajima, J.A.; Silva Filho, E.C. Effective Removal of the Remazol Yellow GR Dye Using Cellulose Functionalized by Basic Groups. Water Air Soil. Pollut. 2018, 229, 1–16. [Google Scholar] [CrossRef]
- Schüll, C.; Frey, H. Grafting of Hyperbranched Polymers: From Unusual Complex Polymer Topologies to Multivalent Surface Functionalization. Polymer 2013, 54, 5443–5455. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, L.; Liao, Q.; Chen, X.; Qian, Z.; Shen, J.; Liang, J.; Yao, J. Functionalization of Cellulose with Hyperbranched Polyethylenimine for Selective Dye Adsorption and Separation. Cellulose 2016, 23, 3785–3797. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Kim, K.H. Hyperbranched Polymers as Superior Adsorbent for the Treatment of Dyes in Water. Adv. Colloid. Interface Sci. 2022, 302, 102633. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, V.; Jouyandeh, M.; Akhi, H.; Mousavi Khadem, S.S.; Ganjali, M.R.; Moradi, H.; Mirsadeghi, S.; Badiei, A.; Esmaeili, A.; Rabiee, N.; et al. Hyperbranched Polyethylenimine Functionalized Silica/Polysulfone Nanocomposite Membranes for Water Purification. Chemosphere 2022, 290, 133363. [Google Scholar] [CrossRef]
- Voit, B.I. Hyperbranched Polymers: A Chance and a Challenge. Comptes Rendus Chimie 2003, 6, 821–832. [Google Scholar] [CrossRef]
- Barakat, M.A.; Ramadan, M.H.; Alghamdi, M.A.; Algarny, S.S.; Woodcock, H.L.; Kuhn, J.N. Remediation of Cu(II), Ni(II), and Cr(III) Ions from Simulated Wastewater by Dendrimer/Titania Composites. J. Environ. Manag. 2013, 117, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, Y.; Wu, M.; Zhang, L.; Wang, L.; Ni, H. Surface Functionalization of Cellulose with Hyperbranched Polyamide for Efficient Adsorption of Organic Dyes and Heavy Metals. J. Clean. Prod. 2019, 232, 774–783. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Luo, Z.; Shen, J.; Ni, Q.; Yao, J. Facile Preparation of a Cellulose-Based Bioadsorbent Modified by HPEI in Heterogeneous System for High-Efficiency Removal of Multiple Types of Dyes. React. Funct. Polym. 2018, 125, 77–83. [Google Scholar] [CrossRef]
- Qin, W.H.; Li, M.X.; Zhang, Y.B.; Li, W.; Jia, R.; Xiong, Y.S.; Lu, H.Q.; Zhang, S.Y. High Capacity and Selective Adsorption of Congo Red by Cellulose-Based Aerogel with Mesoporous Structure: Adsorption Properties and Statistical Data Simulation. Int. J. Biol. Macromol. 2024, 259, 129137. [Google Scholar] [CrossRef] [PubMed]
- Ee, L.Y.; Chia, S.Y.R.; Xue, K.; Chin, S.Y.; Cho, C.A.H.; Tan, X.Y.; Li, S.F.Y. Hyperbranched Nanocellulose Enabling Rapid Boron Removal from Aqueous Environment. Chem. Eng. J. 2023, 454, 140218. [Google Scholar] [CrossRef]
- Mahdavi, H.; Shahalizade, T. Preparation, Characterization and Performance Study of Cellulose Acetate Membranes Modified by Aliphatic Hyperbranched Polyester. J. Memb. Sci. 2015, 473, 256–266. [Google Scholar] [CrossRef]
- Silva, L.S.; Lima, L.C.B.; Silva, F.C.; Matos, J.M.E.; Santos, M.R.M.C.; Santos Júnior, L.S.; Sousa, K.S.; da Silva Filho, E.C. Dye Anionic Sorption in Aqueous Solution onto a Cellulose Surface Chemically Modified with Aminoethanethiol. Chem. Eng. J. 2013, 218, 89–98. [Google Scholar] [CrossRef]
- Vieira, A.P.; Santana, S.A.A.; Bezerra, C.W.B.; Silva, H.A.S.; de Melo, J.C.P.; Filho, E.C.d.S.; Airoldi, C. Copper Sorption from Aqueous Solutions and Sugar Cane Spirits by Chemically Modified Babassu Coconut (Orbignya Speciosa) Mesocarp. Chem. Eng. J. 2010, 161, 99–105. [Google Scholar] [CrossRef]
- Teixeira, T.P.F.; Pereira, S.I.; Aquino, S.F.; Dias, A. Calcined Layered Double Hydroxides for Decolorization of Azo Dye Solutions: Equilibrium, Kinetics, and Recycling Studies. Environ. Eng. Sci. 2012, 29, 685–692. [Google Scholar] [CrossRef]
- Rajabi, M.; Bagheri-Roochi, M.; Asghari, A. Effect of Electrolyte Nature on Kinetics of Remazol Yellow g Removal by Electrocoagulation. Russ. J. Phys. Chem. A 2011, 85, 1820–1824. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Lazaridis, N.K.; Kostoglou, M. On the Simultaneous Adsorption of a Reactive Dye and Hexavalent Chromium from Aqueous Solutions onto Grafted Chitosan. J. Colloid. Interface Sci. 2013, 407, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.O.; de Lourdes Nascimento Santos, M.; Costa, J.A.S.; de Jesus, R.A.; Navickiene, S.; Sussuchi, E.M.; de Mesquita, M.E. Investigating the Potential of Functionalized MCM-41 on Adsorption of Remazol Red Dye. Environ. Sci. Pollut. Res. 2013, 20, 5028–5035. [Google Scholar] [CrossRef] [PubMed]
- Indhu, S.; Muthukumaran, K. Removal and Recovery of Reactive Yellow 84 Dye from Wastewater and Regeneration of Functionalised Borassus Flabellifer Activated Carbon. J. Environ. Chem. Eng. 2018, 6, 3111–3121. [Google Scholar] [CrossRef]
- Gong, R.; Ding, Y.; Li, M.; Yang, C.; Liu, H.; Sun, Y. Utilization of Powdered Peanut Hull as Biosorbent for Removal of Anionic Dyes from Aqueous Solution. Dye. Pigment. 2005, 64, 187–192. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Kinetic Models for the Sorption of Dye from Aqueous Solution by Wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. J. Franklin Inst. 1917, 183, 102–105. [Google Scholar] [CrossRef]
- Herbert Freundlich, V. Über Die Adsorption in Lösungen. Zeitschrift Für Physikalische Chemie 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Temkin, M.J.; Pyzhev, V. Kinetics of Ammonia Synthesis on Promoted Iron Catalysts. Acta Physicochim. U.R.S.S 1940, 12, 217–222. [Google Scholar]
- Satoh, T.; Kinugawa, Y.; Tamaki, M.; Kitajyo, Y.; Sakai, R.; Kakuchi, T. Synthesis, Structure, and Characteristics of Hyperbranched Polyterpene Alcohols. Macromolecules 2008, 41, 5265–5271. [Google Scholar] [CrossRef]
- Sun, M.; Li, J.; Li, B.; Fu, Y.; Bo, Z. Toward High Molecular Weight Triphenylamine-Based Hyperbranched Polymers. Macromolecules 2005, 38, 2651–2658. [Google Scholar] [CrossRef]
- Silva, L.S.; Lima, L.C.B.; Ferreira, F.J.L.; Silva, M.S.; Osajima, J.A.; Bezerra, R.D.S.; Silva Filho, E.C. Sorption of the Anionic Reactive Red RB Dye in Cellulose: Assessment of Kinetic, Thermodynamic, and Equilibrium Data. Open Chem. 2015, 13, 801–812. [Google Scholar] [CrossRef]
- Sadare, O.O.; Nkosi, N.A.; Moothi, K. Preparation and Characterization of Hexadecyl Trimethyl Ammonium Bromide (HDTMA-Br)-Modified Cellulose Nanocrystals (CNCs) Derived from South African Waste Agricultural Residue (Corncobs). Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, S.; Fan, K.; He, H.; Qin, Z. Preparation and Adsorption Properties of Green Cellulose-Based Composite Aerogel with Selective Adsorption of Methylene Blue. Polymer 2022, 258, 125320. [Google Scholar] [CrossRef]
- Da Silva Filho, E.C.; Santana, S.A.A.; Melo, J.C.P.; Oliveira, F.J.V.E.; Airoldi, C. X-Ray Diffraction and Thermogravimetry Data of Cellulose, Chlorodeoxycellulose and Aminodeoxycellulose. J. Therm. Anal. Calorim. 2010, 100, 315–321. [Google Scholar] [CrossRef]
- da Silva Filho, E.C.; da Silva, L.S.; Lima, L.C.B.; de Santos, L.S.; de Santos, M.R.M.C.; de Matos, J.M.E.; Airoldi, C. Thermodynamic Data of 6-(4′-Aminobutylamino)-6-Deoxycellulose Sorbent for Cation Removal from Aqueous Solutions. Sep. Sci. Technol. 2011, 46, 2566–2574. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Al-Bogami, A.S. Influence of Mo(VI) Immobilization and Temperature on As(V) Sorption onto Magnetic Separable Poly p-Phenylenediamine-Thiourea-Formaldehyde Polymer. J. Hazard. Mater. 2018, 342, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Košak, A.; Bauman, M.; Padežnik-Gomilšek, J.; Lobnik, A. Lead (II) Complexation with 3-Mercaptopropyl-Groups in the Surface Layer of Silica Nanoparticles: Sorption, Kinetics and EXAFS/XANES Study. J. Mol. Liq. 2017, 229, 371–379. [Google Scholar] [CrossRef]
- Subbaiah, M.V.; Kim, D.S. Adsorption of Methyl Orange from Aqueous Solution by Aminated Pumpkin Seed Powder: Kinetics, Isotherms, and Thermodynamic Studies. Ecotoxicol. Environ. Saf. 2016, 128, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, J.D.; Berillo, D.; Purkis, J.M.; Byrne, M.L.; Tribolet, A.D.C.C.M.; Warwick, P.E.; Cundy, A.B. Effective 137Cs+ and 90Sr2+ Immobilisation from Groundwater by Inorganic Polymer Resin Clevasol® Embedded within a Macroporous Cryogel Host Matrix. Mater. Today Sustain. 2022, 19, 100190. [Google Scholar] [CrossRef]
- Akköz, Y.; Coşkun, R. Cellulose-Supported Bioadsorbent from Natural Hemp Fiber for Removal of Anionic Dyes from Aqueous Solution. Int. J. Biol. Macromol. 2023, 252, 126447. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.; Panda, P.K.; Lin, T.H.; Wu, M.C.; Yang, P.C. Graphitic Carbon Nitride Nanosheet and Ferroelectric PbTiO3 Nanoplates S-Scheme Heterostructure for Enhancing Hydrogen Production and Textile Dye Degradation. Chem. Eng. Sci. 2024, 295, 120133. [Google Scholar] [CrossRef]
- Leal, A.N.R.; De Lima, A.d.C.A.; Azevedo, M.G.F.d.A.; Santos, D.K.D.d.N.; Zaidan, L.E.M.C.; De Lima, V.F.; Cruz Filho, I.J. Removal of Remazol Black B Dye Using Bacterial Cellulose as an Adsorbent. Scientia Plena 2021, 17, 034201. [Google Scholar] [CrossRef]
- Huang, X.Y.; Mao, X.Y.; Bu, H.T.; Yu, X.Y.; Jiang, G.B.; Zeng, M.H. Chemical Modification of Chitosan by Tetraethylenepentamine and Adsorption Study for Anionic Dye Removal. Carbohydr. Res. 2011, 346, 1232–1240. [Google Scholar] [CrossRef]
- Meas, A.; Wi, E.; Chang, M.; Hwang, H.S. Carboxylmethyl Cellulose Produced from Wood Sawdust for Improving Properties of Sodium Alginate Hydrogel in Dye Adsorption. Sep. Purif. Technol. 2024, 341, 126906. [Google Scholar] [CrossRef]
- Jabli, M.; Sebeia, N.; El-Ghoul, Y.; Soury, R.; Al-Ghamdi, Y.O.; Saleh, T.A. Chemical Modification of Microcrystalline Cellulose with Polyethyleneimine and Hydrazine: Characterization and Evaluation of Its Adsorption Power toward Anionic Dyes. Int. J. Biol. Macromol. 2023, 229, 210–223. [Google Scholar] [CrossRef]
- Furlan, F.R.; de Melo da Silva, L.G.; Morgado, A.F.; de Souza, A.A.U.; Guelli Ulson de Souza, S.M.A. Removal of Reactive Dyes from Aqueous Solutions Using Combined Coagulation/Flocculation and Adsorption on Activated Carbon. Resour. Conserv. Recycl. 2010, 54, 283–290. [Google Scholar] [CrossRef]
- Homagai, P.L.; Poudel, R.; Poudel, S.; Bhattarai, A. Adsorption and Removal of Crystal Violet Dye from Aqueous Solution by Modified Rice Husk. Heliyon 2022, 8, e09261. [Google Scholar] [CrossRef] [PubMed]
- Mhlongo, J.T.; Dlamini, M.L.; Nuapia, Y.; Etale, A. Synthesis and Application of Cationized Cellulose for Adsorption of Anionic Dyes. Mater. Today Proc. 2022, 62, S133–S140. [Google Scholar] [CrossRef]
- Iqbal, D.; Ullah, R.; Zhao, R.; Dou, Y.; Yan, D.; Ning, X. Dye Adsorption and Antimicrobial Performances of Composite Nanofiber Membranes Containing Cuprammonium Cellulose. Sep. Purif. Technol. 2024, 339, 126677. [Google Scholar] [CrossRef]
- Jin, L.; Li, W.; Xu, Q.; Sun, Q. Amino-Functionalized Nanocrystalline Cellulose as an Adsorbent for Anionic Dyes. Cellulose 2015, 22, 2443–2456. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).