Effect of Amylopectin Content on Mechanical, Barrier and Thermal Properties of Plasticized Starch/Chitosan Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Films Preparation
2.3. Characterization
2.3.1. Thermal Analysis
2.3.2. Film Thickness
2.3.3. Water Vapor Permeability (WVP)
2.3.4. Solubility
2.3.5. Tensile Tests
2.3.6. Statistical Analysis
3. Results and Discussion
3.1. Thermal Analysis
3.2. Thickness and Solubility
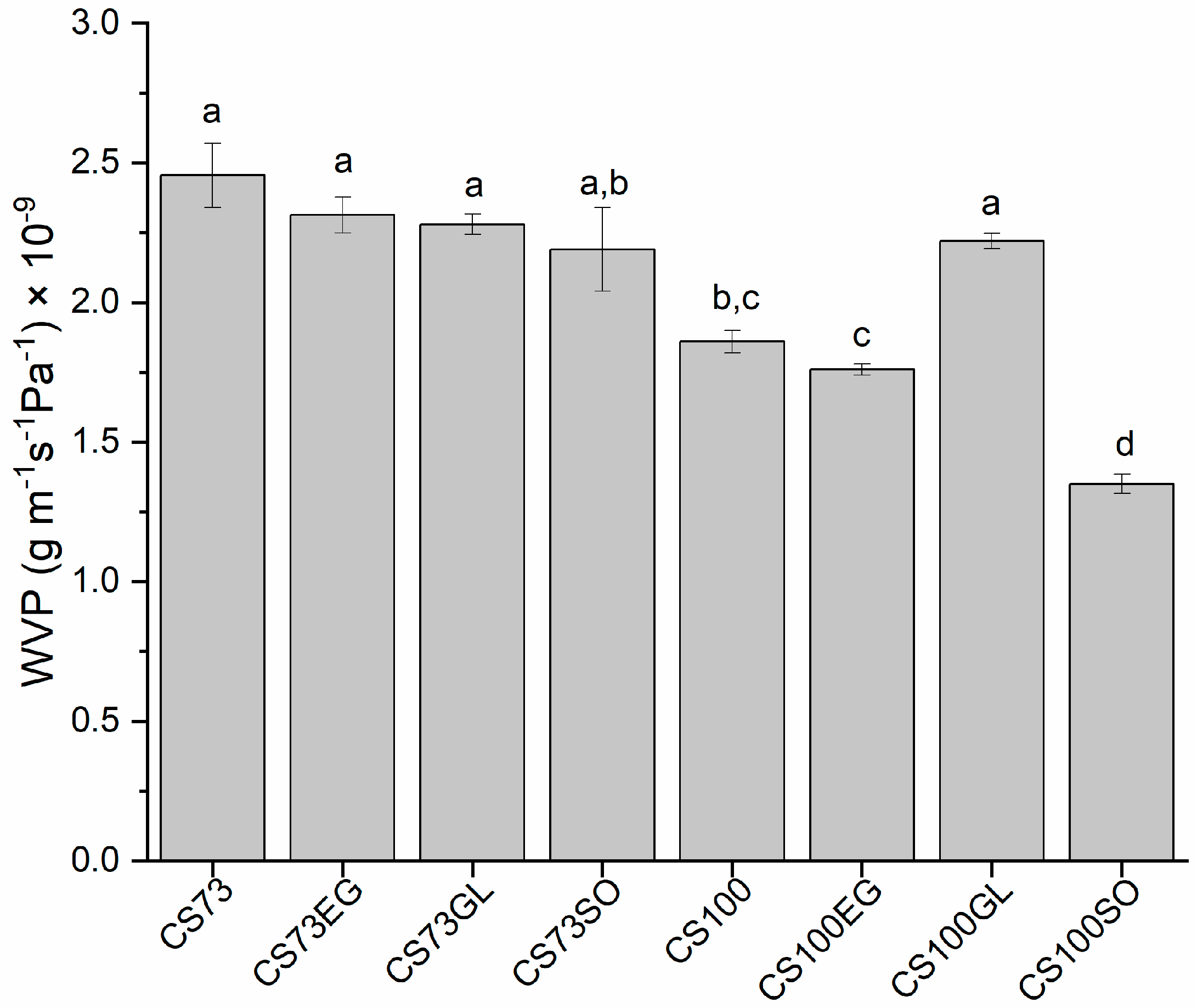
3.3. Water Vapor Permeability (WVP)
3.4. Tensile Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocol. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Aycan, D.; Dolapçı, N.; Karaca, Ö.G.; Alemdar, N. Polysaccharide-based electroconductive films for controlled release of ciprofloxacin. J. Appl. Polym. Sci. 2022, 139, e52761. [Google Scholar] [CrossRef]
- Saraiva, M.M.; Da Campelo, M.S.; Câmara Neto, J.F.; Lima, A.B.N.; de Almeida Silva, G.; de Freitas Figueredo Dias, A.T.; Silva Ricardo, N.M.P.; Kaplan, D.L.; Pinho Ribeiro, M.E.N. Alginate/polyvinyl alcohol films for wound healing: Advantages and challenges. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 220–233. [Google Scholar] [CrossRef]
- Lim, C.; Yusoff, S.; Ng, C.G.; Lim, P.E.; Ching, Y.C. Bioplastic made from seaweed polysaccharides with green production methods. J. Env. Environ. Chem. Eng. 2021, 9, 105895. [Google Scholar] [CrossRef]
- Rahmadi Putri, T.; Adhitasari, A.; Paramita, V.; Endy Yulianto, M.; Dwi Ariyanto, H. Effect of different starch on the characteristics of edible film as functional packaging in fresh meat or meat products: A review. Mater. Today Proc. 2023, in press. [CrossRef]
- Acosta, S.; Chiralt, A.; Santamarina, P.; Rosello, J.; González-Martínez, C.; Cháfer, M. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocol. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S. Nano-cellulose reinforced starch bio composite films- A review on green composites. Int. J. Biol. Macromol. 2021, 185, 849–860. [Google Scholar] [CrossRef]
- van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the Factors Affecting the Solubility of Chitosan in Water. Macromol. Chem. Phys. 2010, 211, 426–433. [Google Scholar] [CrossRef]
- Zou, Y.; Yuan, C.; Cui, B.; Sha, H.; Liu, P.; Lu, L.; Wu, Z. High-Amylose Corn Starch/Konjac Glucomannan Composite Film: Reinforced by Incorporating β-Cyclodextrin. J. Agric. Food Chem. 2021, 69, 2493–2500. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, Y.; Wu, Y.; Li, Y. Characterization of edible starch-chitosan film and its application in the storage of Mongolian cheese. Int. J. Biol. Macromol. 2013, 57, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-Starch Films with Natural Extracts: Physical, Chemical, Morphological and Thermal Properties. Materials 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Tijerina-Ramos, B.I.; García-Hernández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; La Reyes-de Torre, A.I. Antimicrobial, Optical and Mechanical Properties of Chitosan-Starch Films with Natural Extracts. Int. J. Mol. Sci. 2017, 18, 997. [Google Scholar] [CrossRef] [PubMed]
- Tuhin, M.O.; Rahman, N.; Haque, M.E.; Khan, R.A.; Dafader, N.C.; Islam, R.; Nurnabi, M.; Tonny, W. Modification of mechanical and thermal property of chitosan–starch blend films. Radiat. Phys. Chem. 2012, 81, 1659–1668. [Google Scholar] [CrossRef]
- Sun, K.-Q.; Li, F.-Y.; Li, J.-Y.; Li, J.-F.; Zhang, C.-W.; Chen, S.; Sun, X.; Cui, J.-F. Optimisation of compatibility for improving elongation at break of chitosan/starch films. RSC Adv. 2019, 9, 24451–24459. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, S.; Rivadeneira, C.; Castro, M. Edible films based on starch and chitosan. Effect of starch source and concentration, plasticizer, surfactant’s hydrophobic tail and mechanical treatment. Food Hydrocoll. 2015, 49, 89–94. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Suzuki, C.K.; Cereda, M.P.; Scamparini, A. Microstructure and color of starch–gum films: Effect of gum deacetylation and additives. Part 2. Food Hydrocoll. 2005, 19, 1064–1073. [Google Scholar] [CrossRef]
- Horn, M.M.; Martins, V.C.A.; de Guzzi Plepis, A.M. Interaction of anionic collagen with chitosan: Effect on thermal and morphological characteristics. Carbohydr. Polym. 2009, 77, 239–243. [Google Scholar] [CrossRef]
- Garcia, M.A.; Pinotti, A.; Zaritzky, N.E. Physicochemical, Water Vapor Barrier and Mechanical Properties of Corn Starch and Chitosan Composite Films. Starch Stärke 2006, 58, 453–463. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Mathew, S.; Brahmakumar, M.; Abraham, T.E. Microstructural imaging and characterization of the mechanical, chemical, thermal, and swelling properties of starch-chitosan blend films. Biopolymers 2006, 82, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Rusman, R.; Khaldun, I.; Ardana, L.; Mudatsir, M.; Fansuri, H. Active edible sugar palm starch-chitosan films carrying extra virgin olive oil: Barrier, thermo-mechanical, antioxidant, and antimicrobial properties. Int. J. Biol. Macromol. 2020, 163, 766–775. [Google Scholar] [CrossRef]
- Donmez, D.; Pinho, L.; Patel, B.; Desam, P.; Campanella, O.H. Characterization of starch–water interactions and their effects on two key functional properties: Starch gelatinization and retrogradation. Curr. Opin. Food Sci. 2021, 39, 103–109. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Ye, R.; Xiao, J.; Liu, Y.; Ding, J.; Zhang, S.; Liu, A. Mechanical and barrier properties of maize starch-gelatin composite films: Effects of amylose content. J. Sci. Food Agric. 2017, 97, 3613–3622. [Google Scholar] [CrossRef]
- Al-Hassan, A.A.; Norziah, M.H. Starch–gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Dong, M.; Tian, L.; Li, J.; Jia, J.; Dong, Y.; Tu, Y.; Liu, X.; Tan, C.; Duan, X. Improving physicochemical properties of edible wheat gluten protein films with proteins, polysaccharides and organic acid. LWT 2022, 154, 112868. [Google Scholar] [CrossRef]
- Famá, L.; Rojo, P.G.; Bernal, C.; Goyanes, S. Biodegradable starch based nanocomposites with low water vapor permeability and high storage modulus. Carbohydr. Polym. 2012, 87, 1989–1993. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Limsiriwong, N.; Kongjindamunee, R.; Surakit, S. Effect of Agar and Cotton Fiber on Properties of Thermoplastic Waxy Rice Starch Composites. J. Polym. Env. Environ. 2012, 20, 88–95. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.; Biliaderis, C.G.; Ogawa, H.; Kawasaki, N. Biodegradable films made from low-density polyethylene (LDPE), rice starch and potato starch for food packaging applications: Part 1. Carbohydr. Polym. 1998, 36, 89–104. [Google Scholar] [CrossRef]
- Singh, G.P.; Bangar, S.P.; Yang, T.; Trif, M.; Kumar, V.; Kumar, D. Effect on the Properties of Edible Starch-Based Films by the Incorporation of Additives: A Review. Polymers 2022, 14, 1987. [Google Scholar] [CrossRef] [PubMed]
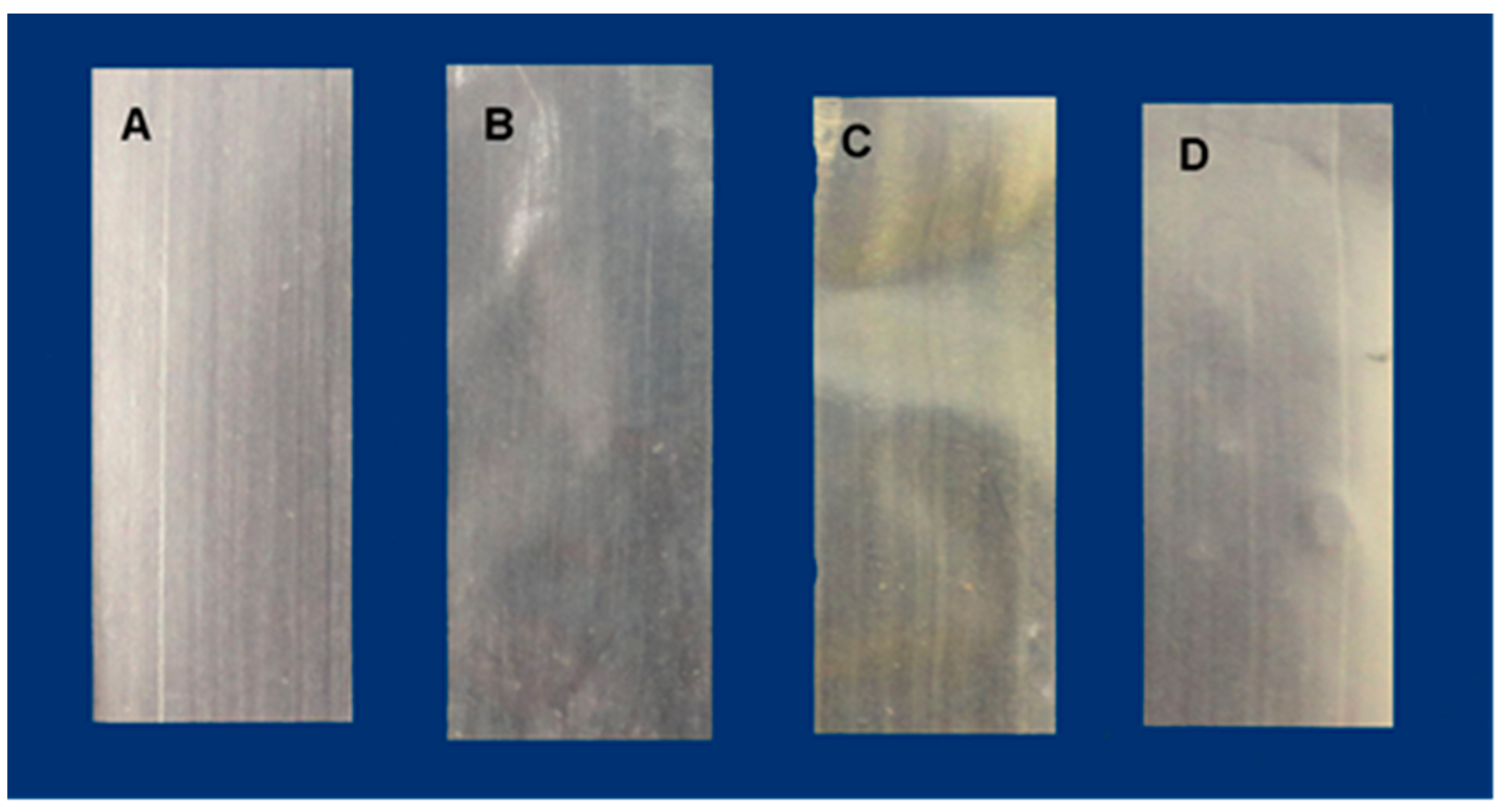
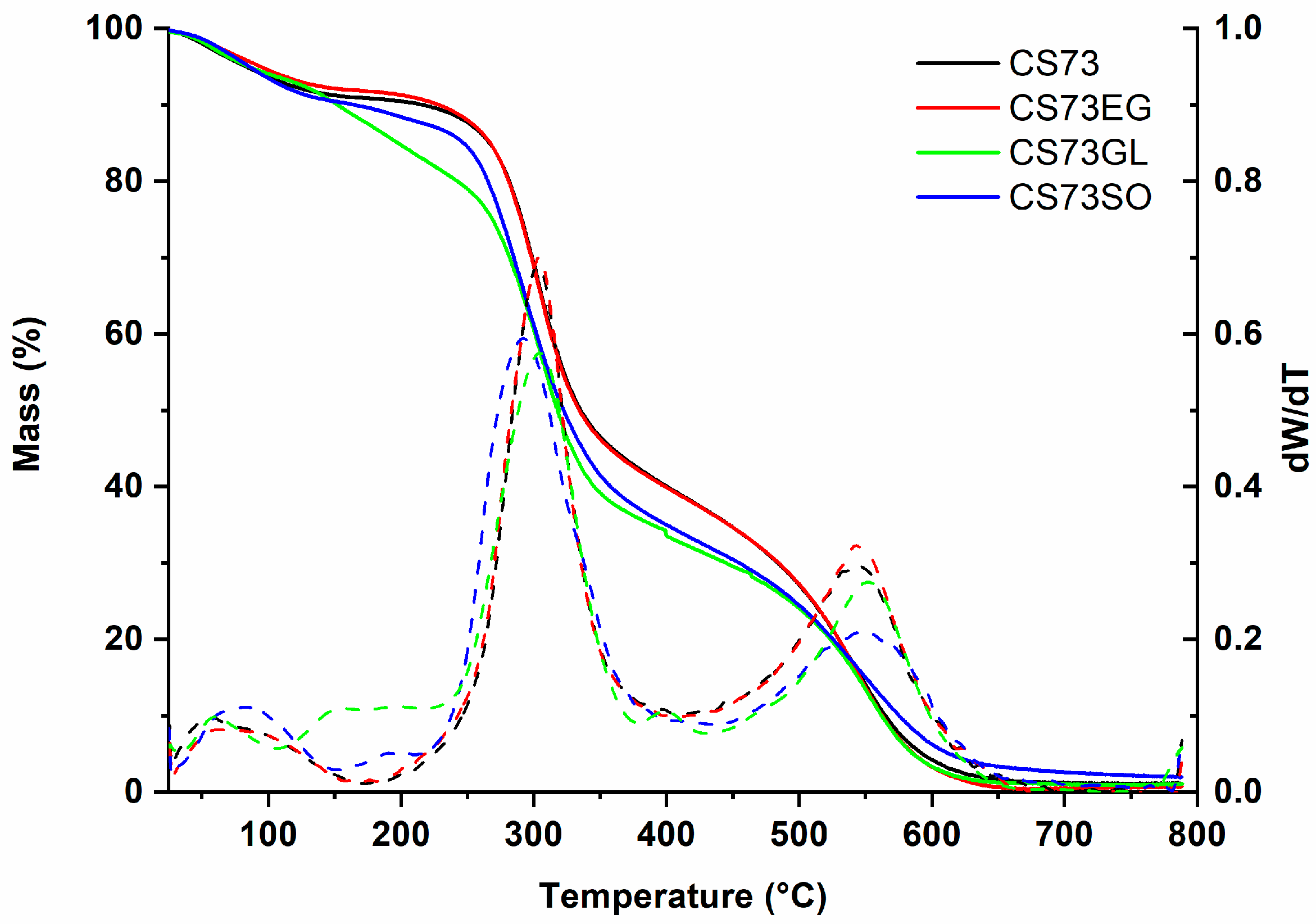
| Film | 1st Stage | 2nd Stage | 3rd Stage | Tonset (°C) |
|---|---|---|---|---|
| (25–200 °C) | (200–400 °C) | (400–750 °C) | ||
| CS73 | 9.2 | 50.4 | 38.9 | 270.4 |
| CS73EG | 8.4 | 51.4 | 39.3 | 264.8 |
| CS73GL | 5.1/11.8 | 50.8 | 32.3 | 269.7 |
| CS73SO | 11.4 | 54.4 | 33.8 | 258.4 |
| CS100 | 10.0 | 50.6 | 38.5 | 267.3 |
| CS100EG | 7.8 | 50.5 | 10.8 | 267.4 |
| CS100GL | 4.7/21.0 | 45.5 | 27.8 | 265.4 |
| CS100SO | 9.7 | 56.8 | 32.7 | 256.3 |
| Film | Temperature (°C) | ΔH (J g−1) |
|---|---|---|
| CS73 | 106.3 | 362.9 |
| CS73EG | 111.1 | 298.7 |
| CS73GL | 119.1 | 206.4 |
| CS73SO | 119.9 | 230.6 |
| CS100 | 91.6 | 334.8 |
| CS100EG | 97.1 | 268.2 |
| CS100GL | 125.3 | 322.9 |
| CS100SO | 116.3 | 316.4 |
| Film | Thickness (mm) | Solubility (%) |
|---|---|---|
| CS73 | 0.066 ± 0.006 a,b | 9.66 ± 0.43 e,f |
| CS73EG | 0.080 ± 0.006 a | 15.90 ± 0.63 d |
| CS73GL | 0.068 ± 0.003 a | 50.58 ± 0.93 a |
| CS73SO | 0.081 ± 0.005 a | 22.34 ± 0.79 c |
| CS100 | 0.042 ± 0.003 c | 7.32 ± 0.54 f |
| CS100EG | 0.045 ± 0.003 c | 12.61 ± 0.60 d,e |
| CS100GL | 0.050 ± 0.002 b,c | 32.50 ± 0.90 b |
| CS100SO | 0.050 ± 0.003 b,c | 34.59 ± 0.93 b |
| Film | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| CS73 | 57.10 ± 0.92 a | 3.04 ± 0.15 b,c | 20.94 ± 0.98 c |
| CS73EG | 60.03 ± 2.72 a | 4.70 ± 0.20 b,c | 26.89 ± 1.42 b |
| CS73GL | 22.78 ± 2.27 c | 7.39 ± 0.40 b | 6.70 ± 0.36 d |
| CS73SO | 32.04 ± 2.87 b | 6.87 ± 0.59 b | 6.41 ± 0.51 d |
| CS100 | 14.75 ± 0.32 d | 2.66 ± 0.11 b,c | 48.04 ± 0.64 a |
| CS100EG | 13.51 ± 1.08 d | 2.04 ± 0.39 c | 27.21 ± 1.23 b |
| CS100GL | 2.54 ± 0.16 e | 20.13 ± 0.29 a | 0.17 ± 0.01 e |
| CS100SO | 14.03 ± 0.72 d | 16.43 ± 1.76 a | 2.04 ± 0.07 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horn, M.M.; Martins, V.C.A.; Plepis, A.M.G. Effect of Amylopectin Content on Mechanical, Barrier and Thermal Properties of Plasticized Starch/Chitosan Films. Polysaccharides 2023, 4, 208-218. https://doi.org/10.3390/polysaccharides4030015
Horn MM, Martins VCA, Plepis AMG. Effect of Amylopectin Content on Mechanical, Barrier and Thermal Properties of Plasticized Starch/Chitosan Films. Polysaccharides. 2023; 4(3):208-218. https://doi.org/10.3390/polysaccharides4030015
Chicago/Turabian StyleHorn, Marilia M., Virginia C. A. Martins, and Ana M. G. Plepis. 2023. "Effect of Amylopectin Content on Mechanical, Barrier and Thermal Properties of Plasticized Starch/Chitosan Films" Polysaccharides 4, no. 3: 208-218. https://doi.org/10.3390/polysaccharides4030015
APA StyleHorn, M. M., Martins, V. C. A., & Plepis, A. M. G. (2023). Effect of Amylopectin Content on Mechanical, Barrier and Thermal Properties of Plasticized Starch/Chitosan Films. Polysaccharides, 4(3), 208-218. https://doi.org/10.3390/polysaccharides4030015






