Formulation and Physical Characterization of a Polysaccharidic Gel for the Vehiculation of an Insoluble Phytoextract for Mucosal Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Perilla Frutescens Phytocomplex Preparation
2.3. Samples Preparation
2.4. Rheological Analyses
2.5. Texture Analyses
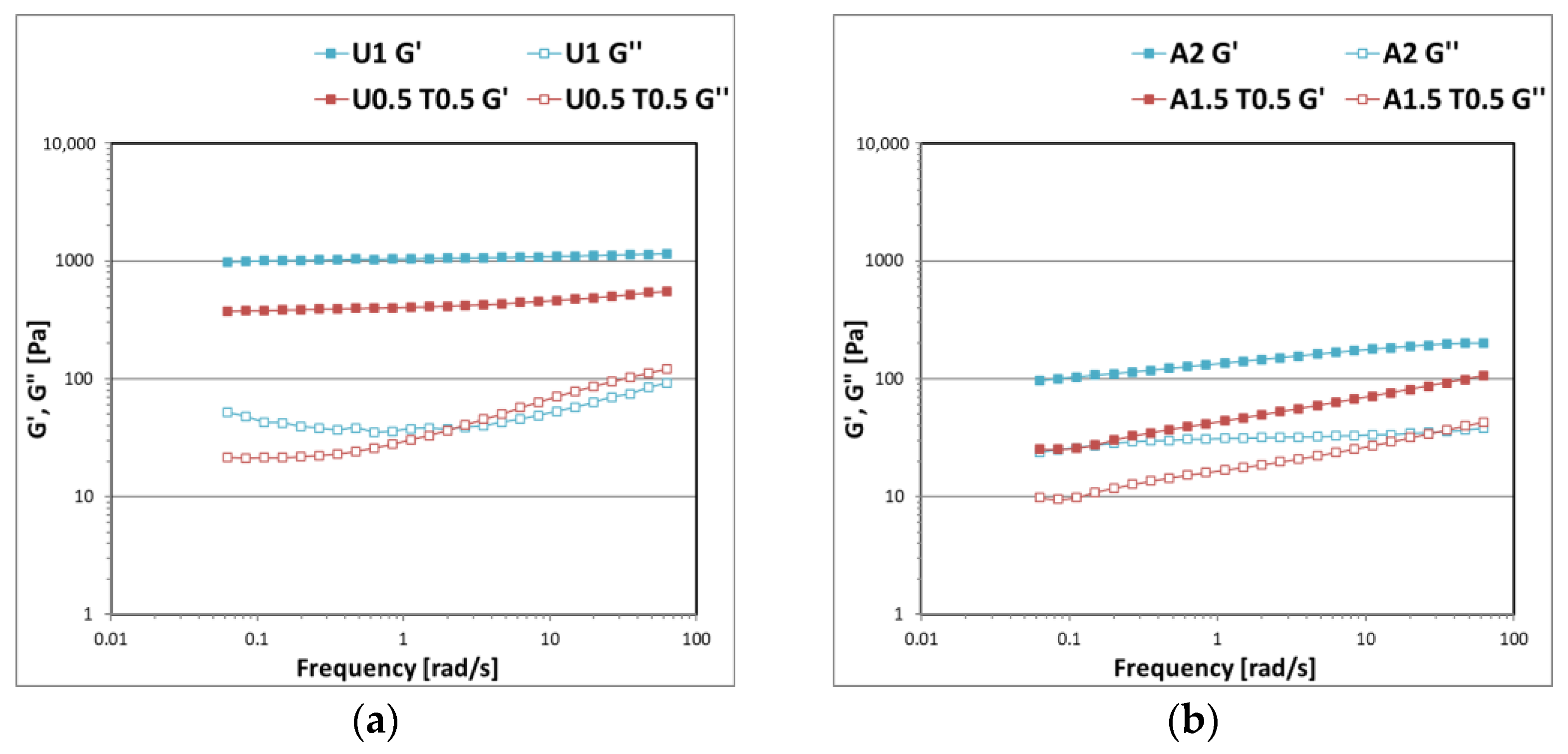
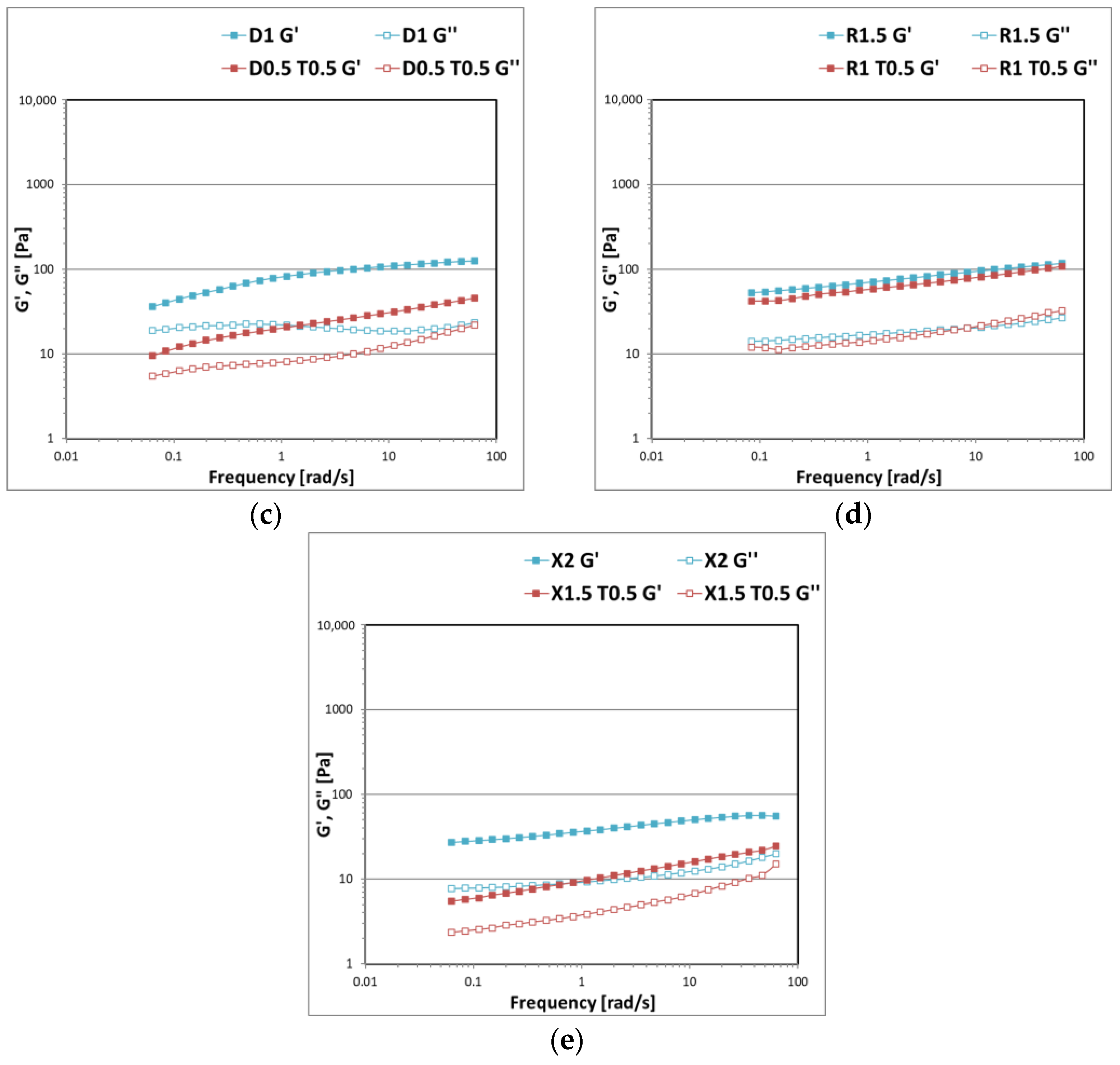
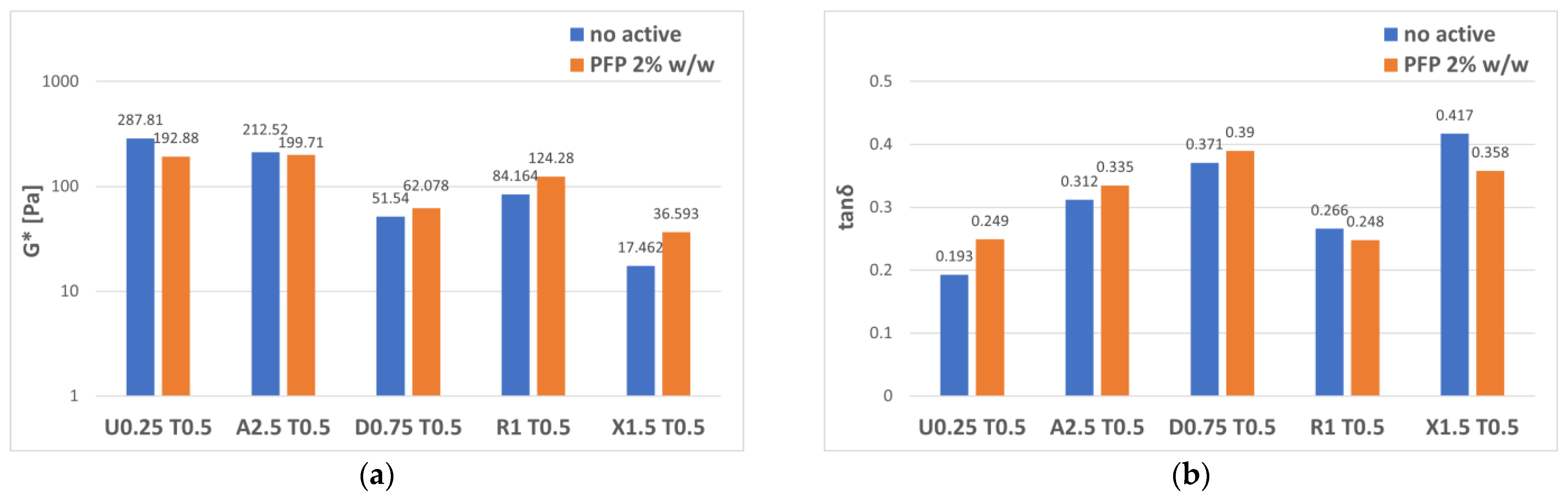
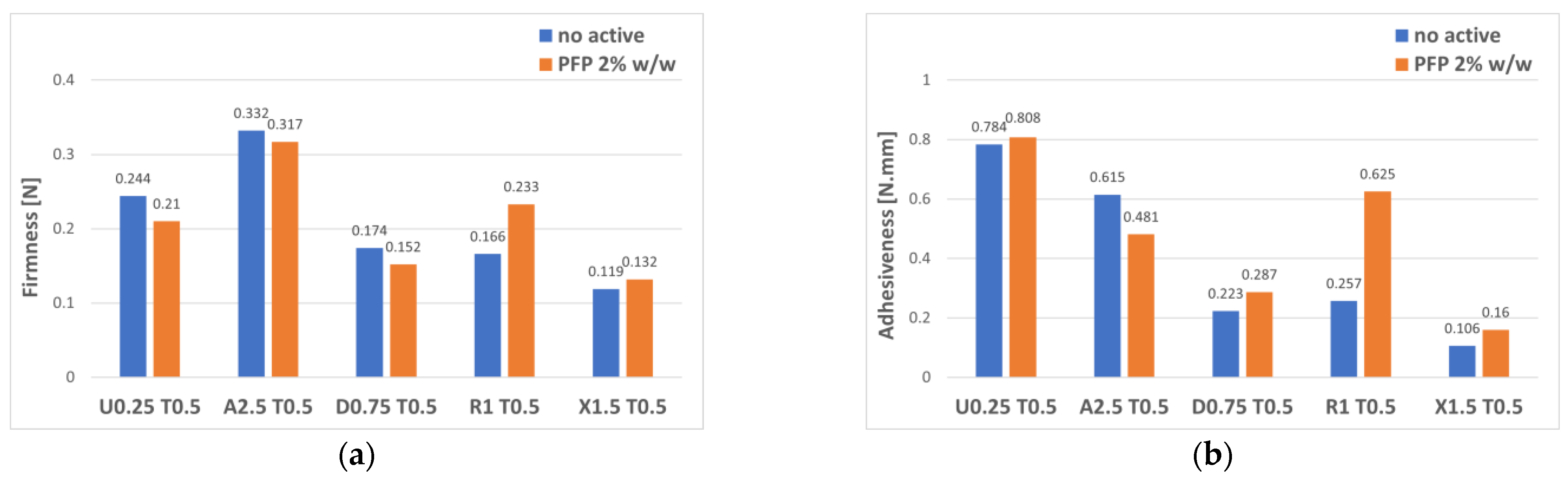
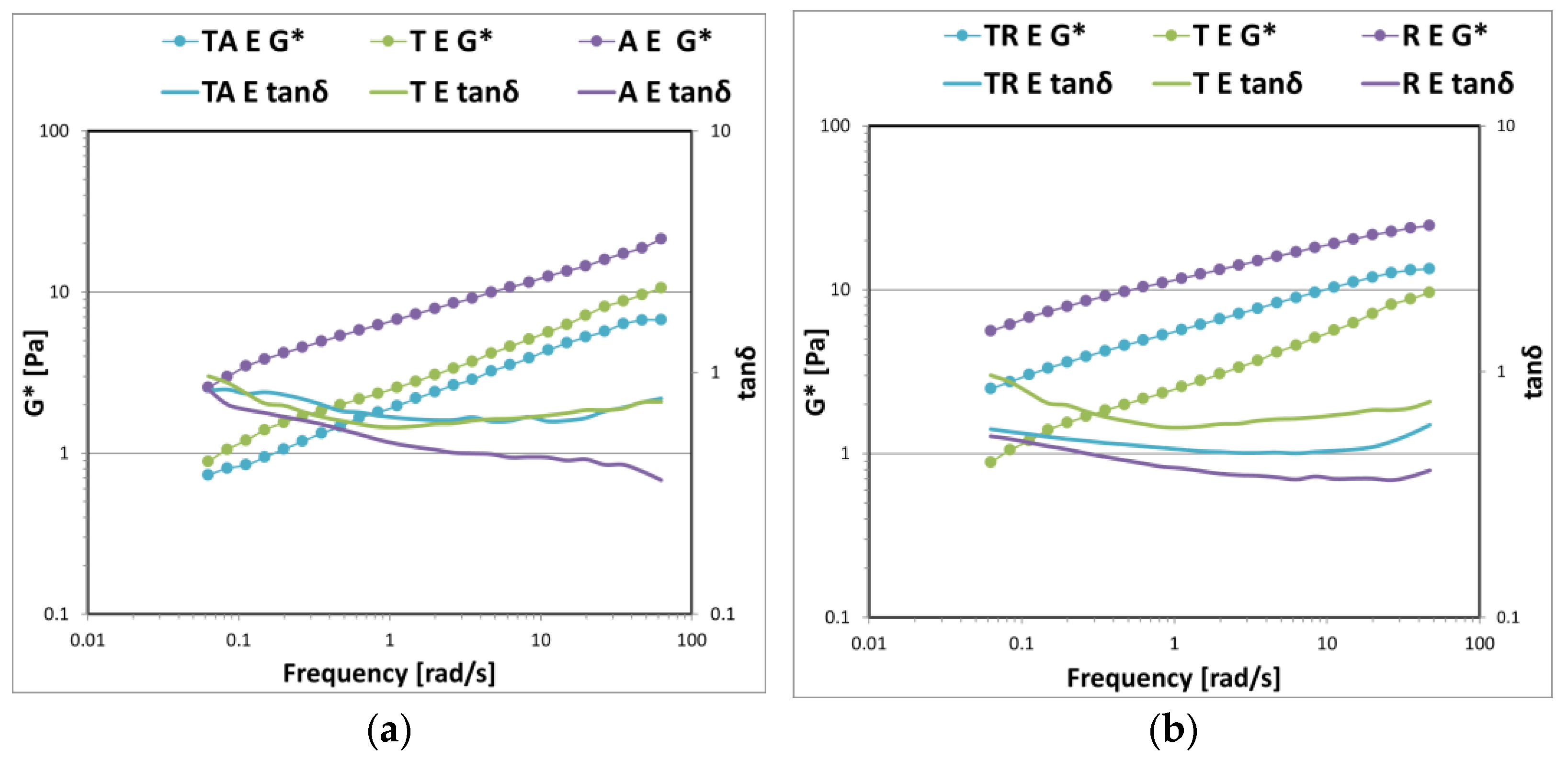
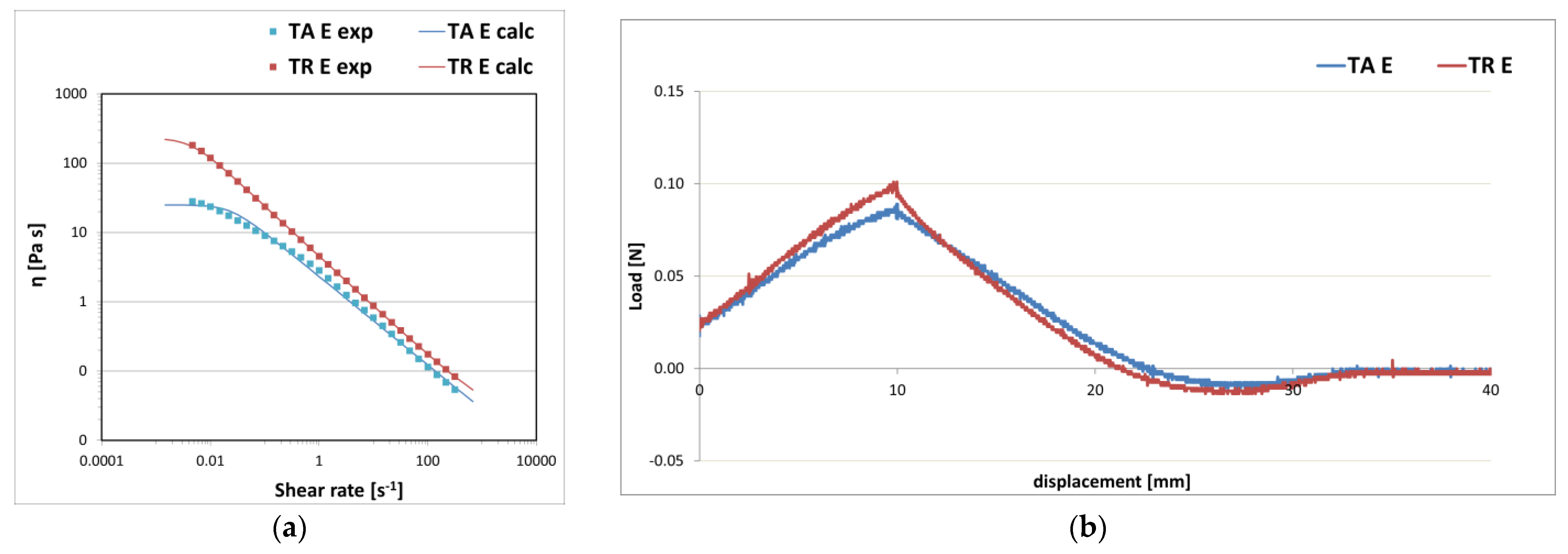
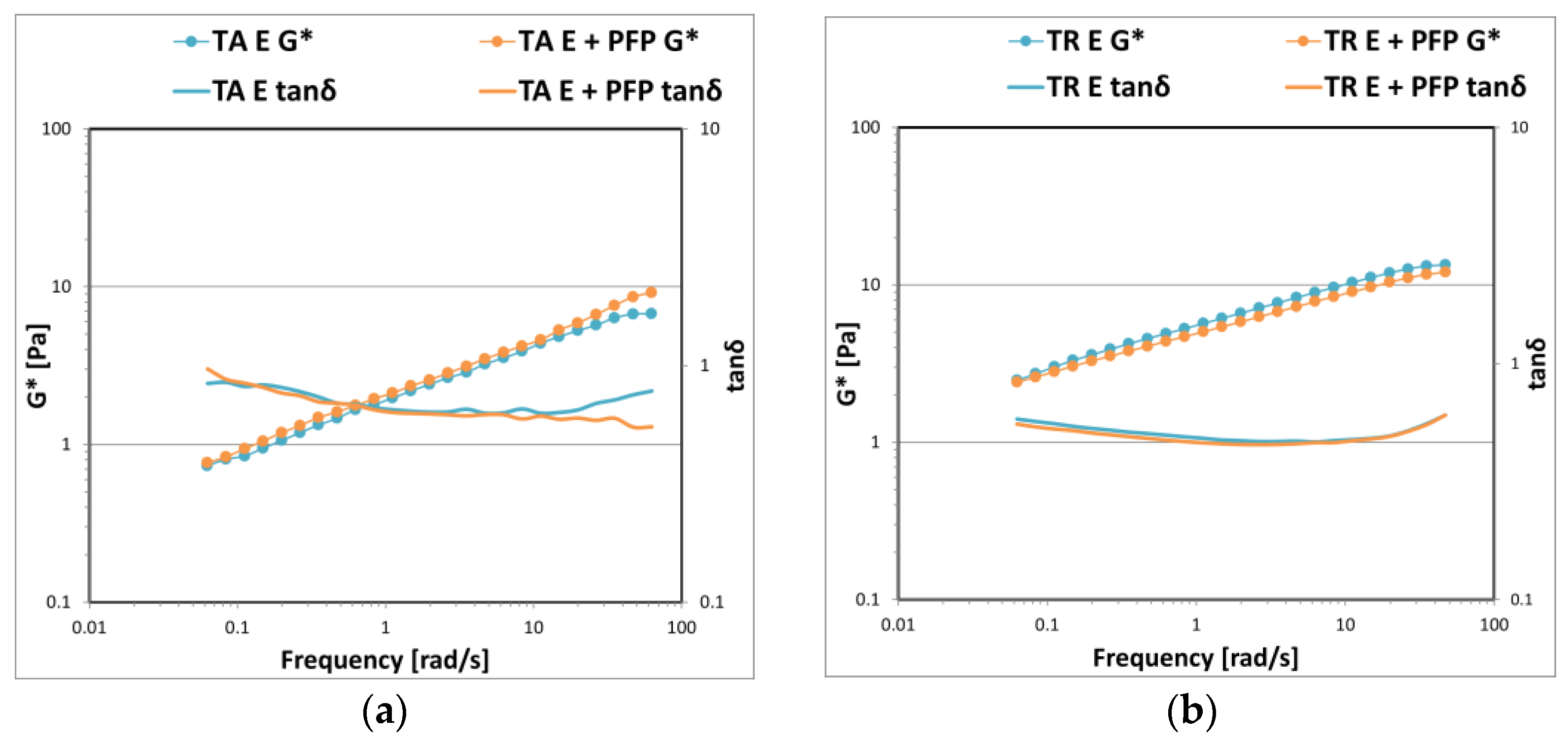
3. Results and Discussion
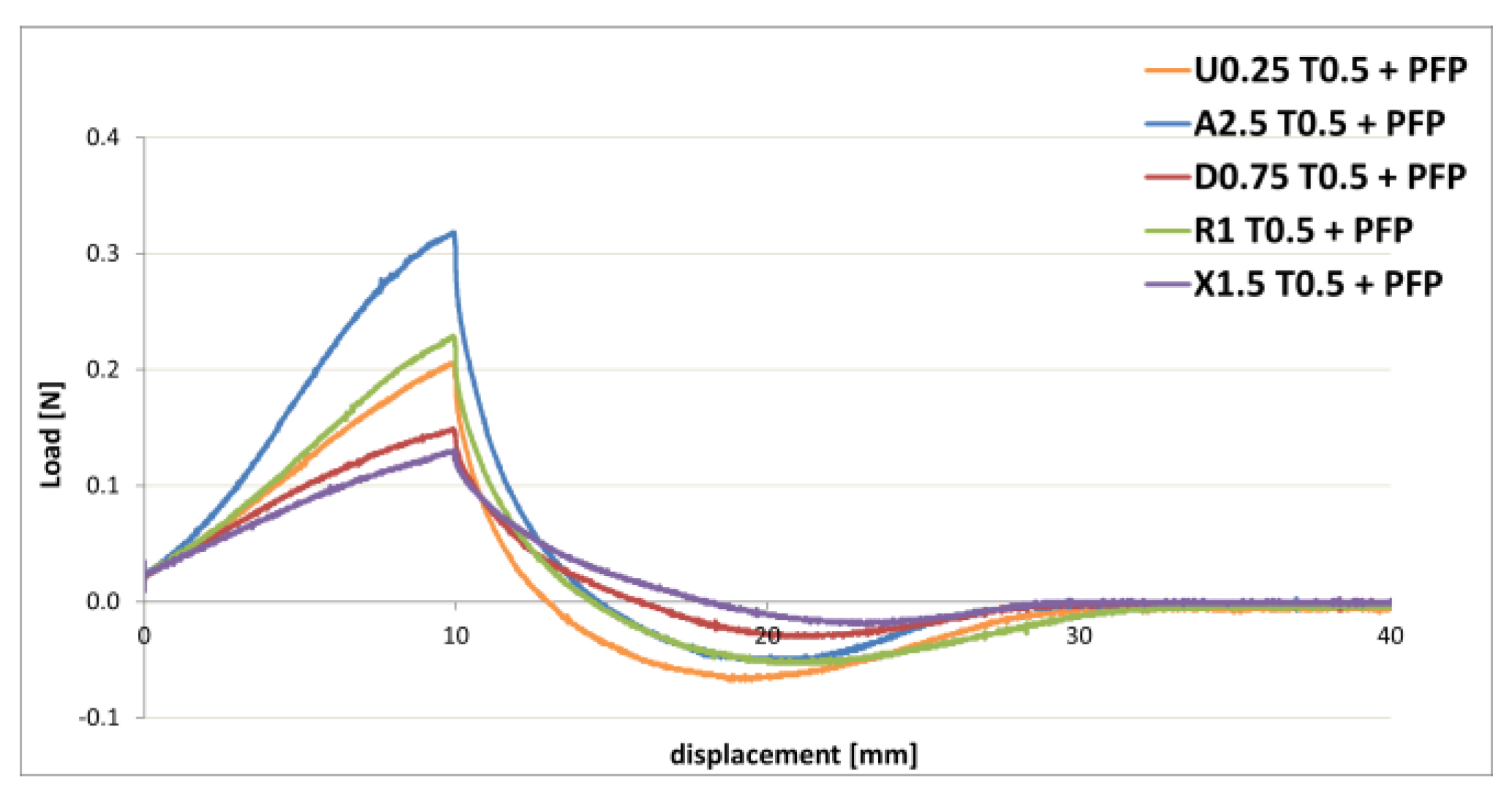
3.1. Gel Formulations
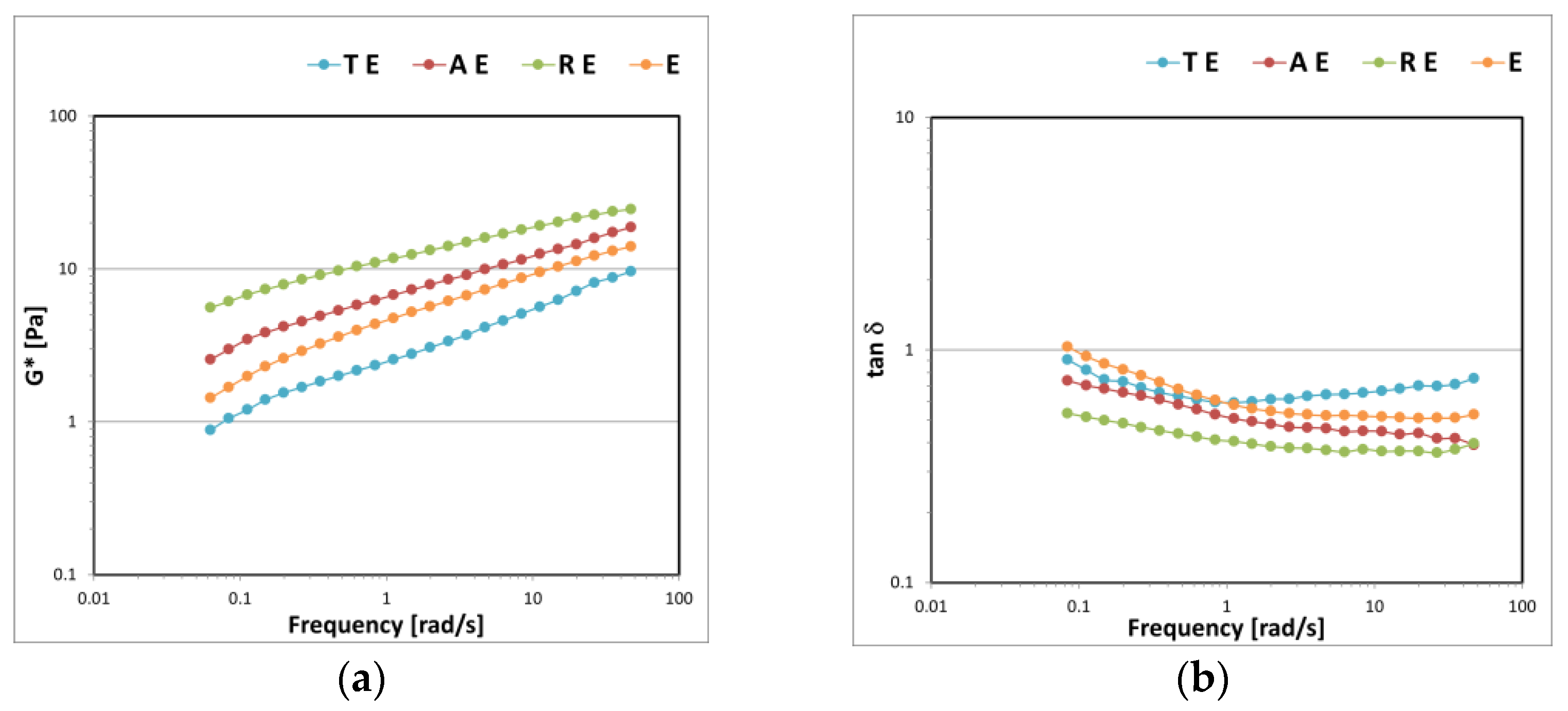
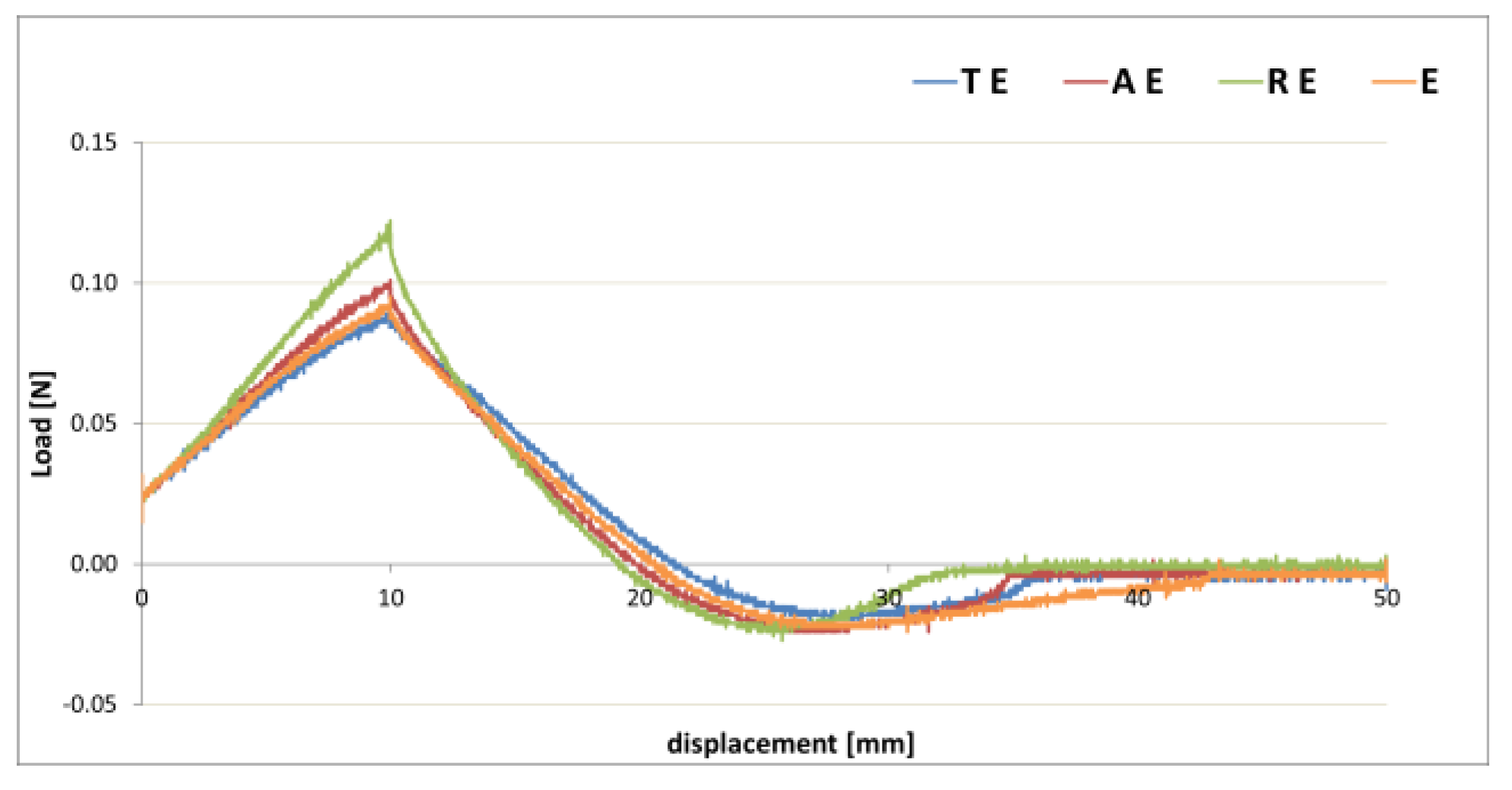
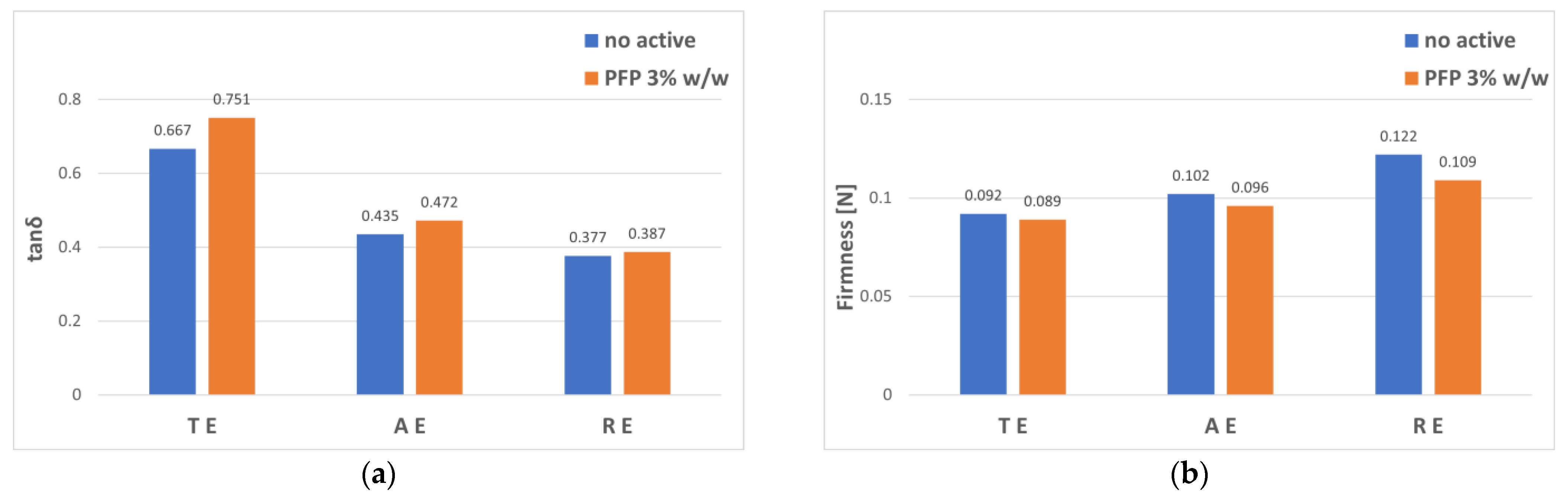
3.2. Gel–Cream Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Amit, G.; Ashawat, M.S.; Shailendra, S.; Swarnlata, S. Phytosome: A novel approach towards functional cosmetics. J. Plant Sci. 2007, 2, 644–649. [Google Scholar] [CrossRef]
- Fkiri, S.; Mezni, F.; Ouarghi, A.; Ghazghazi, H.; Larbi Khouja, M.; Khaldi, A.; Nasr, Z. Variability of phenolic compounds and antioxidant efficacy in needles extracts of Pinus nigra Arn. J. New Sci. 2018, 53, 3528–3535. [Google Scholar]
- Mulabagal, V.; Hsin-Sheng, T. Plant cell cultures—An alternative and efficient source for the production of biologically important secondary metabolites. Int. J. Appl. Sci. Eng. 2004, 2, 29–48. [Google Scholar]
- Hong, D.Y.; Lee, J.S.; Lee, H.G. Chitosan/poly—Glutamic acid nanoparticles improve the solubility of lutein. Int. J. Biol. Macromol. 2016, 85, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.C.d.A.; Barreto, S.M.A.G.; Ostrosky, E.A.; Rocha-Filho, P.A.d.; Veríssimo, L.M.; Ferrari, M. Production and Characterization of Cosmetic Nanoemulsions Containing Opuntia ficus-indica (L.) Mill Extract as Moisturizing Agent. Molecules 2015, 20, 2492–2509. [Google Scholar] [CrossRef]
- Olejnik, A.; Goscianska, J.; Zielinska, A.; Nowak, I. Stability determination of the formulations containing hyaluronic acid. Int. J. Cosmet. Sci. 2015, 37, 401–407. [Google Scholar] [CrossRef]
- Matusiak, J.; Grządka, E.; Bastrzyk, A. Stability, adsorption and electrokinetic properties of the chitosan/silica system. Colloids Surf. A Physiochem. Eng. Asp. 2018, 554, 245–252. [Google Scholar] [CrossRef]
- Filipović, M.; Lukić, M.; Krstonošić, V.; Đorđević, S.; Pantelić, I.; Gledović, A.; Vuleta, G.; Savić, S. Feasibility of a Natural Surfactant as a Stabilizer for Cosmetics with Liposome-Encapsulated Plant Stem Cells: Pre-Formulation and Formulation through Stability Studies. Tenside Surf. Det. 2016, 53, 214–226. [Google Scholar] [CrossRef]
- Semenzato, A.; Costantini, A.; Meloni, M.; Maramaldi, G.; Meneghin, M.; Baratto, G. Formulating O/W Emulsions with Plant-Based Actives: A Stability Challenge for an Effective Product. Cosmetics 2018, 5, 59. [Google Scholar] [CrossRef]
- Goddard, E.D.; Gruber, J.V. Principles of Polymer Science and Technology in Cosmetics and Personal Care. In Cosmetic Science and Technology; Marcel Dekker: New York, NY, USA, 1999. [Google Scholar]
- Varges, P.R.; Costa, C.M.; Fonseca, B.S.; Naccache, M.F.; De Souza Mendes, P.R. Rheological characterization of Carbopol dispersions in water and in water/glycerol. Fluids 2019, 4, 3. [Google Scholar] [CrossRef]
- Kentin, E.; Kaarto, H. An EU ban on microplastics in cosmetic products and the right to regulate. RECIEL Rev. Eur. Comp. Int. Environ. Law 2018, 27, 254–266. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Natural polysaccharides for skin care. In Polysaccharides of Microbial Origin; Oliveira, J., Radhouani, H., Reis, R.L., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–23. [Google Scholar]
- Noralian, z.; Gashti, M.P.; Moghaddam, M.R.; Tayyeb, H.; Erfanian, I. Ultrasonically developed silver/iota-carrageenan/cotton bionanocomposite as an efficient material for biomedical applications. Int J. Biol. Macromol. 2021, 180, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Tafuro, G.; Costantini, A.; Baratto, G.; Francescato, S.; Busata, L.; Semenzato, A. Characterization of Polysaccharidic Associations for Cosmetic Use: Rheology and Texture Analysis. Cosmetics 2021, 8, 62. [Google Scholar] [CrossRef]
- Gilbert, L.; Picard, C.; Savary, G.; Grisel, M. Rheological and textural characterization of cosmetic emulsions containing natural and synthetic polymers: Relationships between both data. Colloids Surf. A Physiochem. Eng. Asp. 2013, 421, 150–163. [Google Scholar] [CrossRef]
- César, F.C.S.; Maia Campos, P.M.B.G. Influence of vegetable oils in the rheology, texture profile and sensory properties of cosmetic formulations based on organogel. Int. J. Cosmet. Sci. 2020, 42, 494–500. [Google Scholar] [CrossRef]
- Gómez, I.; Calvo, F.; Gómez, J.M.; Ricardez-Sandoval, L.; Alvarez, O. A multiscale approach for the integrated design of emulsified cosmetic products. Chem. Eng. Sci. 2022, 251, 117493. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Ptaszek, A.; Kruk, G. Effective tool for assessment of the quality of barrier creams—Relationships between rheological, textural and sensory properties. Regul. Toxicol. Pharmacol. 2019, 103, 113–123. [Google Scholar] [CrossRef]
- Tadros, T. Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 227–258. [Google Scholar] [CrossRef]
- Yokokoji, A.; Kitayama, W.; Wichai, K.; Urakawa, O.; Matsumoto, A.; Vao-Soongnern, V.; Inoue, T. Viscoelastic relaxation of polymerized ionic liquid and lithium salt mixtures: Effect of salt concentration. Polymers 2021, 13, 1772. [Google Scholar] [CrossRef]
- Zainal, N.F.A.; Lai, S.A.; Chan, C.H. Melt rheological behavior and morphology of poly(ethylene oxide)/natural rubber graft-poly(methyl methacrylate) blends. Polymers 2020, 12, 724. [Google Scholar] [CrossRef] [PubMed]
- Calixto, M.S.; Maia Campos, P.M.B.G. Physical–Mechanical characterization of cosmetic formulations and correlation between instrumental measurements and sensorial properties. Int. J. Cosmet. Sci. 2017, 39, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Vergilio, M.M.; de Freitas, A.C.P.; da Rocha-Filho, P.A. Comparative sensory and instrumental analyses and principal components of commercial sunscreens. J. Cosmet. Dermatol. 2022, 21, 729–739. [Google Scholar] [CrossRef]
- Vieira, G.S.; Lavarde, M.; Fréville, V.; Rocha-Filho, P.A.; Pensé-Lhéritier, A. Combining sensory and texturometer parameters to characterize different type of cosmetic ingredients. Int. J. Cosmet. Sci. 2020, 42, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Hiwa, M.A. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules 2019, 24, 102. [Google Scholar]
- Igarashi, M.; Miyazaki, Y. A Review on Bioactivities of Perilla: Progress in Research on the Functions of Perilla as Medicine and Food. Evid. Based Complement. Altern. Med. 2013, 2013, 925342. [Google Scholar] [CrossRef]
- Dhyani, A.; Chopra, R.; Garg, M. A Review on Nutritional Value, Functional Properties and Pharmacological Application of Perilla (Perilla frutescens L.). Biomed. Pharmacol. J. 2019, 12, 649–660. [Google Scholar] [CrossRef]
- Valenta, C. The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 2005, 57, 1692–1712. [Google Scholar] [CrossRef]
- Lam, H.T.; Zupancic, O.; Laffleur, F.; Bernkop-Schnurch, A. Mucoadhesive properties of polyacrylates: Structure–Function relationship. Int. J. Adhes. Adhes. 2021, 107, 102857. [Google Scholar] [CrossRef]
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, B. Polymers in Mucoadhesive Drug-Delivery Systems: A Brief Note. Des. Monomers Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef]
- Lapasin, R.; Pricl, S. Rheology of Industrial Polysaccarides, Theory and Application; Blackie Academic & Professional: London, UK, 1995; pp. 38–39. [Google Scholar]
- Vinarta, S.C.; Francois, N.J.; Daraio, M.E.; Figueroa, L.I.C.; Farina, J.I. Sclerotium rolfsii scleroglucan: The promising behavior of a natural polysaccharide as a drug delivery vehicle, suspension stabilizer and emulsifier. Int. J. Biol. Macrom. 2007, 41, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Tinland, B. Relation between Molecular Structure and Physicochemical Properties for Some Microbial Polysaccharides. In Novel Biodegradable Microbial Polymers; Dawes, E.A., Ed.; Kluwer Academic Publishers: Norwell, MA, USA, 1990; pp. 349–370. [Google Scholar]
- Xu, L.; Gong, H.; Dong, M.; Li, Y. Rheological properties and thickening mechanism of aqueous diutan gum solution: Effects of temperature and salts. Carbohydr. Polym. 2015, 132, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Semenzato, A.; Costantini, A.; Baratto, G. Green Polymers in Personal Care Products: Rheological Properties of Tamarind Seed Polysaccharide. Cosmetics 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Joshi, A.A.; Patil, C.L.; Amale, P.D.; Patel, H.M.; Surana, S.J.; Belgamwar, V.S.; Chaudhari, K.S.; Pardeshi, C.V. Xyloglucan: A functional biomacromolecule for drug delivery applications. Int. J. Biol. Macrom. 2017, 104, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Lapasin, R.; De Lorenzi, L.; Pricl, S.; Torriano, G. Flow properties of hydroxypropyl guar gum and its long-chain hydrophobic derivatives. Carbohydr. Polym. 1995, 28, 195–202. [Google Scholar] [CrossRef]
- Pressi, G.; Bertaiola, O.; Guzzo, F.; Biagi, M. Phytocomplex and Extract of a Meristematic Cell Line Selected of Perilla frutescens. Patent ITA102020000028230/PCT/IB2021/057560, 24 November 2020. [Google Scholar]
- Yasuda, K.; Armstrong, R.C.; Cohen, R.E. Shear flow properties of concentrated solutions of linear and star branched polystyrenes. Rheol. Acta 1981, 20, 163–178. [Google Scholar] [CrossRef]
- Tai, A.; Bianchini, R.; Jachowicz, J. Texture analysis of cosmetic/pharmaceutical raw materials and formulations. Int. J. Cosmet. Sci. 2014, 36, 291–304. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Amarak, M.H.; Sousa Lobo, J.M. Comparison between sensory and instrumental characterization of topical formulations: Impact of thickening agent. Int. J. Cosmet. Sci. 2016, 38, 389–398. [Google Scholar] [CrossRef]
- Bais, D.; Trevisan, A.; Lapasin, R.; Partal, P.; Gallegos, C. Rheological characterization of polysaccharide–surfactant matrices for cosmetic O/W emulsions. J. Colloid Interface Sci. 2005, 290, 546–556. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Calderas, F.; Sanchez-Olivares, G.; Nunez-Ramirez, D.M. Rheology of Sodium Polyacrylate as an Emulsifier Employed in Cosmetic Emulsions. Ind. Eng. Chem. Res. 2014, 53, 18346–18351. [Google Scholar] [CrossRef]


| Phase | Ingredients | Composition % |
|---|---|---|
| A | Water | Add until reaching 100 |
| Glycerin | 3 | |
| Tamarindus indica seed polysaccharides | 0.5 | |
| Rheology modifier | Varies | |
| PFP | 0–3 | |
| B | Phenoxyethanol, Ethyhexylglycerin | 0.9 |
| C | Buffering agent | Add until reaching pH 5–5.5 |
| Phase | Ingredients | Composition % |
|---|---|---|
| A | Water | Add until reaching 100 |
| Glycerin | 3 | |
| Esaflor HM 22 | 0.3–0.5–0.7 | |
| Tamarindus indica seed polysaccharides | 0–0.2 | |
| Rheology modifier | 0–0.2 | |
| B | Propylheptyl Caprylate | 2 |
| Caprylic/capric triglyceride | 0.5 | |
| C | PFP | 0–3 |
| D | Phenoxyethanol, Ethyhexylglycerin | 0.9 |
| E | Buffering agent | Add until reaching pH 5–5.5 |
| Polymer | Polymer % w/w | T % w/w | PFP % w/w | Stability |
|---|---|---|---|---|
| U | 0.25 | 0.5 | 3 | homogeneous |
| 0.5 | homogeneous | |||
| 0.75 | homogeneous | |||
| A | 1 | 0.5 | 3 | sedimentation |
| 1.5 | sedimentation | |||
| 2 | sedimentation | |||
| 2.5 | homogeneous | |||
| D | 0.25 | 0.5 | 3 | sedimentation |
| 0.5 | sedimentation | |||
| 0.75 | slight sedimentation | |||
| R | 0.5 | 0.5 | 3 | sedimentation |
| 0.75 | sedimentation | |||
| 1 | homogeneous | |||
| X | 1 | 0.5 | 3 | sedimentation |
| 1.5 | sedimentation | |||
| 2 | slight sedimentation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pressi, G.; Barbieri, E.; Rizzi, R.; Tafuro, G.; Costantini, A.; Di Domenico, E.; Semenzato, A. Formulation and Physical Characterization of a Polysaccharidic Gel for the Vehiculation of an Insoluble Phytoextract for Mucosal Application. Polysaccharides 2022, 3, 728-744. https://doi.org/10.3390/polysaccharides3040042
Pressi G, Barbieri E, Rizzi R, Tafuro G, Costantini A, Di Domenico E, Semenzato A. Formulation and Physical Characterization of a Polysaccharidic Gel for the Vehiculation of an Insoluble Phytoextract for Mucosal Application. Polysaccharides. 2022; 3(4):728-744. https://doi.org/10.3390/polysaccharides3040042
Chicago/Turabian StylePressi, Giovanna, Elisa Barbieri, Raffaella Rizzi, Giovanni Tafuro, Alessia Costantini, Elisa Di Domenico, and Alessandra Semenzato. 2022. "Formulation and Physical Characterization of a Polysaccharidic Gel for the Vehiculation of an Insoluble Phytoextract for Mucosal Application" Polysaccharides 3, no. 4: 728-744. https://doi.org/10.3390/polysaccharides3040042
APA StylePressi, G., Barbieri, E., Rizzi, R., Tafuro, G., Costantini, A., Di Domenico, E., & Semenzato, A. (2022). Formulation and Physical Characterization of a Polysaccharidic Gel for the Vehiculation of an Insoluble Phytoextract for Mucosal Application. Polysaccharides, 3(4), 728-744. https://doi.org/10.3390/polysaccharides3040042








