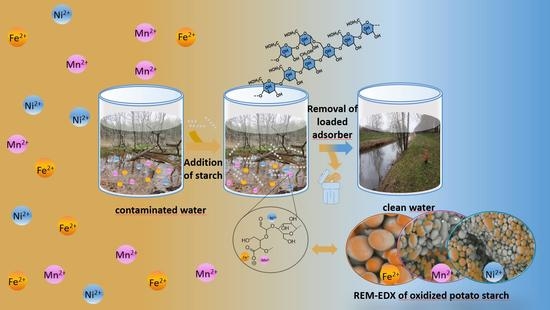
Native and Oxidized Starch for Adsorption of Nickel, Iron, and Manganese Ions from Water
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.1.1. Starches
2.1.2. Materials for Adsorption Experiments
2.1.3. Ultrapure Water
2.1.4. ICP-OES Standard Solutions
2.2. Adsorption Experiments
2.2.1. Adsorption Isotherms
2.2.2. Adsorption in Dependence on Time
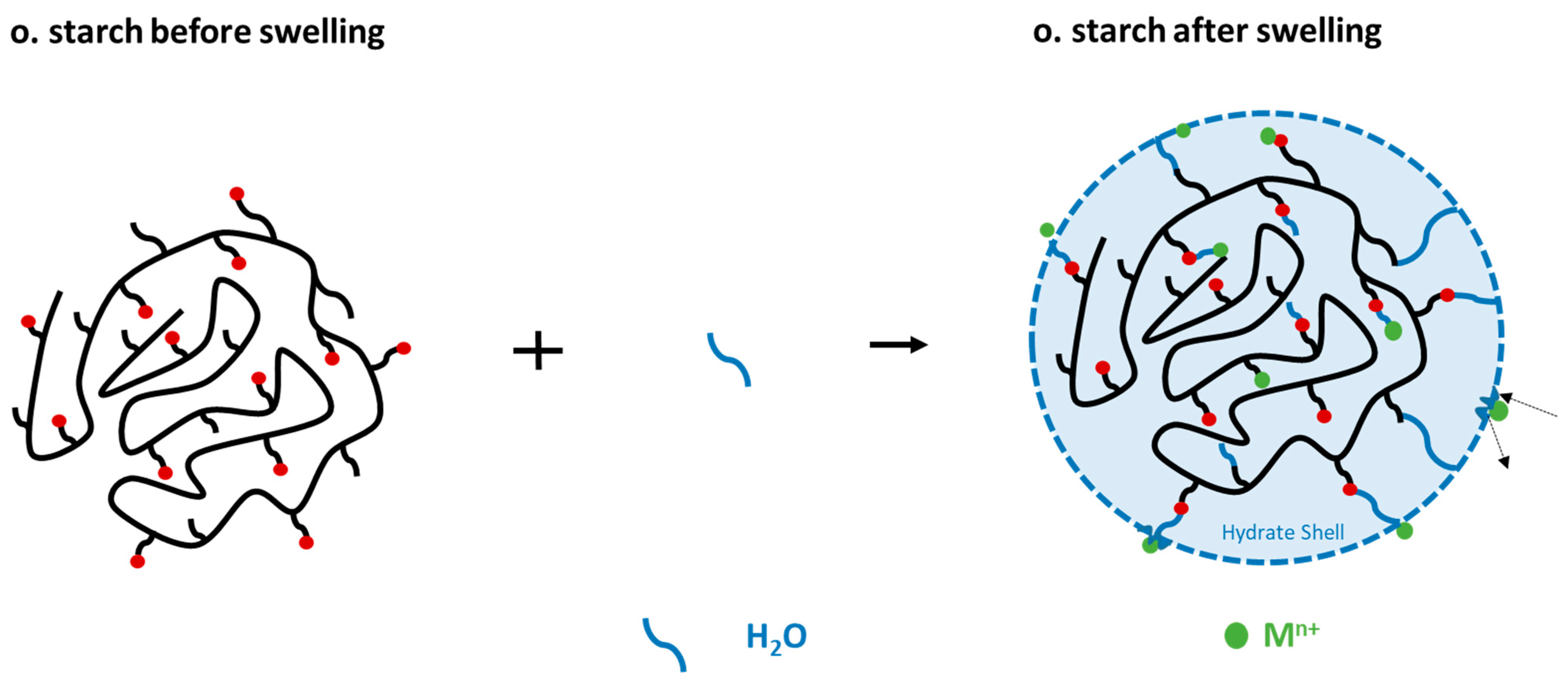
2.2.3. Adsorption in Dependence of Swelling
3. Theoretical Model
3.1. Sorption Rates and Sorption Capacity
3.2. Employed Adsorption Models
4. Results and Discussion
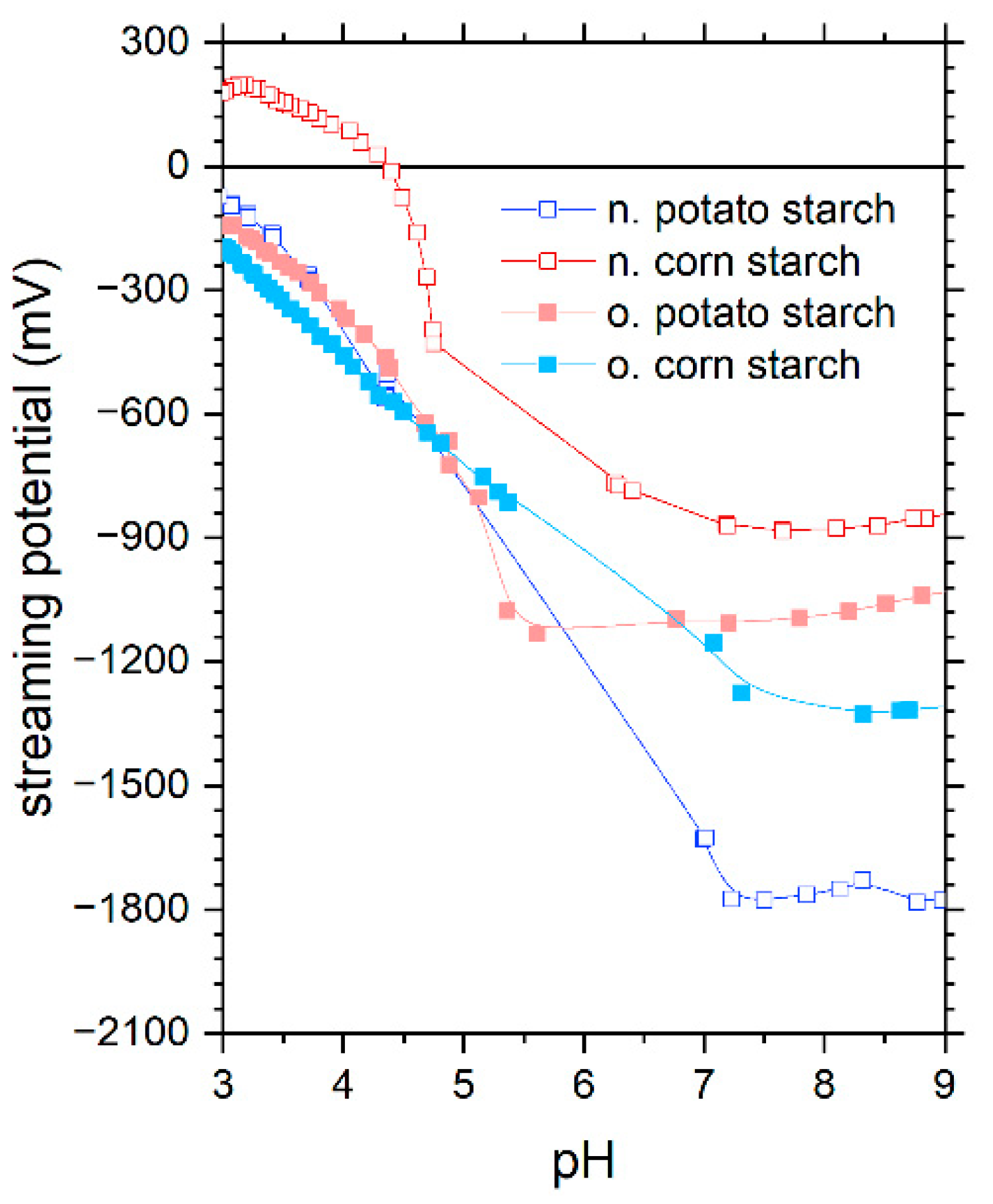
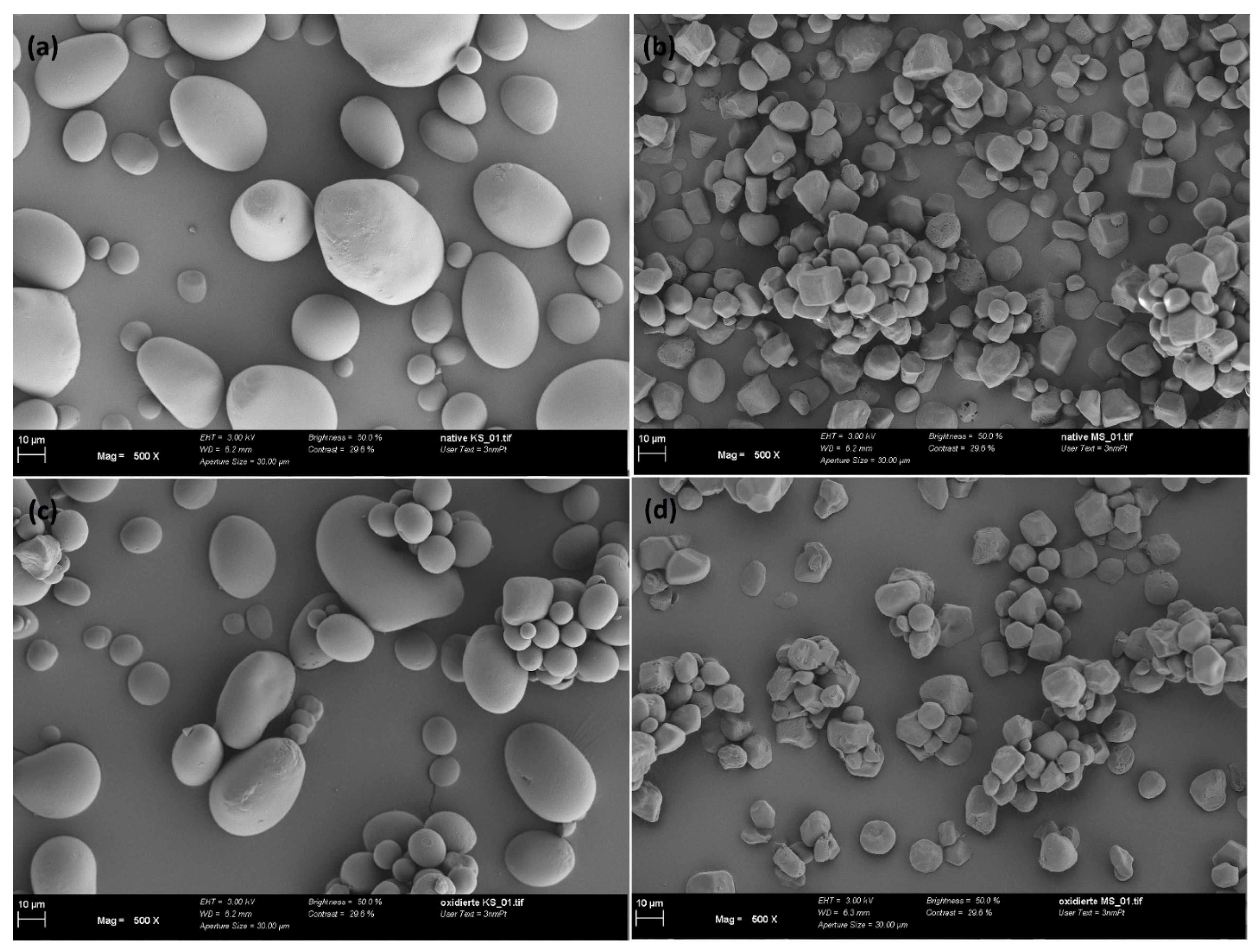
4.1. Characterization of Starch
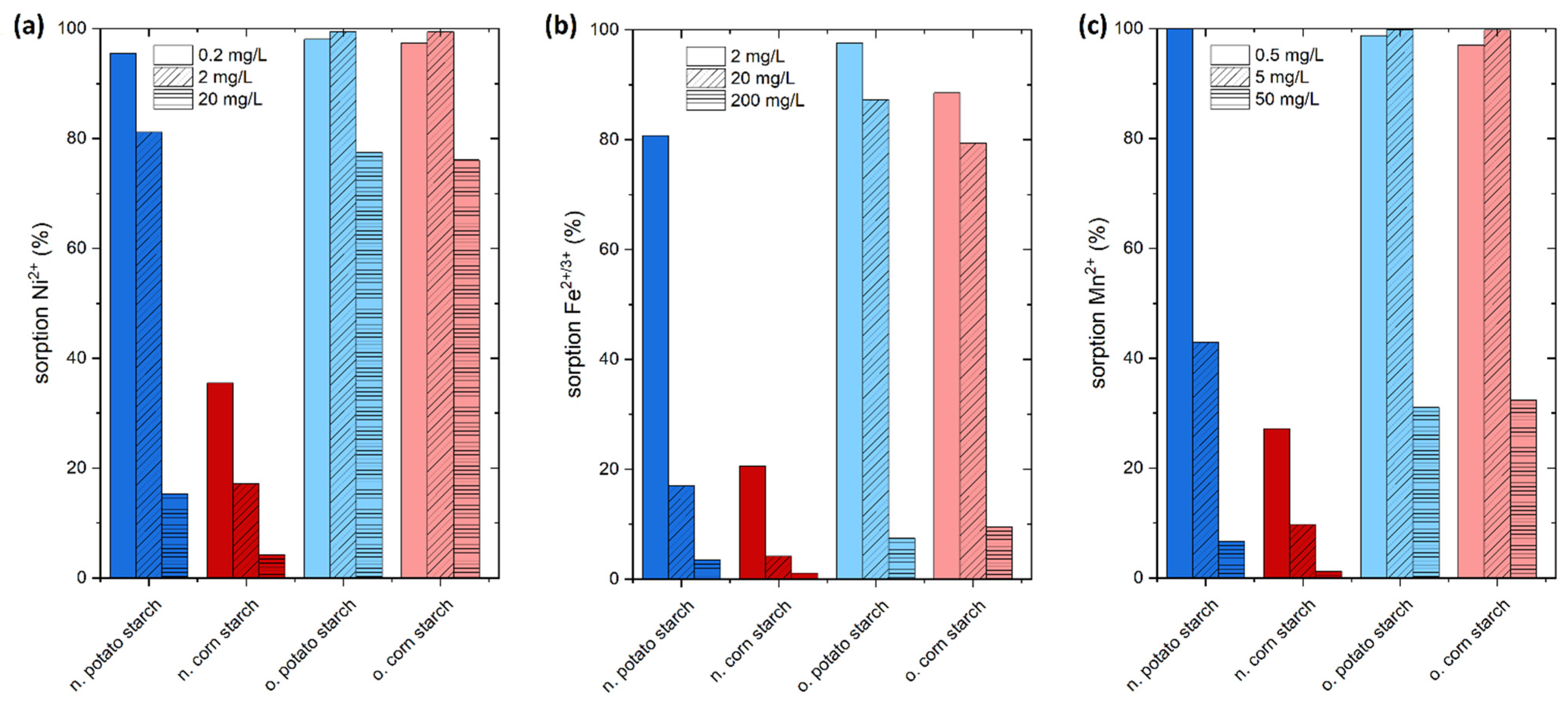
4.2. Preliminary Sorption Experiments
4.3. Kinetic and Isotherm Adsorption Experiments
4.3.1. Kinetic Study of Fe2+/3+ and SO42− Adsorption
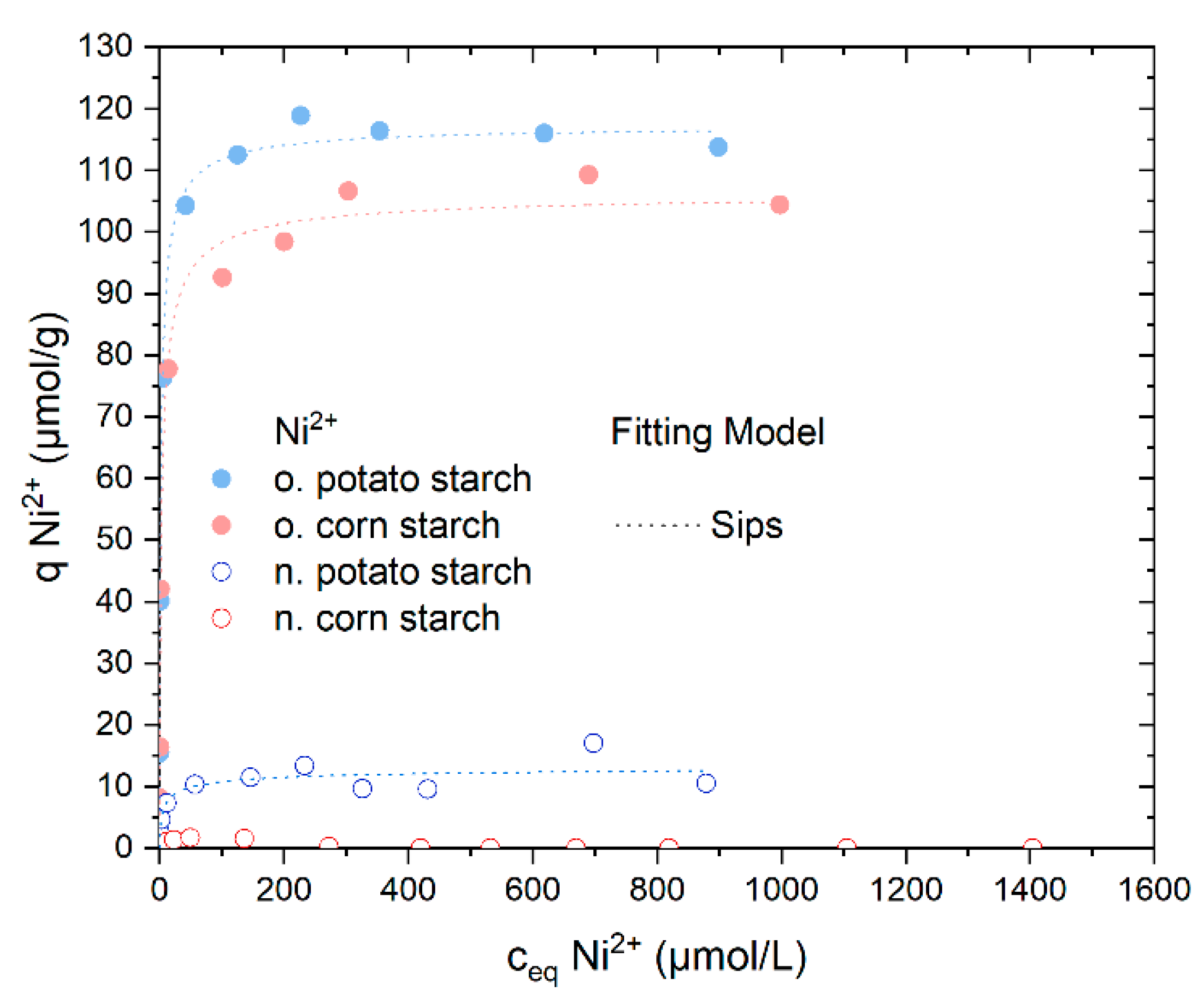
4.3.2. Sorption Isotherm Experiments with Ni2+
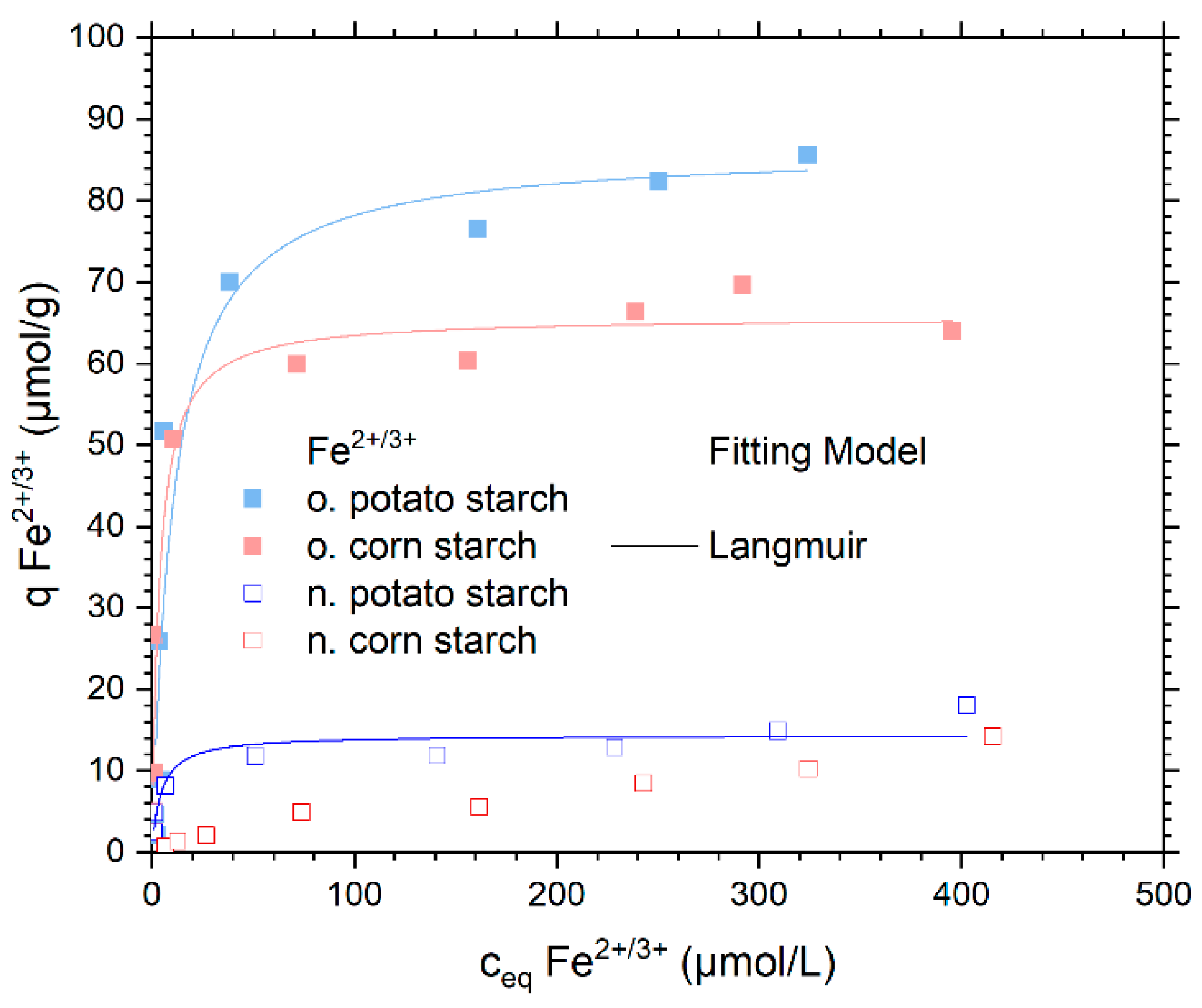
4.3.3. Sorption Isotherm Experiments with Fe2+/3+
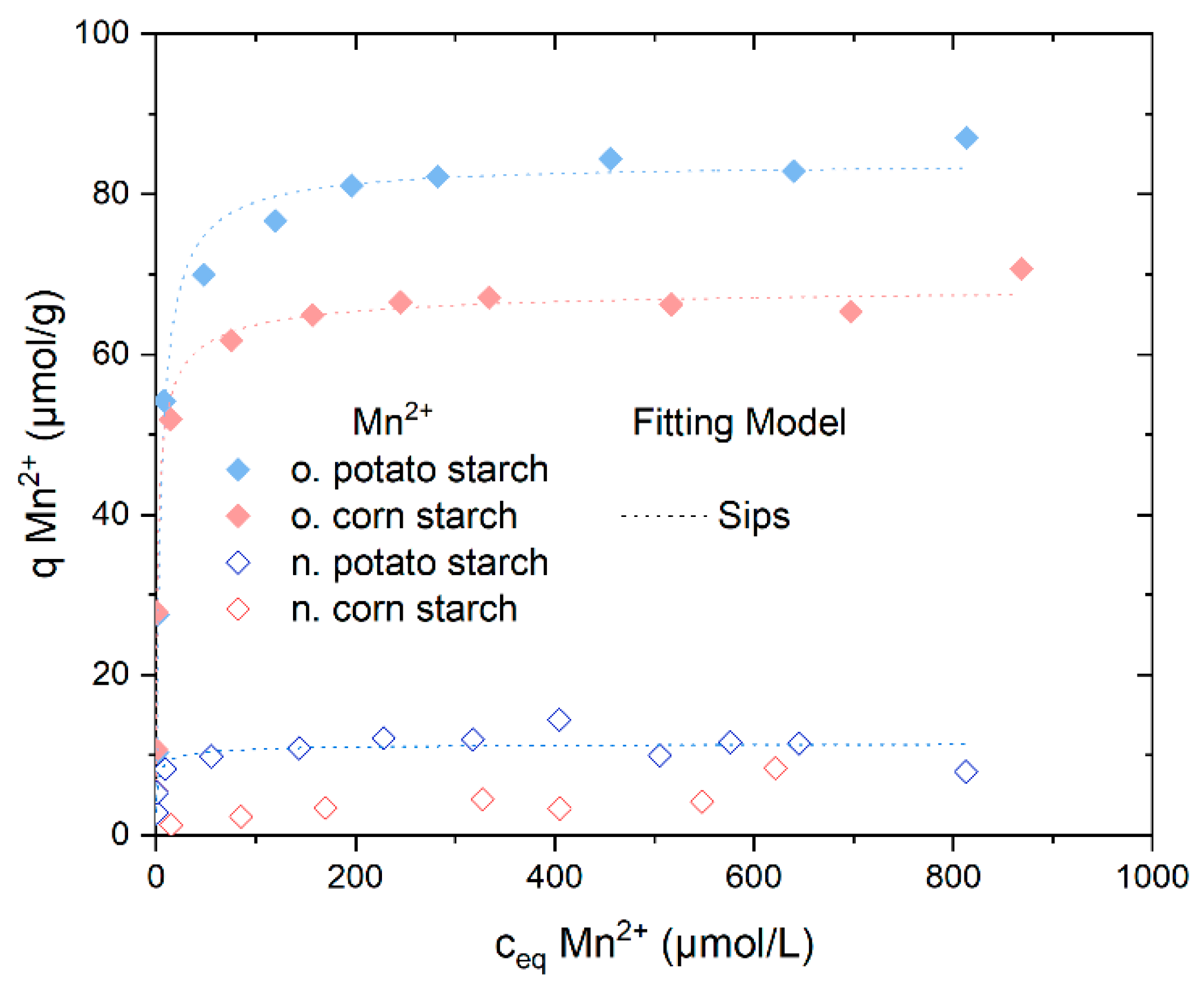
4.3.4. Sorption Isotherm Experiments with Mn2+
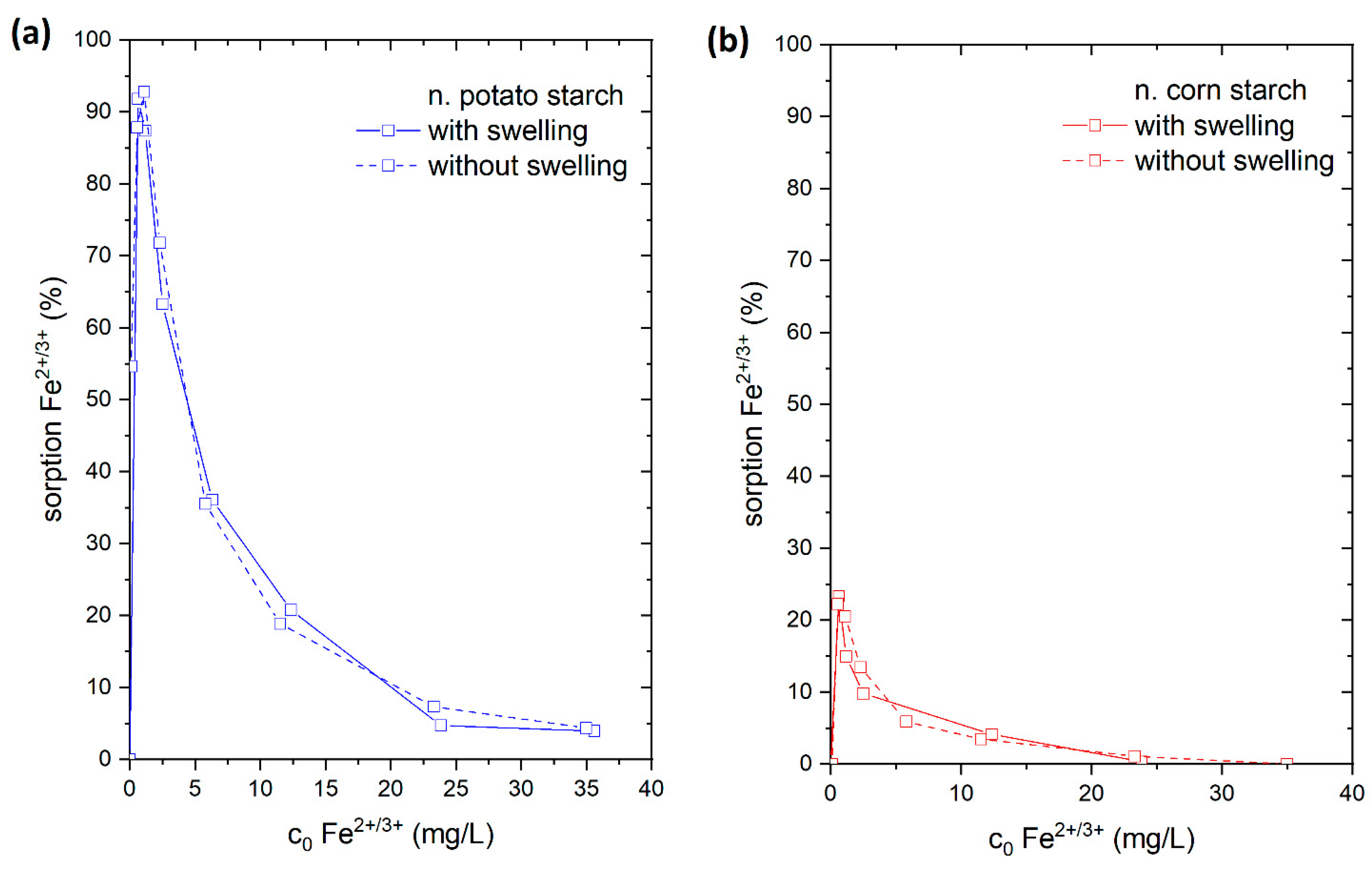
4.3.5. Adsorption of Fe2+/3+ with and without Swelling on Starch
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarz, S.; Steinbach, C.; Schwarz, D.; Mende, M.; Boldt, R. Chitosan—The Application of a Natural Polymer against Iron Hydroxide Deposition. Am. J. Anal. Chem. 2016, 7, 623–632. [Google Scholar] [CrossRef][Green Version]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdisci. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Soto, D.; Urdaneta, J.; Pernía, K.; Leõn, O.; Muñoz-Bonilla, A.; Fernandez-García, M. Removal of Heavy Metal Ions in Water by Starch Esters. Starch/Staerke 2016, 68, 37–46. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Caruso, A.; Capasso, A.; Palladino, C.; Panno, A.; Saturnino, C. Heavy Metals: Toxicity and Carcinogenicity. Pharmacologyonline 2010, 333, 329–333. [Google Scholar]
- Schwarz, S.; Schwarz, D.; Ohmann, W.; Neuber, S. Adsorption and Desorption Studies on Reusing Chitosan as an Efficient Adsorbent. CSEE 2018, 128, 3–6. [Google Scholar] [CrossRef]
- Saito, M.; Arakaki, R.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Molecular Mechanisms of Nickel Allergy. Int. J. Mol. Sci. 2016, 17, 202. [Google Scholar] [CrossRef]
- Qiao, Y.; Ma, L. Quantification of Metal Ion Induced DNA Damage with Single Cell Array Based Assay. Analyst 2013, 138, 5713–5718. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking—Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]
- Baby, J.; Raj, J.; Biby, E.; Sankarganesh, P.; Jeevitha, M.; Ajisha, S.; Rajan, S. Toxic Effect of Heavy Metals on Aquatic Environment. Int. J. Biol. Chem. Sci. 2011, 4, 939–952. [Google Scholar] [CrossRef]
- Lucchini, R.; Placidi, D.; Cagna, G.; Fedrighi, C.; Oppini, M.; Peli, M.; Zoni, S. Manganese and Developmental Neurotoxicity. Adv. Neutrobiol. 2017, 18, 13–34. [Google Scholar] [CrossRef]
- Bundesministerin der Justiz und für Verbraucherschutz-Trinkwasserverordnung, Anlage 2 (Zu § 6 Absatz 2): TrinkwV. 2019. Available online: https://www.gesetze-im-internet.de/trinkwv_2001/anlage_2.html (accessed on 9 July 2022).
- Carrasco, K.H.; Höfgen, E.G.; Brunner, D.; Borchert, K.B.L.; Reis, B.; Steinbach, C.; Mayer, M.; Schwarz, S.; Glas, K.; Schwarz, D. Removal of Iron, Manganese, Cadmium, and Nickel Ions Using Brewers’ Spent Grain. Polysaccharides 2022, 3, 356–379. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Chattopadhyay, D. Coal and Other Mining Operations: Role of Sustainability. In Fossil Energy; Springer: New York, NY, USA, 2020; pp. 333–356. [Google Scholar] [CrossRef]
- Jambor, J.L.; Dutrizac, J.E.; Groat, L.A.; Raudsepp, M. Static Tests of Neutralization Potentials of Silicate and Aluminosilicate Minerals. Environ. Geol. 2002, 43, 1–17. [Google Scholar] [CrossRef]
- Johnston, D.; Potter, H.; Jones, C.; Rolley, S.; Watson, I.; Pritchard, J. Abandoned Mines and the Water Environment; Environment Agenc: London, UK, 2008; ISBN 978-1-84432-894-9. [Google Scholar]
- Borchert, K.B.L.; Boughanmi, R.; Reis, B.; Zimmermann, P.; Steinbach, C.; Graichen, P.; Svirepa, A.; Schwarz, J.; Boldt, R.; Schwarz, S.; et al. Removal of Lead, Cadmium, and Aluminum Sulfate from Simulated and Real Water with Native and Oxidized Starches. Polysaccharides 2021, 2, 429–453. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch Modification through Environmentally Friendly Alternatives: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2482–2505. [Google Scholar] [CrossRef]
- Ma, X.; Liu, X.; Anderson, D.P.; Chang, P.R. Modification of Porous Starch for the Adsorption of Heavy Metal Ions from Aqueous Solution. Food Chem. 2015, 181, 133–139. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part II.-Liquids. J. Franklin Inst. 1917, 184, 721. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Kanellopoulos, N.K. Prediction of Binary Adsorption Isotherms of Cu2+, Cd2+ and Pb2+ on Calcium Alginate Beads from Single Adsorption Data. J. Hazard. Mater. 2009, 162, 1347–1354. [Google Scholar] [CrossRef]
- Dubinin, M. The Equation of the Characteristic Curve of Activated Charcoal. Proc. USSR Acad. Sci. 1947, 55, 327–329. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Zhou, X. Correction to the Calculation of Polanyi Potential from Dubinnin-Rudushkevich Equation. J. Hazard. Mater. 2020, 384, 121101. [Google Scholar] [CrossRef]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Mittal, A.; Ahmad, R.; Hasan, I. Biosorption of Pb2+, Ni2+ and Cu2+ Ions from Aqueous Solutions by L-Cystein-Modified Montmorillonite-Immobilized Alginate Nanocomposite. Desalin. Water Treat. 2016, 57, 17790–17807. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T.; DeCaprio, A.; Barber, D.S. Application of the Hard and Soft, Acids and Bases (HSAB) Theory to Toxicant—Target Interactions. Chem. Res. Toxicol 2012, 25, 239–251. [Google Scholar] [CrossRef]
- Jones, M.M.; Vaughn, W.K. HSAB Theory and Acute Metal Ion Toxicity and Detoxification Processes. J. Inorg. Nucl. Chem. 1978, 40, 2081–2088. [Google Scholar] [CrossRef]
- Persson, I. Hydrated Metal Ions in Aqueous Solution: How Regular Are Their Structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Adsorption Isotherms, Kinetics and Mechanism for the Adsorption of Cationic and Anionic Dyes onto Carbonaceous Particles Prepared from Juglans Regia Shell Biomass. Int. J. Environ. Sci. Technol. 2013, 10, 231–242. [Google Scholar] [CrossRef]
- Keren, Y.; Borisover, M.; Bukhanovsky, N. Sorption Interactions of Organic Compounds with Soils Affected by Agricultural Olive Mill Wastewater. Chemosphere 2015, 138, 462–468. [Google Scholar] [CrossRef]
- Hartman, P.; Chan, H.K. Application of the Periodic Bond Chain (PBC) Theory and Attachment Energy Consideration to Derive the Crystal Morphology of Hexamethylmelamine. Pharm. Res. 1993, 10, 1052–1058. [Google Scholar] [CrossRef]
- Singh, A.K.; Goel, T.C.; Mendiratta, R.G. Effect of Cation Distribution on the Properties of Mn0.2ZnxNi0.8-XFe2O4. Solid State Commun. 2003, 125, 121–125. [Google Scholar] [CrossRef]
- Andreas, S. Einsatz von Cellulose- Und Stärkehaltigen Naturstoffen Zur Abwasserreinigung. Ph.D. Thesis, Technischen Universität Bergakademie Freiberg, Freiberg, Germany, 2005. [Google Scholar]
- Weißpflog, J.; Gündel, A.; Vehlow, D.; Steinbach, C.; Müller, M.; Boldt, R.; Schwarz, S.; Schwarz, D. Solubility and Selectivity Effects of the Anion on the Adsorption of Different Heavy Metal Ions onto Chitosan. Molecules 2020, 25, 2482. [Google Scholar] [CrossRef]


| Heavy Metal Ions | TrinkwV (German Organisation) mg/L | WHO (World Health Organization) mg/L |
|---|---|---|
| Ni2+ | 0.02 | 0.07 |
| Fe2+ | 0.2 | 0.3 |
| Mn2+ | 0.05 | 0.4 |
| Sample | Model | Qm µmol/g | K L/µmol * | ßDR × 10−9 mol2/J2 | n | Eads,DR KJ/mol | R2 (COD) | ΔG° kJ/mol |
|---|---|---|---|---|---|---|---|---|
| Native potato starch | Langmuir | 11.94 ± 0.88 | 0.20 ± 0.12 | -- | -- | 0.847 | −30.3 | |
| Sips | 13.56 ± 3.20 | 0.34 ± 0.18 | -- | 0.52 ± 0.32 | -- | 0.865 | -- | |
| Dubinin–Radushkevich | 19.67 ± 3.39 | -- | 1.29 ± 0.36 | -- | 19.62 ± 2.74 | 0.853 | -- | |
| Oxidized potato starch. | Langmuir | 114.17 ± 2.43 | 0.59 ± 0.11 | -- | -- | -- | 0.988 | −33.0 |
| Sips | 117.50754 ± 2.6239 | 0.57 ± 0.07 | -- | 0.76 ± 0.07 | -- | 0.993 | -- | |
| Dubinin–Radushkevich | 199.07 ± 21.40 | -- | 1.36 ± 0.21 | -- | 19.15 ± 1.44 | 0.930 | -- | |
| Oxidized corn starch | Langmuir | 101.24 ± 3.33 | 0.57 ± 0.16 | -- | -- | -- | 0.975 | −32.9 |
| Sips | 106.68 ± 4.76 | 0.46 ± 0.10 | -- | 0.69 ± 0.11 | -- | 0.982 | -- | |
| Dubinin–Radushkevich | 184.99 ± 20.31 | -- | 1.50 ± 0.22 | -- | 18.25 ± 1.34 | 0.930 | -- |
| Sample | Model | Qm µmol/g | K L/µmol | ßDR × 10−9 mol2/J2 | Eads.DR KJ/mol | R2 (COD) | ΔG° kJ/mol |
|---|---|---|---|---|---|---|---|
| Native potato starch | Langmuir | 10.27 ± 0.90 | 0.32 ± 0.15 | -- | -- | 0.862 | −31.5 |
| Dubinin–Radushkevich | 17.72 ± 5.39 | -- | 1.39 ± 0.2 | 18.95 ± 3.57 | 0.644 | -- | |
| Oxidized potato starch | Langmuir | 87.00 ± 9.13 | 0.04 ± 0.01 | -- | -- | 0.923 | −26.3 |
| Dubinin–Radushkevich | 304.28 ± 113.80 | -- | 3.22 ± 0.7 | 12.44 ± 1.42 | 0.836 | -- | |
| Oxidized corn starch | Langmuir | 65.74 ± 6.37 | 0.14 ± 0.05 | -- | -- | 0.909 | −29.4 |
| Dubinin–Radushkevich | 159.50 ± 49.04 | -- | 2.20 ±0.5 | 15.07 ± 1.91 | 0.804 | -- |
| Sample | Model | Qm µmol/g | K L/µmol * | ßDR × 10−9 mol2/J2 | n | Eads.DR KJ/mol | R2 (COD) | ΔG° kJ/mol |
|---|---|---|---|---|---|---|---|---|
| Native potato starch | Langmuir | 10.97 ± 0.5 | 1.5 ± 0.5 | -- | -- | -- | 0.727 | −31.5 |
| Sips | 11.66 ± 1.2 | 0.9 ± 0.5 | -- | 0.5 ± 0.3 | -- | 0.972 | -- | |
| Dubinin–Radushkevich | 15.39 ± 1.8 | -- | 0.8 ± 0.2 | -- | 24.5 ± 3.5 | 0.672 | -- | |
| Oxidized potato starch | Langmuir | 82.9 ± 1.5 | 0.23 ± 0.03 | -- | -- | -- | 0.989 | −26.3 |
| Sips | 84.0 ± 2.0 | 0.2 ± 0.04 | -- | 0.9 ± 0.1 | -- | 0.990 | -- | |
| Dubinin–Radushkevich | 155.3 ± 17.8 | -- | 0.1 ± 0.02 | -- | 17.6 ± 1.3 | 0.922 | -- | |
| Oxidized corn starch | Langmuir | 64.5 ± 1.5 | 1.5 ± 0.34 | -- | -- | -- | 0.979 | −29.4 |
| Sips | 68.97 ± 2.0 | 07 ± 0.1 | -- | 0.6 ± 0.1 | -- | 0.990 | -- | |
| Dubinin–Radushkevich | 106.6 ± 8.5 | -- | 0.2 ± 1.6 | -- | 20.4 ± 1.3 | 0.944 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boughanmi, R.; Borchert, K.B.L.; Steinbach, C.; Mayer, M.; Schwarz, S.; Svirepa, A.; Schwarz, J.; Mertig, M.; Schwarz, D. Native and Oxidized Starch for Adsorption of Nickel, Iron, and Manganese Ions from Water. Polysaccharides 2022, 3, 556-573. https://doi.org/10.3390/polysaccharides3030033
Boughanmi R, Borchert KBL, Steinbach C, Mayer M, Schwarz S, Svirepa A, Schwarz J, Mertig M, Schwarz D. Native and Oxidized Starch for Adsorption of Nickel, Iron, and Manganese Ions from Water. Polysaccharides. 2022; 3(3):556-573. https://doi.org/10.3390/polysaccharides3030033
Chicago/Turabian StyleBoughanmi, Rahma, Konstantin B. L. Borchert, Christine Steinbach, Martin Mayer, Simona Schwarz, Anastasiya Svirepa, Johannes Schwarz, Michael Mertig, and Dana Schwarz. 2022. "Native and Oxidized Starch for Adsorption of Nickel, Iron, and Manganese Ions from Water" Polysaccharides 3, no. 3: 556-573. https://doi.org/10.3390/polysaccharides3030033
APA StyleBoughanmi, R., Borchert, K. B. L., Steinbach, C., Mayer, M., Schwarz, S., Svirepa, A., Schwarz, J., Mertig, M., & Schwarz, D. (2022). Native and Oxidized Starch for Adsorption of Nickel, Iron, and Manganese Ions from Water. Polysaccharides, 3(3), 556-573. https://doi.org/10.3390/polysaccharides3030033