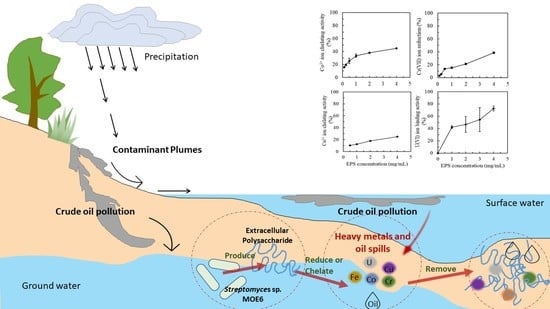
Bioremediation Potential of Streptomyces sp. MOE6 for Toxic Metals and Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Optimization for the Production of MOE6-EPS
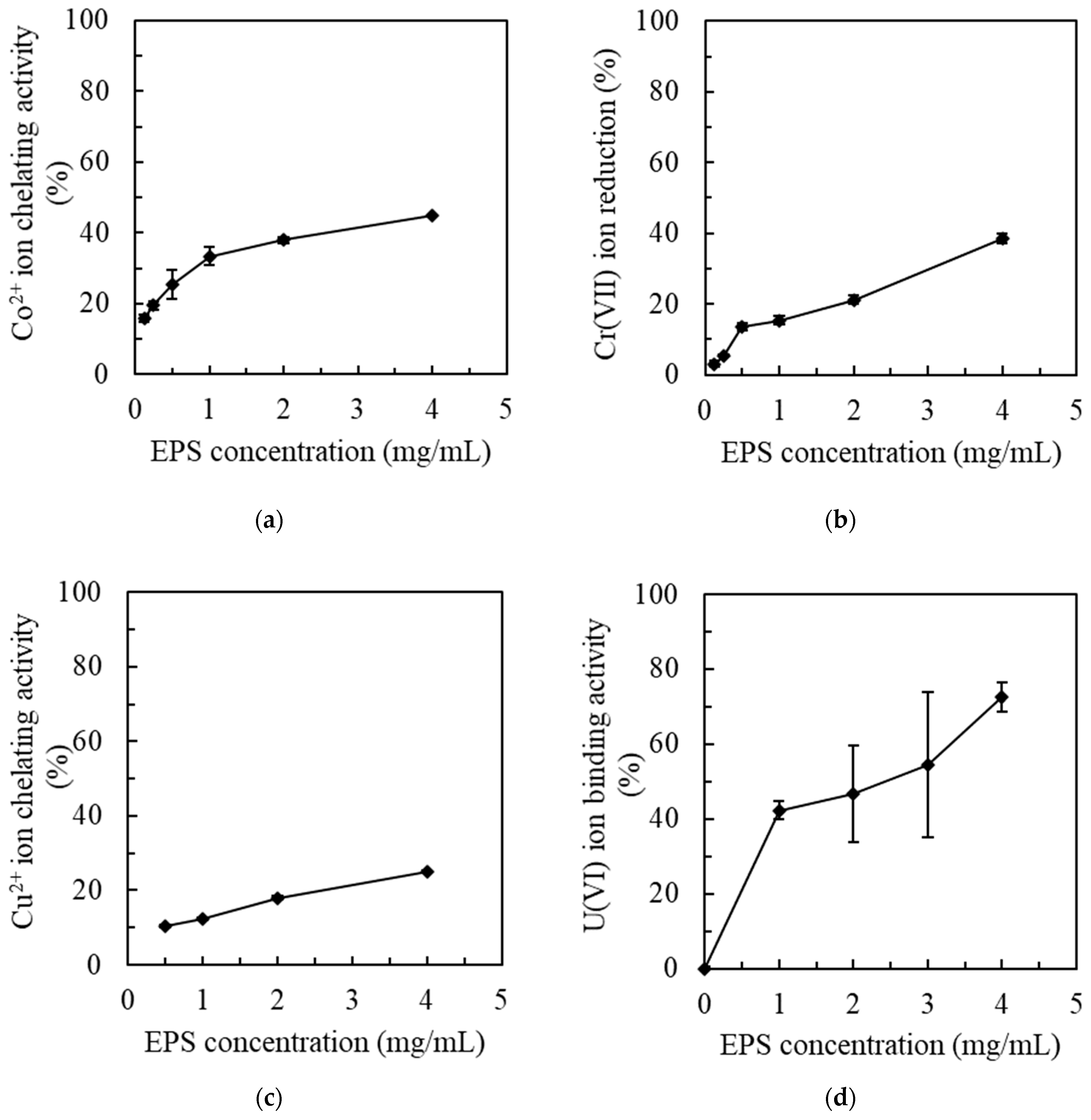
2.3. Metal Removal by MOE6-EPS
2.3.1. Cobalt Removal Assay by MOE6-EPS
2.3.2. Chromium Reduction Assay by MOE6-EPS
2.3.3. Copper Removal Assay by MOE6-EPS
2.3.4. Uranium Removal Assay by MOE6-EPS
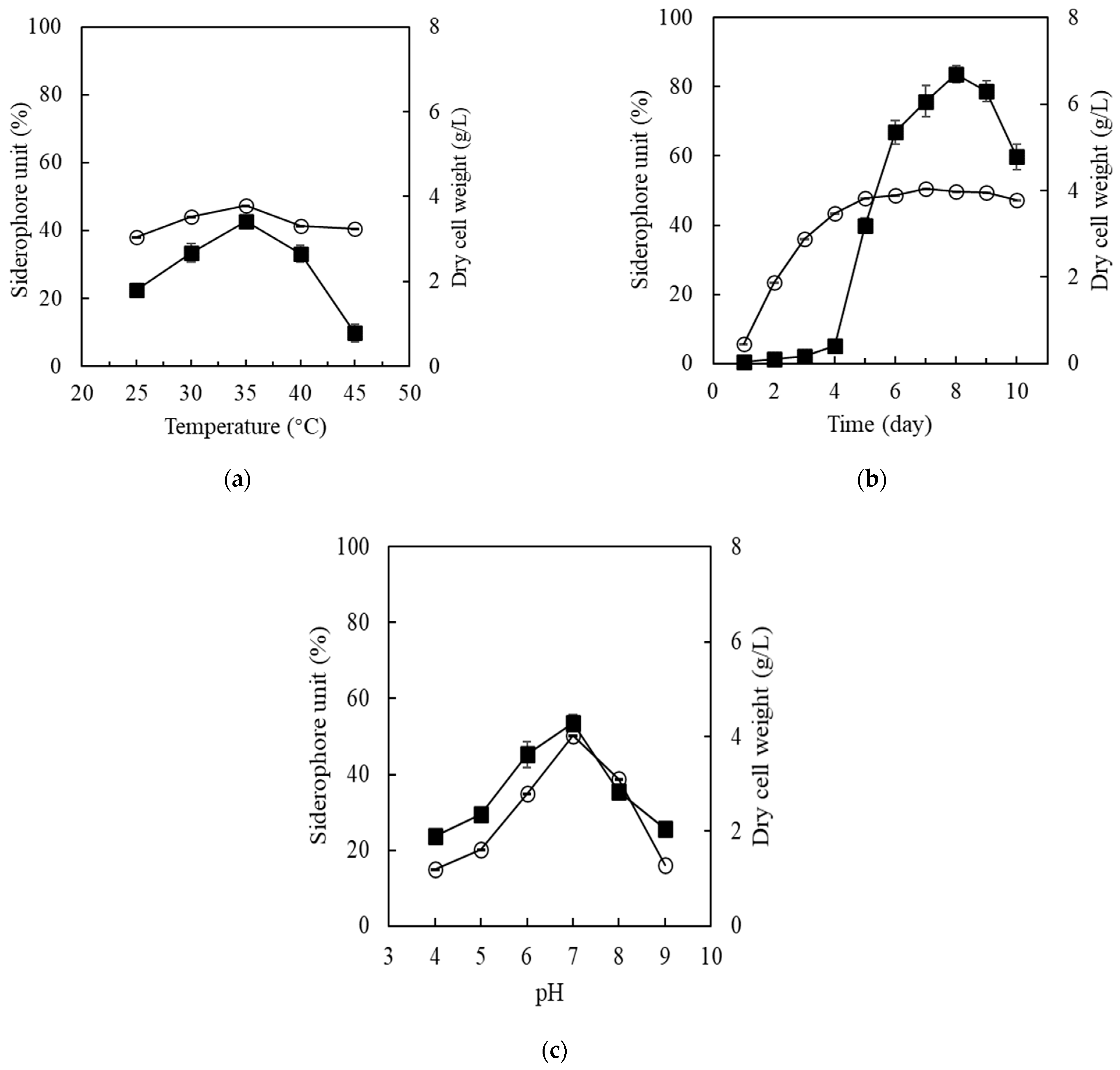
2.4. Qualitative Estimation of Siderophore Produced by Streptomyces sp. MOE6
2.5. Quantitative Estimation of Siderophore Produced by Streptomyces sp. MOE6
2.6. Optimization of Siderophore Production by Streptomyces sp. MOE6
2.7. Emulsifying Activities of MOE6-EPS
3. Results
3.1. Optimization of MOE6-EPS Production
3.2. Metal Sequestration by MOE6-EPS
3.3. Siderophores Production and Activity
3.3.1. Qualitative and Quantitative Detection of Siderophores
3.3.2. Siderophore Optimization
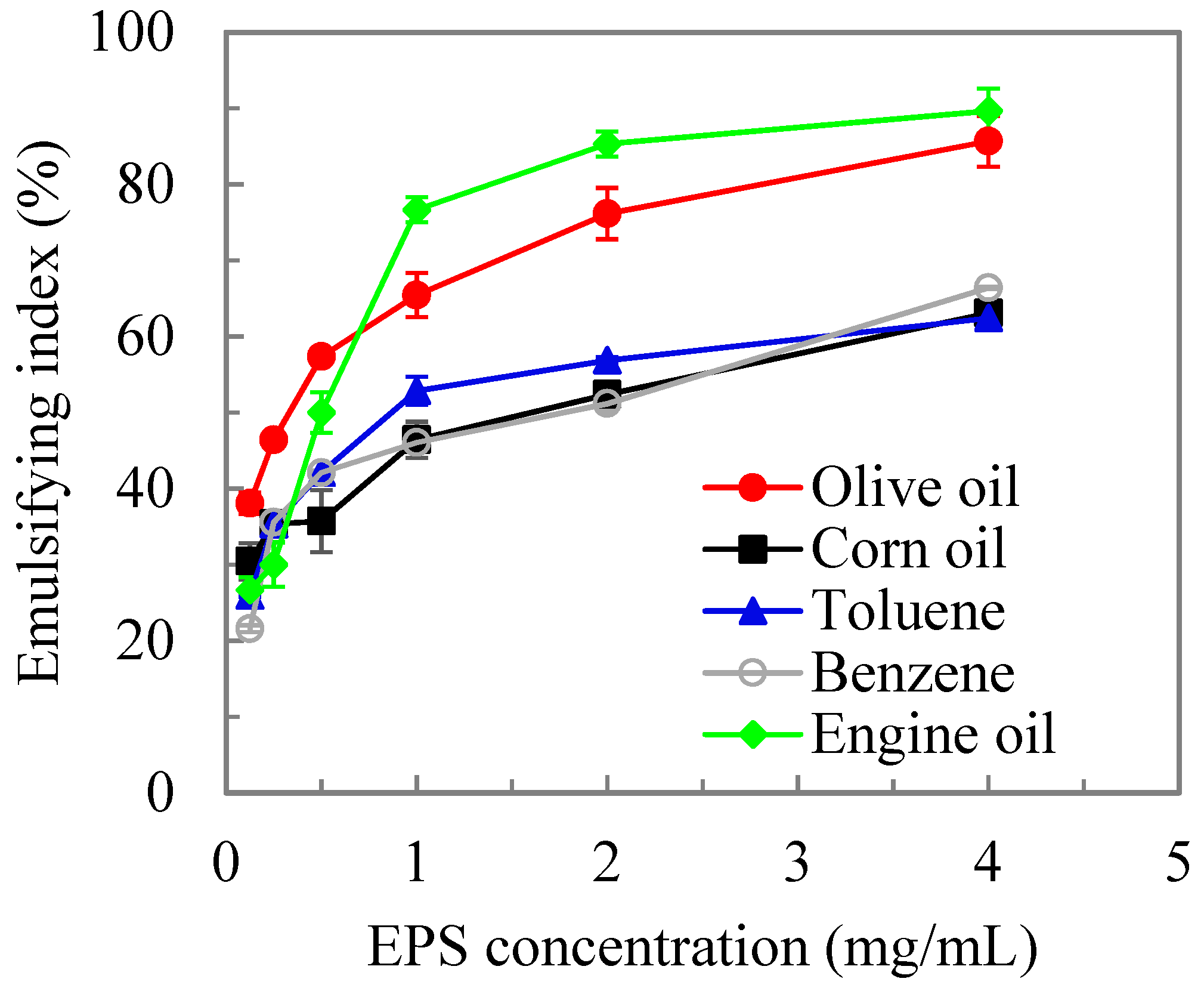
3.4. Emulsification Activity of MOE6-EPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Lakherwal, D. Adsorption of heavy metals: A review. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2016, 13, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Wang, W.-X. Trace metal contamination in estuarine and coastal environments in China. Sci. Total Environ. 2012, 421, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Yu, Z.; Ji, J.; Khan, A.; Li, X. Microbial community structure and function indicate the severity of chromium contamination of the Yellow River. Front. Microbiol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Volesky, B.; Holan, Z. Biosorption of heavy metals. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Removal of heavy metals from the environment by biosorption. Eng. Life Sci. 2004, 4, 219–232. [Google Scholar] [CrossRef]
- Viraraghavan, A.K.A.T. Fungal biosorption-an alternative treatment option for heavy metal bearing waste water. Bioresour. Technol. 1995, 53, 195–206. [Google Scholar] [CrossRef]
- Kapahi, M.; Sachdeva, S. Bioremediation options for heavy metal pollution. J. Health Pollut. 2019, 9, 191203. [Google Scholar] [CrossRef]
- Simeonova, A.G.T.; Ivanova, D.X. Biosorption of heavy metals by dead Streptomyces fradiae. J. Environ. Eng. Sci. 2008, 25, 627–632. [Google Scholar] [CrossRef]
- Hernadez, A.M.R.P.; Martinez, J.L. Metal accumulation and vanadium-induced multidrug resistence by environmental isolates of Escherichia coli and Enterobacter cloacae. Appl. Environ. Microbiol. 1998, 1, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D. Recovery and utilization of chitin and chitosan in food processing waste management. Food Technol. 1991, 45, 114–122. [Google Scholar]
- Hong, K.; Gao, A.-H.; Xie, Q.-Y.; Gao, H.G.; Zhuang, L.; Lin, H.-P.; Yu, H.-P.; Li, J.; Yao, X.-S.; Goodfellow, M. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs 2009, 7, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Albarracín, V.H.; Amoroso, M.J.; Abate, C.M. Isolation and characterization of indigenous copper resistant actinomycete strains. Geochemistry 2005, 65, 145–156. [Google Scholar] [CrossRef]
- Lucas, X.; Senger, C.; Erxleben, A.; Grüning, B.A.; Döring, K.; Mosch, J.; Flemming, S.; Günther, S. StreptomeDB: A resource for natural compounds isolated from Streptomyces species. Nucleic Acids Res. 2013, 41, D1130–D1136. [Google Scholar] [CrossRef]
- Mokrane, S.; Bouras, N.; Sabaou, N.; Mathieu, F. Actinomycetes from saline and non-saline soils of Saharan palm groves: Taxonomy, ecology and antagonistic properties. Afr. J. Microbiol. Res. 2013, 7, 2167–2178. [Google Scholar] [CrossRef][Green Version]
- Schütze, E.; Klose, M.; Merten, D.; Nietzsche, S.; Senftleben, D.; Roth, M.; Kothe, E. Growth of streptomycetes in soil and their impact on bioremediation. J. Hazard. Mater. 2014, 267, 128–135. [Google Scholar] [CrossRef]
- Welman, A.D.; Maddox, I.S. Exopolysaccharides from lactic acid bacteria: Perspectives and challenges. Trends Biotechnol. 2003, 21, 269–274. [Google Scholar] [CrossRef]
- Leigh, J.A.; Coplin, D.L. Exopolysaccharides in plant-bacterial interactions. Annu. Rev. Microbiol. 1992, 46, 307–346. [Google Scholar] [CrossRef]
- Cescutti, P. Bacterial Capsular Polysaccharides and Exopolysaccharides. In Microbial Glycobiology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 93–108. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, T.; Shimmield, T.; Haidon, C.; Black, K.; Green, D.H. Emulsifying and metal ion binding activity of a glycoprotein exopolymer produced by Pseudoalteromonas sp. strain TG12. Appl. Environ. Microbiol. 2008, 74, 4867–4876. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tian, H.; Xiang, D. Stabilizing the oil-in-water emulsions using the mixtures of Dendrobium officinale polysaccharides and gum arabic or propylene glycol alginate. Molecules 2020, 25, 759. [Google Scholar] [CrossRef]
- Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Recent advances in polysaccharides stabilized emulsions for encapsulation and delivery of bioactive food ingredients: A review. Carbohydr. Polym. 2020, 116388. [Google Scholar] [CrossRef]
- Garcıa-Ochoa, F.; Santos, V.; Casas, J.; Gomez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Schütze, E.; Ahmed, E.; Voit, A.; Klose, M.; Greyer, M.; Svatoš, A.; Merten, D.; Roth, M.; Holmström, S.J.; Kothe, E. Siderophore production by streptomycetes—Stability and alteration of ferrihydroxamates in heavy metal-contaminated soil. Environ. Sci. Pollut. Res. 2015, 22, 19376–19383. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Svatoš, A.; Dabrowska, P.; Schmidt, A.; Boland, W.; Kothe, E. Involvement of siderophores in the reduction of metalinduced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 2008, 74, 19–25. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Edberg, F.; Kalinowski, B.; Holmstrom, S.J.; Holm, K. Mobilization of metals from uranium mine waste: The role of pyoverdines produced by Pseudomonas fluorescens. Geobiology 2010, 8, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Elnahas, M.O.; Amin, M.A.; Hussein, M.; Shanbhag, V.C.; Ali, A.E.; Wall, J.D. Isolation, characterization and bioactivities of an extracellular polysaccharide produced from Streptomyces sp. MOE6. Molecules 2017, 22, 1396. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Sivasankar, P.; Venkatesan, J.; Senthilkumar, K.; Sivakumar, K.; Kim, S.K. Production and characterization of an extracellular polysaccharide from Streptomyces violaceus MM72. Int. J. Biol. Macromol. 2013, 59, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Flashka, H.; Barnard, A.J. Chelates in Analytical Chemistry; Marcel Dekker, Inc.: New York, NY, USA, 1969; Volume 5. [Google Scholar] [CrossRef]
- Bermejo-Martinez, F.; Rodriguez-Campos, J. Photometric determination of copper with DTPA. Microchem. J. 1966, 11, 331–342. [Google Scholar] [CrossRef]
- Johnson, D.; Florence, T. Spectrophotometric determination of uranium (VI) with 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol. Anal. Chim. Acta 1971, 53, 73–79. [Google Scholar] [CrossRef]
- Milagres, A.M.; Machuca, A.; Napoleao, D. Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J. Microbiol. Methods 1999, 37, 1–6. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1997, 160, 46–56. [Google Scholar] [CrossRef]
- El Karkouri, A.; Assou, S.A.; El Hassouni, M. Isolation and screening of actinomycetes producing antimicrobial substances from an extreme Moroccan biotope. Pan Afr. Med. J. 2019, 33. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Amodu, O.S.; Ntwampe, S.K.; Ojumu, T.V. Optimization of biosurfactant production by Bacillus licheniformis STK 01 grown exclusively on Beta vulgaris waste using response surface methodology. BioResources 2014, 9, 5045–5065. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Konnova, S.A.; Sigida, E.N.; Lyubun, E.V.; Muratova, A.Y.; Fedonenko, Y.P.; Elbanna, K. Bioremediation potential of a halophilic Halobacillus sp. strain, EG1HP4QL: Exopolysaccharide production, crude oil degradation, and heavy metal tolerance. Extremophiles 2020, 24, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Method 7196A: Chromium, Hexavalent (Colorimetric), Part of Test Methods for Evaluating Solid Waste, Physical/Chemical Methods. 1992. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/7196a.pdf (accessed on 1 August 2016).
- Cerning, J. Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiol. Lett. 1990, 87, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Duboc, P.; Mollet, B. Applications of exopolysaccharides in the dairy industry. Int. Dairy J. 2001, 11, 759–768. [Google Scholar] [CrossRef]
- Rathour, R.; Gupta, J.; Tyagi, B.; Kumari, T.; Thakur, I.S. Biodegradation of pyrene in soil microcosm by Shewanella sp. ISTPL2, a psychrophilic, alkalophilic and halophilic bacterium. Bioresour. Technol. 2018, 4, 129–136. [Google Scholar] [CrossRef]
- Gupta, J.; Rathour, R.; Singh, R.; Thakur, I.S. Production and characterization of extracellular polymeric substances (EPS) generated by a carbofuran degrading strain Cupriavidus sp. ISTL7. Bioresour. Technol. 2019, 282, 417–424. [Google Scholar] [CrossRef]
- Bezawada, J.; Hoang, N.V.; More, T.T.; Yan, S.; Tyagi, N.; Tyagi, R.D.; Surampalli, R.Y. Production of extracellular polymeric substances (EPS) by Serratia sp. 1 using wastewater sludge as raw material and flocculation activity of the EPS produced. J. Environ. Manag. 2013, 128, 83–91. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- Trabelsi, L.; M’sakni, N.H.; Ben Ouada, H.; Bacha, H.; Roudesli, S. Partial characterization of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnol. Bioprocess Eng. 2009, 14, 27–31. [Google Scholar] [CrossRef]
- Chentir, I.; Hamdi, M.; Doumandji, A.; HadjSadok, A.; Ouada, H.B.; Nasri, M.; Jridi, M. Enhancement of extracellular polymeric substances (EPS) production in Spirulina (Arthrospira sp.) by two-step cultivation process and partial characterization of their polysaccharidic moiety. Int. J. Biol. Macromol. 2017, 105, 1412–1420. [Google Scholar] [CrossRef]
- More, T.T.; Yadav, J.S.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances of bacteria and their potential environmental applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hasani, A.; Kariminik, A.; Issazadeh, K. Streptomycetes: Characteristics and their antimicrobial activities. IJABBR 2014, 2, 63–75. [Google Scholar]
- Dezfully, N.K.; Ramanayaka, J.G. Isolation, identification and evaluation of antimicrobial activity of Streptomyces flavogriseus, strain ACTK2 from soil sample of Kodagu, Karnataka State (India). Jundishapur J. Microbiol. 2015, 8, e15107. [Google Scholar] [CrossRef]
- Shu, C.-H.; Lung, M.-Y. Effect of pH on the production and molecular weight distribution of exopolysaccharide by Antrodia camphorata in batch cultures. Process Biochem. 2004, 39, 931–937. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, S.Y.; Lee, H.Y. Bistage control of pH for improving exopolysaccharide production from mycelia of Ganoderma lucidum in an air-lift fermentor. J. Biosci. Bioeng. 1999, 88, 646–650. [Google Scholar] [CrossRef]
- Kim, S.W.; Hwang, H.J.; Xu, C.P.; Sung, J.M.; Choi, J.W.; Yun, J.W. Optimization of submerged culture process for the production of mycelial biomass and exo-polysaccharides by Cordyceps militaris C738. J. Appl. Microbiol. 2003, 94, 120–126. [Google Scholar] [CrossRef]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Scleroglucan. Biopolym. Online 2005. [Google Scholar] [CrossRef]
- Lee, J.W.; Yeomans, W.G.; Allen, A.L.; Deng, F.; Gross, R.A.; Kaplan, D.L. Biosynthesis of novel exopolymers by Aureobasidium pullulans. Appl. Environ. Microbiol. 1999, 65, 5265–5271. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Shi, Y.-P.; Gong, X.-Y. Effects of C/N in the substrate on the simultaneous production of polyhydroxyalkanoates and extracellular polymeric substances by Haloferax mediterranei via kinetic model analysis. RSC Adv. 2017, 7, 18953–18961. [Google Scholar] [CrossRef]
- Miqueleto, A.; Dolosic, C.; Pozzi, E.; Foresti, E.; Zaiat, M. Influence of carbon sources and C/N ratio on EPS production in anaerobic sequencing batch biofilm reactors for wastewater treatment. Bioresour. Technol. 2010, 101, 1324–1330. [Google Scholar] [CrossRef]
- Durmaz, B.; Sanin, F. Effect of carbon to nitrogen ratio on the composition of microbial extracellular polymers in activated sludge. Water Sci. Technol. 2001, 44, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Samadlouie, H.R.; Jahanbin, K.; Jalali, P. Production, medium optimization, and structural characterization of an extracellular polysaccharide produced by Rhodotorula minuta ATCC 10658. Food Sci. Nutr. 2020, 8, 4957–4964. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, A.I.; Chai, X.L.; Katsoyiannis, I.A. The application of bioflocculant for the removal of humic acids from stabilized landfill leachates. J. Environ. Manag. 2004, 70, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Nontembiso, P.; Sekelwa, C.; Leonard, M.V.; Anthony, O.I. Assessment of bioflocculant production by Bacillus sp. Gilbert, a marine bacterium isolated from the bottom sediment of Algoa Bay. Mar. Drugs 2011, 9, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O.O.; Aremu, O.S. Bioflocculant production and heavy metal sorption by metal resistant bacterial isolates from gold mining soil. Chemosphere 2019, 231, 113–120. [Google Scholar] [CrossRef]
- Iyer, A.; Mody, K.; Jha, B. Biosorption of heavy metals by a marine bacterium. Mar. Pollut. Bull. 2005, 50, 340–343. [Google Scholar] [CrossRef]
- Cheung, K.; Gu, J.-D. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: A review. Int. Biodeterior. Biodegrad. 2007, 59, 8–15. [Google Scholar] [CrossRef]
- Polti, M.A.; García, R.O.; Amoroso, M.J.; Abate, C.M. Bioremediation of chromium (VI) contaminated soil by Streptomyces sp. MC1. J. Basic Microbiol. 2009, 49, 285–292. [Google Scholar] [CrossRef]
- Harish, R.; Samuel, J.; Mishra, R.; Chandrasekaran, N.; Mukherjee, A. Bio-reduction of Cr (VI) by exopolysaccharides (EPS) from indigenous bacterial species of Sukinda chromite mine, India. Biodegradation 2012, 23, 487–496. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Shojaosadati, S. Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res. 2003, 37, 4231–4235. [Google Scholar] [CrossRef]
- Hussien, S.S. New studies of uranium biosorption by extracellular polymeric substances (EPSs) of Aspergillus clavatus, using isotherms, thermodynamics and kinetics. Int. J. Environ. Stud. 2020, 1–19. [Google Scholar] [CrossRef]
- Elsalamouny, A.R.; Desouky, O.A.; Mohamed, S.A.; Galhoum, A.A.; Guibal, E. Uranium and neodymium biosorption using novel chelating polysaccharide. Int. J. Biol. Macromol. 2017, 104, 963–968. [Google Scholar] [CrossRef]
- Rashmi, V.; Darshana, A.; Bhuvaneshwari, T.; Saha, S.K.; Uma, L.; Prabaharan, D. Uranium adsorption and oil emulsification by extracellular polysaccharide (EPS) of a halophilic unicellular marine cyanobacterium Synechococcus elongatus BDU130911. Curr. Opin. Green Sustain. Chem. 2020, 100051. [Google Scholar] [CrossRef]
- Santos-Francés, F.; Martínez-Graña, A.; Alonso Rojo, P.; García Sánchez, A. Geochemical background and baseline values determination and spatial distribution of heavy metal pollution in soils of the Andes mountain range (Cajamarca-Huancavelica, Peru). Int. J. Environ. Res. Public Health 2017, 14, 859. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, S.; Mostafa, A. Phytoremediation of contaminated soil with cobalt and chromium. J. Geochem. Explor. 2014, 144, 367–373. [Google Scholar] [CrossRef]
- Nolan, J.; Weber, K.A. Natural uranium contamination in major US aquifers linked to nitrate. Environ. Sci. Technol. Lett. 2015, 2, 215–220. [Google Scholar] [CrossRef]
- Tyagi, B.; Gupta, B.; Thakur, I.S. Biosorption of Cr (VI) from aqueous solution by extracellular polymeric substances (EPS) produced by Parapedobacter sp. ISTM3 strain isolated from Mawsmai cave, Meghalaya, India. Environ. Res. 2020, 191, 110064. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.; Li, A.; Qiu, J.; Feng, L.; Zhou, L.; Zhao, H.-P.; Ma, F. Enhanced recovery of hexavalent chromium by remodeling extracellular polymeric substances through engineering Agrobacterium tumefaciens F2. J. Clean. Prod. 2021, 279, 123829. [Google Scholar] [CrossRef]
- Na, Y.; Lee, J.; Lee, S.H.; Kumar, P.; Kim, J.H.; Patel, R. Removal of heavy metals by polysaccharide: A review. Polym-Plast. Technol. Mater. 2020, 59, 1–21. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, Y.; Li, Z.; Tian, D.; Chen, L.; Chen, P. Chitin nanofibrils for rapid and efficient removal of metal ions from water system. Carbohydr. Polym. 2013, 98, 483–489. [Google Scholar] [CrossRef]
- Pérez, J.A.M.; García-Ribera, R.; Quesada, T.; Aguilera, M.; Ramos-Cormenzana, A.; Monteoliva-Sánchez, M. Biosorption of heavy metals by the exopolysaccharide produced by Paenibacillus jamilae. World J. Microb. Biotechnol. 2008, 24, 2699. [Google Scholar] [CrossRef]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Goyal, P. Reusability of Biomaterial: A Cost-effective Approach. In Novel Biomaterials; Springer: Berlin/Heidelberg, Germany, 2010; pp. 93–96. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Haynes, R.J. A comparison of inorganic solid wastes as adsorbents of heavy metal cations in aqueous solution and their capacity for desorption and regeneration. Water Air Soil Pollut. 2011, 218, 457–470. [Google Scholar] [CrossRef]
- Kanamarlapudi, S.; Chintalpudi, V.K.; Muddada, S. Application of biosorption for removal of heavy metals from wastewater. Biosorption 2018, 18, 69. [Google Scholar] [CrossRef]
- Payne, S.M. Detection, isolation, and characterization of siderophores. In Method Enzymology; Elsevier: Amsterdam, The Netherlands, 1994; Volume 235, pp. 329–344. [Google Scholar] [CrossRef]
- Cruz-Morales, P.; Ramos-Aboites, H.E.; Licona-Cassani, C.; Selem-Mójica, N.; Mejía-Ponce, P.M.; Souza-Saldívar, V.; Barona-Gómez, F. Actinobacteria phylogenomics, selective isolation from an iron oligotrophic environment and siderophore functional characterization, unveil new desferrioxamine traits. FEMS Microbiol. Ecol. 2017, 93, fix086. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Merten, D.; Svatos, A.; Büchel, G.; Kothe, E. Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J. Appl. Microbiol. 2009, 107, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, G. Ecology of siderophores with special reference to the fungi. Biometals 2007, 20, 379. [Google Scholar] [CrossRef] [PubMed]
- Lisiecki, P.; Wysocki, P.; Mikucki, J. Occurrence of siderophores in enterococci. Zent. Bakteriol. 2000, 289, 807–815. [Google Scholar] [CrossRef]
- Sharma, T.; Kumar, N.; Rai, N. Production and optimization of siderophore producing Pseudomonas species isolated from Tarai region of Uttarakhand. Int. J. Pharm. Biol. Sci. 2016, 7, 306–314. [Google Scholar]
- Sacco, L.P.; Castellane, T.C.L.; Polachini, T.C.; de Macedo Lemos, E.G.; Alves, L.M.C. Exopolysaccharides produced by Pandoraea shows emulsifying and anti-biofilm activities. J. Polym. Res. 2019, 26, 91. [Google Scholar] [CrossRef]
- Gunstone, F.D. Fatty acid and Lipid Chemistry; Springer: Berlin/Heidelberg, Germany, 2012; Available online: https://www.springer.com/gp/book/9780751402537 (accessed on 5 January 2020).
- O’Connor, R.T.; Herb, S. Specifications of fatty acid composition for identification of fats and oils by gas liquid chromatography. J. Am. Oil Chem. Soc. 1970, 47, 186A–195A. [Google Scholar] [CrossRef]
- Vinothini, G.; Latha, S.; Arulmozhi, M.; Dhanasekaran, D. Statistical optimization, physio-chemical and bio-functional attributes of a novel exopolysaccharide from probiotic Streptomyces griseorubens GD5. Int. J. Biol. Macromol. 2019, 134, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Satish kumar, R.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Production and purification of a novel exopolysaccharide from lactic acid bacterium Streptococcus phocae PI80 and its functional characteristics activity in vitro. Bioresour. Technol. 2011, 102, 4827–4833. [Google Scholar] [CrossRef] [PubMed]
- Elkhawaga, M.A. Optimization and characterization of biosurfactant from Streptomyces griseoplanus NRRL-ISP5009 (MS1). J. Appl. Microbiol. 2018, 124, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Hayder, N.; Alaa, S.; Alsalim, H. Optimized Conditions for bioemulsifier production by local Streptomyces sp. SS 20 isolated from hydrocarbon contaminated soil. Rom. Biotechnol. Lett. 2014, 19, 8979–8993. [Google Scholar]
- Gutierrez, T.; Berry, D.; Yang, T.; Mishamandani, S.; McKay, L.; Teske, A.; Aitken, M.D. Role of bacterial exopolysaccharides (EPS) in the fate of the oil released during the deepwater horizon oil spill. PLoS ONE 2013, 8, e67717. [Google Scholar] [CrossRef]
- Vane, C.H.; Kim, A.W.; Moss-Hayes, V.; Turner, G.; Mills, K.; Chenery, S.R.; Barlow, T.S.; Kemp, A.C.; Engelhart, S.E.; Hill, T.D.; et al. Organic pollutants, heavy metals and toxicity in oil spill impacted salt marsh sediment cores, Staten Island, New York City, USA. Mar. Pollut. Bull. 2020, 151, 110721. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, J.; Gao, C.; Zhang, Y.; Du, W. A novel exopolysaccharide-producing and long-chain n-alkane degrading bacterium Bacillus licheniformis strain DM-1 with potential application for in-situ enhanced oil recovery. Sci. Rep. 2020, 10, 8519. [Google Scholar] [CrossRef]
- Amani, H.; Müller, M.M.; Syldatk, C.; Hausmann, R. Production of microbial rhamnolipid by Pseudomonas Aeruginosa MM1011 for ex situ enhanced oil recovery. Appl. Biochem. Biotechnol. 2013, 170, 1080–1093. [Google Scholar] [CrossRef]
- Qiu, Y.; Xiao, F.; Wei, X.; Wen, Z.; Chen, S. Improvement of lichenysin production in Bacillus licheniformis by replacement of native promoter of lichenysin biosynthesis operon and medium optimization. Appl. Microbiol. Biotechnol. 2014, 98, 8895–8903. [Google Scholar] [CrossRef]
- Chamanrokh, P.; Assadi, M.M.; Noohi, A.; Yahyai, S. Emulsan analysis produced by locally isolated bacteria and Acinetobacter calcoaceticus RAG-1. J. Environ. Health Sci. Eng. 2008, 5, 101–108. [Google Scholar]
- Karlapudi, A.P.; Venkateswarulu, T.C.; Tammineedi, J.; Kanumuri, L.; Ravuru, B.K.; Dirisala, V.r.; Kodali, V.P. Role of biosurfactants in bioremediation of oil pollution—A review. Petroleum 2018, 4, 241–249. [Google Scholar] [CrossRef]
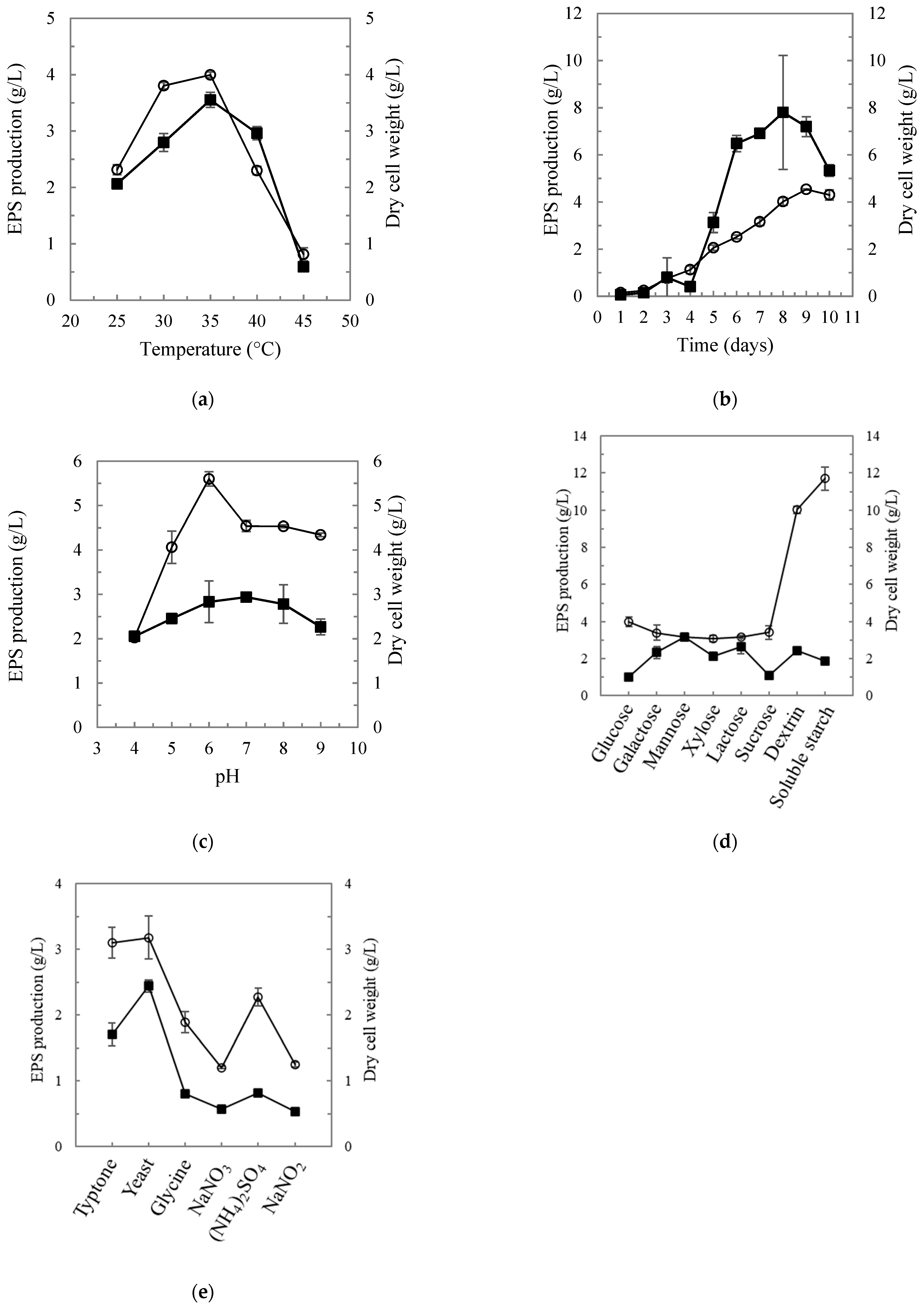

| Carbon Sources (n = 3) | Nitrogen Sources (n = 3) | ||||
|---|---|---|---|---|---|
| Sugars | Crude EPS Yield (g/L) | Biomass Yield (g/L) | Sole N Source | Crude EPS Yield (g/L) | Biomass Yield (g/L) |
| Glucose | 4.0 ± 0.3 | 1.0 ± 0.1 | Tryptone | 3.1 ± 0.2 | 1.7 ± 0.2 |
| Galactose | 3.4 ± 0.4 | 2.3 ± 0.3 | Yeast extract | 3.2 ± 0.3 | 2.4 ± 0.1 |
| Mannose | 3.2 ± 0.1 | 3.2 ± 0.2 | Glycine | 1.9 ± 0.2 | 0.8 ± <0.1 |
| Xylose | 3.1 ± 0.2 | 2.1 ± 0.2 | NaNO3 | 1.2 ± <0.1 | 0.6 ± 0.1 |
| Lactose | 3.2 ± 0.1 | 2.7 ± 0.4 | (NH4)2SO4 | 2.3 ± 0.1 | 0.8 ± <0.1 |
| Sucrose | 3.4 ± 0.4 | 1.1 ± 0.1 | NaNO2 | 1.3 ± <0.1 | 0.5 ± <0.1 |
| Dextrin | 10.0 ± 0.2 | 2.4 ± 0.2 | |||
| Soluble starch | 11.7 ± 0.6 | 1.9 ± 0.1 | |||
| Bio-Based Polymers | Metal Ions | Maximum Biosorption Capacity (mg Metal/g Biopolymer) | Reference |
|---|---|---|---|
| Polysaccharide from Bacillus firmus MS-102 | Pb | 1103 | [73] |
| Cu | 860 | ||
| Zn | 722 | ||
| Extracellular polymeric substances of Aspergillus clavatus | U(VI) | 123.5 | [74] |
| Extracellular polymeric substances of Parapedobacter sp. ISTM3 | Cr(VI) | 33.7 | [80] |
| Extracellular polymeric substances of Agrobacterium tumefaciens F2 | Cr(VI) | 39.9 | [81] |
| Chitin nanofibers | Ni(II) | 134.7 | [83] |
| Zn(II) | 134.0 | ||
| Cd(II) | 330.1 | ||
| Pb(II) | 303.4 | ||
| Cu(II) | 141.0 | ||
| Exopolysaccharide of Paenibacillus jamilae | Pb(II) | 303.0 | [84] |
| Cu(II) | 7.8 | ||
| Zn(II) | 12.3 | ||
| Co(II) | 30.1 | ||
| MOE6-EPS of Streptomyces sp. MOE6 | Co(II) | 13.8 | |
| Cu(II) | 23.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elnahas, M.O.; Hou, L.; Wall, J.D.; Majumder, E.L.-W. Bioremediation Potential of Streptomyces sp. MOE6 for Toxic Metals and Oil. Polysaccharides 2021, 2, 47-68. https://doi.org/10.3390/polysaccharides2010004
Elnahas MO, Hou L, Wall JD, Majumder EL-W. Bioremediation Potential of Streptomyces sp. MOE6 for Toxic Metals and Oil. Polysaccharides. 2021; 2(1):47-68. https://doi.org/10.3390/polysaccharides2010004
Chicago/Turabian StyleElnahas, Marwa O., Liyuan Hou, Judy D. Wall, and Erica L.-W. Majumder. 2021. "Bioremediation Potential of Streptomyces sp. MOE6 for Toxic Metals and Oil" Polysaccharides 2, no. 1: 47-68. https://doi.org/10.3390/polysaccharides2010004
APA StyleElnahas, M. O., Hou, L., Wall, J. D., & Majumder, E. L.-W. (2021). Bioremediation Potential of Streptomyces sp. MOE6 for Toxic Metals and Oil. Polysaccharides, 2(1), 47-68. https://doi.org/10.3390/polysaccharides2010004