Surface Characterization of Powdered Cellulose Activated by Potassium Hydroxide in Dry Condition Through Ball Milling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Powdered Cellulose through Ball Milling
2.3. Co-Milling Powdered Cellulose with Potassium Hydroxide through Ball Milling
2.4. Characterization of Cellulose Powder Samples
2.4.1. X-Ray Photoelectron Spectroscopy (XPS)
2.4.2. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3. Results and Discussion
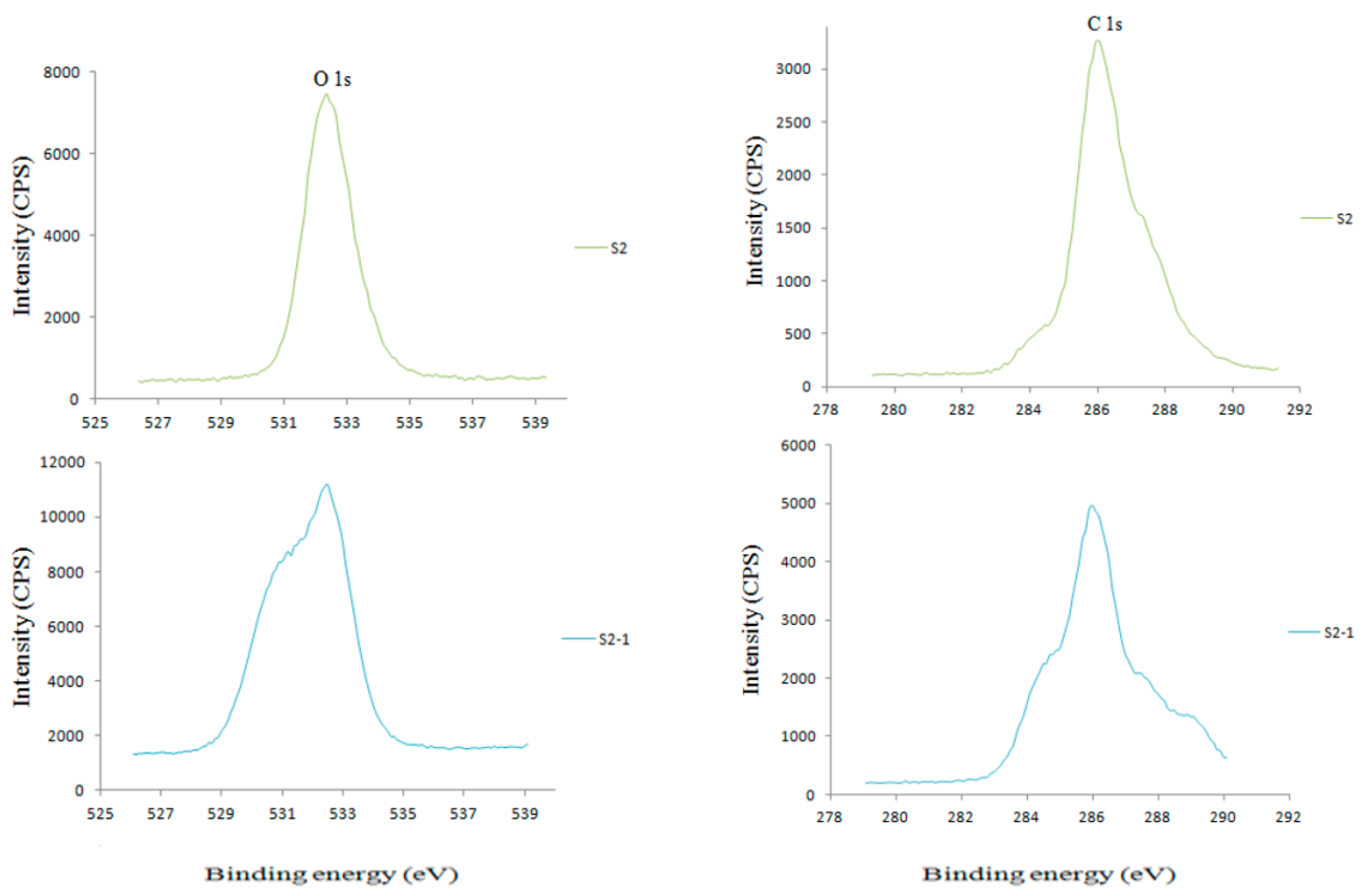
3.1. XPS Analysis
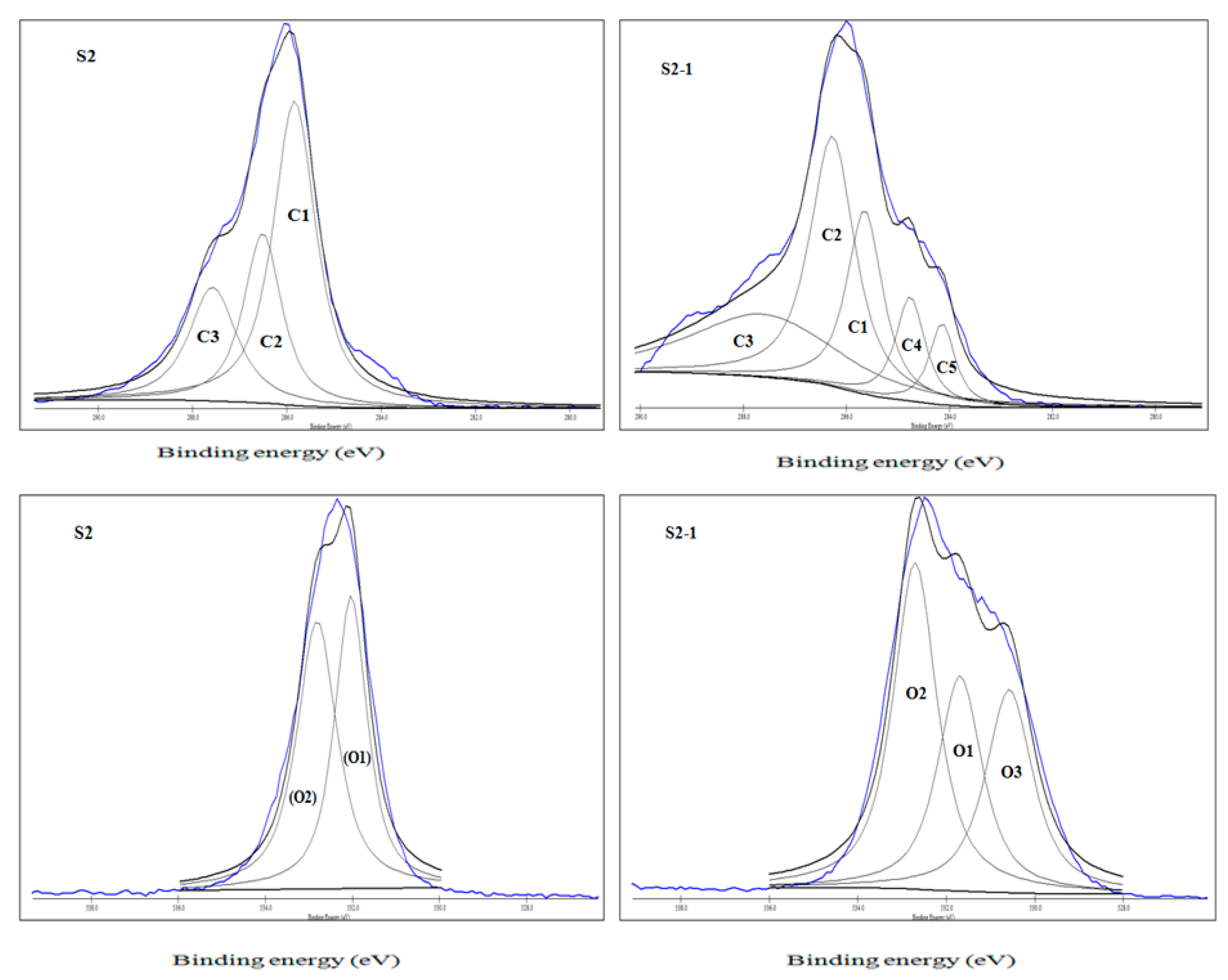
3.1.1. C1s Spectra: Deconvoluted Peaks Analysis
3.1.2. O1s Spectra: Deconvoluted Peaks
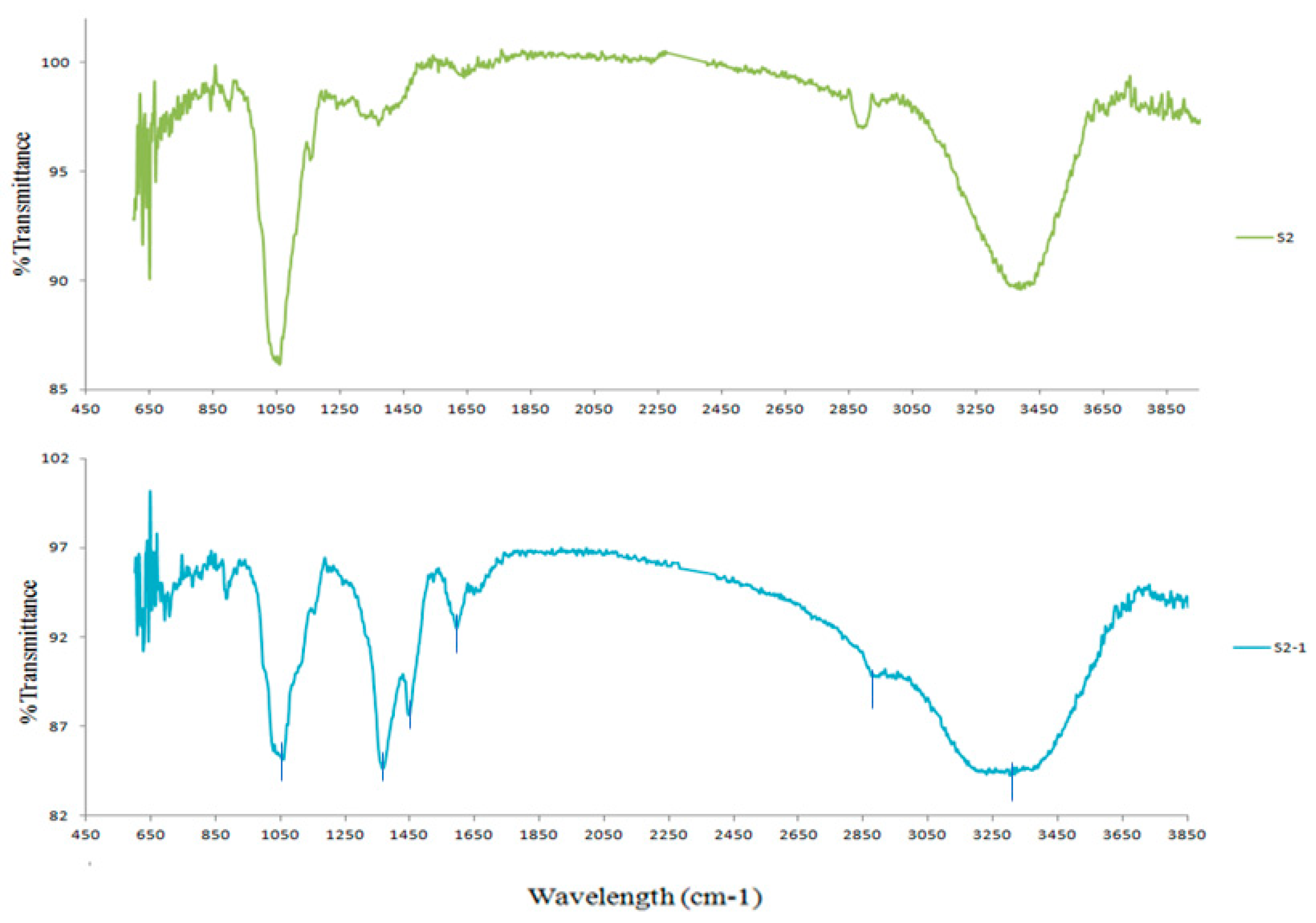
3.2. ATR-FTIR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stütz, A.E. Glycoscience: Epimerisation, Isomerisation and Rearrangement Reactions of Carbohydrates; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Christiansen, J.A. Hypotheses concerning the transition states in mutarotation of pyranoses: To VK La Mer on his seventieth birthday by his friend. J. Colloid Interface Sci. 1966, 1, 1–2. [Google Scholar] [CrossRef]
- Lemieux, R.U.; Stevens, J.D. The proton magnetic resonance spectra and tautomeric equilibria of aldoses in deuterium oxide. Can. J. Chem. 1966, 3, 249–262. [Google Scholar] [CrossRef]
- Ward, P.; Isbell, H. Mutarotation of sugars in solution: Part I: History, basic kinetics, and composition of sugar solutions. Adv. Carbohydr. Chem. 1968, 23, 11–57. [Google Scholar]
- Le Barch, N.; Grossel, J.M.; Looten, P.; Mathlouthi, M. Kinetic study of the mutarotation of D-glucose in concentrated aqueous solution by gas-liquid chromatography. Food Chem. 2001, 1, 119–124. [Google Scholar] [CrossRef]
- Rony, P.R. Polyfunctional catalysis. I. Activation parameters for the mutarotation of tetramethyl-D-glucose in benzene. J. Am. Chem. Soc. 1968, 11, 2824–2831. [Google Scholar] [CrossRef]
- Fiandanese, V.; Naso, F. Benzamidine-catalysed mutarotation of 2, 3, 4, 6-tetra-O-methyl-D-glucose. J. Chem. Soc. Perkin Trans. 1977, 8, 1047–1051. [Google Scholar] [CrossRef]
- Morpurgo, S.; Grandi, A.; Zazza, C.; Bossa, M. A theoretical study on the sugars’ mutarotation: The epimerisation of 2-tetrahydropyranol catalysed by formamidine, benzamidine and by the 2-aminopyridine/2-iminopyridine tautomeric couple. J. Mol. Struct. THEOCHEM 2005, 1, 71–82. [Google Scholar] [CrossRef]
- Rony, P.R.; McCormac, W.E.; Wunderly, S.W. Polyfunctional catalysis. II. General base catalysis of the mutarotation of tetramethyl-D-gluose in benzene and methanol-benzene. J. Am. Chem. Soc. 1969, 15, 4244–4251. [Google Scholar] [CrossRef]
- Brown, G.M.; Levy, H.A. α-d-Glucose: Further refinement based on neutron-diffraction data. Acta Crystallographica Sect. B Struct. Crystallogr. Cryst. Chem. 1979, 3, 656–659. [Google Scholar] [CrossRef]
- Fisher, J. Modern NMR Techniques for Synthetic Chemistry; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Dujardin, N.; Willart, J.F.; Dudognon, E.; Danede, F.; Descamps, M. First obtaining of glass solutions and phase diagram of glucose with fully tunable anomeric concentration. J. Pharm. Sci. 2010, 3, 1476–1483. [Google Scholar] [CrossRef]
- Dujardin, N.; Willart, J.F.; Dudognon, E.; Hédoux, A.; Guinet, Y.; Paccou, L.; Chazallon, B.; Descamps, M. Solid state vitrification of crystalline α and β-D-glucose by mechanical milling. Solid State Commun. 2008, 1, 78–82. [Google Scholar] [CrossRef]
- Willart, J.F.; Carpentier, L.; Danède, F.; Descamps, M. Solid-state vitrification of crystalline griseofulvin by mechanical milling. J. Pharm. Sci. 2012, 4, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Broido, A.; Houminer, Y.; Patai, S. Pyrolytic reactions of carbohydrates. Part I. Mutarotation of molten D-glucose. J. Chem. Soc. B Phys. Org. 1966, 411–414. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Órfão, J.J.; Pereira, M.F.R. Enhanced direct production of sorbitol by cellulose ball-milling. Green Chem. 2015, 5, 2973–2980. [Google Scholar] [CrossRef]
- Paakkari, T.; Serimaa, R.; Fink, H.P. Structure of amorphous cellulose. Acta Polymerica 1989, 12, 731–734. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, J.; Zhang, X.; Wolcott, M. Evaluation of physical structural features on influencing enzymatic hydrolysis efficiency of micronized wood. RSC Adv. 2016, 105, 103026–103034. [Google Scholar] [CrossRef]
- Mikushina, I.V.; Troitskaya, I.B.; Dushkin, A.V.; Bazarnova, N.G. Changes in the chemical composition of wood during mechanochemical treatment. Chem. Sust. Dev. 2002, 4, 441–445. [Google Scholar]
- Azadfar, M.; Graham, M.R.; Wolcott, M.P. Effect of Cellulose Reducing Ends on the Reinforcing Capacity of Powdered Cellulose in Polypropylene Composites. J. Compos. Sci. 2019, 3, 98. [Google Scholar] [CrossRef] [Green Version]
- Yaylayan, V.A.; Ismail, A.A. Investigation of the enolization and carbonyl group migration in reducing sugars by FTIR spectroscopy. Carbohydr. Res. 1995, 2, 253–265. [Google Scholar] [CrossRef]
- Shaffer, P.A.; Friedemann, T.E. Sugar activation by alkali I. Formation of lactic and saccharinic acids. J. Biol. Chem. 1930, 1, 345–374. [Google Scholar]
- Braun, M. Modern Enolate Chemistry: From Preparation to Applications in Asymmetric Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Sinnott, M. Carbohydrate Chemistry and Biochemistry: Structure and Mechanism; Royal Society of Chemistry: London, UK, 2007. [Google Scholar]
- Menuel, S.; Doumert, B.; Saitzek, S.; Ponchel, A.; Delevoye, L.; Monflier, E.; Hapiot, F. Selective secondary face modification of cyclodextrins by mechanosynthesis. J. Org. Chem. 2015, 12, 6259–6266. [Google Scholar] [CrossRef] [PubMed]
- Matuana, L.M.; Balatinecz, J.J.; Park, C.B.; Sodhi, R.N.S. X-ray photoelectron spectroscopy study of silane-treated newsprint-fibers. Wood Sci. Technol. 1999, 4, 259–270. [Google Scholar] [CrossRef]
- Kovac, J. Surface characterization of polymers by XPS and SIMS techniques. Mater. Tehnol. 2011, 45, 191–197. [Google Scholar]
- Azadfar, M.; Gao, A.H.; Bule, M.V.; Chen, S. Structural characterization of lignin: A potential source of antioxidants guaiacol and 4-vinylguaiacol. Int. J. Biol. Macromol. 2015, 75, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Kamdem, D.P.; Riedl, B.; Adnot, A.; Kaliaguine, S. ESCA spectroscopy of poly (methyl methacrylate) grafted onto wood fibers. J. Appl. Polym. Sci. 1991, 10, 1901–1912. [Google Scholar] [CrossRef]
- Johansson, L.S.; Campbell, J.M. Reproducible XPS on biopolymers: Cellulose studies. Surf. Interface Anal. 2004, 8, 1018–1022. [Google Scholar] [CrossRef]
- Johansson, L.S.; Campbell, J.M.; Hänninen, T.; Ganne-Chèdeville, C.; Vuorinen, T.; Hughes, M.; Laine, J. XPS and the medium-dependent surface adaptation of cellulose in wood. Surf. Interface Anal. 2012, 8, 899–903. [Google Scholar] [CrossRef]
- Stark, N.M.; Matuana, L.M. Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy. Polym. Degrad. Stab. 2004, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.P.; Rials, T.G.; Simonsen, J. Relationship of wood surface energy to surface composition. Langmuir 1998, 2, 536–541. [Google Scholar] [CrossRef]
- Nzokou, P.; Kamdem, D.P. X-ray photoelectron spectroscopy study of red oak-(Quercus rubra), black cherry-(Prunus serotina) and red pine-(Pinus resinosa) extracted wood surfaces. Surf. Interface Anal. 2005, 8, 689–694. [Google Scholar] [CrossRef]
- Pizzi, A.; Mittal, K.L. Handbook of Adhesive Technology, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Sun, X.F.; Sun, R.; Sun, J.X. Acetylation of rice straw with or without catalysts and its characterization as a natural sorbent in oil spill cleanup. J. Agric. Food Chem. 2002, 22, 6428–6433. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; Ismail, H.; Rozman, H.D.; Ahmad, M.N. The effect of acetylation on interfacial shear strength between plant fibers and various matrices. Eur. Polym. J. 2001, 5, 1037–1045. [Google Scholar] [CrossRef]
- Huang, L.; Ye, Z.; Berry, R. Modification of cellulose nanocrystals with quaternary ammonium-containing hyperbranched polyethylene ionomers by ionic assembly. ACS Sustain. Chem. Eng. 2016, 9, 4937–4950. [Google Scholar] [CrossRef]

| Sample | % C | % O | % K | O/C |
|---|---|---|---|---|
| S2 | 57.36 | 42.64 | 0.74 | |
| S2-1 | 40.76 | 41.4 | 9.79 | 1.01 |
| Group | Symbol | Carbon Atom or Oxygen Atom Bonded to |
|---|---|---|
| Carbon | ||
| I | C1 | C-C/C-H |
| II | C2 | C-OH |
| III | C3 | C=O, O-C-O |
| IV | C4 | -C*=C(OH)-CHO -C=C*(OH)-CHO -C-C(O)-C*HO |
| V | C5 | |
| Oxygen | ||
| I | O1 | C=O*: (-(C6H10O5)-)n C-O*-C: (-(C6H10O5)-)n |
| II | O2 | |
| III | O3 | -C=C(OH)-CHO* -C-C(O*)-CHO |
| Carbon Type | Binding Energy (eV) | Intensity (CPS) |
|---|---|---|
| S2 | ||
| C1 | 285.834 | 3082 |
| C2 | 286.511 | 2573.33 |
| C3 | 287.568 | 1423.33 |
| S2-1 | ||
| C1 | 285.645 | 3998 |
| C2 | 286.273 | 4782.66 |
| C3 | 287.632 | 2012.66 |
| C4 | 284.761 | 2399.33 |
| C5 | 284.139 | 1748 |
| Oxygen Type | Binding Energy (eV) | Intensity (CPS) |
|---|---|---|
| S2 | ||
| O1 | 532.032 | 6880 |
| O2 | 532.812 | 5944.44 |
| S2-1 | ||
| O1 | 531.691 | 9206.63 |
| O2 | 532.706 | 10,463.55 |
| O3 | 530.577 | 7575.66 |
| Assignment | Cellulose Powder Samples | |
|---|---|---|
| λ (cm−1) | T (%) | |
| –OH stretching vibrations | 3380 (S2), 3290(S2-1) | 89.6(S2), 84.2(S2-1) |
| –CH stretching vibrations (-CH2-, -CH3) | 2900 (S2), 2890 (S2-1) | 97.1 (S2), 89.7 (S2-1) |
| C=C stretching vibrations | 1590 (S2-1) | 92.90 (S2-1) |
| -C-H stretching vibrations | 1440–1370 (S2-1) | 87.6–84.6 (S2-1) |
| CH2 and C-H bending in cellulose | 1370 (S2) | 97.1 (S2) |
| C-O and C-O-C stretching vibrations in cellulose | 1050 (S2), 1050 (S2-1) | 86.2 (S2), 85.2 (S2-1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azadfar, M.; Wolcott, M.P. Surface Characterization of Powdered Cellulose Activated by Potassium Hydroxide in Dry Condition Through Ball Milling. Polysaccharides 2020, 1, 80-89. https://doi.org/10.3390/polysaccharides1010006
Azadfar M, Wolcott MP. Surface Characterization of Powdered Cellulose Activated by Potassium Hydroxide in Dry Condition Through Ball Milling. Polysaccharides. 2020; 1(1):80-89. https://doi.org/10.3390/polysaccharides1010006
Chicago/Turabian StyleAzadfar, Mohammadali, and Michael P. Wolcott. 2020. "Surface Characterization of Powdered Cellulose Activated by Potassium Hydroxide in Dry Condition Through Ball Milling" Polysaccharides 1, no. 1: 80-89. https://doi.org/10.3390/polysaccharides1010006
APA StyleAzadfar, M., & Wolcott, M. P. (2020). Surface Characterization of Powdered Cellulose Activated by Potassium Hydroxide in Dry Condition Through Ball Milling. Polysaccharides, 1(1), 80-89. https://doi.org/10.3390/polysaccharides1010006






