The Effect of Different Extraction Conditions on the Physical Properties, Conformation and Branching of Pectins Extracted from Cucumis melo Inodorus
Abstract
:1. Introduction
2. Materials and Methods
2.1. High-Performance Size Exclusion Chromatography Coupled to a Differential Pressure Viscometer (HPSEC-DPV)
2.2. Estimation of Conformation
2.2.1. Translational Frictional Ratio
2.2.2. Persistence Length (Lp) and Mass Per Unit Length (ML)
2.2.3. Conformation Zoning (Normalised Scaling Relations)
2.3. Statistical Analysis
3. Results and Discussion
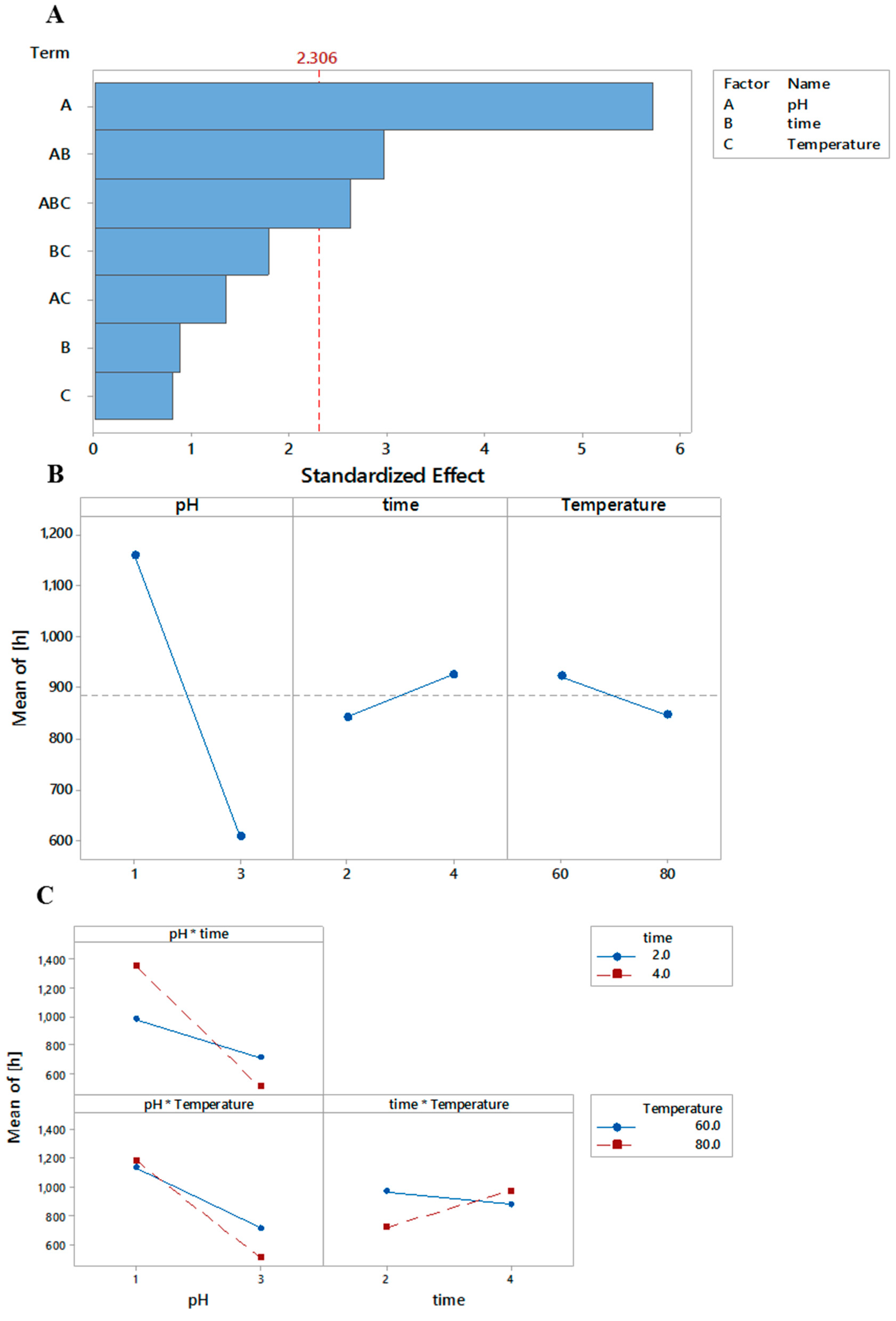
3.1. Intrinsic Viscosity ([η]w)
r2 = 0.87
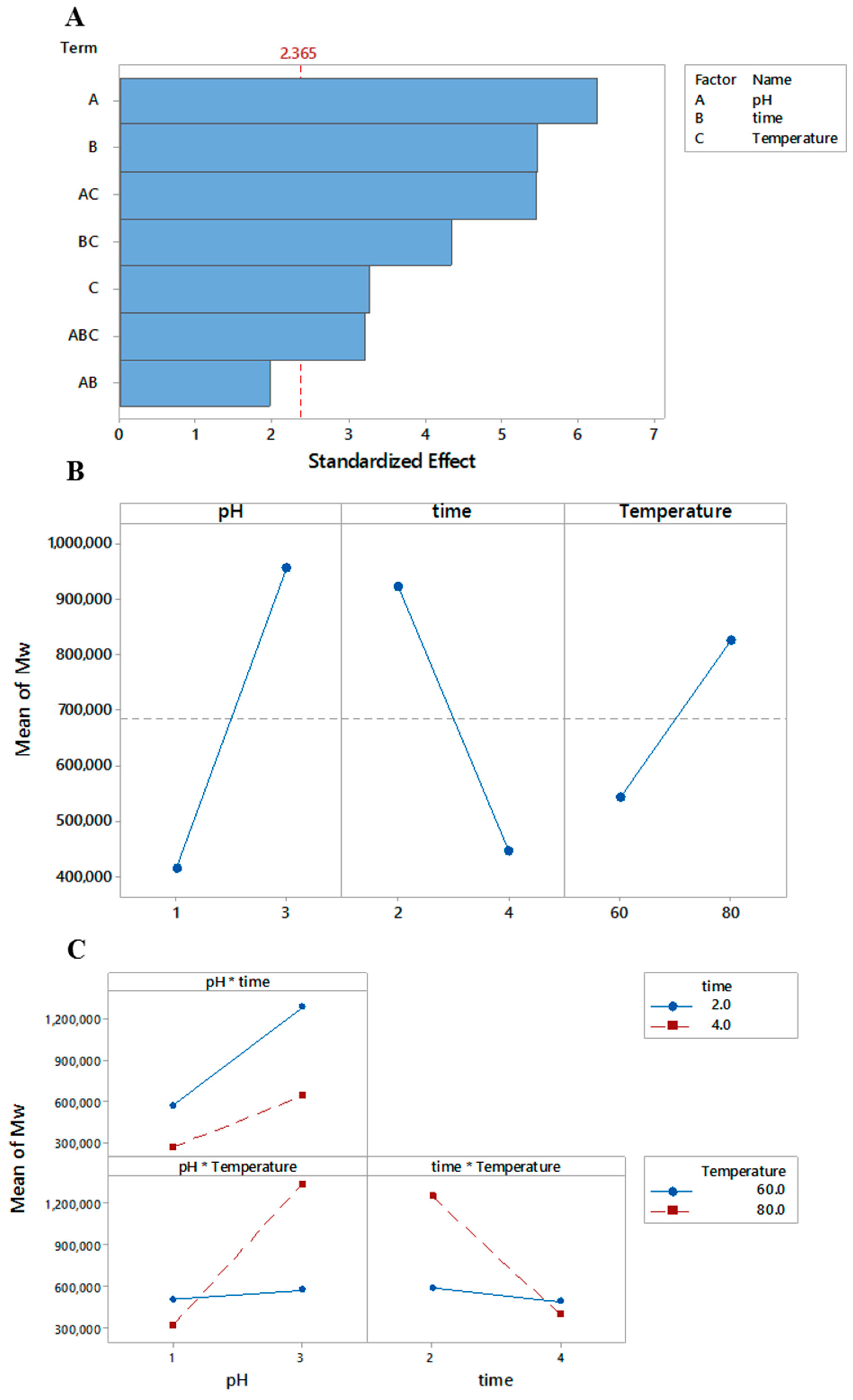
3.2. Molar Mass (Mn)
r2 = 0.93
3.3. Estimation of Conformation
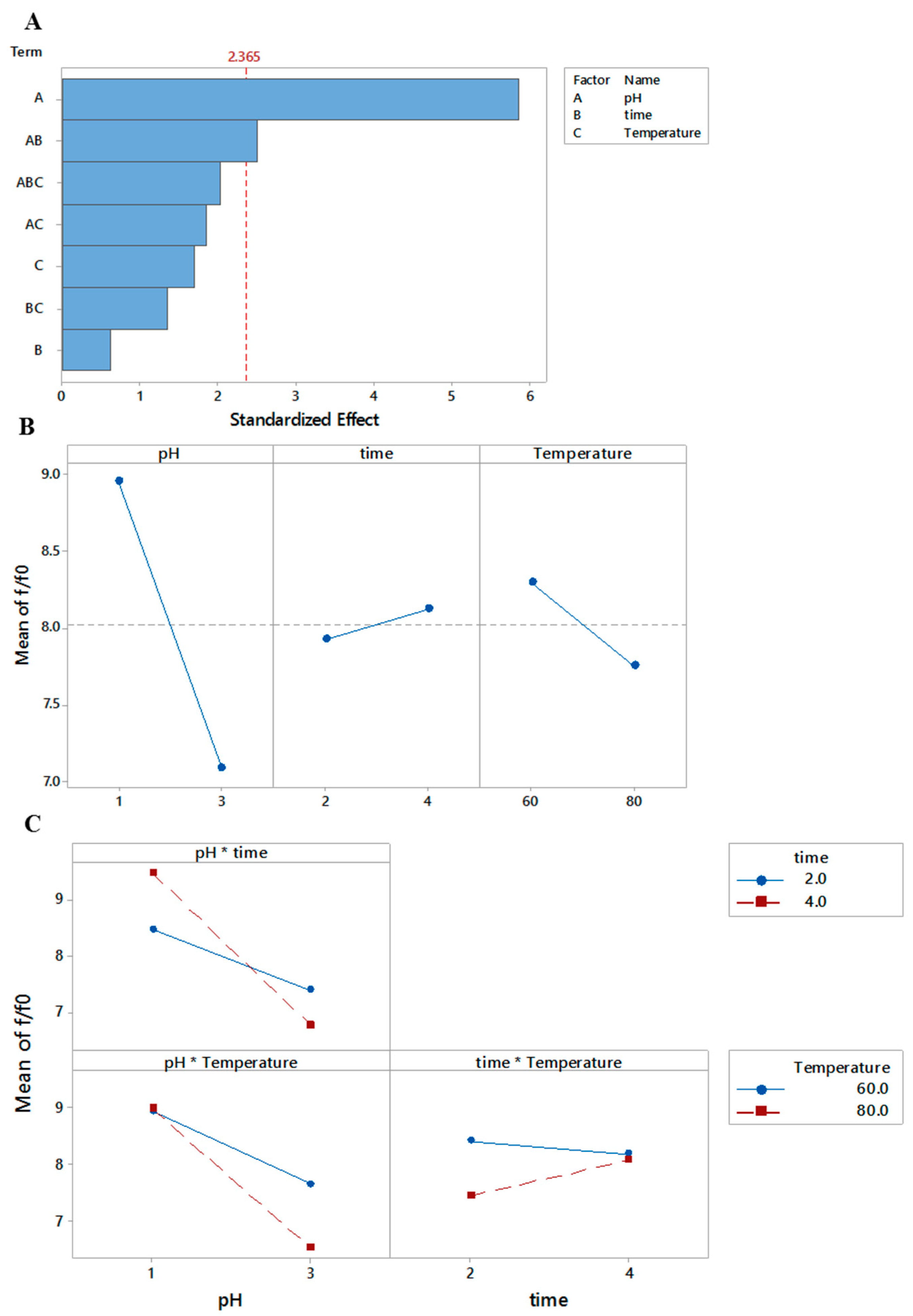
3.3.1. Translational Frictional Ratio
r2 = 0.89
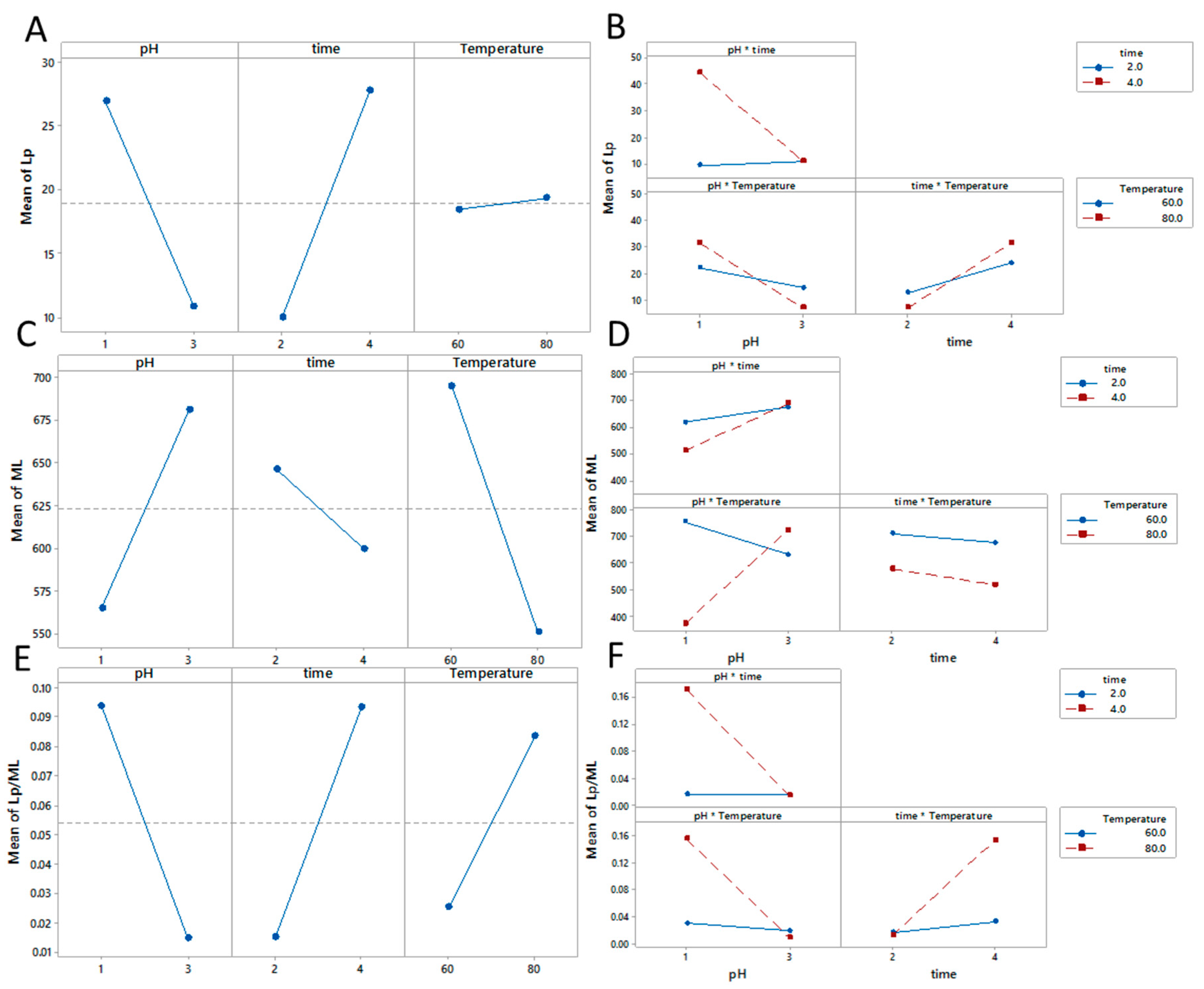
3.3.2. Persistence Length (Lp) and Mass Per Unit length (ML)
r2 = 0.85
r2 = 0.51
r2 = 0.69
3.3.3. Conformation Zoning (Normalised Scaling Relations)
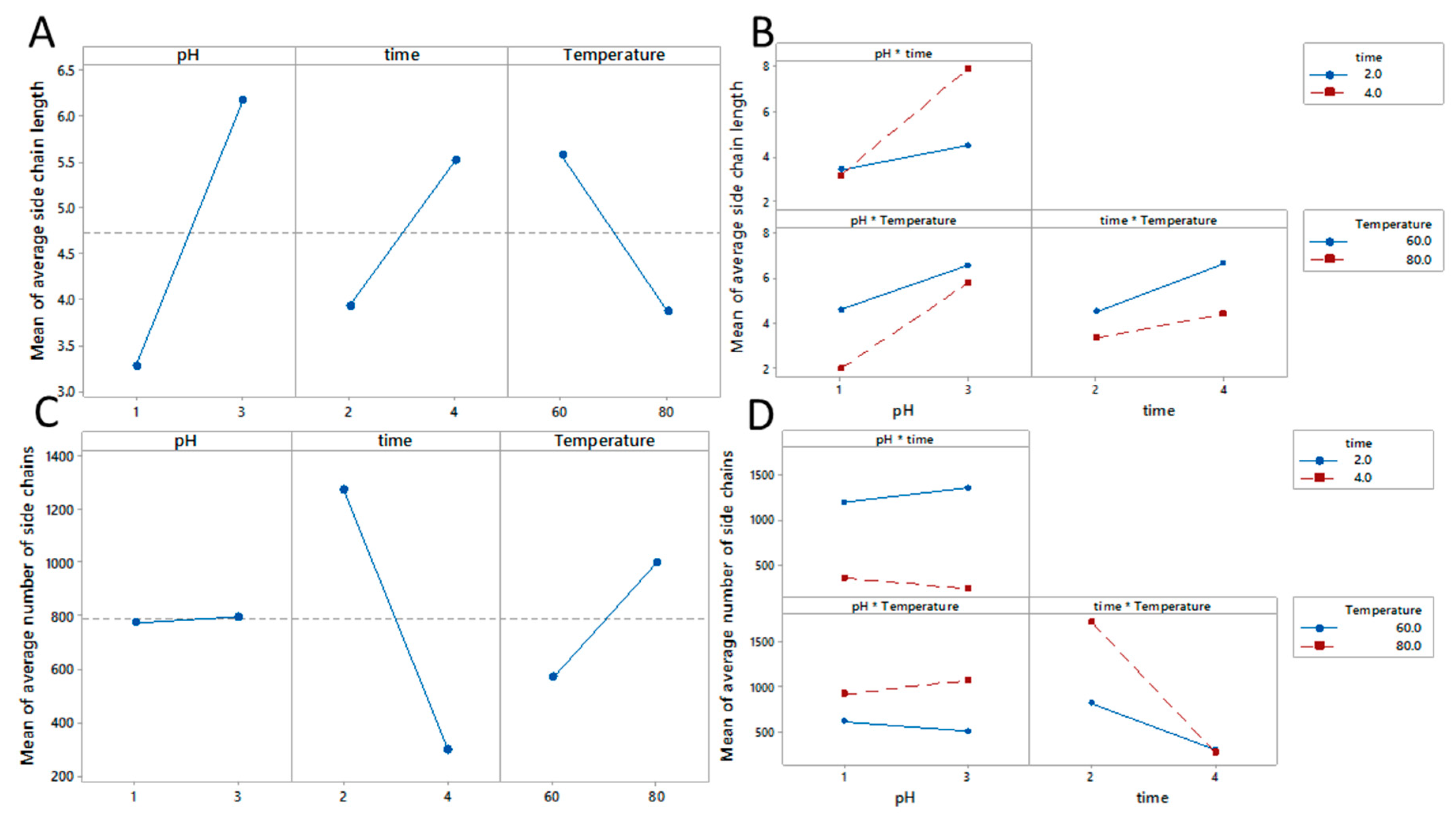
3.4. Estimation of Branching
r2 = 0.75
r2 = 0.58
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harding, S.E.; Tombs, M.; Adams, G.; Smestad Paulsen, B.; Inngjerdingen, K.; Barsett, H. An Introduction to Polysaccharide Biotechnology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Willats, W.G.T.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Comparative study of the cell wall composition of broccoli, carrot, and tomato: Structural characterization of the extractable pectins and hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Zhang, F.; Yang, X.; Ni, L.; Zhang, W.; Liu, Z.; Zhang, Y. Improving viscosity and gelling properties of leaf pectin by comparing five pectin extraction methods using green tea leaf as a model material. Food Hydrocoll. 2020, 98, 105246. [Google Scholar] [CrossRef]
- Pi, F.; Liu, Z.; Guo, X.; Guo, X.; Meng, H. Chicory root pulp pectin as an emulsifier as compared to sugar beet pectin. Part 1: Influence of structure, concentration, counterion concentration. Food Hydrocoll. 2019, 89, 792–801. [Google Scholar] [CrossRef]
- Fracasso, A.F.; Perussello, C.A.; Carpiné, D.; Petkowicz, C.L.d.O.; Haminiuk, C.W.I. Chemical modification of citrus pectin: Structural, physical and rheologial implications. Int. J. Biol. Macromol. 2018, 109, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U.S.; Koch, L.; Rentschler, C.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Effect of Molecular Weight Reduction, Acetylation and Esterification on the Emulsification Properties of Citrus Pectin. Food Biophys. 2015, 10, 217–227. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Zhao, S.; Tian, G.; Wang, F.; Li, C.; Wang, F.; Zheng, J. Alkali + cellulase-extracted citrus pectins exhibit compact conformation and good fermentation properties. Food Hydrocoll. 2020, 106079. [Google Scholar] [CrossRef]
- Nergard, C.S.; Kiyohara, H.; Reynolds, J.C.; Thomas-Oates, J.E.; Matsumoto, T.; Yamada, H.; Michaelsen, T.E.; Diallo, D.; Paulsen, B.S. Structure-immunomodulating activity relationships of a pectic arabinogalactan from Vernonia kotschyana Sch. Bip. ex Walp. Carbohydr. Res. 2005, 340, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, Y.N.; Paulsen, B.S.; Kiyohara, H.; Ciz, M.; Ognyanov, M.H.; Vasicek, O.; Rise, F.; Denev, P.N.; Lojek, A.; Batsalova, T.G.; et al. Tilia tomentosa pectins exhibit dual mode of action on phagocytes as β-glucuronic acid monomers are abundant in their rhamnogalacturonans I. Carbohydr. Polym. 2017, 175, 178–191. [Google Scholar] [CrossRef]
- Huang, C.; Yao, R.; Zhu, Z.; Pang, D.; Cao, X.; Feng, B.; Paulsen, B.S.; Li, L.; Yin, Z.; Chen, X.; et al. A pectic polysaccharide from water decoction of Xinjiang Lycium barbarum fruit protects against intestinal endoplasmic reticulum stress. Int. J. Biol. Macromol. 2019, 130, 508–514. [Google Scholar] [CrossRef]
- Zou, Y.F.; Zhang, Y.Y.; Fu, Y.P.; Inngjerdingen, K.T.; Paulsen, B.S.; Feng, B.; Zhu, Z.K.; Li, L.X.; Jia, R.Y.; Huang, C.; et al. A polysaccharide isolated from codonopsis pilosula with immunomodulation effects both in vitro and in vivo. Molecules 2019, 24, 3632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.F.; Zhang, Y.Y.; Paulsen, B.S.; Rise, F.; Chen, Z.L.; Jia, R.Y.; Li, L.X.; Song, X.; Feng, B.; Tang, H.Q.; et al. Structural features of pectic polysaccharides from stems of two species of Radix Codonopsis and their antioxidant activities. Int. J. Biol. Macromol. 2020, 159, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.K.; Cucheval, A.; Ström, A.; Ralet, M.C. Electrophoretic behavior of copolymeric galacturonans including comments on the information content of the intermolecular charge distribution. Biomacromolecules 2009, 10, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ropartz, D.; Cui, L.; Shi, H.; Ralet, M.C.; Zhou, Y. Structural characterization of rhamnogalacturonan domains from Panax ginseng C. A. Meyer. Carbohydr. Polym. 2019, 203, 119–127. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263. [Google Scholar] [CrossRef] [Green Version]
- Axelos, M.A.V.; Thibault, J.F. Influence of the substituents of the carboxyl groups and of the rhamnose content on the solution properties and flexibility of pectins. Int. J. Biol. Macromol. 1991, 13, 77–82. [Google Scholar] [CrossRef]
- Seixas, F.L.; Fukuda, D.L.; Turbiani, F.R.B.; Garcia, P.S.; Petkowicz, C.L.D.O.; Jagadevan, S.; Gimenes, M.L. Extraction of pectin from passion fruit peel (Passiflora edulis f.flavicarpa) by microwave-induced heating. Food Hydrocoll. 2014, 38, 186–192. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Pagán, J.; Ibarz, A. Extraction and rheological properties of pectin from fresh peach pomace. J. Food Eng. 1999, 39, 193–201. [Google Scholar] [CrossRef]
- Pagán, J.; Ibarz, A.; Llorca, M.; Coll, L. Quality of industrial pectin extracted from peach pomace at different pH and temperatures. J. Sci. Food Agric. 1999, 79, 1038–1042. [Google Scholar] [CrossRef]
- Pagán, J.; Ibarz, A.; Llorca, M.; Pagán, A.; Barbosa-Cánovas, G.V. Extraction and characterization of pectin from stored peach pomace. Food Res. Int. 2001, 34, 605–612. [Google Scholar] [CrossRef]
- Denman, L.J.; Morris, G.A. An experimental design approach to the chemical characterisation of pectin polysaccharides extracted from Cucumis melo Inodorus. Carbohydr. Polym. 2015, 117, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Kliemann, E.; De Simas, K.N.; Amante, E.R.; Prudêncio, E.S.; Teófilo, R.F.; Ferreira, M.M.C.; Amboni, R.D.M.C. Optimisation of pectin acid extraction from passion fruit peel (Passiflora edulis flavicarpa) using response surface methodology. Int. J. Food Sci. Technol. 2009, 44, 476–483. [Google Scholar] [CrossRef]
- Kumar, M.; Chauhan, A.K.R.; Kumar, S.; Kumar, A.; Malik, S. Design and evaluation of pectin based metrics for transdermal patches of meloxicam. J. Pharm. Res. Health Care 2010, 2, 244–247. [Google Scholar]
- Levigne, S.; Ralet, M.C.; Thibault, J.F. Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohydr. Polym. 2002, 49, 145–153. [Google Scholar] [CrossRef]
- Samavati, V. Polysaccharide extraction from Abelmoschus esculentus: Optimization by response surface methodology. Carbohydr. Polym. 2013, 95, 588–597. [Google Scholar] [CrossRef]
- Samavati, V. Central composite rotatable design for investigation of microwave-assisted extraction of okra pod hydrocolloid. Int. J. Biol. Macromol. 2013, 61, 142–149. [Google Scholar] [CrossRef]
- Sudhakar, D.V.; Maini, S.B. Isolation and characterization of mango peel pectins. J. Food Process. Preserv. 2000, 24, 209–227. [Google Scholar] [CrossRef]
- Ayora-Talavera, T.; Ramos-Chan, C.; Covarrubias-Cárdenas, A.; Sánchez-Contreras, A.; García-Cruz, U.; Pacheco, L.N. Evaluation of Pectin Extraction Conditions and Polyphenol Profile from Citrus x lantifolia Waste: Potential Application as Functional Ingredients. Agriculture 2017, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Happi Emaga, T.; Ronkart, S.N.; Robert, C.; Wathelet, B.; Paquot, M. Characterisation of pectins extracted from banana peels (Musa AAA) under different conditions using an experimental design. Food Chem. 2008, 108, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Wu, D.; Wei, C.; Tao, W.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Reconsidering conventional and innovative methods for pectin extraction from fruit and vegetable waste: Targeting rhamnogalacturonan I. Trends Food Sci. Technol. 2019, 94, 65–78. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, J.; Mao, G.; Hu, W.; Ye, X.; Linhardt, R.J.; Chen, S. Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health. Crit. Rev. Food Sci. Nutr. 2019, 1–23. [Google Scholar] [CrossRef]
- Harding, S.E.; Berth, G.; Ball, A.; Mitchell, J.R.; de la Torre, J.G. The molecular weight distribution and conformation of citrus pectins in solution studied by hydrodynamics. Carbohydr. Polym. 1991, 16, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Stokke, B.T.; Smidsrød, O.; Elgsaeter, A. Electron microscopy of native xanthan and xanthan exposed to low ionic strength. Biopolymers 1989, 28, 617–637. [Google Scholar] [CrossRef]
- Morris, G.A.; de al Torre, J.C.; Ortega, A.; Castile, J.; Smith, A.; Harding, S.E. Molecular flexibility of citrus pectins by combined sedimentation and viscosity analysis. Food Hydrocoll. 2008, 22, 1435–1442. [Google Scholar] [CrossRef] [Green Version]
- Theisen, A.; Johann, C.; Deacon, M.P.; Harding, S.E. Refractive Increment Data-Book for Polymer and Biomolecular Scientists; Nottingham University Press: Nottingham, UK, 2000. [Google Scholar]
- Grubisic, Z.; Rempp, P.; Benoit, H. A universal calibration for gel permeation chromatography. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1707–1713. [Google Scholar] [CrossRef]
- Tanford, C. Physical Chemistry of Macromolecules; John Willey and Sons Inc.: Hoboken, NJ, USA, 1961. [Google Scholar]
- Morris, G.A.; Foster, T.J.; Harding, S.E. The effect of the degree of esterification on the hydrodynamic properties of citrus pectin. Food Hydrocoll. 2000, 14, 227–235. [Google Scholar] [CrossRef]
- Harding, S.E. The intrinsic viscosity of biological macromolecules. Progress in measurement, interpretation and application to structure in dilute solution. Prog. Biophys. Mol. Biol. 1997, 68, 207–262. [Google Scholar] [CrossRef]
- Kratky, O.; Porod, G. Röntgenuntersuchung gelöster Fadenmoleküle. Recl. Trav. Chim. Pays-Bas 1949, 68, 1106–1122. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Korneeva, E.V.; Harding, S.E.; Vichoreva, G.A. Dilute solution properties of carboxymethylchitins in high ionic-strength solvent. Polymer 1998, 39, 6951–6961. [Google Scholar] [CrossRef]
- Ortega, A.; García de la Torre, J. Equivalent radii and ratios of radii from solution properties as indicators of macromolecular conformation, shape, and flexibility. Biomacromolecules 2007, 8, 2464–2475. [Google Scholar] [CrossRef]
- Bushin, S.V.; Tsvetkov, V.N.; Lysenko, E.B.; Emel’yanov, V.N. Conformational properties and rigidity of molecules of ladder polyphenylsiloxane in solutions according the data of sedimentation-diffusion analysis and viscometry. Vysokomol. Soedin. Ser. A 1981, 23, 2494–2503. [Google Scholar]
- Bohdanecky, M. New Method for Estimating the Parameters of the Wormlike Chain Model from the Intrinsic Viscosity of Stiff-Chain Polymers. Macromolecules 1983, 16, 1483–1492. [Google Scholar] [CrossRef]
- Ralet, M.C.; Crépeau, M.J.; Lefèbvre, J.; Mouille, G.; Höfte, H.; Thibault, J.F. Reduced number of homogalacturonan domains in pectins of an Arabidopsis mutant enhances the flexibility of the polymer. Biomacromolecules 2008, 9, 1454–1460. [Google Scholar] [CrossRef]
- Morris, G.A.; Ralet, M.C.; Bonnin, E.; Thibault, J.F.; Harding, S.E. Physical characterisation of the rhamnogalacturonan and homogalacturonan fractions of sugar beet (Beta vulgaris) pectin. Carbohydr. Polym. 2010, 82, 1161–1167. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, G.M.; Harding, S.E.; Rowe, A.J. Normalized scaling relations as a natural classification of linear macromolecules according to size. In Progress in Colloid and Polymer Science; Springer: Berlin, Germany, 1999; Volume 113, pp. 76–80. [Google Scholar]
- Pavlov, G.M.; Rowe, A.J.; Harding, S.E. Conformation zoning of large molecules using the analytical ultracentrifuge. TrAC Trends Anal. Chem. 1997, 16, 401–405. [Google Scholar] [CrossRef]
- Gadalla, H.H.; El-Gibaly, I.; Soliman, G.M.; Mohamed, F.A.; El-Sayed, A.M. Amidated pectin/sodium carboxymethylcellulose microspheres as a new carrier for colonic drug targeting: Development and optimization by factorial design. Carbohydr. Polym. 2016, 153, 526–534. [Google Scholar] [CrossRef]
- Teja, S.P.S.; Damodharan, N. 23 Full Factorial Model for Particle Size Optimization of Methotrexate Loaded Chitosan Nanocarriers: A Design of Experiments (DoE) Approach. BioMed Res. Int. 2018, 2018, 7834159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Millett, R.; Lee, C.S.; Yakubov, G.; Harding, S.E.; Binner, E. Investigating the influence of pectin content and structure on its functionality in bio-flocculant extracted from okra. Carbohydr. Polym. 2020, 241, 116414. [Google Scholar] [CrossRef] [PubMed]
- Israel, L.L.; Lellouche, E.; Kenett, R.S.; Green, O.; Michaeli, S.; Lellouche, J.P. Ce3/4+ cation-functionalized maghemite nanoparticles towards siRNA-mediated gene silencing. J. Mater. Chem. B 2014, 2, 6215–6225. [Google Scholar] [CrossRef] [PubMed]
- Urias-Orona, V.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Carvajal-Millán, E.; Gardea, A.A.; Ramírez-Wong, B. A novel pectin material: Extraction, characterization and gelling properties. Int. J. Mol. Sci. 2010, 11, 3686–3695. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Luo, J.P.; Yang, X.F.; Zha, X.Q. Structural analysis of a rhamnoarabinogalactan from the stems of Dendrobium nobile Lindl. Food Chem. 2010, 122, 572–576. [Google Scholar] [CrossRef]
- Li, D.-Q.; Jia, X.; Wei, Z.; Liu, Z.-Y. Box–Behnken experimental design for investigation of microwave-assisted extracted sugar beet pulp pectin. Carbohydr. Polym. 2012, 88, 342–346. [Google Scholar] [CrossRef]
- Morris, G.A.; Ralet, M.C. The effect of neutral sugar distribution on the dilute solution conformation of sugar beet pectin. Carbohydr. Polym. 2012, 88, 1488–1491. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.A.; Patel, T.R.; Picout, D.R.; Ross-Murphy, S.B.; Ortega, A.; Garcia de la Torre, J.; Harding, S.E. Global hydrodynamic analysis of the molecular flexibility of galactomannans. Carbohydr. Polym. 2008, 72, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Paniagua, C.; Posé, S.; Morris, V.J.; Kirby, A.R.; Quesada, M.A.; Mercado, J.A. Fruit softening and pectin disassembly: An overview of nanostructural pectin modifications assessed by atomic force microscopy. Ann. Bot. 2014, 114, 1375–1383. [Google Scholar] [CrossRef] [Green Version]
- Santos, E.E.; Amaro, R.C.; Bustamante, C.C.C.; Guerra, M.H.A.; Soares, L.C.; Froes, R.E.S. Extraction of pectin from agroindustrial residue with an ecofriendly solvent: Use of FTIR and chemometrics to differentiate pectins according to degree of methyl esterification. Food Hydrocoll. 2020, 107, 105921. [Google Scholar] [CrossRef]
- Yamada, H.; Kiyohara, H. Complement-activating polysaccharides from medicinal herbs. Immunomodul. Agents Plants 1999, 161–202. [Google Scholar] [CrossRef]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Oduro, I.N.; Morris, G.A.; Kontogiorgos, V. Structure-Function Relationships in Pectin Emulsification. Food Biophys. 2018, 13, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buergy, A.; Rolland-Sabaté, A.; Leca, A.; Renard, C.M.G.C. Pectin modifications in raw fruits alter texture of plant cell dispersions. Food Hydrocoll. 2020, 107, 105962. [Google Scholar] [CrossRef]
- Deng, L.Z.; Pan, Z.; Zhang, Q.; Liu, Z.L.; Zhang, Y.; Meng, J.S.; Gao, Z.J.; Xiao, H.W. Effects of ripening stage on physicochemical properties, drying kinetics, pectin polysaccharides contents and nanostructure of apricots. Carbohydr. Polym. 2019, 222, 114980. [Google Scholar] [CrossRef]
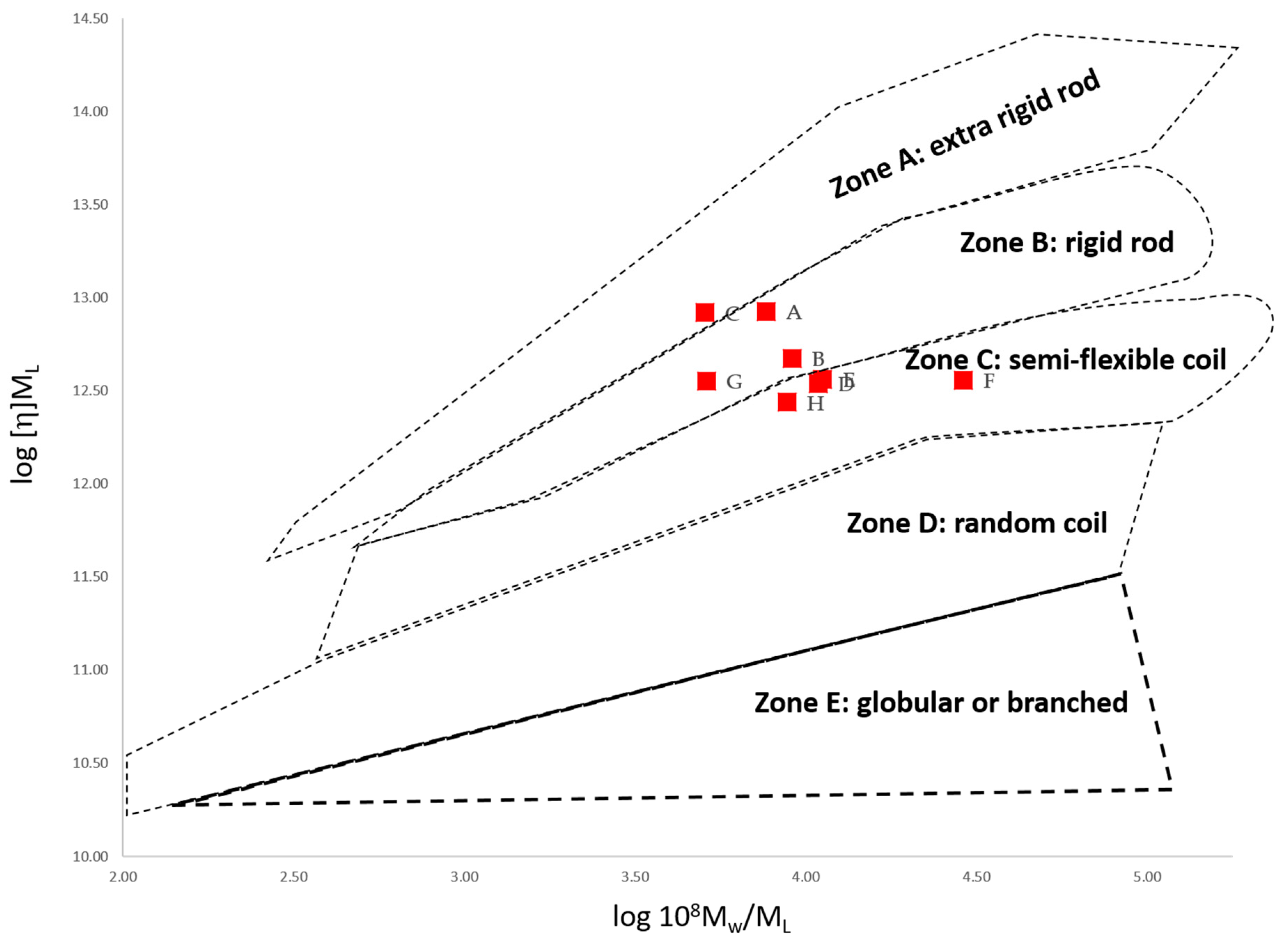


| Sample (Extraction Conditions) | Mn (g/mol) | [η]w (mL/g) | f/f0 | ML (g/mol nm) | Lp (nm) | Lp/ML (nm2 mol/g) |
|---|---|---|---|---|---|---|
| A (pH 1, 2 h, 60 °C) | 610,000 b | 1160 a,b | 9 a,b | 760 a | 9 b | 0.012 |
| B (pH 3, 2 h, 60 °C) | 570,000 b | 770 b,c | 8 a,b,c | 660 a | 17 a,b | 0.022 |
| C (pH 1, 2 h, 80 °C) | 405,000 b | 1110 a,b,c | 9 a,b | 750 a | 35 a,b | 0.048 |
| D (pH 3, 2 h, 80 °C) | 580,000 b | 650 b,c | 7 b,c | 610 a | 13 b | 0.018 |
| E (pH 1, 4 h, 60 °C) | 520,000 b | 790 b,c | 8 a,b,c | 475 a | 9 b | 0.018 |
| F (pH 3, 4 h, 60 °C) | 2,000,000 a | 650 b,c | 7 b,c | 690 a | 5 b | 0.007 |
| G (pH 1, 4 h, 80 °C) | 115,000 b | 1580 a | 10 a | 275 a | 54 a | 0.295 |
| H (pH 3, 4 h, 80 °C) | 680,000 b | 360 c | 6 c | 770 a | 9 b | 0.012 |
| Sample | HG: RG-I | HG Mn (g/mol) | HG ML (g/mol nm) | RG-I Mn (g/mol) | RG-I ML (g/mol nm) | a Mean Side Chain Length | a Mean Number of Side Chains |
|---|---|---|---|---|---|---|---|
| A | 0.3 | 140,000 | 367 | 470,000 | 887 | 5 | 800 |
| B | 0.6 | 205,000 | 368 | 370,000 | 831 | 4 | 850 |
| C | 0.3 | 90,000 | 368 | 315,000 | 876 | 4 | 450 |
| D | 1.1 | 300,000 | 371 | 280,000 | 1494 | 9 | 180 |
| E | 0.3 | 135,000 | 363 | 390,000 | 516 | 2 | 1590 |
| F | 0.5 | 725,000 | 373 | 1,300,000 | 868 | 5 | 1850 |
| G | 0.7 | 45,000 | 365 | 70,000 | 472 | 2 | 260 |
| H | 1.1 | 370,000 | 372 | 315,000 | 1228 | 7 | 300 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynolds, D.C.; Denman, L.J.; Binhamad, H.A.S.; Morris, G.A. The Effect of Different Extraction Conditions on the Physical Properties, Conformation and Branching of Pectins Extracted from Cucumis melo Inodorus. Polysaccharides 2020, 1, 3-20. https://doi.org/10.3390/polysaccharides1010002
Reynolds DC, Denman LJ, Binhamad HAS, Morris GA. The Effect of Different Extraction Conditions on the Physical Properties, Conformation and Branching of Pectins Extracted from Cucumis melo Inodorus. Polysaccharides. 2020; 1(1):3-20. https://doi.org/10.3390/polysaccharides1010002
Chicago/Turabian StyleReynolds, Danielle C., Laura J. Denman, Hana A. S. Binhamad, and Gordon A. Morris. 2020. "The Effect of Different Extraction Conditions on the Physical Properties, Conformation and Branching of Pectins Extracted from Cucumis melo Inodorus" Polysaccharides 1, no. 1: 3-20. https://doi.org/10.3390/polysaccharides1010002
APA StyleReynolds, D. C., Denman, L. J., Binhamad, H. A. S., & Morris, G. A. (2020). The Effect of Different Extraction Conditions on the Physical Properties, Conformation and Branching of Pectins Extracted from Cucumis melo Inodorus. Polysaccharides, 1(1), 3-20. https://doi.org/10.3390/polysaccharides1010002





