Multicomponent-Type High-Intensity Interval Training Improves Vitamin D Status in Adults with Overweight/Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Multicomponent-Type High-Intensity Interval Training (m-HIIT)
2.3. Assessment of Body Composition
2.4. Physical Activity Monitoring
2.5. Assessment of Dietary Intake
2.6. Biochemical Analysis
2.7. Statistical Analysis
3. Results
3.1. Internal Load During m-HIIT Sessions
3.2. Participants’ Dietary Intake and Physical Activity Level During the 12-Week Training Intervention
3.3. Body Composition
3.4. Systemic Indices of Liver and Bone Metabolism
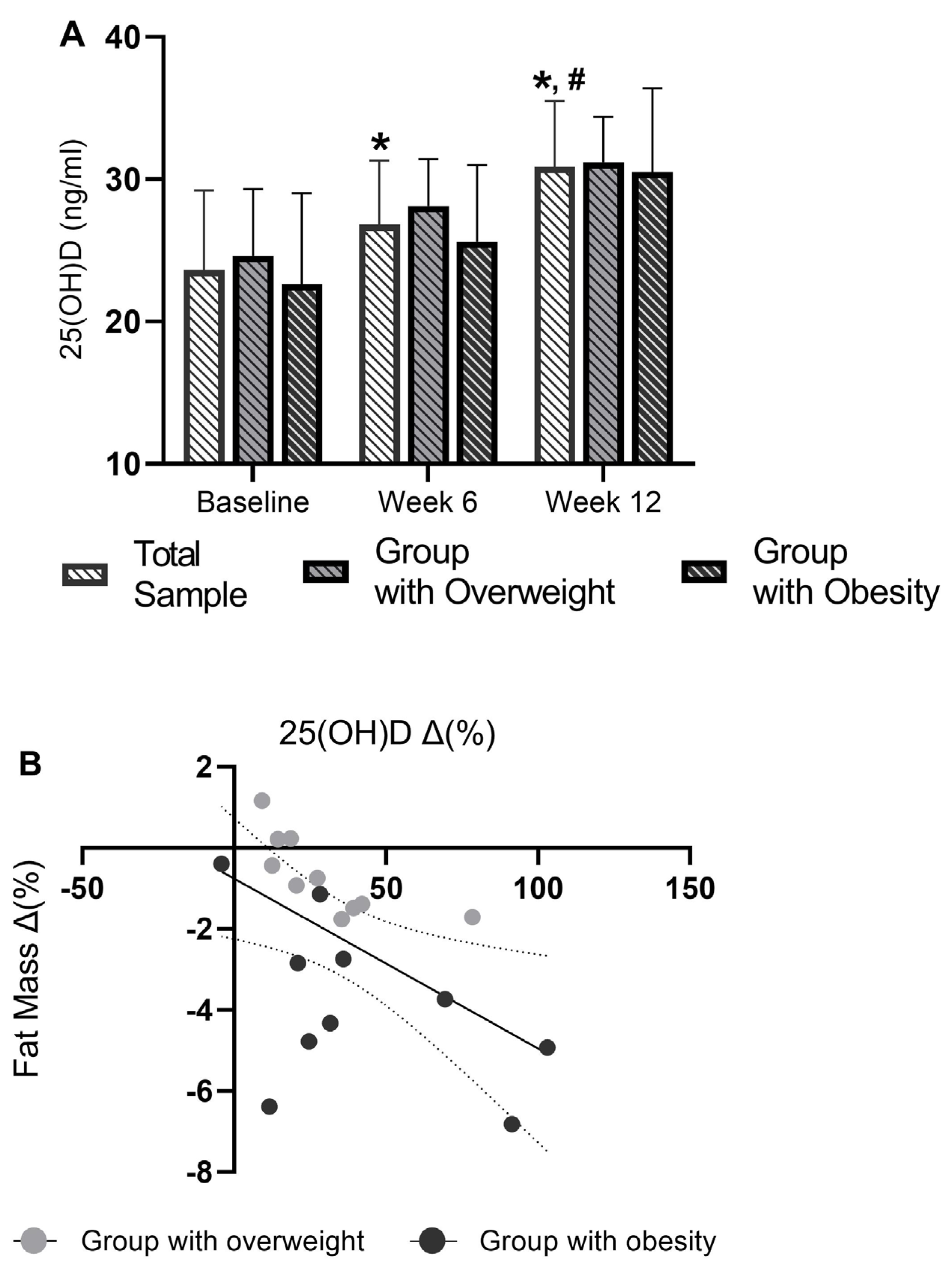
3.5. Serum 25(OH)D
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holick, M.F.; Chen, T.C. Vitamin D Deficiency: A Worldwide Problem with Health Consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and Vitamin D Deficiency: A Systematic Review and Meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, A.; De Toni, L.; Sabovic, I.; Rocca, M.S.; De Filippis, V.; Opocher, G.; Azzena, B.; Vettor, R.; Plebani, M.; Foresta, C. Impaired Release of Vitamin D in Dysfunctional Adipose Tissue: New Cues on Vitamin D Supplementation in Obesity. J. Clin. Endocrinol. Metab. 2017, 102, 2564–2574. [Google Scholar] [CrossRef] [PubMed]
- Hengist, A.; Perkin, O.; Gonzalez, J.T.; Betts, J.A.; Hewison, M.; Manolopoulos, K.N.; Jones, K.S.; Koulman, A.; Thompson, D. Mobilising Vitamin D from Adipose Tissue: The Potential Impact of Exercise. Nutr. Bull. 2019, 44, 25–35. [Google Scholar] [CrossRef]
- Chatzinikolaou, A.; Fatouros, I.; Petridou, A.; Jamurtas, A.; Avloniti, A.; Douroudos, I.; Mastorakos, G.; Lazaropoulou, C.; Papassotiriou, I.; Tournis, S.; et al. Adipose Tissue Lipolysis Is Upregulated in Lean and Obese Men During Acute Resistance Exercise. Diabetes Care 2008, 31, 1397–1399. [Google Scholar] [CrossRef]
- Petridou, A.; Chatzinikolaou, A.; Avloniti, A.; Jamurtas, A.; Loules, G.; Papassotiriou, I.; Fatouros, I.; Mougios, V. Increased Triacylglycerol Lipase Activity in Adipose Tissue of Lean and Obese Men During Endurance Exercise. J. Clin. Endocrinol. Metab. 2017, 102, 3945–3952. [Google Scholar] [CrossRef]
- Petridou, A.; Mougios, V. Acute Changes in Triacylglycerol Lipase Activity of Human Adipose Tissue during Exercise. J. Lipid Res. 2002, 43, 1331–1334. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Z.-B. Exercise: A Possibly Effective Way to Improve Vitamin D Nutritional Status. Nutrients 2022, 14, 2652. [Google Scholar] [CrossRef]
- Perkin, O.J.; Davies, S.E.; Hewison, M.; Jones, K.S.; Gonzalez, J.T.; Betts, J.A.; Jenkinson, C.; Lindsay, M.A.; Meadows, S.R.; Parkington, D.A.; et al. Exercise without Weight Loss Prevents Seasonal Decline in Vitamin D Metabolites: The VitaDEx Randomized Controlled Trial. Adv. Sci. 2025. [Google Scholar] [CrossRef]
- Batrakoulis, A.; Jamurtas, A.Z.; Draganidis, D.; Georgakouli, K.; Tsimeas, P.; Poulios, A.; Syrou, N.; Deli, C.K.; Papanikolaou, K.; Tournis, S.; et al. Hybrid Neuromuscular Training Improves Cardiometabolic Health and Alters Redox Status in Inactive Overweight and Obese Women: A Randomized Controlled Trial. Antioxidants 2021, 10, 1601. [Google Scholar] [CrossRef]
- Batrakoulis, A.; Jamurtas, A.Z.; Metsios, G.S.; Perivoliotis, K.; Liguori, G.; Feito, Y.; Riebe, D.; Thompson, W.R.; Angelopoulos, T.J.; Krustrup, P.; et al. Comparative Efficacy of 5 Exercise Types on Cardiometabolic Health in Overweight and Obese Adults: A Systematic Review and Network Meta-Analysis of 81 Randomized Controlled Trials. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e008243. [Google Scholar] [CrossRef] [PubMed]
- Batrakoulis, A.; Loules, G.; Georgakouli, K.; Tsimeas, P.; Draganidis, D.; Chatzinikolaou, A.; Papanikolaou, K.; Deli, C.K.; Syrou, N.; Comoutos, N.; et al. High-intensity Interval Neuromuscular Training Promotes Exercise Behavioral Regulation, Adherence and Weight Loss in Inactive Obese Women. Eur. J. Sport. Sci. 2020, 20, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Colleluori, G.; Aguirre, L.; Phadnis, U.; Fowler, K.; Armamento-Villareal, R.; Sun, Z.; Brunetti, L.; Hyoung Park, J.; Kaipparettu, B.A.; Putluri, N.; et al. Aerobic Plus Resistance Exercise in Obese Older Adults Improves Muscle Protein Synthesis and Preserves Myocellular Quality Despite Weight Loss. Cell Metab. 2019, 30, 261–273.e6. [Google Scholar] [CrossRef]
- Díaz-Buschmann, I.; Jaureguizar, K.V.; Calero, M.J.; Aquino, R.S. Programming Exercise Intensity in Patients on Beta-Blocker Treatment: The Importance of Choosing an Appropriate Method. Eur. J. Prev. Cardiol. 2014, 21, 1474–1480. [Google Scholar] [CrossRef]
- Coates, A.M.; Joyner, M.J.; Little, J.P.; Jones, A.M.; Gibala, M.J. A Perspective on High-Intensity Interval Training for Performance and Health. Sports Med. 2023, 53, 85–96. [Google Scholar] [CrossRef]
- Cipryan, L.; Laursen, P.B.; Plews, D.J. Cardiac Autonomic Response Following High-intensity Running Work-to-rest Interval Manipulation. Eur. J. Sport. Sci. 2016, 16, 808–817. [Google Scholar] [CrossRef]
- Draganidis, D.; Jamurtas, A.; Stampoulis, T.; Laschou, V.; Deli, C.; Georgakouli, K.; Papanikolaou, K.; Chatzinikolaou, A.; Michalopoulou, M.; Papadopoulos, C.; et al. Disparate Habitual Physical Activity and Dietary Intake Profiles of Elderly Men with Low and Elevated Systemic Inflammation. Nutrients 2018, 10, 566. [Google Scholar] [CrossRef]
- Marshall, R.H.; Eissa, M.; Bluth, E.I.; Gulotta, P.M.; Davis, N.K. Hepatorenal Index as an Accurate, Simple, and Effective Tool in Screening for Steatosis. Am. J. Roentgenol. 2012, 199, 997–1002. [Google Scholar] [CrossRef]
- Cohen, P.; Cohen, P.; West, S.G.; Aiken, L.S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences; Psychology Press: London, UK, 2014; ISBN 9781135468255. [Google Scholar]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.-S.; Magkos, F.; Dalamaga, M. Vitamin D and Obesity: Current Evidence and Controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, M.; Wagenpfeil, S.; Schöpe, J.; Vieth, R.; Vogt, T.; Reichrath, J. Meta-Analysis of European Clinical Trials Characterizing the Healthy-Adult Serum 25-Hydroxyvitamin D Response to Vitamin D Supplementation. Nutrients 2023, 15, 3986. [Google Scholar] [CrossRef] [PubMed]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. Correction to: The Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2022, 33, 2243. [Google Scholar] [CrossRef]
- Gao, H.; Peng, X.; Li, N.; Gou, L.; Xu, T.; Wang, Y.; Qin, J.; Liang, H.; Ma, P.; Li, S.; et al. Emerging Role of Liver-Bone Axis in Osteoporosis. J. Orthop. Transl. 2024, 48, 217–231. [Google Scholar] [CrossRef]
- Katrinaki, M.; Kampa, M.; Margioris, A.; Castanas, E.; Malliaraki, N. Vitamin D Levels in a Large Mediterranean Cohort: Reconsidering Normal Cut-off Values. Hormones 2016, 15, 205–223. [Google Scholar] [CrossRef]
- Papadakis, G.; Keramidas, I.; Kakava, K.; Pappa, T.; Villiotou, V.; Triantafillou, E.; Drosou, A.; Tertipi, A.; Kaltzidou, V.; Pappas, A. Seasonal Variation of Serum Vitamin D among Greek Female Patients with Osteoporosis. In Vivo 2015, 29, 409–413. [Google Scholar]
| Baseline | Week 6 | Week 12 | Fvalues | p Value | Effect Size | |
|---|---|---|---|---|---|---|
| Body Mass (kg) | ||||||
| Total sample (n = 20) | 99.7 ± 13.1 | 100.0 ± 12.9 | 99.3 ± 12.9 | F(2,38) = 3.067 | p = 0.058 | η2 = 0.140 |
| Group with overweight (n = 10) | 88.3 ± 3.1 | 88.7 ± 3.0 | 87.8 ± 2.7 | F(2.36) = 0.216 | p = 0.807 | η2 = 0.012 |
| Group with obesity (n = 10) | 112.2 ± 7.9 ¥ | 111.3 ± 7.7 ¥ | 110.7 ± 7.4 ¥ | |||
| Fat mass (kg) | ||||||
| Total sample (n = 20) | 34.6 ± 8.2 | 34.1 ± 7.8 | 33.7 ± 7.6 * | F(2.38) = 5.244 | p = 0.010 | η2 = 0.249 |
| Group with overweight (n = 10) | 27.8 ± 2.4 | 27.5 ± 1.8 | 27.6 ± 2.3 | F(2.36) = 3.572 | p = 0.038 | η2 = 0.166 |
| Group with obesity (n = 10) | 41.4 ± 5.8 ¥ | 40.6 ± 5.5 ¥ | 39.9 ± 5.8 *,¥ | |||
| Android fat (kg) | ||||||
| Total sample (n = 20) | 4.29 ± 0.96 | 4.13 ± 0.89 * | 4.10 ± 0.92 * | F(2.38) = 7.823 | p = 0.001 | η2 = 0.316 |
| Group with overweight (n = 10) | 3.78 ± 0.87 | 3.70 ± 0.86 | 3.68 ± 0.94 | F(2.36) = 2.188 | p = 0.127 | η2 = 0.108 |
| Group with obesity (n = 10) | 4.81 ± 0.78 ¥ | 4.55 ± 0.71 ¥ | 4.53 ± 0.70 ¥ | |||
| Lean mass (kg) | ||||||
| Total sample (n = 20) | 61.8 ± 5.6 | 62.6 ± 5.7 | 62.2 ± 5.9 | F(2.38) = 2.475 | p = 0.098 | η2 = 0.128 |
| Group with overweight (n = 10) | 57.3 ± 3.0 | 58.1 ± 3.1 | 57.0 ± 3.1 | F(2.36) = 2.212 | p = 0.124 | η2 = 0.109 |
| Group with obesity (n = 10) | 66.2 ± 3.6 ¥ | 67.1 ± 3.6 ¥ | 67.3 ± 2.0 ¥ | |||
| Hepatorenal Index | ||||||
| Total sample (n = 20) | 1.31 ± 0.15 | 1.26 ± 0.18 | 1.20 ± 0.14 * | F(2.38) = 3.948 | p = 0.028 | η2 = 0.175 |
| Group with overweight (n = 10) | 1.29 ± 0.14 | 1.21 ± 0.17 | 1.16 ± 0.12 | F(2.36) = 0.421 | p = 0.660 | η2 = 0.023 |
| Group with obesity (n = 10) | 1.33 ± 0.17 | 1.32 ± 0.17 | 1.24 ± 0.17 |
| Baseline | Week 6 | Week 12 | F2,38 | p Value | Effect Size | |
|---|---|---|---|---|---|---|
| SGPT (U/L) | ||||||
| Total sample (n = 20) | 39.9 ± 12.6 | 37.6 ± 9 | 42.4 ± 7.3 | F(2.38) = 2.475 | p = 0.098 | η2 = 0.116 |
| Group with overweight (n = 10) | 35.5 ± 7.0 | 34.0 ± 5.4 | 38.8 ± 2.1 | F(2.36) = 0.12 | p = 0.890 | η2 = 0.006 |
| Group with obesity (n = 10) | 44.5 ± 15.5 | 41.2 ± 10.7 | 46.0 ± 8.9 | |||
| SGOT (U/L) | ||||||
| Total sample (n = 20) | 36.8 ± 11.7 | 32.9 ± 9.9 | 43.2 ± 12.6 # | F(2.38) = 8.526 | <0.001 | η2 = 0.312 |
| Group with overweight (n = 10) | 36.0 ± 9.3 | 31.4 ± 6.6 | 43.3 ± 11.3 | F(2.36) = 0.21 | p = 0.811 | η2 = 0.012 |
| Group with obesity (n = 10) | 37.7 ± 14.2 | 34.6 ± 12.6 | 43.2 ± 13.8 | |||
| SGPT/SGOT | ||||||
| Total sample (n = 20) | 1.12 ± 0.27 | 1.20 ± 0.38 | 1.05 ± 0.34 | F(2.38) = 2.033 | p = 0.145 | η2 = 0.097 |
| Group with overweight (n = 10) | 1.03 ± 0.24 | 1.15 ± 0.42 | 0.97 ± 0.33 | F(2.36) = 0.13 | p = 0.879 | η2 = 0.007 |
| Group with obesity (n = 10) | 1.22 ± 0.29 | 1.26 ± 0.35 | 1.14 ± 0.36 | |||
| ALP (U/L) | ||||||
| Total sample (n = 20) | 167.6 ± 31.2 | 178.2 ± 44 * | 163.8 ± 37 # | F(2.38) = 6.851 | p = 0.003 | η2 = 0.304 |
| Group with overweight (n = 10) | 157.1 ± 16.3 | 167.6 ± 27.4 | 162.2 ± 28.8 | F(2.36) = 3.83 | p = 0.031 | η2 = 0.176 |
| Group with obesity (n = 10) | 178.2 ± 39.2 | 188.8 ± 55.7 | 162.2 ± 28.8 | |||
| γ-GT (U/L) | ||||||
| Total sample (n = 20) | 27.9 ± 11 | 24.5 ± 9.1 | 24.9 ± 9.5 | F(2.38) = 1.779 | p = 0.183 | η2 = 0.086 |
| Group with overweight (n = 10) | 26.0 ± 10.8 | 22.7 ± 5.7 | 21.9 ± 7.7 | F(2.36) = 0.2 | p = 0.821 | η2 = 0.011 |
| Group with obesity (n = 10) | 30.0 ± 11.5 | 26.4 ± 11.7 | 28.0 ± 10.4 | |||
| Fetuin-A (ng/mL) | ||||||
| Total sample (n = 20) | 24.2 ± 3 | 26.6 ± 4.5 * | 25.7 ± 4.7 | F(2.38) = 3.395 | p = 0.044 | η2 = 0.156 |
| Group with overweight (n = 10) | 23.7 ± 2.0 | 26.3 ± 4.9 | 26.1 ± 5.8 | F(2.36) = 0.65 | p = 0.529 | η2 = 0.035 |
| Group with obesity (n = 10) | 24.7 ± 3.8 | 26.9 ± 4.3 | 25.2 ± 3.4 | |||
| Calcium (mg/dL) | ||||||
| Total sample (n = 20) | 8.90 ± 0.71 | 8.64 ± 0.62 | 8.97 ± 0.96 | F(2.38) = 2.045 | p = 0.159 | η2 = 0.100 |
| Group with overweight (n = 10) | 9.01 ± 0.47 | 8.72 ± 0.38 | 8.94 ± 0.5 | F(2.36) = 0.5 | p = 0.608 | η2 = 0.027 |
| Group with obesity (n = 10) | 8.79 ± 0.9 | 8.57 ± 0.81 | 9.07 ± 1.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protopapa, M.; Draganidis, D.; Avloniti, A.; Fatouros, I.G.; Stampoulis, T.; Pantazis, D.; Balampanos, D.; Retzepis, N.O.; Poulios, A.; Zaras, N.; et al. Multicomponent-Type High-Intensity Interval Training Improves Vitamin D Status in Adults with Overweight/Obesity. Obesities 2025, 5, 51. https://doi.org/10.3390/obesities5030051
Protopapa M, Draganidis D, Avloniti A, Fatouros IG, Stampoulis T, Pantazis D, Balampanos D, Retzepis NO, Poulios A, Zaras N, et al. Multicomponent-Type High-Intensity Interval Training Improves Vitamin D Status in Adults with Overweight/Obesity. Obesities. 2025; 5(3):51. https://doi.org/10.3390/obesities5030051
Chicago/Turabian StyleProtopapa, Maria, Dimitrios Draganidis, Alexandra Avloniti, Ioannis G. Fatouros, Theodoros Stampoulis, Dimitrios Pantazis, Dimitrios Balampanos, Nikolaos Orestis Retzepis, Athanasios Poulios, Nikolaos Zaras, and et al. 2025. "Multicomponent-Type High-Intensity Interval Training Improves Vitamin D Status in Adults with Overweight/Obesity" Obesities 5, no. 3: 51. https://doi.org/10.3390/obesities5030051
APA StyleProtopapa, M., Draganidis, D., Avloniti, A., Fatouros, I. G., Stampoulis, T., Pantazis, D., Balampanos, D., Retzepis, N. O., Poulios, A., Zaras, N., Bampali, M., Karakasiliotis, I., Mastorakos, G., Angelopoulos, T. J., Michalopoulou, M., Kambas, A., Jamurtas, A. Z., & Chatzinikolaou, A. (2025). Multicomponent-Type High-Intensity Interval Training Improves Vitamin D Status in Adults with Overweight/Obesity. Obesities, 5(3), 51. https://doi.org/10.3390/obesities5030051













