1. Introduction
Short sleep duration has been linked to increased body mass index in many studies. The cause of this relationship may be due to neuroendocrine changes, which can impact appetite cravings, which could ultimately lead to weight gain and obesity. Sleep deprivation can potentially lead to weight gain and obesity through the regulation of appetite hormones [
1].
Good sleep is essential for our health and well-being. It is a restorative process that helps with brain function and physical health. Sleep is also critically involved in our metabolism, appetite regulation, immune and hormone function, and cardiovascular system [
2]. A third of adults in the United States say that they routinely sleep less than the recommended amount [
3].
Sleep deprivation refers to “abnormal sleep conditions that exhibit deficient sleep quantity, structure, and/or quality [
4].” Partial sleep deprivation can be defined as less than 6 h of sleep in a 24 h period, and total sleep deprivation is 0 h of sleep in a 24 h period [
5]. Both forms of sleep deprivation have been shown to have negative consequences on appetite regulation and hunger hormones.
Sleep deprivation can also impact blood glucose values, particularly in individuals with diabetes that may cause issues with blood glucose management. Indeed, previous studies have found that poor sleep quality or sleep deprivation cause greater variability in blood glucose values in individuals with type 1 diabetes mellitus [
1].
Research has suggested that sleep duration can directly impact appetite by regulating our hunger hormones [
6]. Many studies have investigated this relationship between sleep and appetite regulation [
7,
8,
9]. Appetite hormones such as leptin and ghrelin act on the arcuate nucleus (ARC) in order to manage our appetite and our energy expenditure [
6].
Leptin is called the “satiety hormone.” An increase in adipose deposits associated with positive energy balance will increase leptin production, and this in turn signals to the hypothalamus to reduce nutrient intake and increase energy expenditure [
6,
10,
11].
Ghrelin is called the “hunger hormone.” It is secreted from the stomach and released into the bloodstream, similar to leptin [
10,
11]. Ghrelin has been found to have an inverse relationship with BMI, where it is upregulated in malnourished states such as anorexia nervosa and is downregulated in obesity [
11]. The downregulation of ghrelin in obesity can suggest a potential sensitivity to ghrelin because of feelings of hunger and overeating.
Sleep loss has been linked to changes in these appetite hormones. This is because sleep plays a major role in energy homeostasis [
12]. Leptin and ghrelin work together as a sort of feedback loop to control energy homeostasis and maintain body weight [
13]. If this loop is disrupted, it can lead to conditions such as obesity or anorexia [
12].
Many recent research studies have discovered that after just one night of inadequate sleep, circulating ghrelin levels increase, while leptin levels decrease [
14,
15,
16]. However, these findings are inconsistent through a variety of different study designs [
17]. Research has shown that there is a link between sleep loss and obesity [
18], but there have been inconsistent results regarding its impact on the hunger hormones leptin and ghrelin in laboratory studies, most likely due to variations in study design.
Therefore, a primary aim for this review is to determine the role sleep loss plays on the regulation of hunger hormones leptin and ghrelin. The objectives of the current study were (1) to examine the effects of sleep deprivation on the circulating levels of appetite-regulating hormones, specifically leptin and ghrelin, and (2) to assess the potential role of sleep deprivation in the pathophysiology of obesity through its effects on appetite-regulating hormones.
2. Materials and Methods
2.1. Study Selection
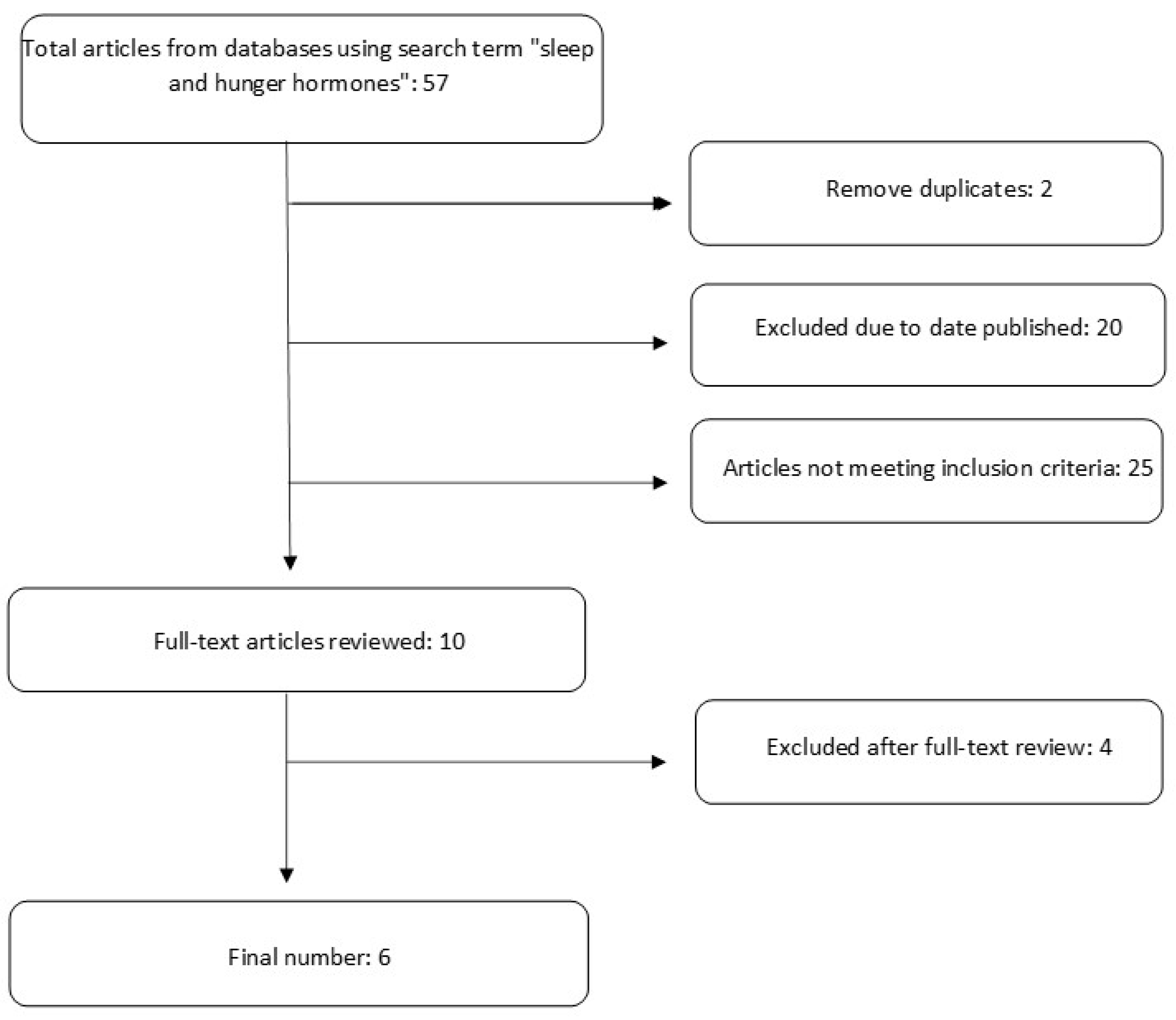
This systematic review was conducted using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [
19]. Databases including PubMed, CINAHL, and Google Scholar were searched for primary studies. A detailed search strategy is depicted in
Figure 1.
2.2. Inclusion and Exclusion Criteria
Studies were included if they (1) recruited participants that were 18 years or older; (2) were randomized controlled trials (RCTs) published in peer-reviewed journals with at least 10 participants per experimental group; (3) were published in English; (4) were published within the last 10 years (2013–2023); (5) experimentally manipulated sleep duration in study participants; and (6) measured leptin and ghrelin levels by blood samples. Studies were excluded if they (1) included participants with sleep disorders such as sleep apnea, insomnia, narcolepsy, sleepless leg syndrome, or any others that impact sleep duration; (2) used drugs or medications for sleep deprivation; (3) included participants with acute or chronic medical conditions that could impact sleep duration; (4) were narrative or systematic reviews or animal studies. This review has been submitted to Prospero for registration. Details provided in
Figure S1.
2.3. Data Extraction
Relevant data extracted for investigation included the first authors’ first and last name, article title and year published, number of participants, age and gender of participants, BMI, study design, methods used to evaluate sleep duration (survey, questionnaire, polysomnography), methods used to experimentally manipulate sleep duration, and methods used to evaluate leptin and ghrelin levels (ELISA, radioimmunoassay).
2.4. Risk of Bias
Research articles were assessed for bias using the Evidence Analysis Library Worksheet and Quality Criteria Checklist (QCC) created by the Academy of Nutrition and Dietetics [
20]. Only articles that received a positive rating were included in this meta-analysis.
2.5. Statistical Analysis
All data was organized into RStudio and was analyzed using the metafor package Version 2024.12.1 [
21]. All data was analyzed using a random effects model, mearing standardized mean differences and 95% confidence intervals. A random-effects model allows for heterogeneity by assuming underlying effects follow a normal distribution [
22]. Standard deviations were reported in all six studies; therefore, the standard deviations from the pre-test values were used. Effect sizes from repeated measures were calculated using the following equation [
23]:
3. Results
Of the six studies assessed for final analysis, five included data on leptin levels and all six on changes in ghrelin levels. Sample sizes ranged from 12 to 44 participants. All studies were classified as randomized crossover trials. Trial duration varied from 10 days to 15 weeks. Sleep was mainly restricted by about 4–5 h, with one study restricting sleep all night, keeping participants up for 24 h. A descriptive summary of study characteristics can be found in
Table 1 and
Table 2.
Table 3 shows the risk of bias assessment conducted for all studies. All studies presented low risk in terms of selection bias, missing data, randomization process, factors other than the intervention that may influence outcome, and measurement of the outcomes.
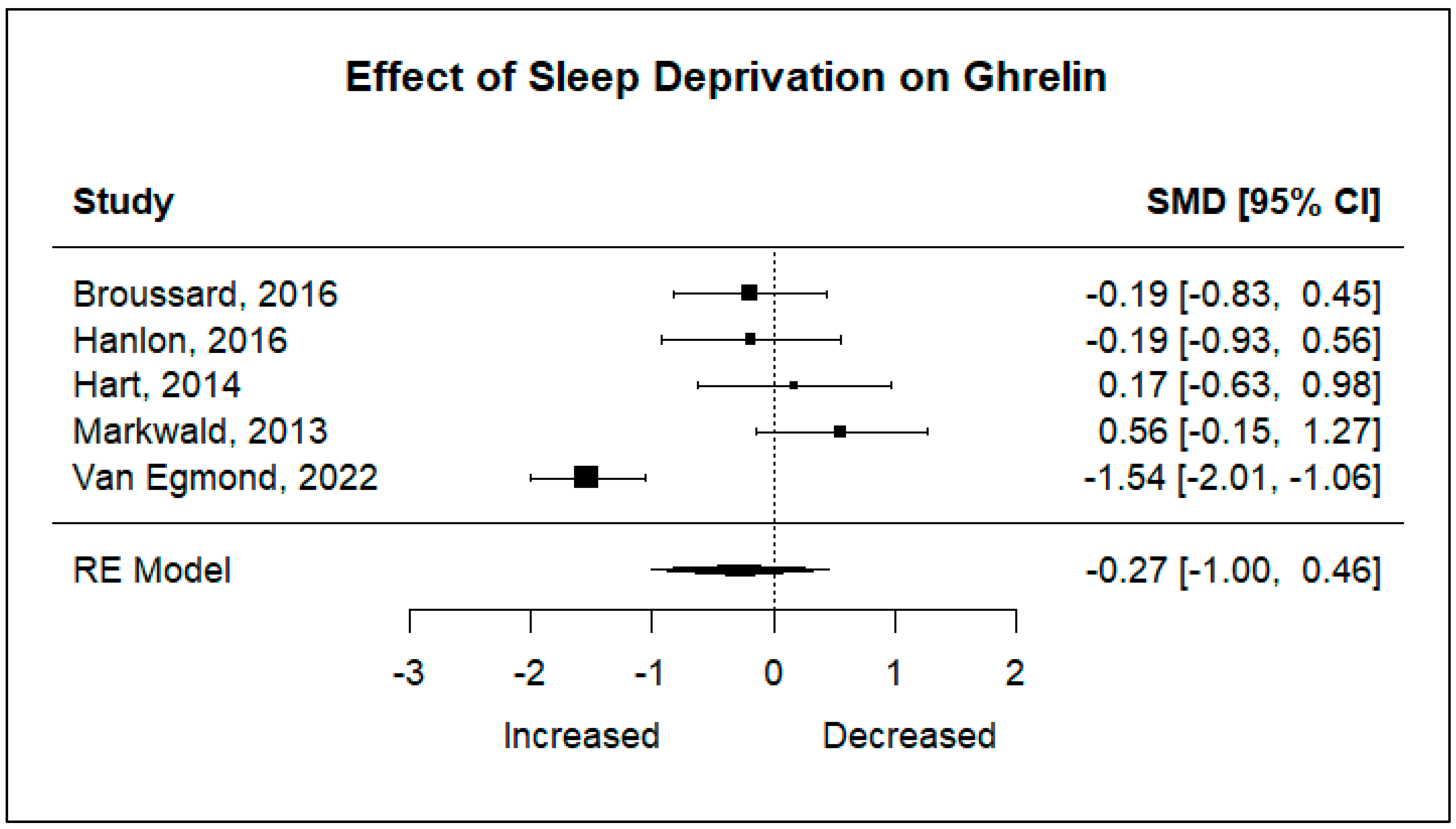
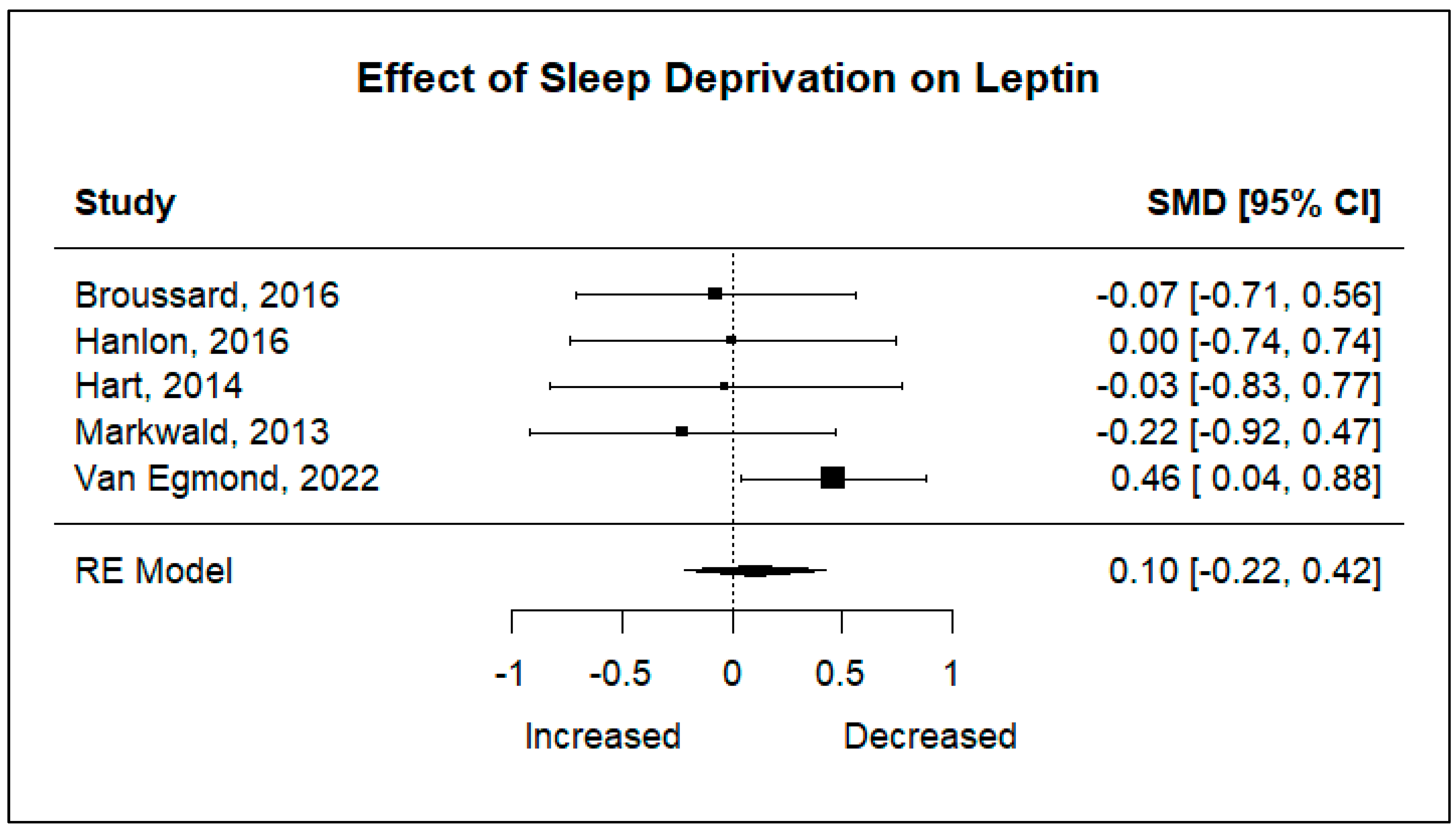
Five studies including 105 participants were analyzed to determine the effect sleep deprivation would have on the hunger hormones ghrelin and leptin. Significant heterogeneity was found for ghrelin (I2 = 83.83%, p < 0.001), and non-significant heterogeneity was found for leptin (I2 = 21.86%, p = 0.4049). Using a random-effects model, the results showed that there are no statistically significant changes in ghrelin levels after a night of restricted sleep, nor in leptin levels after a night of restricted sleep.
The pooled effect sizes of both primary outcomes are shown in
Figure 2 and
Figure 3. The standard mean deviations (SMDs) of both ghrelin (SMD: −0.27, 95% CI: −1.00, 0.46,
p = 0.4712), and leptin (SMD: 0.10, 95% CI: −0.22, 0.42,
p = 0.5266) show that there is no significant difference in changes in hormone levels after sleep deprivation.
Figure 2.
Forest plot of the effects of sleep deprivation on ghrelin levels [
16,
17,
24,
25,
26].
Figure 2.
Forest plot of the effects of sleep deprivation on ghrelin levels [
16,
17,
24,
25,
26].
Figure 3.
Forest plot of the effects of sleep deprivation on leptin levels [
16,
17,
24,
25,
26].
Figure 3.
Forest plot of the effects of sleep deprivation on leptin levels [
16,
17,
24,
25,
26].
Table 1.
Baseline characteristics of studies included in the meta-analysis. Data is presented as mean ± SD.
Table 1.
Baseline characteristics of studies included in the meta-analysis. Data is presented as mean ± SD.
|
Author (Year)
|
Age (Years)
|
Participants
|
Sex (Number of M/F)
|
BMI (kg/m2)
|
|---|
| Broussard (2016) [25] | 23.5 ± 0.7 | 19 | 19/0 | 23.1 ± 0.4 |
| Hanlon (2016) [24] | 23.4 ± 0.8 | 14 | 11/3 | 23.9 ± 0.7 |
| Hart (2014) [26] | 41.7 ± 10.3 | 12 | 0/12 | 31.0 ± 4.2 |
| Markwald (2013) [17] | 22.4 ± 4.8 | 16 | 8/8 | 22.9 ± 2.4 |
| Van Egmond (2023) [16] | 24.9 ± 2.9 | 44 | 24/20 | 27.4 ± 6.4 |
Table 2.
Outcome measures of studies included in the meta-analysis. Data is presented as mean ± SD.
Table 2.
Outcome measures of studies included in the meta-analysis. Data is presented as mean ± SD.
|
Author (Year)
|
Group
|
Leptin
|
Ghrelin
|
|---|
| Broussard (2016) [25] | Sleep-Deprived
Control | 3.8 ± 3.1 ng/mL
3.6 ± 2.6 ng/mL | 704 ± 227 pg/mL
658 ± 235 pg/mL |
| Hanlon (2016) [24] | Sleep-Deprived
Control | 9.5 ± 2.7 ng/mL
9.5 ± 2.4 ng/mL | 790 ± 458 pg/mL
732 ± 303 pg/mL |
| Hart (2014) [26] | Sleep-Deprived
Control | 36.3 ± 13.1 ng/mL
35.9 ± 12.3 ng/mL | 379.4 ± 103 pg/mL
404.7 ± 140.6 pg/mL |
| Markwald (2013) [17] | Sleep-Deprived
Control | 6.7 ± 5.1 ng/mL
5.5 ± 5.2 ng/mL | 660.2 ± 235.4 pg/mL
794.6 ± 233.8 pg/mL |
| Van Egmond (2023) [16] | Sleep-Deprived
Control | 17.3 ± 2.6 ng/mL
18.6 ± 2.8 ng/mL | 839.4 ± 77.5 pg/mL
741.4 ± 63.2 pg/mL |
Table 3.
Descriptive summary of studies included in the meta-analysis.
Table 3.
Descriptive summary of studies included in the meta-analysis.
|
Author (Year)
|
Study Design
|
Sleep Restriction
|
Control
|
Duration
|
Outcomes
|
Results
|
Risk of Bias
|
|---|
| Broussard (2016) [25] | Randomized Crossover Trial | 4.5 h | 8.5 h | 6 weeks | Leptin, Ghrelin | Leptin no change
Ghrelin increased | low |
| Hanlon (2016) [24] | Randomized Crossover Trial | 4.5 h | 8.5 h | 6 weeks | Leptin, Ghrelin | Leptin no change
Ghrelin increased | low |
| Hart (2014) [26] | Randomized Crossover Trial | 5 h | 9 h | 2 weeks | Leptin, Ghrelin | No significant
changes | low |
| Markwald (2013) [17] | Randomized Crossover Trial | 5 h | 9 h | 15 days | Leptin, Ghrelin | Ghrelin decreased | low |
| Van Egmond (2023) [16] | Randomized Crossover Trial | 0 h | 8 h | 2 weeks | Leptin, Ghrelin | Leptin decreased
Ghrelin increased | low |
4. Discussion
The purpose of the current study was to determine the effect sleep deprivation has on circulating levels of the hunger hormones leptin and ghrelin via meta-analysis. There is no significant evidence that sleep deprivation plays a role in raising or lowering circulating hunger hormones. These results are surprising because of the amount of research suggesting that hunger hormones may play a role in the relationship between sleep loss and obesity.
Results from the Broussard et al. study, Hanlon et al. study, and Van Egmond et al. study found that ghrelin levels increased after a night of sleep deprivation [
16,
24,
25]. Results from the Hart et al. study and Markwald et al. study show that ghrelin levels decrease after a night of sleep deprivation [
17,
26,
27]. Significant variability is found in the studies that were selected for this meta-analysis. The heterogeneity score for ghrelin was considerable, with a high percentage (I
2 = 93.36%). This indicates that results from these studies were not similar enough to determine reliable conclusions.
Results from the forest plot for ghrelin were extremely variable. This could be because there were many differences in study design. Blood samples in Van Egmond et al. were taken at 7:30 a.m. for both study groups, whereas in Broussard et al., blood samples were taken in 15–30 min intervals for 24 h starting the evening of the third night of each sleep condition. Again, it is important to take note of differences in study design. In Van Egmond et al., blood samples were taken after just a single night of sleep or sleep restriction, whereas in Broussard et al., participants participated in four consecutive nights of adequate sleep or sleep restriction [
16,
25]. Many of the studies also took blood samples in hourly [
17,
27] or 15–30 min intervals [
24,
25] and therefore used the mean of these values, rather than just a single point in time [
16,
26]. These differences in timing of blood sampling may be causing the significant amount of heterogeneity seen in the results of the forest plots for both leptin and ghrelin.
Another thing to note is that people with obesity have higher circulating concentrations of leptin. The leptin in your blood is directly proportional to adiposity [
28]. High circulating leptin levels can in turn cause leptin resistance [
29]. Hart et al. had an average BMI of (31.0 ± 4.2), and Van Egmond et al. had an average BMI of (27.4 ± 6.4), whereas all the other studies had an average BMI that fell within the healthy BMI range of 18–24.9. The baseline circulating leptin levels for Hart et al. were (35.9 ± 12.3) and for Van Egmond et al. circulating levels were (18.6 ± 2.8). Baseline leptin levels increase with BMI. The variation in BMI between studies could have contributed to the variability seen in the results.
There are many strengths and limitations to this meta-analysis. A detailed search for reputable articles was conducted, and the risk of bias was low for each article selected. This is one of the first meta-analyses looking at the effects of sleep deprivation on circulating leptin and ghrelin levels. All studies included in this meta-analysis were randomized crossover trials, which can reduce variability in results because each participant acts as their own control. A limitation of the study includes a low number of research articles found for the meta-analysis. Only six studies fit the inclusion and exclusion criteria. Another limitation was that many of the articles chosen had small experimental groups, with Hart et al. having only 12 participants in total. There were also varying durations of study, with four articles having durations of around 2 weeks and two articles having 6-week durations. Sampling varied among the articles, with some samples being taken directly after a night of sleep or sleep deprivation and some studies using averages of blood samples taken frequently throughout a 24 h period.
For future research, it is important that studies examining the role of sleep deprivation on hunger hormones and other parameters have not only similar designs, but also similar durations, timing of measurements, and populations.
5. Conclusions
In conclusion, this meta-analysis aimed to evaluate the impact of sleep deprivation on the circulating levels of hunger hormones, specifically leptin and ghrelin, and their potential role in obesity. Despite the extensive research suggesting that sleep deprivation might disrupt appetite regulation through these hormones, our findings indicate no significant evidence that sleep loss directly alters the levels of either leptin or ghrelin. These results were unexpected, given the established theoretical relationship between sleep duration, hunger hormones, and obesity.
One possible explanation for the lack of consistent findings is the significant variability in study designs, including differences in sample size, timing of blood sample collection, and study duration. The considerable heterogeneity found in the ghrelin data further suggests that the differences in methodology across studies may have influenced the outcomes. Additionally, factors like BMI, which directly affects leptin levels, might also contribute to variations in hormone responses to sleep deprivation.
While the current body of evidence does not support a clear link between sleep deprivation and changes in hunger hormone levels, the complexity of this relationship underscores the need for more rigorous, standardized studies in this field. Future research should focus on controlling variables such as measurement timing, population characteristics, and study design in order to clarify the role of sleep deprivation in appetite regulation and its contribution to weight gain and obesity. Until then, further investigation is needed to fully understand the neuroendocrine mechanisms through which sleep deprivation may affect metabolism and body weight.
Author Contributions
Conceptualization, M.M.-B. and D.G.; methodology, M.M.-B., D.G., K.M. and S.L.; software, K.M. and S.L.; validation, M.M.-B., D.G., K.M. and S.L.; formal analysis, M.M.-B., D.G., K.M. and S.L.; investigation, M.M.-B., D.G., K.M. and S.L.; data curation, D.G.; writing—original draft preparation, D.G.; writing—review and editing, M.M.-B., D.G., K.M. and S.L.; supervision, M.M.-B., K.M. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bayon, V.; Leger, D.; Gomez-Merino, D.; Vecchierini, M.; Chennaoui, M. Sleep debt and obesity. Ann. Med. 2014, 46, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Consensus Conference Panel; Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.; et al. Joint consensus statement of the American academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: Methodology and discussion. Sleep 2015, 38, 1161–1183. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Sleep and Sleep Disorders. Available online: https://www.cdc.gov/sleep/index.html (accessed on 2 November 2023).
- Krause, A.J.; Ben Simon, E.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Salfi, F.; Lauriola, M.; Tempesta, D.; Calanna, P.; Socci, V.; De Gennaro, L.; Ferrara, M. Effects of total and partial sleep deprivation on reflection impulsivity and risk-taking in deliberative decision-making. Nat. Sci. Sleep 2020, 12, 309–324. [Google Scholar] [CrossRef]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Models Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef]
- Meyhöfer, S.; Chamorro, R.; Hallschmid, M.; Spyra, D.; Klinsmann, N.; Schultes, B.; Lehnert, H.; Meyhöfer, S.M.; Wilms, B. Late, but not early, night sleep loss compromises neuroendocrine appetite regulation and the desire for food. Nutrients 2023, 15, 2035. [Google Scholar] [CrossRef]
- Nocturnal Ghrelin, ACTH, GH and Cortisol Secretion After Sleep Deprivation in Humans—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0306453006000886?via%3Dihub (accessed on 20 June 2024).
- St-Onge, M.; O’Keeffe, M.; Roberts, A.L.; RoyChoudhury, A.; Laferrère, B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep 2012, 35, 1503–1510. [Google Scholar] [CrossRef]
- Mosavat, M.; Mirsanjari, M.; Arabiat, D.; Smyth, A.; Whitehead, L. The role of sleep curtailment on leptin levels in obesity and diabetes mellitus. Obes. Facts 2021, 14, 214–221. [Google Scholar] [CrossRef]
- Cui, H.; López, M.; Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017, 13, 338–351. [Google Scholar] [CrossRef]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef]
- Ghrelin and Leptin. News-Medical.net. Available online: https://www.news-medical.net/health/Ghrelin-and-Leptin.aspx (accessed on 2 November 2023).
- Schmid, S.M.; Hallschmid, M.; Jauch-Chara, K.; Born, J.; Schultes, B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J. Sleep Res. 2008, 17, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef]
- van Egmond, L.T.; Meth, E.M.S.; Engström, J.; Ilemosoglou, M.; Keller, J.A.; Vogel, H.; Benedict, C. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: A laboratory study. Obesity 2023, 31, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P., Jr. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. [Google Scholar] [CrossRef]
- Brondel, L.; Romer, M.A.; Nougues, P.M.; Touyarou, P.; Davenne, D. Acute partial sleep deprivation increases food intake in healthy men. Am. J. Clin. Nutr. 2010, 91, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- PRISMA. Prisma-statement.org. 2020. Available online: http://www.prisma-statement.org/ (accessed on 20 February 2024).
- EAL. Available online: https://www.andeal.org/evidence-analysis-manual (accessed on 20 February 2024).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 20 February 2024).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.4; Cochrane: London, UK, 2023. Available online: www.training.cochrane.org/handbook (accessed on 20 February 2024).
- Morris, S.B.; DeShon, R.P. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol. Methods 2002, 7, 105–125. [Google Scholar] [CrossRef]
- Hanlon, E.C.; Tasali, E.; Leproult, R.; Stuhr, K.L.; Doncheck, E.; de Wit, H.; Hillard, C.J.; Van Cauter, E. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep 2016, 39, 653–664. [Google Scholar] [CrossRef]
- Broussard, J.L.; Kilkus, J.M.; Delebecque, F.; Abraham, V.; Day, A.; Whitmore, H.R.; Tasali, E. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity 2016, 24, 132–138. [Google Scholar] [CrossRef]
- Hart, C.N.; Carskadon, M.A.; Demos, K.E.; Van Reen, E.; Sharkey, K.M.; Raynor, H.A.; Considine, R.V.; Jones, R.N.; Wing, R.R. Acute changes in sleep duration on eating behaviors and appetite-regulating hormones in overweight/obese adults. Behav. Sleep Med. 2014, 13, 424–436. [Google Scholar] [CrossRef]
- Depner, C.M.; Melanson, E.L.; Eckel, R.H.; A Higgins, J.; Bergman, B.C.; Perreault, L.; A Knauer, O.; Birks, B.R.; Wright, K.P. Effects of ad libitum food intake, insufficient sleep and weekend recovery sleep on energy balance. Sleep 2021, 44, zsab136. [Google Scholar] [CrossRef]
- Cleveland Clinic. Leptin: What It Is, Function & Levels. Available online: https://my.clevelandclinic.org/health/articles/22446-leptin (accessed on 20 June 2024).
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).