Mirabegron and Physical Exercise Is a Potential Strategical for BAT Activation in Obesity
Abstract
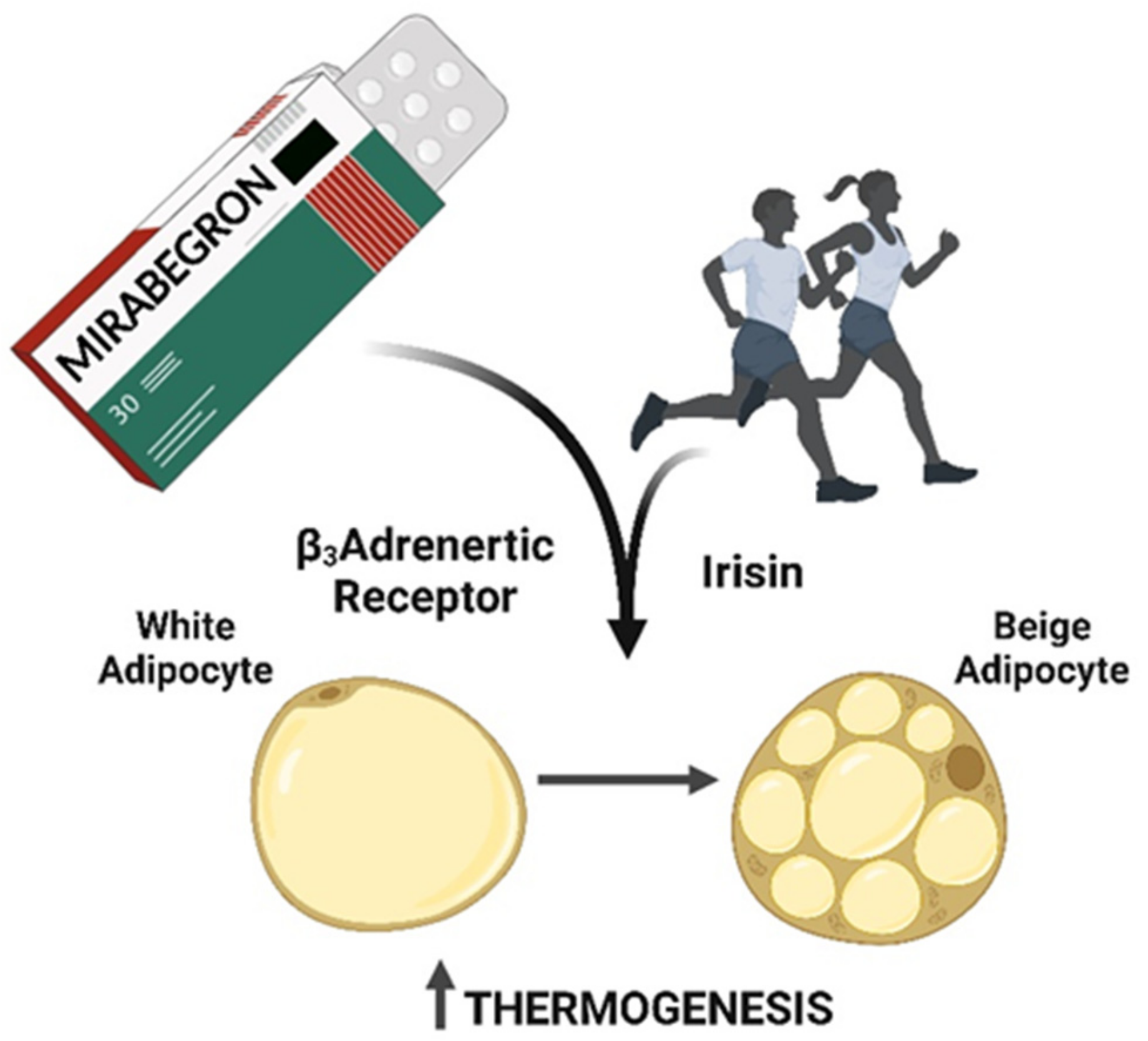
1. Introduction
2. Mirabegron Mechanism of Action and Safety
3. Mirabegron and Obesity
4. Physical Exercise, BAT Activation and Obesity
5. Cold Exposure
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaspar, R.C.; Veiga, C.B.; Bessi, M.P.; Dátilo, M.N.; Sant’Ana, M.R.; Rodrigues, P.; de Moura, L.P.; da Silva, A.S.R.; Santos, G.A.; Catharino, R.R.; et al. Unsaturated fatty acids from flaxseed oil and exercise modulate GPR120 but not GPR40 in the liver of obese mice: A new anti-inflammatory approach. J. Nutr. Biochem. 2019, 66, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.C.; Pauli, J.R.; Shulman, G.I.; Muñoz, V.R. An update on brown adipose tissue biology: A discussion of recent findings. Am. J. Physiol. Metab. 2021, 320, E488–E495. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Labbé, S.M.; Tingelstad, H.C.; Noll, C.; Kunach, M.; Phoenix, S.; Guérin, B.; Turcotte, É.E.; Carpentier, A.C.; Richard, D.; et al. Increased Brown Adipose Tissue Oxidative Capacity in Cold-Acclimated Humans. J. Clin. Endocrinol. Metab. 2014, 99, E438–E446. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Shen, M.; Yadav, H.; Thakali, K.M. Novel Browning Agents, Mechanisms, and Therapeutic Potentials of Brown Adipose Tissue. BioMed Res. Int. 2016, 2016, 2365609. [Google Scholar] [CrossRef]
- Benn, T.; Kim, B.; Park, Y.-K.; Wegner, C.J.; Harness, E.; Nam, T.-G.; Kim, D.-O.; Lee, J.S.; Lee, J.-Y. Mitochondrial dysfunction plays an essential role in remodeling aging adipose tissue. Mech. Ageing Dev. 2021, 200, 111598. [Google Scholar] [CrossRef]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Elía, E.F.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of Human Brown Adipose Tissue by a β3-Adrenergic Receptor Agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef]
- Pinto, Y.O.; Festuccia, W.T.L.; Magdalon, J. The involvement of the adrenergic nervous system in activating human brown adipose tissue and browning. Hormones 2022, 21, 195–208. [Google Scholar] [CrossRef]
- Lin, J.; Goosen, T.; Tse, S.; Yamagami, H.; Malhotra, B. Physiologically Based Pharmacokinetic Modeling Suggests Limited Drug-Drug Interaction for Fesoterodine When Coadministered With Mirabegron. J. Clin. Pharmacol. 2019, 59, 1505–1518. [Google Scholar] [CrossRef]
- O’Kane, M.; Robinson, D.; Cardozo, L.; Wagg, A.; Abrams, P. Mirabegron in the Management of Overactive Bladder Syndrome. Int. J. Women’s Health 2022, 14, 1337–1350. Available online: https://www.dovepress.com/mirabegron-in-the-management-of-overactive-bladder-syndrome-peer-reviewed-fulltext-article-IJWH (accessed on 27 October 2022).
- Sui, W.; Li, H.; Yang, Y.; Jing, X.; Xue, F.; Cheng, J.; Dong, M.; Zhang, M.; Pan, H.; Chen, Y.; et al. Bladder drug mirabegron exacerbates atherosclerosis through activation of brown fat-mediated lipolysis. Proc. Natl. Acad. Sci. USA 2019, 116, 10937–10942. [Google Scholar] [CrossRef]
- Peres Valgas da Silva, C.; Calmasini, F.; Alexandre, E.C.; Raposo, H.F.; Delbin, M.A.; Monica, F.Z.; Zanesco, A. The effects of mirabegron on obesity-induced inflammation and insulin resistance are associated with brown adipose tissue activation but not beiging in the subcutaneous white adipose tissue. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1477–1487. [Google Scholar] [CrossRef]
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [CrossRef]
- Becker, M.; Serr, I.; Salb, V.K.; Ott, V.B.; Mengel, L.; Blüher, M.; Weigmann, B.; Hauner, H.; Tschöp, M.H.; Daniel, C. Short-term cold exposure supports human Treg induction in vivo. Mol. Metab. 2019, 28, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [PubMed]
- Bea, J.W.; Lohman, T.G.; Cussler, E.C.; Going, S.B.; Thompson, P.A. Lifestyle Modifies the Relationship Between Body Composition and Adrenergic Receptor Genetic Polymorphisms, ADRB2, ADRB3 and ADRA2B: A Secondary Analysis of a Randomized Controlled Trial of Physical Activity Among Postmenopausal Women. Behav. Genet. 2010, 40, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Unelius, L.; Bengtsson, T.; Cannon, B.; Nedergaard, J. Coexisting beta-adrenoceptor subtypes: Significance for thermogenic process in brown fat cells. Am. J. Physiol. Physiol. 1994, 267, C969–C979. [Google Scholar] [CrossRef]
- Singh, R.; Barrios, A.; Dirakvand, G.; Pervin, S. Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues. Cells 2021, 10, 3030. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Nielsen, S.; Kuipers, E.N.; Severinsen, M.C.; Jensen, V.H.; Miard, S.; Jespersen, N.Z.; Kooijman, S.; Boon, M.R.; Fortin, M.; et al. Human Brown Adipocyte Thermogenesis Is Driven by β2-AR Stimulation. Cell Metab. 2020, 32, 287–300.e7. [Google Scholar] [CrossRef]
- Baskin, A.S.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Anflick-Chames, E.; Cero, C.; Johnson, J.W.; O’Mara, A.E.; Fletcher, L.A.; Leitner, B.P.; et al. Regulation of Human Adipose Tissue Activation, Gallbladder Size, and Bile Acid Metabolism by a β3-Adrenergic Receptor Agonist. Diabetes 2018, 67, 2113–2125. [Google Scholar] [CrossRef]
- Chapple, C.R.; Dvorak, V.; Radziszewski, P.; Van Kerrebroeck, P.; Wyndaele, J.J.; Bosman, B.; Boerrigter, P.; Drogendijk, T.; Ridder, A.; Yamaguchi, O.; et al. A phase II dose-ranging study of mirabegron in patients with overactive bladder. Int. Urogynecology J. 2013, 24, 1447–1458. [Google Scholar] [CrossRef]
- Loh, R.K.C.; Formosa, M.F.; La Gerche, A.; Reutens, A.T.; Kingwell, B.A.; Carey, A.L. Acute metabolic and cardiovascular effects of mirabegron in healthy individuals. Diabetes, Obes. Metab. 2018, 21, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.-H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Scott, S.; Abbasi, M.; Zu, Y.; Khan, S.H.; Yang, Y.; Wu, D.; Zhao, L.; Wang, S. Beneficial Metabolic Effects of Mirabegron In Vitro and in High-Fat Diet-Induced Obese Mice. J. Pharmacol. Exp. Ther. 2019, 369, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Finlin, B.S.; Memetimin, H.; Zhu, B.; Confides, A.L.; Vekaria, H.J.; El Khouli, R.H.; Johnson, Z.R.; Westgate, P.M.; Chen, J.; Morris, A.J.; et al. The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J. Clin. Investig. 2020, 130, 2319–2331. [Google Scholar] [CrossRef]
- Paśko, P.; Rodacki, T.; Domagała-Rodacka, R.; Owczarek, D. A short review of drug–food interactions of medicines treatingoveractive bladder syndrome. Int. J. Clin. Pharm. 2018, 36, 1350–1356. [Google Scholar]
- Yang, L.; Zhao, D.; Yin, R.; Ma, Y.; Zhang, N. Combined effects of voluntary running and liraglutide on glucose homeostasis, fatty acid composition of brown adipose tissue phospholipids, and white adipose tissue browning in db/db mice. Chin. J. Physiol. 2022, 65, 117. [Google Scholar] [CrossRef]
- Ma, X.; Li, M.; Liu, L.; Lei, F.; Wang, L.; Xiao, W.; Tan, Y.; He, B.; Ruan, S. A randomized controlled trial of Baduanjin exercise to reduce the risk of atherosclerotic cardiovascular disease in patients with prediabetes. Sci. Rep. 2022, 12, 19338. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Moslehi, E.; Minasian, V.; Sadeghi, H. Subcutaneous Adipose Tissue Browning, Serum Orexin-A, and Insulin Resistance Following Aerobic Exercise in High-Fat Diet Obesity Male Wistar Rats. Int. J. Prev. Med. 2021, 12, 132. [Google Scholar]
- Haghighi, A.H.; Hajinia, M.; Askari, R.; Abbasian, S.; Goldfied, G. Effect of high-intensity interval training and high-intensity resistance training on irisin and fibroblast growth factor 21 in men with overweight and obesity. Physiol. Pharmacol. 2022, 100, 937–944. [Google Scholar] [CrossRef]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Lehnig, A.C.; Stanford, K.I. Exercise-induced adaptations to white and brown adipose tissue. J. Exp. Biol. 2018, 7 (Suppl. 1), 221. Available online: https://journals.biologists.com/jeb/article/221/Suppl_1/jeb161570/33955/Exercise-induced-adaptations-to-white-and-brown (accessed on 27 October 2022).
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1111–1120.e3. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1550413118302419 (accessed on 27 October 2022). [CrossRef] [PubMed]
- Wu, M.V.; Bikopoulos, G.; Hung, S.; Ceddia, R.B. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: Impact on whole-body energy expenditure. J. Biol. Chem. 2014, 289, 34129–34140. [Google Scholar] [CrossRef]
- Vosselman, M.J.; Hoeks, J.; Brans, B.; Pallubinsky, H.; Nascimento, E.B.M.; Van Der Lans, A.A.J.J.; Broeders, E.P.M.; Mottaghy, F.M.; Schrauwen, P.; Van Marken Lichtenbelt, W.D. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int. J. Obes. 2015, 39, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Motiani, P.; Virtanen, K.A.; Motiani, K.K.; Eskelinen, J.J.; Middelbeek, R.J.; Goodyear, L.J.; Savolainen, A.M.; Kemppainen, J.; Jensen, J.; Din, M.U.; et al. Decreased insulin-stimulated brown adipose tissue glucose uptake after short-term exercise training in healthy middle-aged men. Diabetes, Obes. Metab. 2017, 19, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Kolimechkov, S.; Seijo, M.; Swaine, I.; Thirkell, J.; Colado, J.C.; Naclerio, F. Physiological effects of microcurrent and its application for maximising acute responses and chronic adaptations to exercise. Eur. J. Appl. Physiol. 2022, 121, 1–15. [Google Scholar] [CrossRef]
- van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J. Cold-Activated Brown Adipose Tissue in Healthy Men. New Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Bel, J.S.; Tai, T.; Khaper, N.; Lees, S.J. Mirabegron: The most promising adipose tissue beiging agent. Physiol. Rep. 2021, 9, e14779. [Google Scholar] [CrossRef]
- Jiang, S.; Bae, J.-H.; Wang, Y.; Song, W. The Potential Roles of Myokines in Adipose Tissue Metabolism with Exercise and Cold Exposure. Int. J. Mol. Sci. 2022, 23, 11523. [Google Scholar] [CrossRef]
- Sugimoto, S.; Mena, H.A.; Sansbury, B.E.; Kobayashi, S.; Tsuji, T.; Wang, C.-H.; Yin, X.; Huang, T.L.; Kusuyama, J.; Kodani, S.D.; et al. Brown adipose tissue-derived MaR2 contributes to cold-induced resolution of inflammation. Nat. Metab. 2022, 4, 775–790. [Google Scholar] [CrossRef]
- Hanssen, M.J.W.; Hoeks, J.; Brans, B.; Van Der Lans, A.A.J.J.; Schaart, G.; Van Den Driessche, J.J.; Jörgensen, J.A.; Boekschoten, M.V.; Hesselink, M.K.C.; Havekes, B.; et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 2015, 21, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Gencoglu, C.; Ulupinar, S.; Ozbay, S.; Altinkaynak, K.; Sebin, E.; Oymak, B. Exercise in the cold causes greater irisin release but may not be enough for adropin. Chin. J. Physiol. 2021, 64, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Goto, K. Myokine secretion following moderate-intensity endurance exercise under different environmental temperatures. Cytokine 2021, 144, 155553. [Google Scholar] [CrossRef] [PubMed]
- Aldiss, P.; Lewis, J.E.; Lupini, I.; Bloor, I.; Chavoshinejad, R.; Boocock, D.J.; Miles, A.K.; Ebling, F.J.P.; Budge, H.; Symonds, M.E. Cold Exposure Drives Weight Gain and Adiposity following Chronic Suppression of Brown Adipose Tissue. Int. J. Mol. Sci. 2022, 23, 1869. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunes, G.C.; Macêdo, A.P.A.; Conceição, L.R.; Pauli, J.R. Mirabegron and Physical Exercise Is a Potential Strategical for BAT Activation in Obesity. Obesities 2022, 2, 380-388. https://doi.org/10.3390/obesities2040032
Antunes GC, Macêdo APA, Conceição LR, Pauli JR. Mirabegron and Physical Exercise Is a Potential Strategical for BAT Activation in Obesity. Obesities. 2022; 2(4):380-388. https://doi.org/10.3390/obesities2040032
Chicago/Turabian StyleAntunes, Gabriel Calheiros, Ana Paula Azevêdo Macêdo, Luciana Renata Conceição, and José Rodrigo Pauli. 2022. "Mirabegron and Physical Exercise Is a Potential Strategical for BAT Activation in Obesity" Obesities 2, no. 4: 380-388. https://doi.org/10.3390/obesities2040032
APA StyleAntunes, G. C., Macêdo, A. P. A., Conceição, L. R., & Pauli, J. R. (2022). Mirabegron and Physical Exercise Is a Potential Strategical for BAT Activation in Obesity. Obesities, 2(4), 380-388. https://doi.org/10.3390/obesities2040032







