Triglyceride-to-High-Density-Lipoprotein-Cholesterol Ratio as a Predictor of Metabolic Syndrome According to Stage of Life at Obesity Onset in Women with Severe Obesity—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Measurements
2.3.1. Anthropometric Measurements
2.3.2. Blood Analysis
2.4. MetS Classification
2.5. Statistical Analysis
3. Results
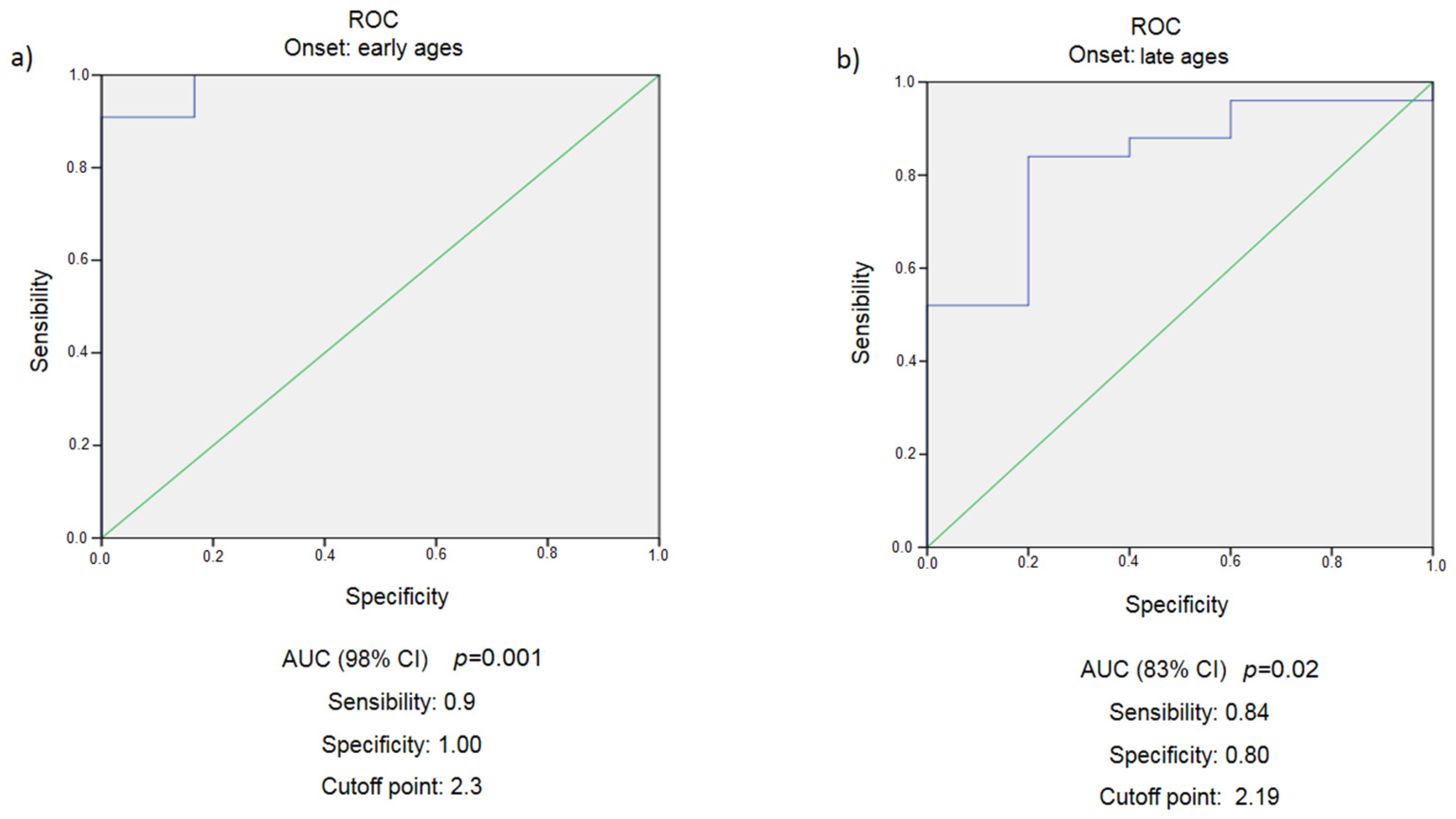
3.1. Early Age Obesity Development (<18 Years)
3.2. Late Age Obesity Development (>18 Years)
3.3. Difference between Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Al-Qawasmeh, R.H.; Tayyem, R.F. Dietary and lifestyle risk factors and metabolic syndrome: Literature review. Curr. Res. Nutr. Food Sci. 2018, 6, 594–608. [Google Scholar] [CrossRef]
- Lloyd, L.J.; Langley-Evans, S.C.; McMullen, S. Childhood obesity and risk of the adult metabolic syndrome: A systematic review. Int. J. Obes. 2012, 36, 1–11. [Google Scholar] [CrossRef]
- International Diabetes Federation. Consensus Worldwide Definition of the Metabolic Syndrome. 2006. Available online: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html (accessed on 11 January 2022).
- Du, T.; Yuan, G.; Zhang, M.; Zhou, X.; Sun, X.; Yu, X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc. Diabetol. 2014, 13, 146. [Google Scholar] [CrossRef]
- Borrayo, G.; Basurto, L.; González-Escudero, E.; Diaz, A.; Vázquez, A.; Sánchez, L.; Hernández-González, G.; Barrera, S.; Degollado, J.; Córdova, N.; et al. TG/HDL-C ratio as cardio-metabolic biomarker even in normal weight women. Acta Endocrinol. 2018, 14, 261–267. [Google Scholar] [CrossRef]
- Uruska, A.; Zozulinska-Ziolkiewicz, D.; Niedzwiecki, P.; Pietrzak, M.; Wierusz-Wysocka, B. TG/HDL-C ratio and visceral adiposity index may be useful in assessment of insulin resistance in adults with type 1 diabetes in clinical practice. J. Clin. Lipidol. 2018, 12, 734–740. [Google Scholar] [CrossRef]
- Young, K.A.; Maturu, A.; Lorenzo, C.; Langefeld, C.D.; Wagenknecht, L.E.; Chen, Y.-D.I.; Taylor, K.D.; Rotter, J.I.; Norris, J.M.; Rasouli, N. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance, β-cell function, and diabetes in Hispanics and African Americans. J. Diabetes Complicat. 2019, 33, 118–122. [Google Scholar] [CrossRef]
- Chen, B.-D.; Yang, Y.-N.; Ma, Y.-T.; Pan, S.; He, C.-H.; Liu, F.; Ma, X.; Fu, Z.-Y.; Li, X.-M.; Xie, X.; et al. Waist-to-height ratio and triglycerides/high-density lipoprotein cholesterol were the optimal predictors of metabolic syndrome in Uighur men and women in Xinjiang, China. Metab. Syndr. Relat. Disord. 2015, 13, 214–220. [Google Scholar] [CrossRef]
- Radetti, G.; Grugni, G.; Lupi, F.; Fanolla, A.; Caroli, D.; Bondesan, A.; Sartorio, A. High Tg/HDL-Cholesterol Ratio Highlights a Higher Risk of Metabolic Syndrome in Children and Adolescents with Severe Obesity. J. Clin. Med. 2022, 11, 4488. [Google Scholar] [CrossRef]
- Nur Zati Iwani, A.K.; Jalaludin, M.Y.; Yahya, A.; Mansor, F.; Md Zain, F.; Hong, J.Y.H.; Wan Mohd Zin, R.M.; Mokhtar, A.H. TG: HDL-C Ratio as Insulin Resistance Marker for Metabolic Syndrome in Children With Obesity. Front. Endocrinol. 2022, 13, 852290. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, Z.; Liu, Y.; Zhao, Y.; Fu, J.; Zhang, Y.; Liu, Y.; Fan, Z. Triglyceride to high-density lipoprotein cholesterol ratio and cardiovascular events in the general population: A systematic review and meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 318–329. [Google Scholar] [CrossRef]
- SBD- Sociedade Brasileira de Diabetes. Diretrizes da Sociedade Brasileira de Diabetes. São Paulo, 2017–2018. Available online: https://diretriz.diabetes.org.br/ (accessed on 30 December 2019).
- Allain-Regnault, M.; Bwibo, N.O.; Chigier, E. WHO Study Group on Young People and ‘Health for All by the Year 2000’ & World Health Organization. Young People’s Health-a Challenge for Society: Report of a WHO Study Group on Young People and “Health for All by the Year 2000” [Meeting Held in Geneva from 4 to 8 June 1984]. World Health Organization. 1986. Available online: https://apps.who.int/iris/handle/10665/41720 (accessed on 15 December 2019).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Stern, S.E.; Williams, K.; Ferrannini, E.; DeFronzo, R.A.; Bogardus, C.; Stern, M.P. Using Routine Clinical Measurements. Diabetes 2005, 54, 333–339. [Google Scholar] [CrossRef]
- Chu, S.-Y.; Jung, J.-H.; Park, M.-J.; Kim, S.-H. Risk assessment of metabolic syndrome in adolescents using the triglyceride/high-density lipoprotein cholesterol ratio and the total cholesterol/high-density lipoprotein cholesterol ratio. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 41–48. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley & Sons, Inc.: New York City, NY, USA, 2000; ISBN 0-471-72214-6. [Google Scholar]
- Tests, D. Basic Principles of ROC Analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar]
- Rezapour, M.; Shahesmaeili, A.; Hossinzadeh, A.; Zahedi, R.; Najafipour, H.; Gozashti, M.H. Comparison of Lipid Ratios to Identify Metabolic Syndrome. Arch. Iran. Med. 2018, 21, 572–577. [Google Scholar]
- Gasevic, D.; Frohlich, J.; Mancini, G.J.; Lear, S.A. Clinical usefulness of lipid ratios to identify men and women with metabolic syndrome: A cross-sectional study. Lipids Health Dis. 2014, 13, 159. [Google Scholar] [CrossRef]
- Huang, W.; Shu, L.; Zhao, H.; Chen, S.; Zhang, H.; Song, G. Association of the Triglyceride to High-Density Lipoprotein Ratio and the Visceral Adiposity Index with Metabolic Syndrome in Diabetic Susceptible Population. Horm. Metab. Res. 2020, 52, 95–103. [Google Scholar] [CrossRef]
- Gu, Z.; Zhu, P.; Wang, Q.; He, H.; Xu, J.; Zhang, L.; Li, D.; Wang, J.; Hu, X.; Ji, G. Obesity and lipid-related parameters for predicting metabolic syndrome in Chinese elderly population. Lipids Health Dis. 2018, 17, 289. [Google Scholar] [CrossRef]
- De Cosmi, V.; Scaglioni, S.; Agostoni, C. Early taste experiences and later food choices. Nutrients 2017, 9, 107. [Google Scholar] [CrossRef]
- Joshi, S.M.; Katre, P.A.; Kumaran, K.; Joglekar, C.; Osmond, C.; Bhat, D.S.; Lubree, H.; Pandit, A.; Yajnik, C.S.; Fall, C.H.D. Tracking of cardiovascular risk factors from childhood to young adulthood-The Pune Children’s Study. Int. J. Cardiol. 2014, 175, 176–178. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Wrzosek, M.; Wiśniewska, K.; Sawicka, A.; Tałałaj, M.; Nowicka, G. Early Onset of Obesity and Adult Onset of Obesity as Factors Affecting Patient Characteristics Prior to Bariatric Surgery. Obes. Surg. 2018, 28, 3902–3909. [Google Scholar] [CrossRef]
- Wrzosek, M.; Sawicka, A.; Wrzosek, M.; Piątkiewicz, P.; Tałałaj, M.; Nowicka, G. Age at onset of obesity, transcription factor 7-like 2 (TCF7L2) rs7903146 polymorphism, adiponectin levels and the risk of type 2 diabetes in obese patients. Arch. Med. Sci. 2019, 15, 321–329. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Giordano, A.; Murano, I.; Mondini, E.; Perugini, J.; Smorlesi, A.; Severi, I.; Barazzoni, R.; Scherer, P.E.; Cinti, S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res. 2013, 54, 2423–2436. [Google Scholar] [CrossRef]
- Murano, I.; Rutkowski, J.; Wang, Q.; Cho, Y.-R.; Scherer, P.; Cinti, S. Time course of histomorphological changes in adipose tissue upon acute lipoatrophy. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 723–731. [Google Scholar] [CrossRef]
- Baez-Duarte, B.G.; Zamora-Ginez, I.; González-Duarte, R.; Torres-Rasgado, E.; Ruiz-Vivanco, G.; Pérez-Fuentes, R. Triglyceride/high-density lipoprotein cholesterol (TG/HDL-C) index as a reference criterion of risk for metabolic syndrome (MetS) and low insulin sensitivity in apparently healthy subjects. Gac. Med. Mex. 2017, 153, 152–158. [Google Scholar]
| Variable | Total Sample (n = 47) (Mean + SD) | % of MetS Parameters Altered * |
|---|---|---|
| Age (years) | 40.68 ± 8.63 | - |
| Weight (kg) | 122.22 ± 17.74 | - |
| Height (m) | 1.59 ± 0.60 | - |
| BMI (kg/m2) | 48.38 ± 6.72 | 100% |
| WC (cm) | 130.93 ± 12.34 | 100% |
| HC (cm) | 145.24 ± 13.45 | - |
| WHR | 0.903 ± 0.06 | - |
| Glucose (mg/dL) | 114.55 ± 48.34 | 46.8% |
| Fasting Insulin (μUI/mL) | 27.10 ± 14.27 | - |
| HOMA-IR | 7.06 ± 3.77 | - |
| HBA1c (%) | 6.315 ± 1.30 | - |
| QUICKI | 0.29 ± 0.02 | - |
| TG (mg/dL) | 146 ± 65.60 | 42.6% |
| HDL-c (mg/dL) | 46.79 ± 10.35 | 66% |
| TG/HDL-c | 3.32 ± 1,87 | - |
| SBP (mmHg) | 138.42 ± 14.63 | 82.2% |
| DBP (mmHg) | 85.80 ± 9.57 | 44.4% |
| Variable | Early (n = 17) (Mean + SD) | Late (n = 30) (Mean + SD) | p * | Effect Size |
|---|---|---|---|---|
| Weight (kg) | 131.17 ± 20.61 | 117.15 ± 13.83 | 0.008 | 0.800 |
| Height (m) | 1.60 ± 0.05 | 1.58 ± 0.06 | 0.366 | 0.362 |
| BMI (kg/m2) | 51.16 ± 7.26 | 46.81 ± 5.95 | 0.031 | 0.655 |
| WC (cm) | 135.40 ± 14.54 | 128.39 ± 10.32 | 0.061 | 0.556 |
| HC (cm) | 150.66 ± 14.89 | 142.17 ± 11.73 | 0.036 | 0.633 |
| WHR | 0.901 ± 0.08 | 0.905 ± 0.058 | 0.866 | 0.096 |
| Glucose (mg/dL) | 106.41 ± 35.07 | 119.17 ± 54.47 | 0.391 | 0.278 |
| Fasting Insulin (μUI/mL) | 33.48 ± 19.03 | 23.49 ± 9.26 | 0.055 | 0.667 |
| QUICKI | 0.29 ± 0.016 | 0.29 ± 0.02 | 0.159 | 0 |
| HOMA-IR | 7.23 ± 3.55 | 6.96 ± 3.94 | 0.814 | 0.072 |
| HBA1c (%) | 6.147 ± 1.09 | 6.410 ± 1.42 | 0.513 | 2.077 |
| SBP (mmHg) | 140.31 ± 13.32 | 137.38 ± 15.44 | 0.526 | 0.203 |
| DBP (mmHg) | 86.19 ± 7.952 | 85.59 ± 10.49 | 0.843 | 0.010 |
| TG (mg/dL) | 137.12 ± 61.37 | 151.03 ± 68.38 | 0.491 | 0.214 |
| HDL-c | 48.41 ± 12.96 | 45.87 ± 8.65 | 0.424 | 0.230 |
| Presence | Absence | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Early | Late | Total | Early | Late | Total | p |
| Diabetes | 17.6% | 30% | 25.5% | 82.4% | 70% | 74.5% | 0.351 |
| Glucose * | 29.4% | 56.7% | 46.8% | 70.6% | 43.3% | 53.2% | 0.072 |
| SAH | 47.1% | 66.7% | 59.6% | 52.9% | 33.3% | 40.4% | 0.188 |
| SBP * | 81.3% | 82.8% | 82.2% | 18.8% | 17.2% | 17.8% | 0.899 |
| DBP * | 43.8% | 44.8% | 44.4% | 56.3% | 55.2% | 55.6% | 0.944 |
| TG * | 47.1% | 40% | 42.6% | 52.9% | 60% | 57.4% | 0.638 |
| TG/HDL-c ** | 41.2% | 46.7% | 44.7% | 58.8% | 53.3% | 55.3% | 0.716 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, N.; de Oliveira Queiroz, M.; Lopes, K.L.S.; Oliveira, L.; Dâmaso, A.R.; de Melo, P.R.E.; de Souza Abreu, V.; Mota, J.F.; Horst, M.A.; Corgosinho, F.C. Triglyceride-to-High-Density-Lipoprotein-Cholesterol Ratio as a Predictor of Metabolic Syndrome According to Stage of Life at Obesity Onset in Women with Severe Obesity—A Pilot Study. Obesities 2022, 2, 361-371. https://doi.org/10.3390/obesities2040030
Figueiredo N, de Oliveira Queiroz M, Lopes KLS, Oliveira L, Dâmaso AR, de Melo PRE, de Souza Abreu V, Mota JF, Horst MA, Corgosinho FC. Triglyceride-to-High-Density-Lipoprotein-Cholesterol Ratio as a Predictor of Metabolic Syndrome According to Stage of Life at Obesity Onset in Women with Severe Obesity—A Pilot Study. Obesities. 2022; 2(4):361-371. https://doi.org/10.3390/obesities2040030
Chicago/Turabian StyleFigueiredo, Nayra, Marcela de Oliveira Queiroz, Karem Lays Soares Lopes, Luciana Oliveira, Ana Raimunda Dâmaso, Paulo Reis Esselin de Melo, Valéria de Souza Abreu, João Felipe Mota, Maria Aderuza Horst, and Flávia Campos Corgosinho. 2022. "Triglyceride-to-High-Density-Lipoprotein-Cholesterol Ratio as a Predictor of Metabolic Syndrome According to Stage of Life at Obesity Onset in Women with Severe Obesity—A Pilot Study" Obesities 2, no. 4: 361-371. https://doi.org/10.3390/obesities2040030
APA StyleFigueiredo, N., de Oliveira Queiroz, M., Lopes, K. L. S., Oliveira, L., Dâmaso, A. R., de Melo, P. R. E., de Souza Abreu, V., Mota, J. F., Horst, M. A., & Corgosinho, F. C. (2022). Triglyceride-to-High-Density-Lipoprotein-Cholesterol Ratio as a Predictor of Metabolic Syndrome According to Stage of Life at Obesity Onset in Women with Severe Obesity—A Pilot Study. Obesities, 2(4), 361-371. https://doi.org/10.3390/obesities2040030






