Cyclic VLCKD Meal Replacement in a Patient with Obesity and Mild Chronic Kidney Disease following Kidney Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical History
2.2. Dietary Intervention
2.3. Data Collection and Analysis
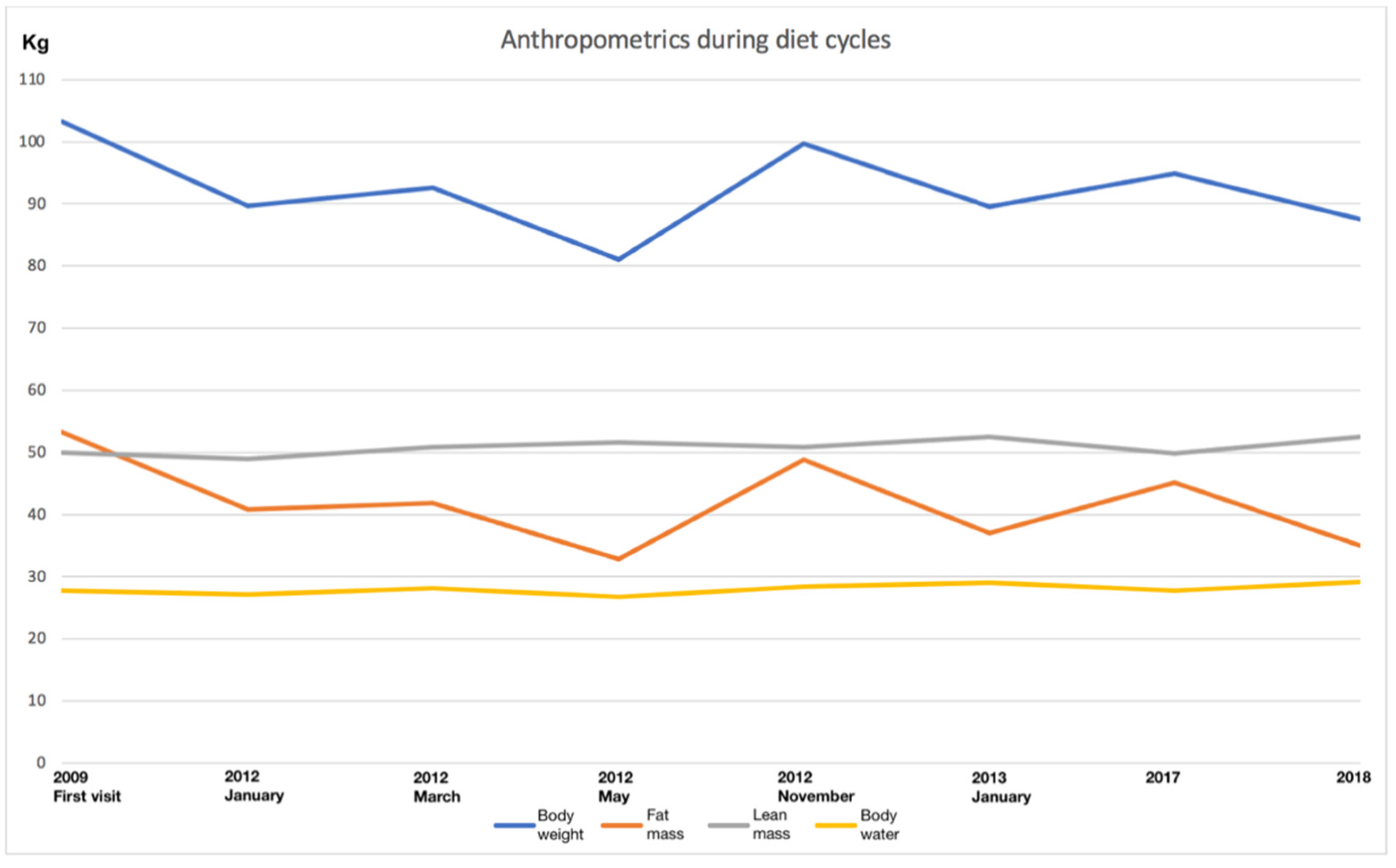
3. Results
3.1. Hypocaloric Diet
3.2. First VLCKD
3.3. Second VLCKD
3.4. Third VLCKD
3.5. Fourth VLCKD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- OECD. The Heavy Burden of Obesity: The Economics of Prevention, OECD Health Policy Studies; OECD Publishing: Paris, France, 2019. [Google Scholar] [CrossRef]
- Bray, G.A.; Fruhbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef]
- Kargin, D.; Tomaino, L.; Serra-Majem, L. Experimental Outcomes of the Mediterranean Diet: Lessons Learned from the Predimed Randomized Controlled Trial. Nutrients 2019, 11, 2991. [Google Scholar] [CrossRef]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO). European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes Facts. 2021, 14, 222–245. [Google Scholar] [CrossRef]
- Gudzune, K.; Doshi, R.; Metha, A.; Chaudhry, Z.W.; Jacobs, D.K.; Vakil, R.M.; Lee, C.J.; Bleich, S.N.; Clark, J.M. Efficacy of commercial weight loss programs: An updated systematic review. Ann. Intern. Med. 2015, 162, 501–512. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion EFSA 2015 sulla composizione essenziale dei sostituenti dietetici per il controllo del peso. Panel NDA on dietetic products, nutrition and allergies. EFSA J. 2015, 13, 3957. [Google Scholar]
- McLester, C.N.; Nickerson, B.S.; Kliszczewicz, B.M.; McLester, J.R. Reliability and Agreement of Various InBody Body Composition Analyzers as Compared to Dual-Energy X-Ray Absorptiometry in Healthy Men and Women. J. Clin. Densitom. 2020, 23, 443–450. [Google Scholar] [CrossRef]
- Vigna, L.; Barberi, C.; Sommaruga, D.; Ghio, L.; Belingheri, M.; Sala, A.; Riboldi, L. Obesità in post-trapianto di rene: Efficacia e sicurezza di una dieta chetogenica VLCD. Progr. Nutr. 2013, 15, 58–64. [Google Scholar]
- National Clinical Guideline Centre (UK). Obesity: Identification, Assessment and Management of Overweight and Obesity in Children, Young People and Adults: Partial Update of CG43; National Institute for Health and Care Excellence (UK): London, UK, 2014; (NICE Clinical Guidelines, No. 189.) 6, Very-Low-Calorie Diets. Available online: https://www.ncbi.nlm.nih.gov/books/NBK311324/ (accessed on 2 June 2022).
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef] [PubMed]
- Moriconi, E.; Camajani, E.; Fabbri, A.; Lenzi, A.; Caprio, M. Very-Low-Calorie Ketogenic Diet as a Safe and Valuable Tool for Long-Term Glycemic Management in Patients with Obesity and Type 2 Diabetes. Nutrients 2021, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Biacchi, E.; Procino, F.; Casanueva, F.F.; Trimboli, P. Very-low-calorie ketogenic diet for the management of obesity, overweight and related disorders. Minerva Endocrinol. 2021, 46, 161–167. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Bahceci, S.; Lucan, B.; Ryan, M. A practical guide for the use of very low calorie diets in adults with chronic kidney disease. Nephrology 2019. epub ahead of print. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar]
- Bruci, A.; Tuccinardi, D.; Tozzi, R.; Balena, A.; Santucci, S.; Frontani, R.; Mariani, S.; Basciani, S.; Spera, G.; Gnessi, L.; et al. Very Low-Calorie Ketogenic Diet: A Safe and Effective Tool for Weight Loss in Patients With Obesity and Mild Kidney Failure. Nutrients 2020, 12, 333. [Google Scholar] [CrossRef]
- Nordmann, A.J.; Nordmann, A.; Briel, M.; Keller, U.; Yancy, W.S.J.; Brehm, B.J.; Bucher, H.C. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006, 166, 285–293, Erratum in Arch. Intern. Med. 2006, 166, 932. [Google Scholar] [CrossRef]
- Bezerra Bueno, N.; Vieira de Melo, I.S.; Lima de Oliveira, S.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Moreno, B.; Bellido, D.; Sajoux, I.; Goday, A.; Saavedra, D.; Crujeiras, A.B.; Casanueva, F.F. Comparison of a very low-calorie- ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine 2014, 47, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Moreno, B.; Crujeiras, A.B.; Bellido, D.; Sajoux, I.; Casanueva, F.F. Obesity treatment by very low-calorie-ketogenic diet at two years: Reduction in visceral fat and on the burden of disease. Endocrine 2016, 54, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Webber, L.; Divajeva, D.; Marsh, T.; McPherson, K.; Brown, M.; Galea, G.; Breda, J. The future burden of obesity-related diseases in the 53 WHO European-Region countries and the impact of effective interventions: A modelling study. BMJ Open 2014, 4, e004787. [Google Scholar] [CrossRef]
- Mustajoki, P.; Pekkarinen, T. Very low energy diets in the treatment of obesity. Obes. Rev. 2001, 2, 61–72. [Google Scholar] [CrossRef]
- Brehm, B.J.; Seeley, R.J.; Daniels, S.R.; D’Alessio, D.A. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 1617–1623. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Nieuwenhuizen, A.; Tomé, D.; Soenen, S.; Westerterp, K.R. Dietary protein, weight loss, and weight maintenance. Annu. Rev. Nutr. 2009, 29, 21–41. [Google Scholar] [CrossRef]
- Noakes, M.; Foster, P.R.; Keogh, J.B.; Clifton, P.M. Meal replacements are as effective as structured weight-loss diets for treating obesity in adults with features of metabolic syndrome. J. Nutr. 2004, 134, 1894–1899. [Google Scholar] [CrossRef]
- Delbridge, E.; Proietto, J. State of the science: VLED (very low energy diet) for obesity. Asia Pac. J. Clin. Nutr. 2006, 15, 49–54. [Google Scholar]
- Basciani, S.; Costantini, D.; Contini, S.; Persichetti, A.; Watanabe, M.; Mariani, S.; Lubrano, C.; Spera, G.; Lenzi, A.; Gnessi, L. Safety and efficacy of a multiphase dietetic protocol with meal replacements including a step with very low calorie diet. Endocrine 2015, 48, 863–870. [Google Scholar] [CrossRef]
- Vigna, L.M.; Tomaino, L.; Lotito, V.; Di Pierro, F.; Ingenito, M.R.; Sommaruga, D.; Piontini, A.; Marsili, A. Killing three birds with one stone: VLCKD in a female patient with obesity, celiac disease and migraine. Prog. Nutr. 2021, 23, e2021331. [Google Scholar]
- Tragni, E.; Vigna, L.; Ruscica, M.; Macchi, C.; Casula, M.; Santelia, A.; Catapano, A.; Magni, P. Reduction of Cardio-Metabolic Risk and Body Weight through a Multiphasic Very-Low Calorie Ketogenic Diet Program in Women with Overweight/Obesity: A Study in a Real-World Setting. Nutrients 2021, 13, 1804. [Google Scholar] [CrossRef]

| First Cycle | Second Cycle | Third Cycle | Fourth Cycle | |||||
|---|---|---|---|---|---|---|---|---|
| Start | End | Start | End | Start | End | Start | End | |
| Glycaemia, mg/dL | 106 | 94 | 95 | 99 | 93 | 86 | 91 | 84 |
| Insulin, mIU/L | 23.9 | 10.9 | 15.8 | 10.3 | 21.9 | 16.1 | 19.2 | 12.1 |
| HOMA IR | 6.26 | 2.52 | 3.70 | 2.51 | 5.02 | 3.41 | 4.31 | 2.50 |
| Triglycerides, mg/dL | 172 | 100 | 117 | 118 | 169 | 97 | 160 | 150 |
| Total cholesterol, mg/dL | 130 | 135 | 163 | 169 | 179 | 192 | 159 | 194 |
| HDL cholesterol, mg/dL | 34 | 32 | 41 | 43 | 40 | 40 | 40 | 39 |
| LDL cholesterol, mg/dL | 110 | 103 | 98 | 104 | 129 | 139 | 124 | 131 |
| Uric Acid, mg/dL | 7.1 | 6.62 | 5.8 | 5.9 | 5.63 | 6.46 | 6.15 | 5.93 |
| Creatininemia, mg/dL | 1.11 | 1.2 | 1.05 | 1.11 | 1.23 | 1.33 | 1.31 | 1.27 |
| GFR (CKD-EPI), ml/min/1.73 m2 | 91.2 | 83.0 | 91.2 | 79.0 | 78.8 | 71.7 | 72.5 | 75.3 |
| Urinary proteins, mg/dL | 0 | 0 | 0 | 0 | 20 | 20 | 0 | 10 |
| First Cycle | Second Cycle | Third Cycle | Fourth Cycle | |||||
|---|---|---|---|---|---|---|---|---|
| Start | End | Start | End | Start | End | Start | End | |
| Body weight, kg | 103.2 | 89.7 | 92.6 | 81.0 | 99.7 | 89.5 | 94.9 | 87.5 |
| BMI, kg/m2 | 41.9 | 36.4 | 37.6 | 32.9 | 40.4 | 36.3 | 38.5 | 35.5 |
| BMR, kcal | 1448 | 1426 | 1466 | 1440 | 1470 | 1504 | 1446 | 1504 |
| Fat mass, kg | 53.3 | 40.8 | 41.8 | 32.8 | 48.8 | 37.0 | 45.1 | 35.0 |
| Lean mass, kg | 49.9 | 48.9 | 50.8 | 51.6 | 50.9 | 52.5 | 49.8 | 52.5 |
| Muscular mass, kg | 27.7 | 27.1 | 28.2 | 26.8 | 28.4 | 29.0 | 27.7 | 29.1 |
| Body water, kg | 36.7 | 35.9 | 37.4 | 36.8 | 37.4 | 38.6 | 36.7 | 38.6 |
| Before Ketogenic Diet Cycles | After 4 Ketogenic Diet Cycles | % of Variation | |
|---|---|---|---|
| Body weight, kg | 103.2 | 87.5 | −15.21% |
| BMI, kg/m2 | 41.9 | 35.5 | −15.27% |
| Fat mass, kg | 53.3 | 35 | −34.33% |
| Lean mass, kg | 49.9 | 52.5 | +5.21% |
| Muscular mass, kg | 27.7 | 29.1 | +5.05% |
| Body water, kg | 36.7 | 38.6 | +5.17% |
| Positive | Negative |
|---|---|
| Quick and easy weight loss | Reduction of social life |
| Fairly varied diet with assorted products | Sense of unease and discouragement towards people who eat normally |
| Dietary products already weighed and easy to consume | Costs |
| Loss of sense of hunger during the diet | |
| Positive psychological state | |
| Transition phase from the protein diet to the normal diet quite simple to organize |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigna, L.; Tomaino, L.; Lotito, V.; Ingenito, M.R.; Piontini, A.; Marsili, A. Cyclic VLCKD Meal Replacement in a Patient with Obesity and Mild Chronic Kidney Disease following Kidney Transplantation. Obesities 2022, 2, 342-349. https://doi.org/10.3390/obesities2040028
Vigna L, Tomaino L, Lotito V, Ingenito MR, Piontini A, Marsili A. Cyclic VLCKD Meal Replacement in a Patient with Obesity and Mild Chronic Kidney Disease following Kidney Transplantation. Obesities. 2022; 2(4):342-349. https://doi.org/10.3390/obesities2040028
Chicago/Turabian StyleVigna, Luisella, Laura Tomaino, Veronica Lotito, Maria Rosaria Ingenito, Alessandra Piontini, and Alessandro Marsili. 2022. "Cyclic VLCKD Meal Replacement in a Patient with Obesity and Mild Chronic Kidney Disease following Kidney Transplantation" Obesities 2, no. 4: 342-349. https://doi.org/10.3390/obesities2040028
APA StyleVigna, L., Tomaino, L., Lotito, V., Ingenito, M. R., Piontini, A., & Marsili, A. (2022). Cyclic VLCKD Meal Replacement in a Patient with Obesity and Mild Chronic Kidney Disease following Kidney Transplantation. Obesities, 2(4), 342-349. https://doi.org/10.3390/obesities2040028






