Transitions among BMI States: A Test of Competing Hypotheses
Abstract
:1. Introduction
2. Hypotheses
3. Method
3.1. Sample
3.2. Measures
3.3. Analytic Strategy
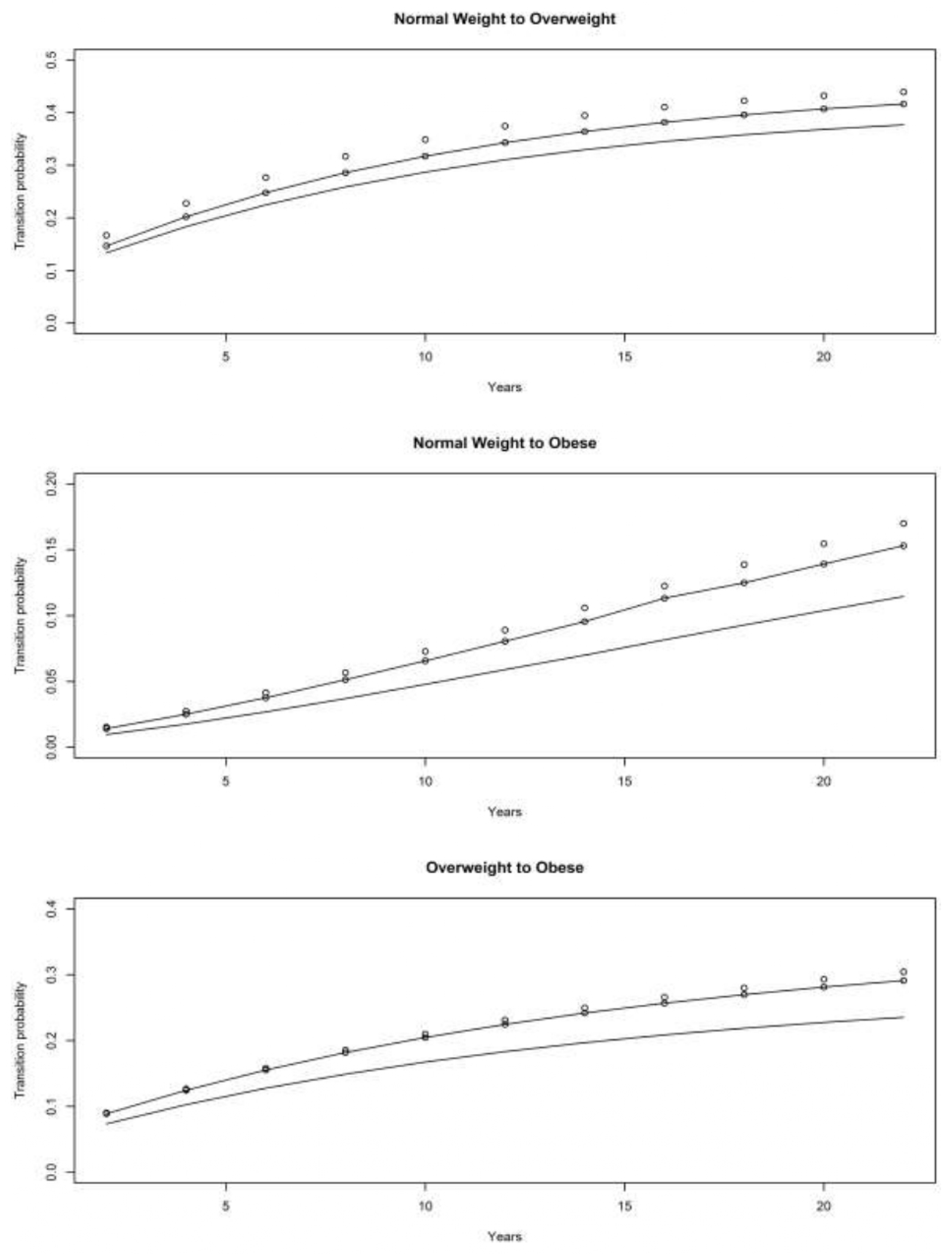
4. Results
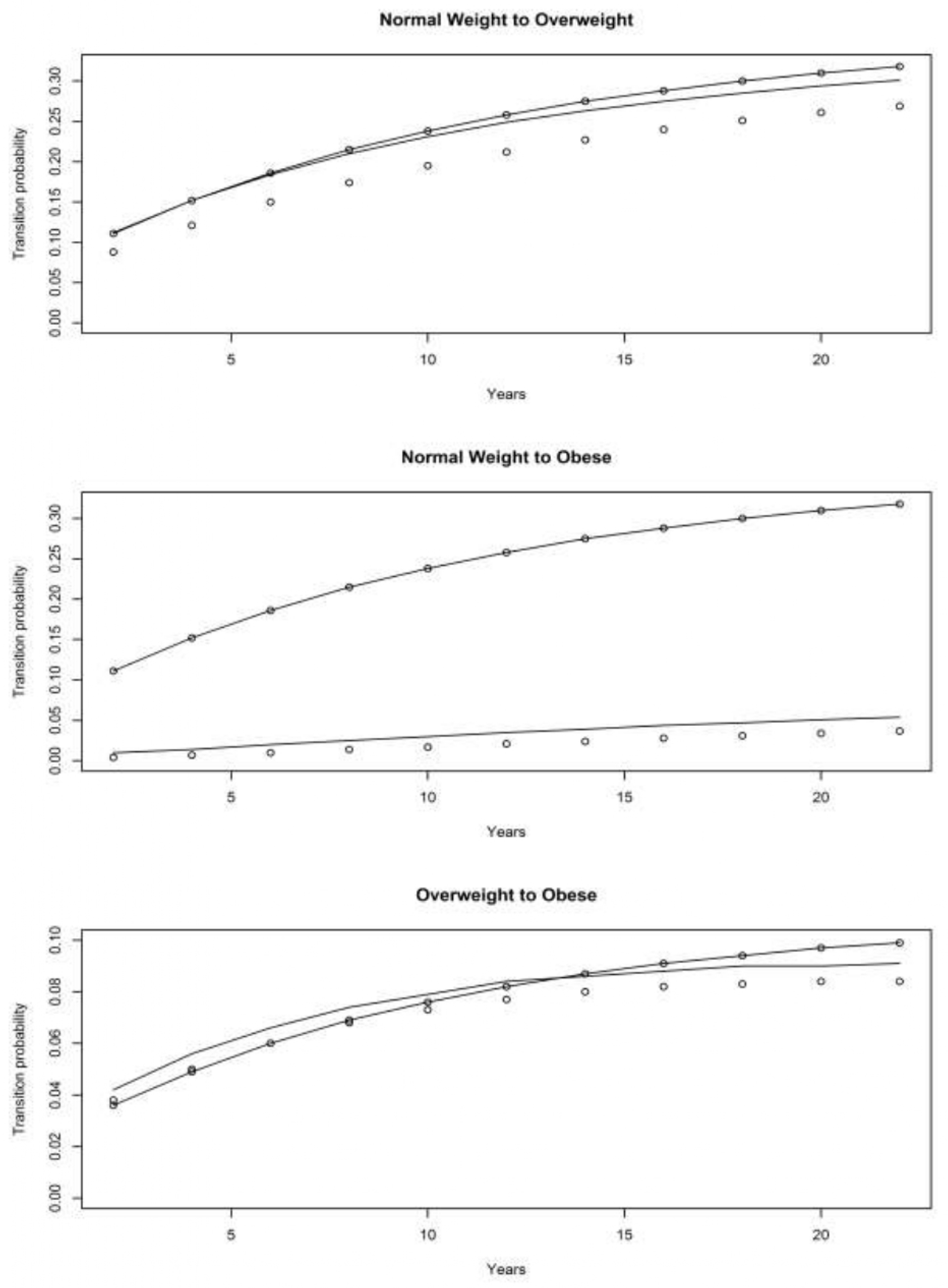
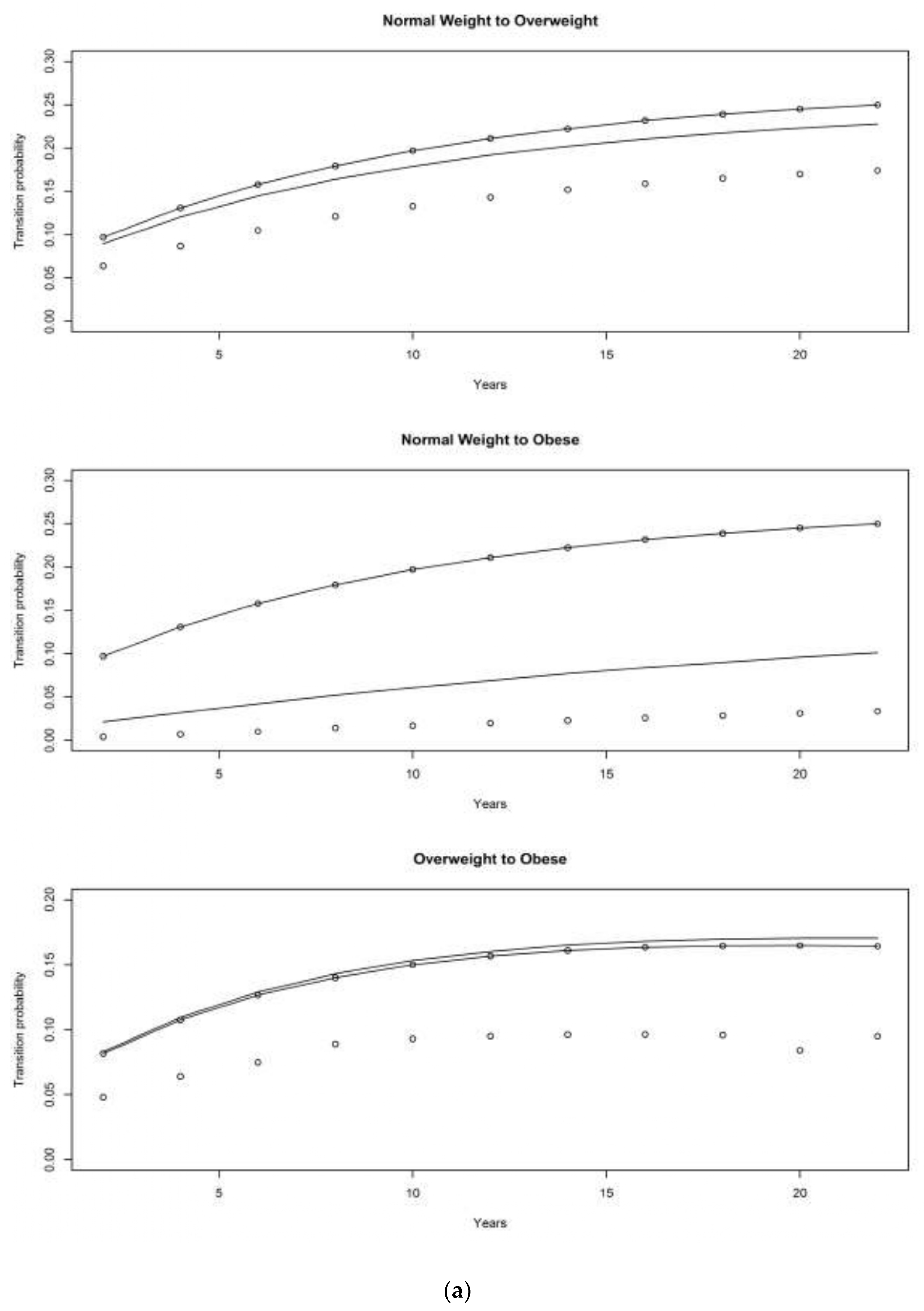
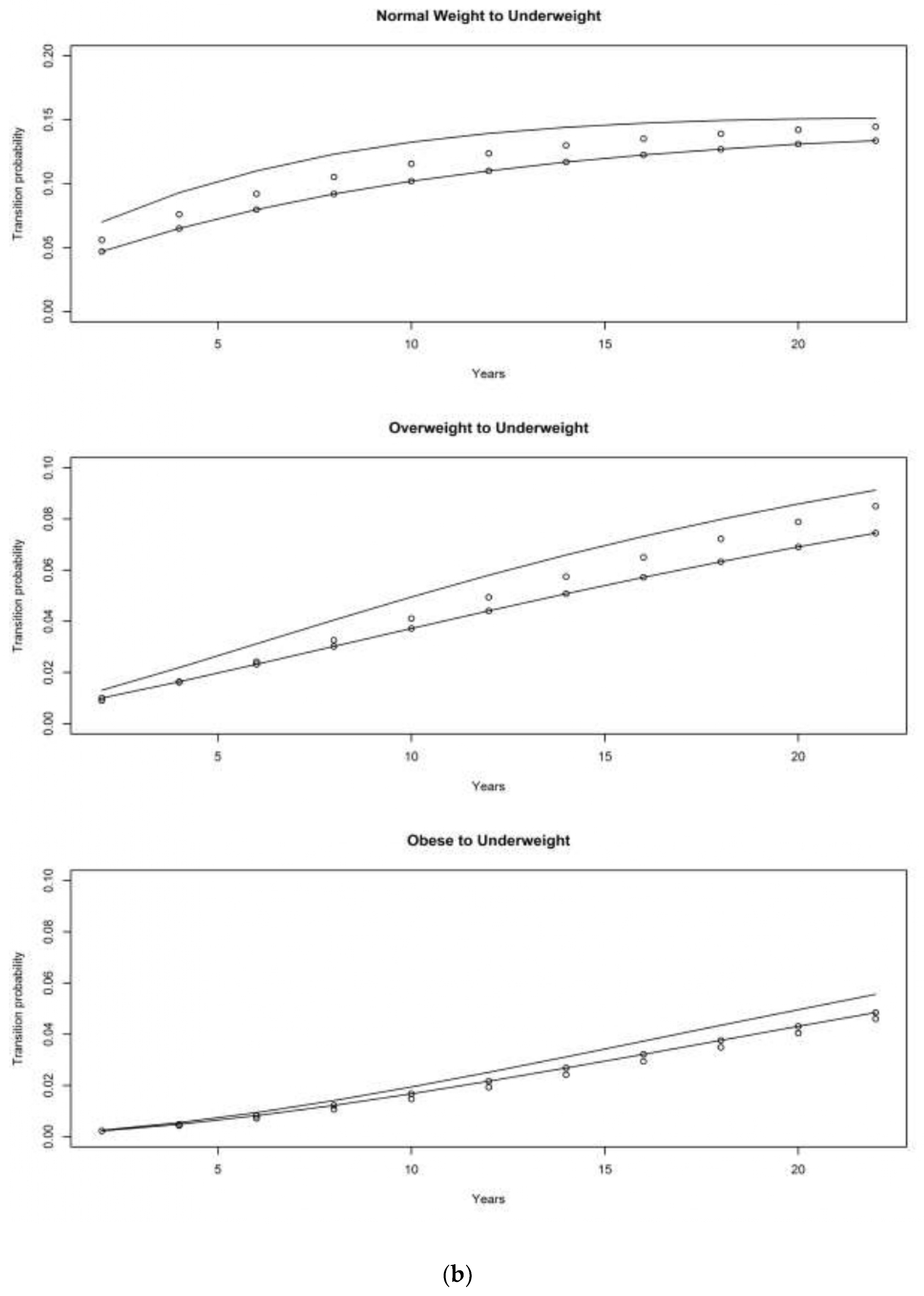
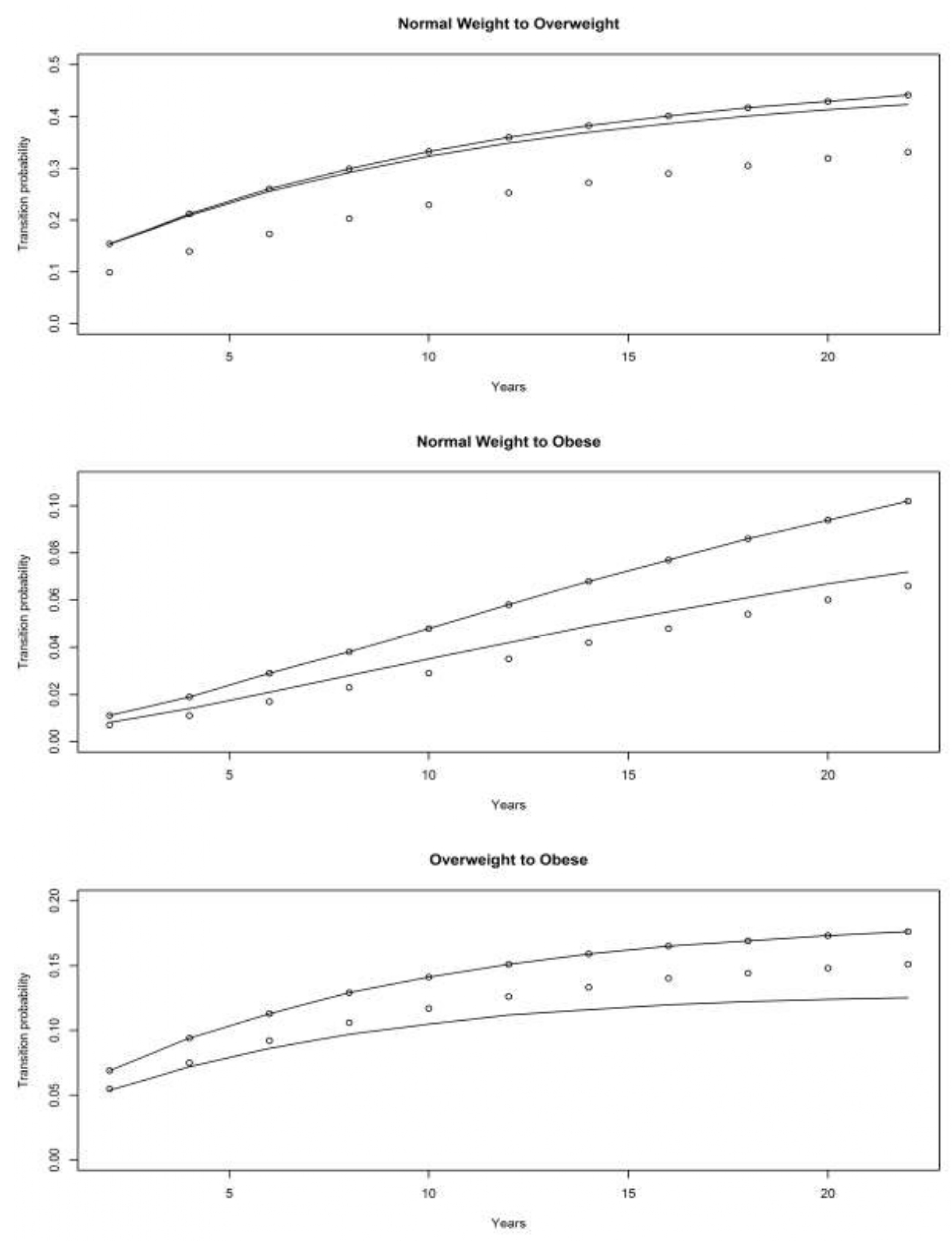
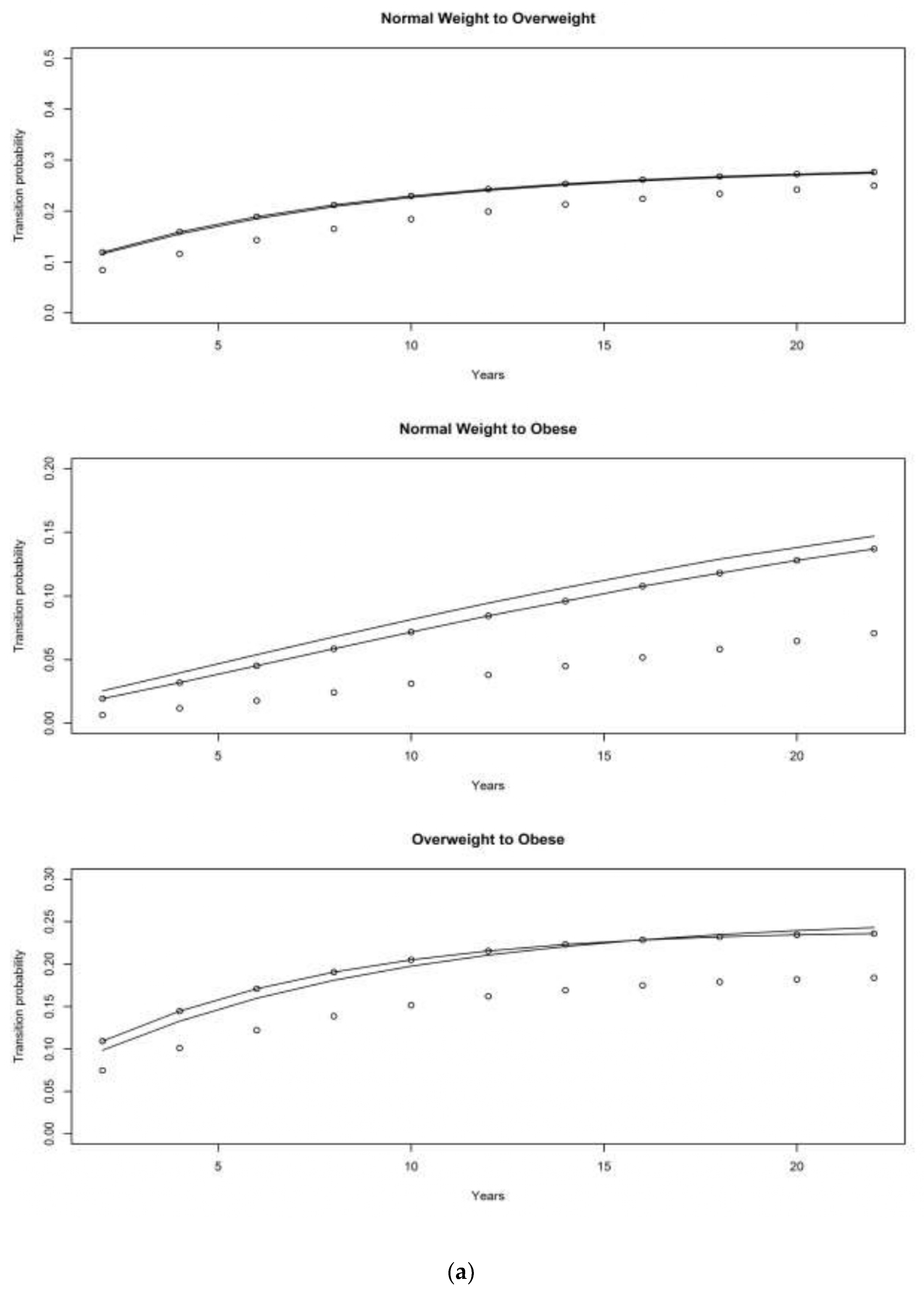
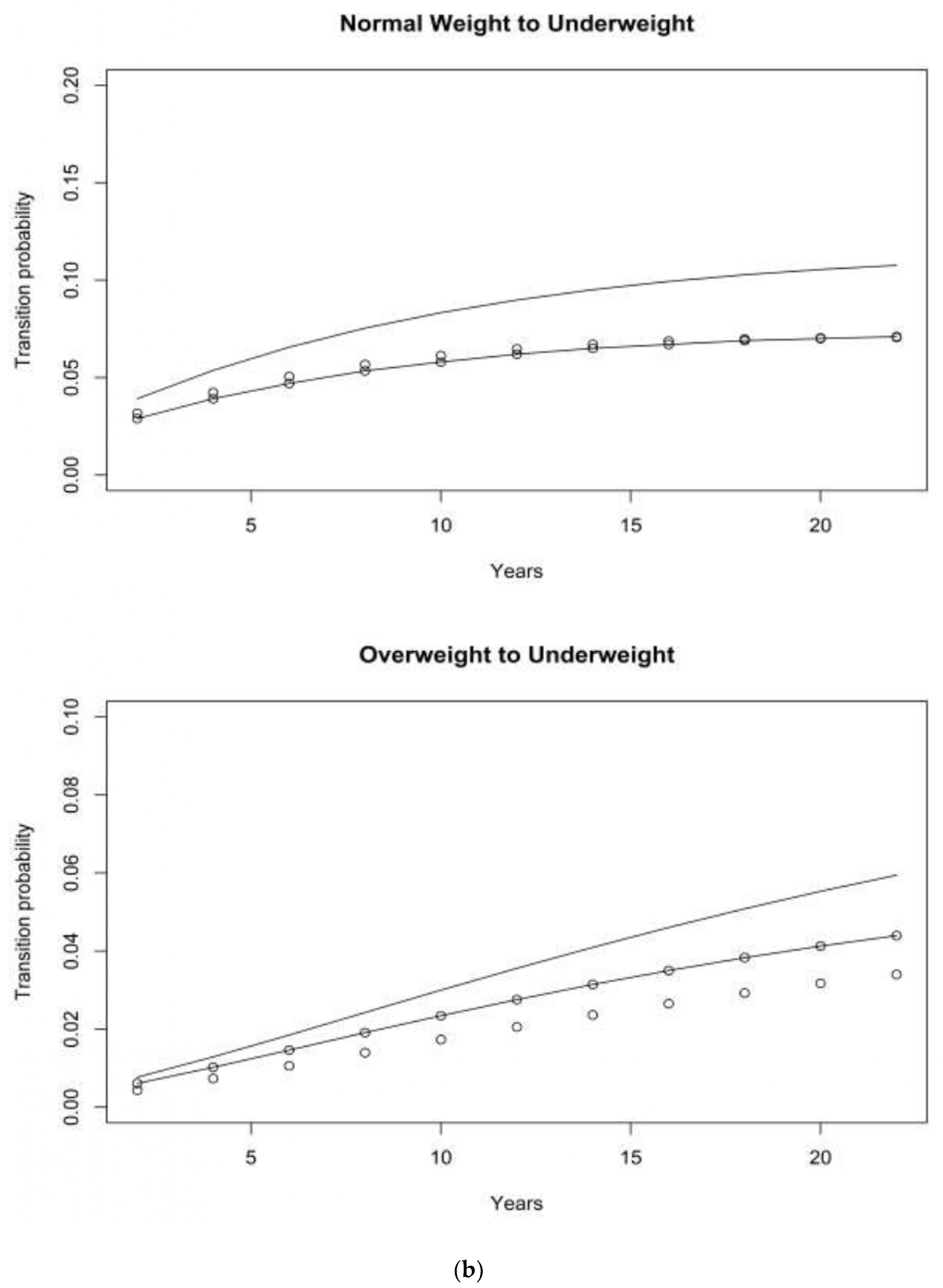
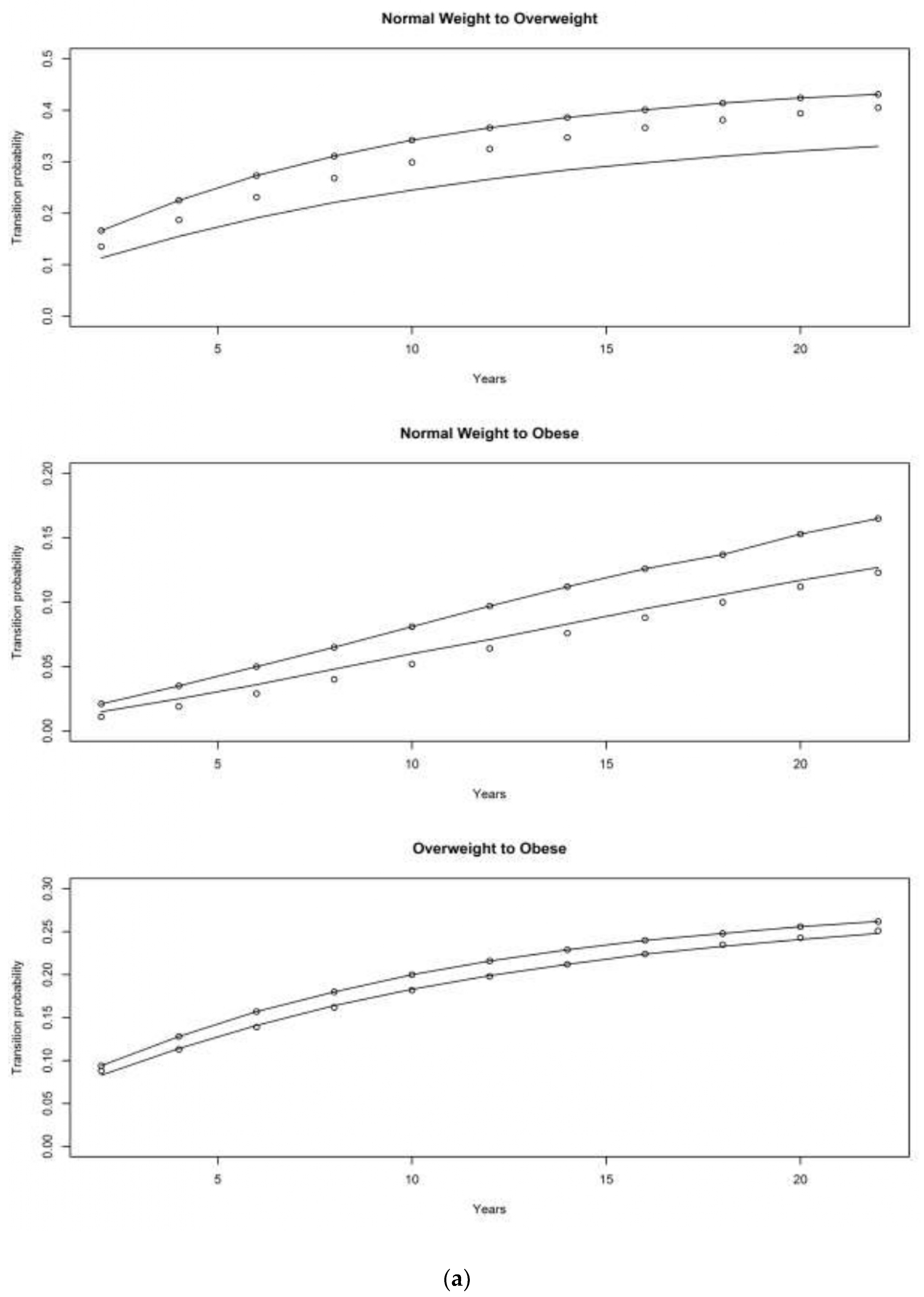
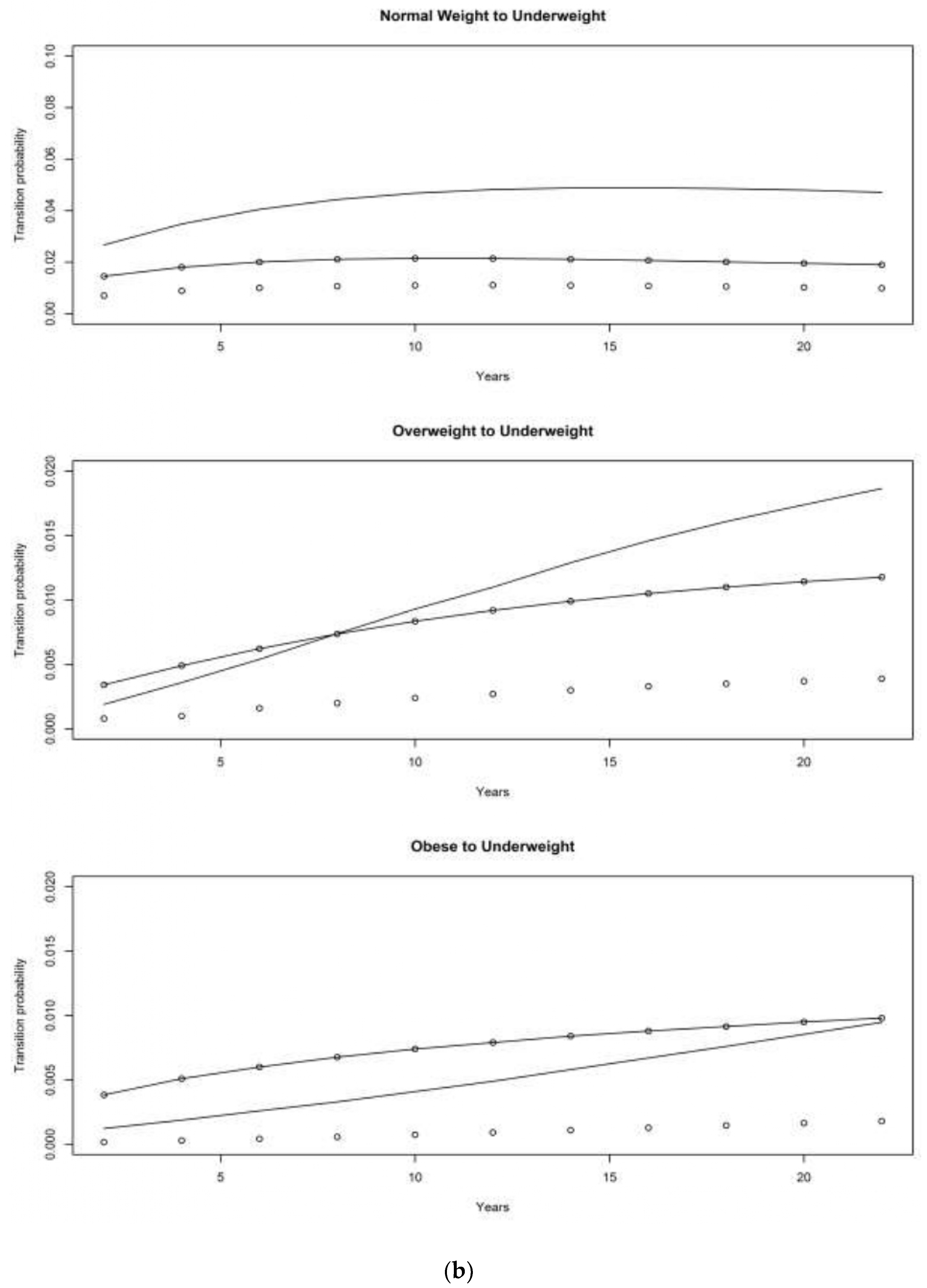
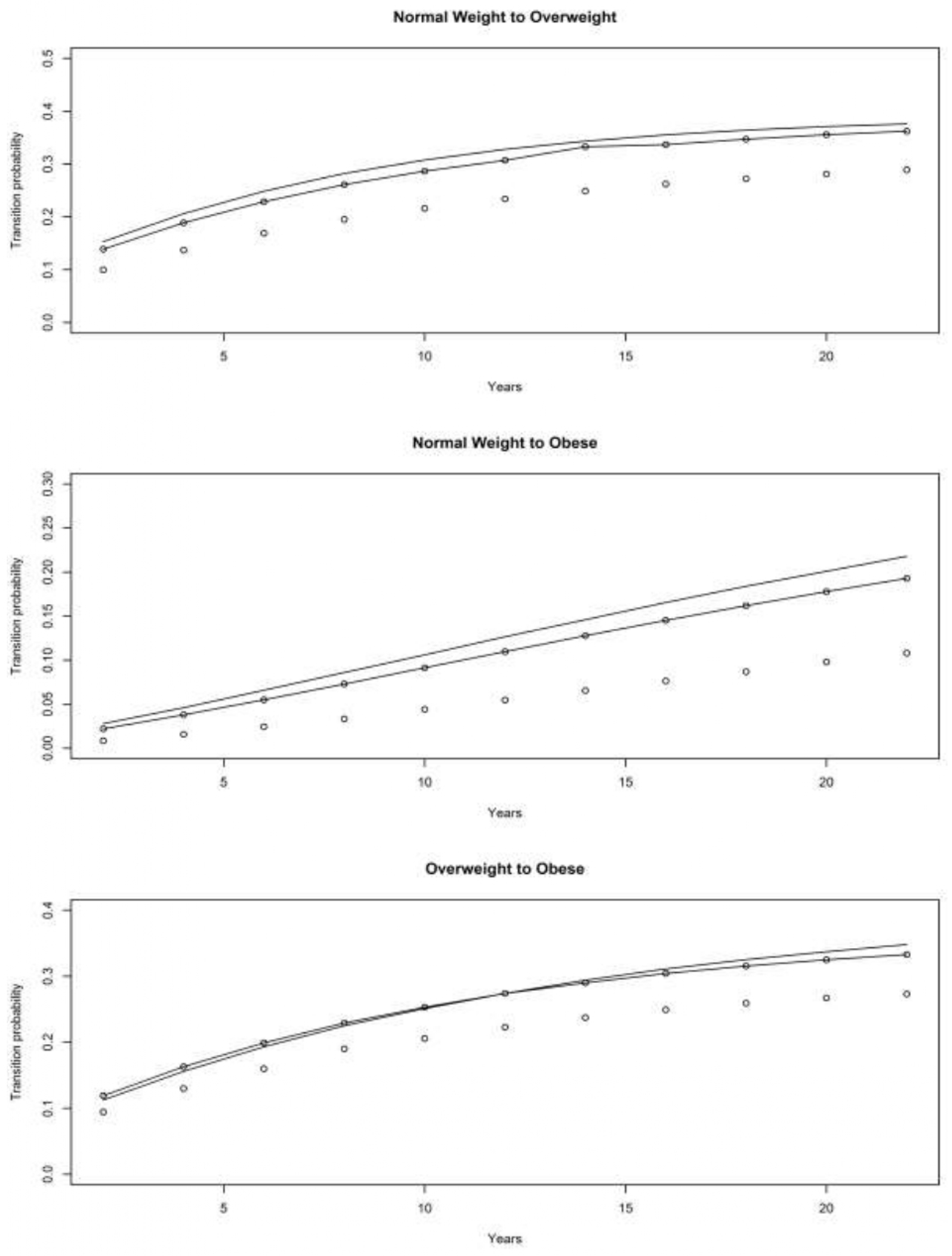
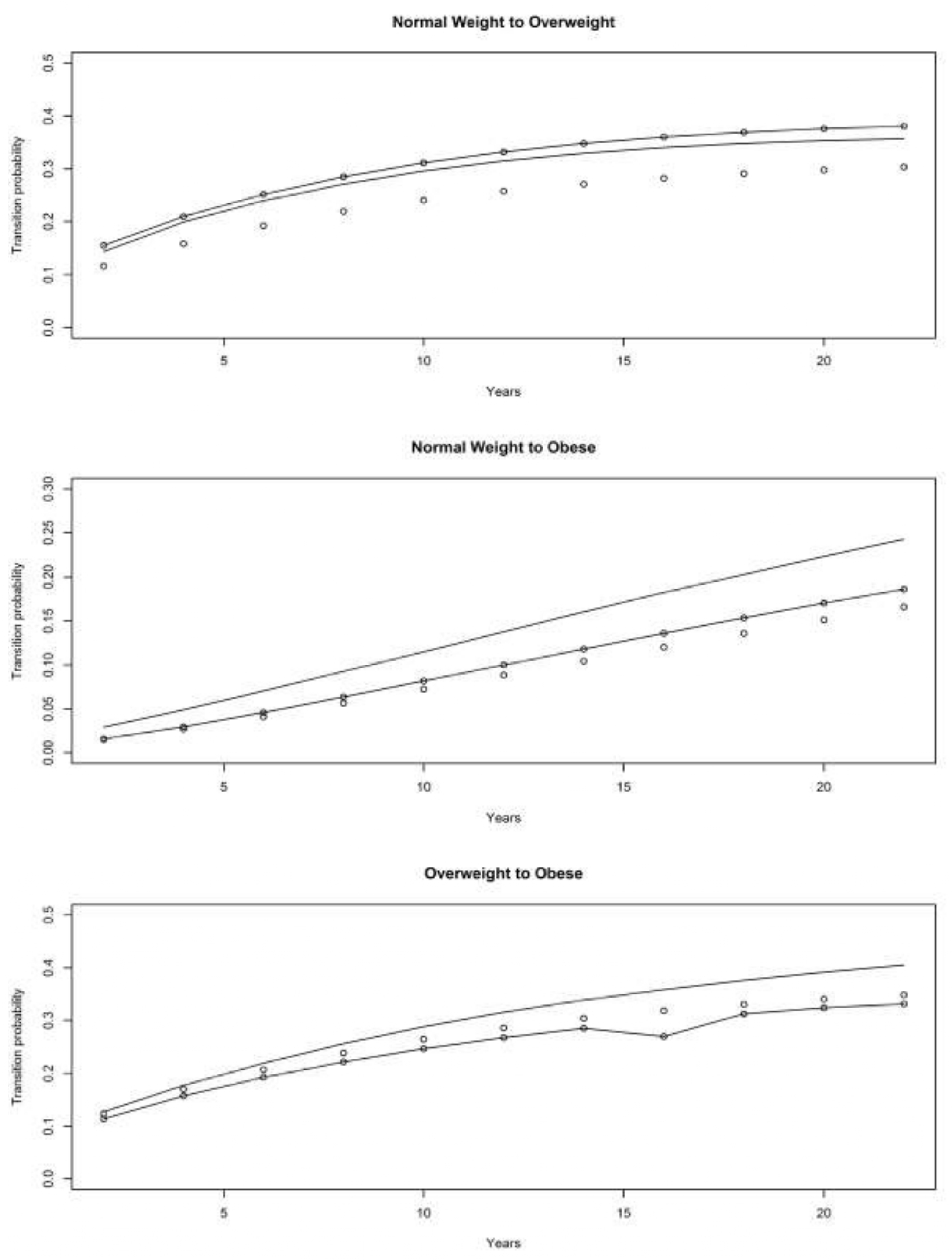
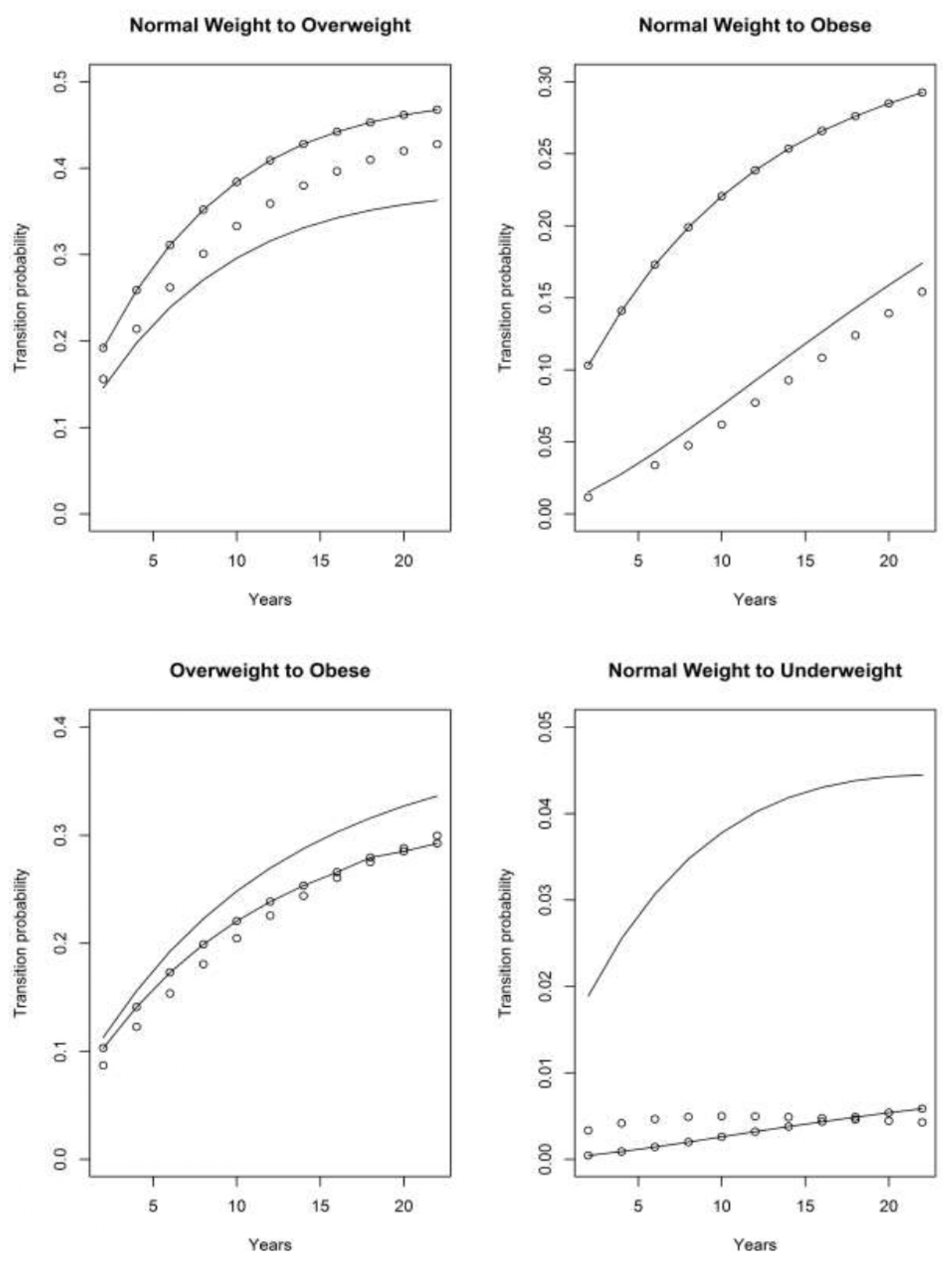
4.1. Asset and Health Dynamics among the Oldest Old (AHEAD) Cohort
4.2. Children of Depression (CODA) Cohort
4.3. The Original HRS Cohort
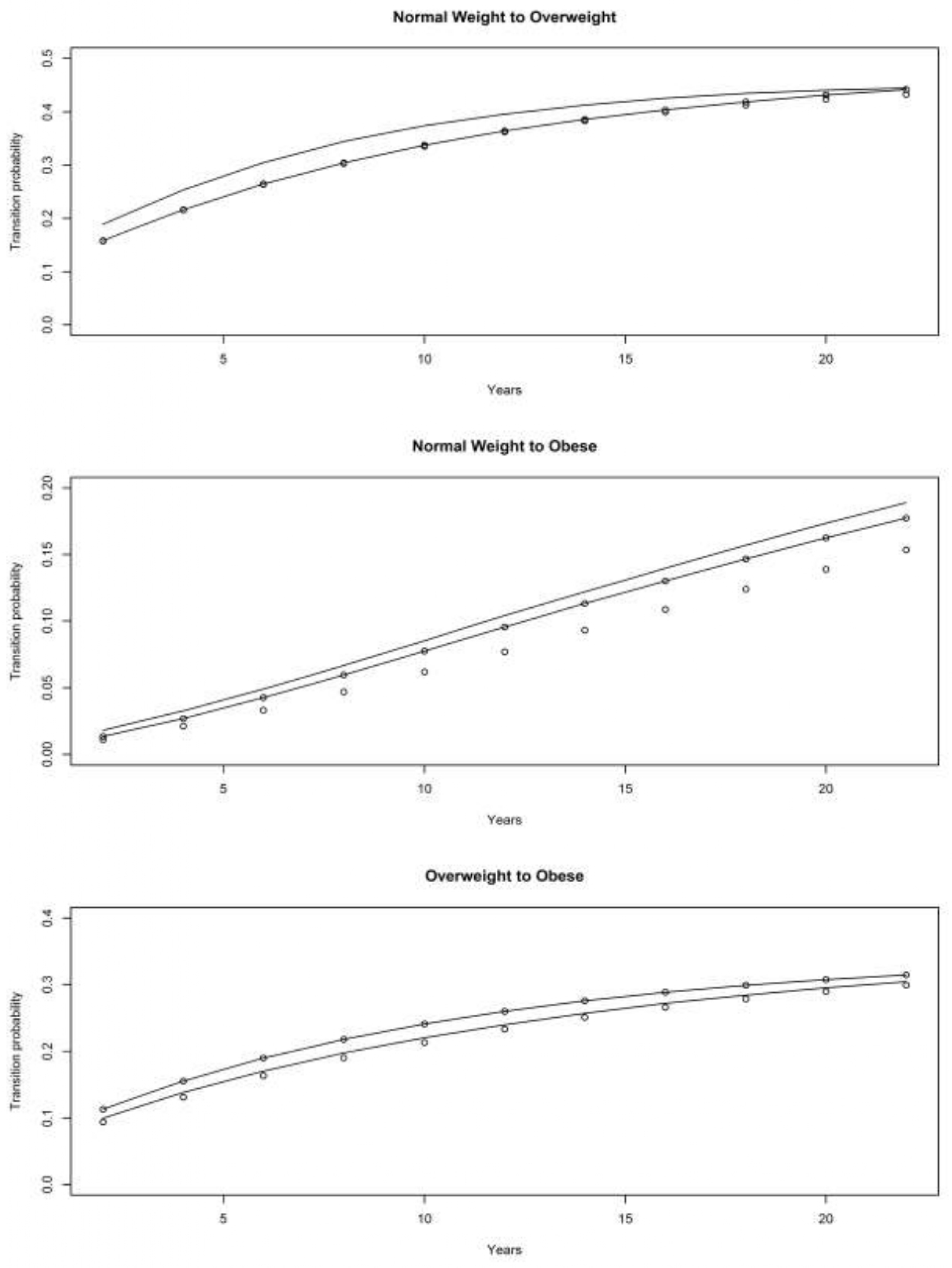
4.3.1. War Baby (WB) Cohort
4.3.2. Early Baby Boomer (EBB) Cohort
4.3.3. Mid Baby Boomer (MBB) Cohort
5. Conclusions
6. Potential Limitations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variables | AHEAD (1890–1923) | CODA (1924–1930) | HRS (1931–1941) |
| Race/Ethnicity | 0.243% | 1.426% | 1.494% |
| Gender | 0.000% | 0.000% | 0.000% |
| N | 7819 | 4277 | 10,645 |
| Variables | WB (1942–1947) | EBB (1948–1953) | MBB (1954–1959) |
| Race/Ethnicity | 1.374% | 2.183% | 3.541% |
| Gender | 0.081% | 0.041% | 0.019% |
| N | 3712 | 4901 | 5224 |
References
- Fakhouri, T.H.; Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of obesity among older adults in the United States, 2007–2010. NCHS Data Brief. 2012, 106, 1–8. [Google Scholar]
- Kritchevsky, S.B. Taking Obesity in Older Adults Seriously. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 57–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Beydoun, M.A. The obesity epidemic in the United States—Gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol. Rev. 2007, 29, 6–28. [Google Scholar] [CrossRef] [PubMed]
- Chapman, I.M. Obesity paradox during aging. In Body Composition and Aging; Mobb, C.V., Hof, P.R., Eds.; Karger Publishers: New York, NY, USA, 2010; pp. 20–36. [Google Scholar]
- Janssen, I. Morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity 2007, 15, 1827–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.L.; Molnar, M.Z.; Naseer, A.; Mikkelsen, M.K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Association of age and BMI with kidney function and mortality: A cohort study. Lancet Diabetes Endocrinol. 2015, 3, 704–714. [Google Scholar] [CrossRef] [Green Version]
- Sardinha, L.B.; Cyrino, E.S.; Dos Santos, L.; Ekelund, U.; Santos, D.A. Fitness but not weight status is associated with projected physical independence in older adults. Age 2016, 38, 54. [Google Scholar] [CrossRef] [Green Version]
- Atti, A.R.; Palmer, K.; Volpato, S.; Winblad, B.; De Ronchi, D.; Fratiglioni, L. Late-life body mass index and dementia incidence: Nine-year follow-up data from the Kungsholmen Project. J. Am. Geriatr. Soc. 2008, 56, 111–116. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, T.F.; Yip, P.K.; Hu, C.Y.; Chu, Y.M.; Chen, J.H. Body mass index (BMI) at an early age and the risk of dementia. Arch. Gerontol. Geriatr. 2010, 50, S48–S52. [Google Scholar] [CrossRef]
- Franklin, C.A.; Karkeck, J. Weight loss and senile dementia in an institutionalized elderly population. J. Am. Diet. Assoc. 1989, 89, 790–792. [Google Scholar]
- De Laet, C.; Kanis, J.A.; Odén, A.; Johanson, H.; Johnell, O.; Delmas, P.; Eisman, J.A.; Kroger, H.; Fujiwara, S.; Garnero, P.; et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 1330–1338. [Google Scholar] [CrossRef]
- Pruzansky, M.E.; Turano, M.; Luckey, M.; Senie, R. Low body weight as a risk factor for hip fracture in both black and white women. J. Orthop. Res. 1989, 7, 192–197. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Shi, Y. Body weight status and onset of functional limitations in US middle-aged and older adults. Disabil. Health J. 2015, 8, 336–344. [Google Scholar] [CrossRef]
- Park, D.; Lee, J.H.; Han, S. Underweight: Another risk factor for cardiovascular disease? A cross-sectional 2013 Behavioral Risk Factor Surveillance System (BRFSS) study of 491,773 individuals in the USA. Medicine 2017, 96, e8769. [Google Scholar] [CrossRef] [PubMed]
- Coin, A.; Sergi, G.; Beninca, P.; Lupoli, L.; Cinti, G.; Ferrara, L.; Benedetti, G.; Tomasi, G.; Pisent, C.; Enzi, G. Bone mineral density and body composition in underweight and normal elderly subjects. Osteoporos. Int. 2000, 11, 1043–1050. [Google Scholar] [CrossRef]
- Lee, H.S. Prevalence of osteopenia/osteoporosis and related risk factors of men aged 50 years and older: Korea National Health and Nutrition Examination Survey 2010–2011 data. J. Korean Diet. Assoc. 2016, 22, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Kim, S.M.; Han, K.D.; Son, J.W.; Lee, S.S.; Oh, S.W.; Lee, W.Y.; Yoo, S.J.; Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Change in weight and body mass index associated with all-cause mortality in Korea: A nationwide longitudinal study. J. Clin. Endocrinol. Metab. 2017, 102, 4041–4050. [Google Scholar] [CrossRef] [PubMed]
- Roh, L.; Braun, J.; Chiolero, A.; Bopp, M.; Rohrmann, S.; Faeh, D. Mortality risk associated with underweight: A census-linked cohort of 31,578 individuals with up to 32 years of follow-up. BMC Public Health 2014, 14, 371. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Yi, Y.; Roebothan, B.; Colbourne, J.; Maddalena, V.; Sun, G.; Wang, P.P. Trajectories of body mass index among Canadian seniors and associated mortality risk. BMC Public Health 2017, 17, 929. [Google Scholar] [CrossRef]
- Tran, M.K.; Krueger, P.M.; McCormick, E.; Davidson, A.; Main, D.S. Body mass transitions through childhood and early adolescence: A multistate life table approach. Am. J. Epidemiol. 2016, 183, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Basu, A. Forecasting distribution of body mass index in the United States: Is there more room for growth? Med. Decis. Mak. 2010, 30, E1–E11. [Google Scholar] [CrossRef]
- Hillemeier, M.M.; Weisman, C.S.; Chuang, C.; Downs, D.S.; McCall-Hosenfeld, J.; Camacho, F. Transition to overweight or obesity among women of reproductive age. J. Womens Health 2011, 20, 703–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddah, M.; Eshraghian, M.R.; Djazayery, A.; Mirdamadi, R. Association of body mass index with educational level in Iranian men and women. Eur. J. Clin. Nutr. 2003, 57, 819–823. [Google Scholar] [CrossRef]
- Kuchibhatla, M.N.; Fillenbaum, G.G.; Kraus, W.E.; Cohen, H.J.; Blazer, D.G. Trajectory classes of body mass index in a representative elderly community sample. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 68, 699–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zajacova, A.; Huzurbazar, S.; Greenwood, M.; Nguyen, H. Long-Term BMI trajectories and health in older adults: Hierarchical clustering of functional curves. J. Aging Health 2015, 27, 1443–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Yi, Y.; Roebothan, B.; Colbourne, J.; Maddalena, V.; Wang, P.P.; Sun, G. Body mass index trajectories among middle-aged and elderly Canadians and associated health outcomes. J. Environ. Public Health 2016, 2016, 7014857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murayama, H.; Liang, J.; Bennett, J.M.; Shaw, B.A.; Botoseneanu, A.; Kobayashi, E.; Fukaya, T.; Sinkai, S. Socioeconomic status and the trajectory of body mass index among older Japanese: A nationwide cohort study of 1987–2006. J. Gerontol. B Psychol. Sci. Soc. Sci. 2015, 71, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.F.; Tang, Z.; Guo, J.; Tao, L.X.; Liu, L.; Li, H.B.; Li, D.T.; Guo, X.H.; Yang, X.H. BMI and BMI Changes to All-cause Mortality among the Elderly in Beijing: A 20-year Cohort Study. Biomed. Environ. Sci. 2017, 30, 79–87. [Google Scholar]
- Wei, L.; Wu, B. Racial and ethnic differences in obesity and overweight as predictors of the onset of functional impairment. J. Am. Geriatr. Soc. 2014, 62, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Jones-Johnson, G.; Johnson, W.R.; Frishman, N. Race and gender differences in obesity and disease. Sociol. Mind 2014, 4, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Shuey, K.M.; Willson, A.E. Cumulative disadvantage and black-white disparities in life-course health trajectories. Res. Aging 2008, 30, 200–225. [Google Scholar] [CrossRef]
- Warner, D.F.; Brown, T.H. Understanding how race/ethnicity and gender define age-trajectories of disability: An intersectionality approach. Soc. Sci. Med. 2011, 72, 1236–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupre, M.E. Educational differences in age-related patterns of disease: Reconsidering the cumulative disadvantage and age-as-leveler hypotheses. J. Health Soc. Behav. 2007, 48, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.P.; Pescatello, A. Marianismo: The Other Face of Machismo in Latin America; University of Pittsburgh Press: Pittsburgh, PA, USA, 1973; pp. 89–101. [Google Scholar]
- Gallo, L.C.; Fortmann, A.L.; McCurley, J.L.; Isasi, C.R.; Penedo, F.J.; Davglus, M.L.; Roesh, S.C.; Talavera, G.A.; Gouskova, N.; Gonzalez, F., III; et al. Associations of structural and functional social support with diabetes prevalence in US Hispanics/Latinos: Results from the HCHS/SOL Sociocultural Ancillary Study. J. Behav. Med. 2015, 38, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigers, M.E.; Sherraden, M.S. A Critical Examination of Acculturation: The Impact of Health Behaviors, Social Support and Economic Resources on Birth Weight among Women of Mexican Descent 1. Int. Migr. Rev. 2001, 35, 804–839. [Google Scholar] [CrossRef]
- Sautter, J.M.; Thomas, P.A.; Dupre, M.E.; George, L.K. Socioeconomic status and the Black–White mortality crossover. Am. J. Public Health 2012, 102, 1566–1571. [Google Scholar] [CrossRef] [Green Version]
- Health and Retirement Study. Sample Sizes and Response Rates. Available online: http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf (accessed on 14 June 2020).
- Flicker, L.; McCaul, K.A.; Hankey, G.J.; Jamrozik, K.; Brown, W.J.; Byles, J.E.; Almeida, O.P. Body mass index and survival in men and women aged 70 to 75. J. Am. Geriatr. Soc. 2010, 58, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.E.; Taylor, J.Y.; Wu, C.Y.; Smith, J.A. Alternative methods for measuring obesity in African American women. Yale J. Biol. Med. 2013, 86, 29–39. [Google Scholar]
- Hougaard, P. Multi-state models: A review. Lifetime Data Anal. 1999, 5, 239–264. [Google Scholar] [CrossRef]
- Schoen, R. The multistate life table. In Modeling Multigroup Populations; Schoen, R., Ed.; Springer: Boston, MA, USA, 1988; pp. 63–105. [Google Scholar]
- Jackson, C. Multi-State Markov and Hidden Markov Model in Continuous Time. Available online: https://cran.r-project.org/web/packages/msm/msm.pdf (accessed on 14 June 2020).
- Ortman, J.M.; Velkoff, V.A.; Hogan, H. An aging nation: The older population in the United States. Available online: https://www.census.gov/content/dam/Census/library/publications/2014/demo/p25-1140.pdf (accessed on 14 June 2020).
- Baruth, M.; Schlaff, R.A. Behavioral Mediators of Weight Loss in Two Group-based Behavioral Interventions in Older Adults. Am. J. Health Educ. 2017, 48, 108–115. [Google Scholar] [CrossRef]
- Batsis, J.A.; Gill, L.E.; Masutani, R.K.; Adachi-Mejia, A.M.; Blunt, H.B.; Bagley, P.J.; Lopez-Jimenez, F.; Bartels, S.J. Weight loss interventions in older adults with obesity: A systematic review of randomized controlled trials since 2005. J. Am. Geriatr. Soc. 2017, 65, 257–268. [Google Scholar] [CrossRef]
- Villareal, D.T.; Apovian, C.M.; Kushner, R.F.; Klein, S. Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obesity 2005, 13, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA. 2013, 309, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidell, J.C.; Pérusse, L.; Després, J.P.; Bouchard, C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: The Quebec Family Study. Am. J. Clin. Nutr. 2001, 74, 315–321. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Moore, S.C.; Koster, A.; Harris, T.B.; Park, Y.; Hollenbeck, A.; Schatzkin, A. Waist circumference as compared with body-mass index in predicting mortality from specific causes. PLoS ONE 2011, 6, e18582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flegal, K.M.; Ogden, C.L.; Fryar, C.; Afful, J.; Klein, R.; Huang, D.T. Comparisons of Self-Reported and Measured Height and Weight, BMI, and Obesity Prevalence from National Surveys: 1999–2016. Obesity 2019, 27, 1711–1719. [Google Scholar] [CrossRef]
- Wen, M.; Kowaleski-Jones, L. Sex and ethnic differences in validity of self-reported adult height, weight and body mass index. Ethn. Dis. 2012, 22, 72–78. [Google Scholar] [PubMed]
- Fernández-Rhodes, L.; Robinson, W.R.; Sotres-Alvarez, D.; Franceschini, S.F.; Buelna, C.; Moncrieft, A.; Llabre, M.; Daviglus, M.L.; Qi, Q.; Agarwal, A.; et al. Accuracy of Self-reported Weight in Hispanic/Latino Adults of the Hispanic Community Health Study/Study of Latinos. Epidemiology 2017, 28, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Michaud, P.C.; Kapteyn, A.; Smith, J.P.; Van Soest, A. Temporary and permanent unit non-response in follow-up interviews of the Health and Retirement Study. Longit. Life Course Stud. 2011, 2, 145–169. [Google Scholar]
- Hendley, Y.; Zhao, L.; Coverson, D.L.; Din-Dzietham, R.; Morris, A.; Quyyumi, A.A.; Gibbons, G.H.; Vaccarino, V. Differences in weight perception among blacks and whites. J. Womens Health 2011, 20, 1805–1811. [Google Scholar] [CrossRef] [Green Version]
- Chithambo, T.P.; Huey, S.J. Black/white differences in perceived weight and attractiveness among overweight women. J. Obes. 2013, 2013, 320–326. [Google Scholar] [CrossRef]


| Variables | Mean, Median, N or (Range) | ||
|---|---|---|---|
| Age | 76 (50 to 98) | ||
| Race/Ethnicity/Gender | |||
| White Male | 11,414 | ||
| White Female | 13,641 | ||
| Black Male | 2579 | ||
| Black Female | 3715 | ||
| Hispanic Male | 1725 | ||
| Hispanic Female | 2115 | ||
| Race | |||
| White | 68.37% | ||
| Black | 17.68% | ||
| Hispanic | 11.08% | ||
| Other | 2.87% | ||
| Sex | |||
| Male | 43.97% | ||
| Female | 56.03% | ||
| BMI | All | Male | Female |
| 1992 | 26.5 (12.8–102.7) | 26.6 (13.6–102.7) | 25.8 (12.8–60.6) |
| 1994 | 25.8 (12.6–92.2) | 26.1 (12.6–92.2) | 25.5 (12.8–74.5) |
| 1996 | 25.8 (10.8–75.5) | 26.4 (10.8–54.9) | 25.6 (11.9–75.5) |
| 1998 | 26.2 (9.6–74.5) | 26.6 (12.8–65.0) | 25.8 (9.6–74.5) |
| 2000 | 26.4 (11.5–75.5) | 26.6 (11.7–64.6) | 25.8 (11.5–75.5) |
| 2002 | 26.5 (9.5–70.9) | 26.6 (9.5–59.1) | 26.2 (11.1–70.9) |
| 2004 | 26.6 (9.6–71.3) | 26.9 (13.6–57.4) | 26.5 (9.6–71.3) |
| 2006 | 27.1 (10.6–82.7) | 27.2 (12.2–61.1) | 26.2 (10.6–82.7) |
| 2008 | 27.3 (10.6–74.4) | 27.3 (10.6–60.3) | 27.0 (10.9–74.4) |
| 2010 | 27.5 (7.0–79.1) | 27.6 (7.0–60.8) | 27.4 (9.3–79.1) |
| 2012 | 27.5 (8.9–83.0) | 27.6 (9.4–59.2) | 27.5 (8.9–83.0) |
| 2014 | 27.5 (11.0–76.6) | 27.7 (12.2–62.2) | 27.5 (11.0–76.6) |
| Variables | AHEAD | CODA | HRS | WB | EBB | MBB |
|---|---|---|---|---|---|---|
| n | 7846 | 4134 | 10,255 | 3557 | 4609 | 4788 |
| Years born | (1890–1923) | (1924–1930) | (1931–1941) | (1942–1947) | (1948–1953) | (1954–1959) |
| Ages observed | 91–109 | 84–90 | 73–83 | 67–72 | 61–66 | 55–60 |
| Race | ||||||
| White | 80.6% | 82.8% | 72.7% | 75.1% | 57.5% | 53.0% |
| Black | 13.5% | 10.3% | 17.6% | 15.9% | 24.4% | 27.3% |
| Hispanic | 5.9% | 6.9% | 9.7% | 9.0% | 18.1% | 19.7% |
| Sex | ||||||
| Male | 41.5% | 48.8% | 47.5% | 40.4% | 44.4% | 43.8% |
| Female | 58.5% | 51.2% | 52.5% | 59.6% | 55.6% | 56.2% |
| Education | ||||||
| No schooling | 42.2% | 30.8% | 26.8% | 17.3% | 16.5% | 16.2% |
| GED | 2.7% | 4.4% | 5.0% | 4.8% | 5.1% | 6.2% |
| High school | 42.4% | 45.7% | 48.0% | 50.0% | 45.9% | 45.7% |
| 2-year college | 1.7% | 2.7% | 3.4% | 5.1% | 7.1% | 8.6% |
| 4-year college | 6.7% | 10.0% | 9.5% | 12.5% | 15.0% | 14.8% |
| Master’s degree | 3.0% | 4.4% | 5.3% | 7.7% | 8.3% | 6.6% |
| Professional/terminal degree | 1.2% | 2.0% | 2.0% | 2.5% | 2.1% | 2.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liew, H. Transitions among BMI States: A Test of Competing Hypotheses. Obesities 2021, 1, 1-25. https://doi.org/10.3390/obesities1010001
Liew H. Transitions among BMI States: A Test of Competing Hypotheses. Obesities. 2021; 1(1):1-25. https://doi.org/10.3390/obesities1010001
Chicago/Turabian StyleLiew, Hui. 2021. "Transitions among BMI States: A Test of Competing Hypotheses" Obesities 1, no. 1: 1-25. https://doi.org/10.3390/obesities1010001
APA StyleLiew, H. (2021). Transitions among BMI States: A Test of Competing Hypotheses. Obesities, 1(1), 1-25. https://doi.org/10.3390/obesities1010001




