A Review of Caprock Integrity in Underground Hydrogen Storage Sites: Implication of Wettability, Interfacial Tension, and Diffusion
Abstract
1. Introduction
2. Porosity and Permeability
| Rock/Mineral | Permeability (mD) | Reference |
|---|---|---|
| Evaporite | 10−9 to 10−3 | [29] |
| Salt (Halite) | 10−8 to 10−4 | [30] |
| Shales | 9 × 10−6 to 6 × 10−3 | [25,31] |
| Mudstone | 2 × 10−7 to 2 × 10−1 | [32] |
| Unfractured metamorphic and igneous rocks | 10−6 to 10−4 | [26] |
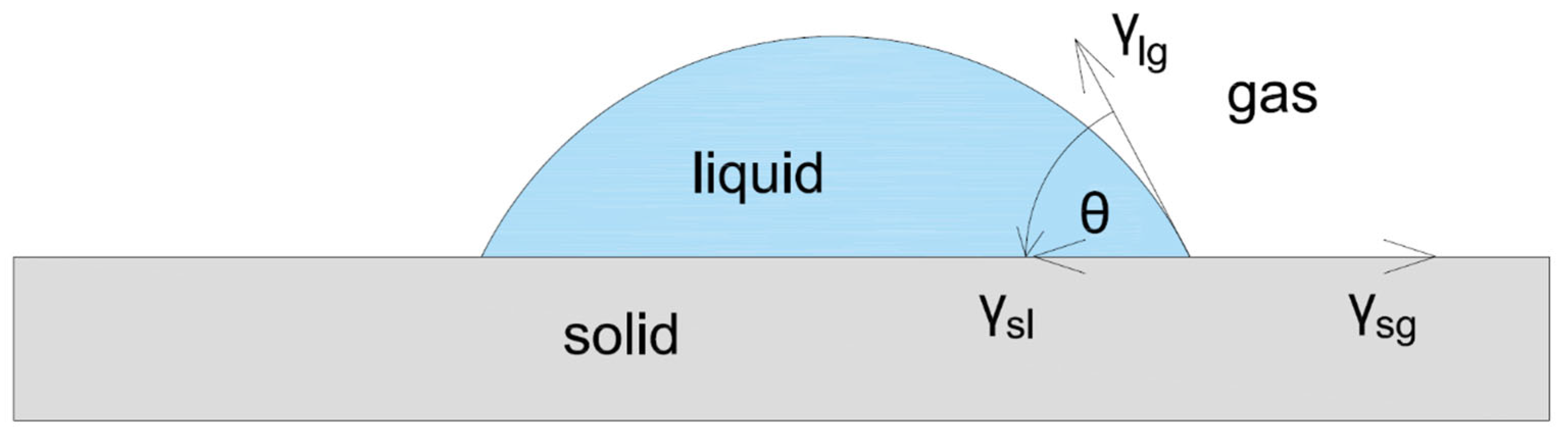
3. Wettability
3.1. Pressure
3.2. Temperature
3.3. Presence of Organic Material and Bacteria
3.4. Salinity
3.5. Gas Mixture
4. Interfacial Tension
4.1. Pressure
4.2. Temperature
4.3. Organic Content
4.4. Salinity
4.5. Gas Composition
5. Diffusion
Diffusion Coefficient
| Rock/Mineral | Temperature (°C) | Pressure (MPa) | Diffusion Coefficient (m2/s) | Reference |
|---|---|---|---|---|
| Werra rock salt | 25 | 1 | 1.40 × 10−9 | [76] |
| Opalinus clay | 1.20 10−9 | |||
| Shale (TOC 3.91%) | 30 to 60 | 4 | 1.30 × 10−8 to 2.40 × 10−8 | [16] |
| 30 | 0 to 20 | 1.80 × 10−8 to 8.00 × 10−9 | ||
| Boom clay | 21 | 1 | (⊥) 2.64 × 10−10 | [77] |
| (‖) 7.25 × 10−10 | ||||
| (‖) 5.51× 10−10 | ||||
| Shale (TOC 13%) | 30 | 1.5 to 4.5 | 7.37 × 10−10 to 3.49 × 10−10 | [15] |
| 60 | 1.5 to 4.5 | 1.04 × 10−10 to 1.27 × 10−11 | ||
| Shale (TOC 18%) | 30 | 1.5 to 4.5 | 2.45 × 10−9 to 7.49 × 10−9 | |
| 60 | 1.5 to 4.5 | 4.49 × 10−11 to 9.61 × 10−8 | ||
| Mudstone | 45 | 1 | 1.00 × 10−10 | [78] |
| Australian anthracite coal | 20 to 60 | 1.3 | 0.99 × 10−9 to 6.77 × 10−9 | [79] |
| Albite | 25 | 4.4 | 1.5 × 10−11 | [70] |
| Quartz | 2.5 × 10−11 | |||
| Illite | 10−11 | |||
| Calcite | 2.5 × 10−11 | |||
| Anhydrite | 1.5 × 10−11 | |||
| Glauberite | 1.6 × 10−11 | |||
| Polyhalite | 1.8 × 10−11 | |||
| Halite | 3 × 10−11 | |||
| Mudstone | 2.1 × 10−11 | |||
| Glauberite-bearing salt | 2.5 × 10−11 |
6. Discussion and Conclusions
- Poses low porosity, preferably unconnected or with a highly complicated pore system, and low permeability.
- The sealing geological formation should be strongly water-wet, regardless of pressure and temperature conditions.
- In order for the previous criterion to be met, the caprock should present low rock IFT, while exhibiting high rock–gas and liquid–gas IFT.
- Its effective diffusion coefficient should be the smallest possible, which is highly dependent on caprock properties such as porosity and tortuosity.
- The organic content of the caprock formation should be minimal, as the literature revealed that the greater the TOC values, the less the wettability (less water-wet).
- Concerning microbial action, it may have a double role in wettability, as it could reduce the formation’s wettability or increase it by developing a biofilm.
- The wettability of other potential caprock formations under various pressures, temperatures, microbial conditions, and gas mixture compositions should be investigated.
- More experimental data should be generated on the wettability behavior of different rock types under long-term injection/production cycles of large- and medium-scale projects.
- Further TOC and mineralogy, as well as comparative experiments on various rock types, should be performed in order to assess the influence of organic and mineral content on wettability.
- Since, IFT reduction varies in rate and extent with increasing pressure across various rocks and temperatures, caution should be given, and more targeted studies should be performed.
- The effective diffusion coefficient should be further examined for a wider variety of geological formations under various pressure and temperature conditions.
- Finally, the use of surfactants, which enhance rock hydrophilicity, should be examined in depth along with their economic and other possible side effects (e.g., environmental impact).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. Available online: https://www.iea.org/reports/global-energy-review-2025/co2-emissions (accessed on 25 June 2025).
- European Commission. Available online: https://energy.ec.europa.eu/topics/eus-energy-system/hydrogen_en (accessed on 25 June 2025).
- Federal Energy Regulatory Commission. Available online: https://www.ferc.gov/industries-data/natural-gas/overview/natural-gas-storage/natural-gas-storage-background (accessed on 25 June 2025).
- Foh, S.; Novil, M.; Rockar, E.; Randolph, P. Underground Hydrogen Storage Final Report; Department of Energy and Environment: New York, NY, USA, 1979.
- Małachowska, A.; Łukasik, N.; Mioduska, J.; Gębicki, J. Hydrogen Storage in Geological Formations—The Potential of Salt Caverns. Energies 2022, 15, 5038. [Google Scholar] [CrossRef]
- Sambo, C.; Dudun, A.; Samuel, S.A.; Esenenjor, P.; Muhammed, N.S.; Haq, B. A Review on Worldwide Underground Hydrogen Storage Operating and Potential Fields. Int. J. Hyd. Energy 2022, 47, 22840–22880. [Google Scholar] [CrossRef]
- Lankof, L.; Luboń, K.; Le Gallo, Y.; Tarkowski, R. The Ranking of Geological Structures in Deep Aquifers of the Polish Lowlands for Underground Hydrogen Storage. Int. J. Hyd. Energy 2024, 62, 1089–1102. [Google Scholar] [CrossRef]
- Raza, A.; Arif, M.; Glatz, G.; Mahmoud, M.; Al Kobaisi, M.; Alafnan, S.; Iglauer, S. A Holistic Overview of Underground Hydrogen Storage: Influencing Factors, Current Understanding, and Outlook. Fuel 2022, 330, 125636. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’connell, J.P.; York, N.; San, C.; Lisbon, F.; Madrid, L.; City, M.; Delhi, M.N.; Juan, S. The Properties of Gases and Liquids, 5th ed.; McGRAW-HILL: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Zeng, L.; Sarmadivaleh, M.; Saeedi, A.; Chen, Y.; Zhong, Z.; Xie, Q. Storage Integrity during Underground Hydrogen Storage in Depleted Gas Reservoirs. Earth-Sci. Rev. 2023, 247, 104625. [Google Scholar] [CrossRef]
- Aghaei, H.; Al-Yaseri, A.; Toorajipour, A.; Shahsavani, B.; Yekeen, N.; Edlmann, K. Host-Rock and Caprock Wettability during Hydrogen Drainage: Implications of Hydrogen Subsurface Storage. Fuel 2023, 351, 129048. [Google Scholar] [CrossRef]
- Ali, M.; Jha, N.K.; Al-Yaseri, A.; Zhang, Y.; Iglauer, S.; Sarmadivaleh, M. Hydrogen Wettability of Quartz Substrates Exposed to Organic Acids; Implications for Hydrogen Geo-Storage in Sandstone Reservoirs. J. Pet. Sci. Eng. 2021, 207, 109081. [Google Scholar] [CrossRef]
- Aftab, A.; Al-Yaseri, A.; Nzila, A.; Al Hamad, J.; Amao, A.O.; Sarmadivaleh, M. Quartz-H2-Brine Bacterium Wettability under Realistic Geo-Conditions: Towards Geological Hydrogen Storage. Energy Fuels 2023, 37, 5623–5631. [Google Scholar] [CrossRef]
- Toorajipour, A.; Aghaei, H.; Shahsavani, B.; Gholami, R.; Yekeen, N.; Al-Yaseri, A. Hydrogen Wettability of Limestone, Dolomite, and Anhydrite in Binary Mixtures of CH4 and CO2. Ore Energy Resour. Geol. 2025, 18, 100087. [Google Scholar] [CrossRef]
- Alanazi, A.; Rasool Abid, H.; Abu-Mahfouz, I.S.; Bawazeer, S.A.; Matamba, T.; Keshavarz, A.; Iglauer, S.; Hoteit, H. Hydrogen Adsorption Kinetics in Organic-Rich Shale Reservoir Rocks for Seasonal Geological Storage. Fuel 2025, 379, 132964. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Wu, R.; Bi, J.; Zhang, K. Shale Reservoir Storage of Hydrogen: Adsorption and Diffusion on Shale. Fuel 2024, 357, 129919. [Google Scholar] [CrossRef]
- Esfandyari, H.; Sarmadivaleh, M.; Esmaeilzadeh, F.; Ali, M.; Iglauer, S.; Keshavarz, A. Experimental Evaluation of Rock Mineralogy on Hydrogen-Wettability: Implications for Hydrogen Geo-Storage. J. Energy Storage 2022, 52, 104866, Erratum in J. Energy Storage 2023, 57, 106162. [Google Scholar] [CrossRef]
- Ali, M.; Yekeen, N.; Ali, M.; Alanazi, A.; Shahzad Kamal, M.; Keshavarz, A.; Hoteit, H. Hydrogen Wettability of Saudi Arabian Basalt: Implications for H2 Geo-Storage. Fuel 2024, 371, 132045. [Google Scholar] [CrossRef]
- Ahmed, H. Reservoir Engineering Handbook, 3rd ed.; Gulf Professional Publishing: Houston, TX, USA, 2006. [Google Scholar]
- Sekar, L.K.; Indro, A.P.; Matey-Korley, V.G.; Ikeokwu, C.C.; Okoroafor, E.R. Advancing Underground Hydrogen Storage: Geological Insights from Natural Hydrogen Occurrences in Porous Media. Geoenergy Sci. Eng. 2025, 257, 214208. [Google Scholar] [CrossRef]
- Prinzhofer, A.; Sidy, C.; Diallo, A.B. Discovery of a Large Accumulation of Natural Hydrogen in Bourakebougou (Mali). Int. J. Hyd. Energy 2018, 43, 19315–19326. [Google Scholar] [CrossRef]
- Arman, H.; Gabr, A.; Lwisa, E.; Paramban, S. Initial Study on Porosity and Permeability of Evaporitic Rock Samples from Abu Dhabi Coastal Area, United Arab Emirates. In World Multidisciplinary Civil Engineering-Architecture-Urban Planning Symposium Wmcaus 2022; IOP Publishing: Bristol, UK, 2023; p. 090009. [Google Scholar] [CrossRef]
- Adamopoulos, G.; Vakalas, I.; Bellas, S.; Kokkalas, S.; Perraki, M.; Gaganis, V.; Stamatakis, E. Initial Assessment of the Sealing Capacity of Caprocks in the Ionian Zone Reservoirs. In Seventh International Conference on Fault and Top Seals; European Association of Geoscientists & Engineers Greece: Bunnik, The Netherlands, 2025; Volume 14. [Google Scholar]
- Wang, J.; Zhang, Q.; Song, Z.; Liu, X.; Wang, X.; Zhang, Y. Microstructural Variations and Damage Evolvement of Salt Rock under Cyclic Loading. Int. J. Rock Mech. Min. Sci. 2022, 152, 105078. [Google Scholar] [CrossRef]
- Li, Q.; Gates, I.D.; Hejazi, S.H. Experimental Investigation of Caprock Sealing Capacity for Underground Hydrogen Storage. Int. J. Hyd. Energy 2025, 165, 150858. [Google Scholar] [CrossRef]
- Jasim, A.; Hemmings, B.; Mayer, K.; Scheu, B. Groundwater Flow and Volcanic Unrest. In Advances in Volcanology; Springer Science and Business Media Deutschland GmbH: Berlin, Germany, 2019; pp. 83–99. [Google Scholar] [CrossRef]
- Jia, S.; Wen, C.; Fu, X.; Liu, T.; Xi, Z. A Caprock Evaluation Methodology for Underground Gas Storage in a Deep Depleted Gas Reservoir: A Case Study for the X9 Lithologic Trap of Langgu Sag, Bohai Bay Basin, China. Energies 2022, 15, 4351. [Google Scholar] [CrossRef]
- Schlumberger Limited. Available online: https://glossary.slb.com/en/terms/c/caprock (accessed on 27 May 2025).
- Saltwork Consultants Pty Ltd. Available online: https://www.saltworkconsultants.com/evaporites-as-seals/ (accessed on 7 April 2025).
- Peach, C. Influence of Deformation on the Fluid Transport Properties of Salt Rocks. Ph.D. Thesis, Faculteit Aardwetenschappen Utrecht University, Utrecht, The Netherlands, 1991. [Google Scholar]
- Mbia, E.N.; Fabricius, I.L.; Krogsbøll, A.; Frykman, P.; Dalhoff, F. Permeability, Compressibility and Porosity of Jurassic Shale from the Norwegian-Danish Basin. Pet. Geosci. 2014, 20, 257–281. [Google Scholar] [CrossRef]
- Yang, Y.; Aplin, A.C. A Permeability-Porosity Relationship for Mudstones. Mar. Pet. Geol. 2010, 27, 1692–1697. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M. A Critical Review of Breakthrough Pressure for Tight Rocks and Relevant Factors. J. Nat. Gas Sci. Eng. 2022, 100, 104456. [Google Scholar] [CrossRef]
- Lassin, A.; Dymitrowska, M.; Azaroual, M. Hydrogen Solubility in Pore Water of Partially Saturated Argillites: Application to Callovo-Oxfordian Clayrock in the Context of a Nuclear Waste Geological Disposal. Phys. Chem. Earth 2011, 36, 1721–1728. [Google Scholar] [CrossRef]
- Goodman, A.; Kutchko, B.; Lackey, G.; Gulliver, D.; Strazisar, B.; Tinker, K.; Wright, R.; Haeri, F.; Huerta, N.; Baek, S.; et al. Subsurface Hydrogen and Natural Gas Storage: State of Knowledge and Research Recommendations Report SHASTA: Subsurface Hydrogen Assessment, Storage, and Technology Acceleration Project. U.S. Department of Energy: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Hashemi, M.; Sedaee, B. Understanding Caprock Integrity in Underground Hydrogen Storage: A Geochemical Study of Mineral Alteration and Sealing Efficiency. Int. J. Hyd. Energy 2025, 154, 150300. [Google Scholar] [CrossRef]
- Zeng, L.; Vialle, S.; Ennis-King, J.; Esteban, L.; Sarmadivaleh, M.; Sarout, J.; Dautriat, J.; Giwelli, A.; Xie, Q. Role of Geochemical Reactions on Caprock Integrity during Underground Hydrogen Storage. J. Energy Storage 2023, 65, 107414. [Google Scholar] [CrossRef]
- Guancheng, J. Gas Wettability of Reservoir Rock Surfaces with Porous Media; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Hosseini, M.; Ali, M.; Fahimpour, J.; Keshavarz, A.; Iglauer, S. Basalt-H2-Brine Wettability at Geo-Storage Conditions: Implication for Hydrogen Storage in Basaltic Formations. J. Energy Storage 2022, 52, 104745. [Google Scholar] [CrossRef]
- Ali, M.; Yekeen, N.; Pal, N.; Keshavarz, A.; Iglauer, S.; Hoteit, H. Influence of Pressure, Temperature and Organic Surface Concentration on Hydrogen Wettability of Caprock; Implications for Hydrogen Geo-Storage. Energy Rep. 2021, 7, 5988–5996. [Google Scholar] [CrossRef]
- Alanazi, A.; Yekeen, N.; Ali, M.; Ali, M.; Abu-Mahfouz, I.S.; Keshavarz, A.; Iglauer, S.; Hoteit, H. Influence of Organics and Gas Mixing on Hydrogen/Brine and Methane/Brine Wettability Using Jordanian Oil Shale Rocks: Implications for Hydrogen Geological Storage. J. Energy Storage 2023, 62, 106865. [Google Scholar] [CrossRef]
- Iglauer, S.; Ali, M.; Keshavarz, A. Hydrogen Wettability of Sandstone Reservoirs: Implications for Hydrogen Geo-Storage. Geophys. Res. Lett. 2021, 48, e2020GL090814. [Google Scholar] [CrossRef]
- Higgs, S.; Da Wang, Y.; Sun, C.; Ennis-King, J.; Jackson, S.J.; Armstrong, R.T.; Mostaghimi, P. In-Situ Hydrogen Wettability Characterisation for Underground Hydrogen Storage. Int. J. Hyd. Energy 2022, 47, 13062–13075. [Google Scholar] [CrossRef]
- Esfandyari, H.; Hosseini, M.; Ali, M.; Iglauer, S.; Haghighi, M.; Keshavarz, A. Assessment of the Interfacial Properties of Various Mineral/Hydrogen/Water Systems. J. Energy Storage 2023, 60, 106637. [Google Scholar] [CrossRef]
- Al-Mukainah, H.; Al-Yaseri, A.; Yekeen, N.; Hamad, J.; Mahmoud, M. Wettability of Shale–Brine–H2 System and H2-Brine Interfacial Tension for Assessment of the Sealing Capacities of Shale Formations during Underground Hydrogen Storage. Energy Rep. 2022, 8, 8830–8843. [Google Scholar] [CrossRef]
- Hashemi, L.; Boon, M.; Glerum, W.; Farajzadeh, R.; Hajibeygi, H. A Comparative Study for H2–CH4 Mixture Wettability in Sandstone Porous Rocks Relevant to Underground Hydrogen Storage. Adv. Water Resour. 2022, 163, 104165. [Google Scholar] [CrossRef]
- Hosseini, M.; Fahimpour, J.; Ali, M.; Keshavarz, A.; Iglauer, S. Capillary Sealing Efficiency Analysis of Caprocks: Implication for Hydrogen Geological Storage. Energy Fuels 2022, 36, 4065–4075. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Wolff-Boenisch, D.; Fauziah, C.A.; Iglauer, S. Hydrogen Wettability of Clays: Implications for Underground Hydrogen Storage. Int. J. Hyd. Energy 2021, 46, 34356–34361. [Google Scholar] [CrossRef]
- Ali, M.; Yekeen, N.; Pal, N.; Keshavarz, A.; Iglauer, S.; Hoteit, H. Influence of Organic Molecules on Wetting Characteristics of Mica/H2/Brine Systems: Implications for Hydrogen Structural Trapping Capacities. J. Colloid Interface Sci. 2022, 608, 1739–1749. [Google Scholar] [CrossRef]
- Hosseini, M.; Fahimpour, J.; Ali, M.; Keshavarz, A.; Iglauer, S. Hydrogen Wettability of Carbonate Formations: Implications for Hydrogen Geo-Storage. J. Colloid Interface Sci. 2022, 614, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.; Ali, M.; Bawazeer, S.A.; Ali, M.; Abu-Mahfouz, I.S.; Tariq, Z.; Aljeban, N.; Abid, H.R.; Keshavarz, A.; Iglauer, S.; et al. Enhancing Hydrogen Storage Efficiency in Organic-Rich Shales Using Silica Nanofluids: A Comprehensive Study on Wettability Alteration. Energy Fuels 2025, 39, 10628–10648. [Google Scholar] [CrossRef]
- Aftab, A.; Al-Yaseri, A.; Nzila, A.; Hamad, J.A.l.; Sarmadivaleh, M. Microbial Impact on Basalt-Water-Hydrogen System: Insights into Wettability, Capillary Pressure, and Interfacial Tension for Subsurface Hydrogen Storage. Greenh. Gases Sci. Technol. 2024, 14, 546–560. [Google Scholar] [CrossRef]
- Ali, M.; Arif, M.; Sedev, R.; Sánchez-Román, M.; Keshavarz, A.; Iglauer, S. Underground Hydrogen Storage: The Microbiotic Influence on Rock Wettability. J. Energy Storage 2023, 72, 108405. [Google Scholar] [CrossRef]
- Esfandyari, H.; Jozani, R.J.; Hassanpouryouzband, A.; Hemmatzadeh, F.; Haghighi, M.; Iglauer, S.; Keshavarz, A.; Zeinijahromi, A. The Microbial Factor in Subsurface Hydrogen Behavior: Implications for Wettability and Interfacial Dynamics. Adv. Colloid Interface Sci. 2025, 346, 103647. [Google Scholar] [CrossRef]
- Hou, J.; Lin, S.; Zhang, M.; Li, W. Salinity, Temperature and Pressure Effect on Hydrogen Wettability of Carbonate Rocks. Int. J. Hyd. Energy 2023, 48, 11303–11311. [Google Scholar] [CrossRef]
- Zhang, S.; Tan, D.; Zhu, H.; Zhang, W. Molecular Dynamic Simulations on the Hydrogen Wettability of Caprock: Considering Effects of Mineralogy, Pressure, Temperature and Salinity. Int. J. Hyd. Energy 2025, 109, 367–382. [Google Scholar] [CrossRef]
- Ali, M.; Pan, B.; Yekeen, N.; Al-Anssari, S.; Al-Anazi, A.; Keshavarz, A.; Iglauer, S.; Hoteit, H. Assessment of Wettability and Rock-Fluid Interfacial Tension of Caprock: Implications for Hydrogen and Carbon Dioxide Geo-Storage. Int. J. Hyd. Energy 2022, 47, 14104–14120. [Google Scholar] [CrossRef]
- Yekeen, N.; Al-Yaseri, A.; Negash, B.M.; Ali, M.; Giwelli, A.; Esteban, L.; Sarout, J. Clay-Hydrogen and Clay-Cushion Gas Interfacial Tensions: Implications for Hydrogen Storage. Int. J. Hyd. Energy 2022, 47, 19155–19167. [Google Scholar] [CrossRef]
- Chow, Y.T.F.; Maitland, G.C.; Trusler, J.P.M. Interfacial Tensions of (H2O + H2) and (H2O + CO2 + H2) Systems at Temperatures of (298–448) K and Pressures up to 45 MPa. Fluid Phase Equilibria 2018, 475, 37–44. [Google Scholar] [CrossRef]
- Hosseini, M.; Fahimpour, J.; Ali, M.; Keshavarz, A.; Iglauer, S. H2−brine Interfacial Tension as a Function of Salinity, Temperature, and Pressure; Implications for Hydrogen Geo-Storage. J. Pet. Sci. Eng. 2022, 213, 110441. [Google Scholar] [CrossRef]
- Janjua, A.N.; Ali, M.; Murtaza, M.; Patil, S.; Kamal, M.S. Effects of Salinity, Temperature, and Pressure on H2–Brine Interfacial Tension: Implications for Underground Hydrogen Storage. J. Energy Storage 2024, 95, 112510. [Google Scholar] [CrossRef]
- Pan, B.; Yin, X.; Iglauer, S. Rock-Fluid Interfacial Tension at Subsurface Conditions: Implications for H2, CO2 and Natural Gas Geo-Storage. Int. J. Hyd. Energy 2021, 46, 25578–25585. [Google Scholar] [CrossRef]
- Hosseini, M.; Ali, M.; Fahimpour, J.; Keshavarz, A.; Iglauer, S. Calcite-Fluid Interfacial Tension: H2 and CO2 Geological Storage in Carbonates. Energy Fuels 2023, 37, 5986–5994. [Google Scholar] [CrossRef]
- Isfehani, Z.D.; Sheidaie, A.; Hosseini, M.; Fahimpour, J.; Iglauer, S.; Keshavarz, A. Interfacial Tensions of (Brine + H2 + CO2) Systems at Gas Geo-Storage Conditions. J. Mol. Liq. 2023, 374, 121279. [Google Scholar] [CrossRef]
- Omrani, S.; Ghasemi, M.; Singh, M.; Mahmoodpour, S.; Zhou, T.; Babaei, M.; Niasar, V. Interfacial Tension-Temperature-Pressure-Salinity Relationship for the Hydrogen-Brine System under Reservoir Conditions: Integration of Molecular Dynamics and Machine Learning. Langmuir 2023, 39, 12680–12691. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Cole, D.R.; Striolo, A. Cushion Gas Effects on Clay-Hydrogen-Brine Wettability at Conditions Relevant to Underground Gas Storage. Int. J. Hyd. Energy 2024, 58, 668–677. [Google Scholar] [CrossRef]
- Kantzas, A.; Bryan, J.; Taheri, S. Fundamentals of Fluid Flow in Porous Media; PERM Inc.: Calgary, AB, Canada.
- Khajooie, S.; Gaus, G.; Seemann, T.; Ahrens, B.; Hua, T.; Littke, R. Exploring Effective Diffusion Coefficients in Water-Saturated Reservoir Rocks via the Pressure Decay Technique: Implications for Underground Hydrogen Storage. Transp. Porous Media 2025, 152, 12. [Google Scholar] [CrossRef]
- Alafnan, S. Factors Influencing Hydrogen Migration in Cap Rocks: Establishing New Screening Criteria for the Selection of Underground Hydrogen Storage Locations. Int. J. Hyd. Energy 2024, 83, 1099–1106. [Google Scholar] [CrossRef]
- Song, R.; Song, Y.; Liu, J.; Yang, C. Multiscale Experimental and Numerical Study on Hydrogen Diffusivity in Salt Rocks and Interlayers of Salt Cavern Hydrogen Storage. Int. J. Hyd. Energy 2024, 79, 319–334. [Google Scholar] [CrossRef]
- Yuan, L.; Stanley, A.; Dehghanpour, H.; Reed, A. Measurement of Helium Diffusion in Lotsberg Salt Cores: A Proxy to Evaluate Hydrogen Diffusion. Int. J. Hyd. Energy 2024, 52, 686–702. [Google Scholar] [CrossRef]
- Banerjee, T.; Balasubramanian, G. Hydrogen Diffusion Induced Dislocation Transformations in a Nickel Superalloy. Fuel 2025, 384, 134064. [Google Scholar] [CrossRef]
- Poletaev, G.M.; Zorya, I.V.; Rakitin, R.Y.; Iliina, M.A. Interatomic Potentials for Describing Impurity Atoms of Light Elements in FCC Metals. Mater. Phys. Mech. 2019, 42, 380–388. [Google Scholar] [CrossRef]
- Kim, C.; Devegowda, D.; Dang, S.T.; Mehana, M. Modeling the Diffusivity of Hydrogen and the Associated Cushion Gas in Depleted Hydrocarbon Reservoir Caprocks. Int. J. Hyd. Energy 2025, 105, 248–257. [Google Scholar] [CrossRef]
- Xie, C.; Huang, J.; Jiang, S.; Li, Y.; Yin, X.; Zhao, H. Hydrogen Leakage through Organic-Rich Caprock: A Molecular Simulation Study under Water and Cushion Gas Conditions. Fuel 2025, 402, 135980. [Google Scholar] [CrossRef]
- Strauch, B.; Pilz, P.; Hierold, J.; Zimmer, M. Experimental Simulations of Hydrogen Migration through Potential Storage Rocks. Int. J. Hydrog. Energy 2023, 48, 25808–25820. [Google Scholar] [CrossRef]
- Jacops, E.; Wouters, K.; Volckaert, G.; Moors, H.; Maes, N.; Bruggeman, C.; Swennen, R.; Littke, R. Measuring the Effective Diffusion Coefficient of Dissolved Hydrogen in Saturated Boom Clay. Appl. Geochem. 2015, 61, 175–184. [Google Scholar] [CrossRef]
- Borello, E.S.; Bocchini, S.; Chiodoni, A.; Coti, C.; Fontana, M.; Panini, F.; Peter, C.; Pirri, C.F.; Tawil, M.; Mantegazzi, A.; et al. Underground Hydrogen Storage Safety: Experimental Study of Hydrogen Diffusion through Caprocks. Energies 2024, 17, 394. [Google Scholar] [CrossRef]
- Keshavarz, A.; Abid, H.; Ali, M.; Iglauer, S. Hydrogen Diffusion in Coal: Implications for Hydrogen Geo-storage. J. Colloid Interface Sci. 2022, 608, 1457–1462. [Google Scholar] [CrossRef]
- Dehghani, M.R.; Ghazi, S.F.; Kazemzadeh, Y. Interfacial Tension and Wettability Alteration during Hydrogen and Carbon Dioxide Storage in Depleted Gas Reservoirs. Sci. Rep. 2024, 14, 11594. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Liu, K.; Ren, B.; Zhang, M.; Ju, Y.; Gu, J.; Zhang, X.; Clarkson, C.R.; Edlmann, K.; Zhu, W.; et al. Impacts of Relative Permeability Hysteresis, Wettability, and Injection/Withdrawal Schemes on Underground Hydrogen Storage in Saline Aquifers. Fuel 2023, 333, 126516. [Google Scholar] [CrossRef]
- Ali, M.; Isah, A.; Yekeen, N.; Hassanpouryouzband, A.; Sarmadivaleh, M.; Okoroafor, E.R.; Al Kobaisi, M.; Mahmoud, M.; Vahrenkamp, V.; Hoteit, H. Recent Progress in Underground Hydrogen Storage. Energy Environ. Sci. 2025, 18, 5740–5810. [Google Scholar] [CrossRef]
- Lüddeke, C.T.; Hagemann, B.; Ganzer, L. Conversion of Underground Gas Storages to Hydrogen: Impact on Storage Integrity, Capacity and Deliverability. In EAGE GET 2022; European Association of Geoscientists & Engineers: Bunnik, The Netherlands, 2022; Volume 2022, pp. 1–5. [Google Scholar] [CrossRef]

| Rock/Mineral | Porosity (%) | Reference |
|---|---|---|
| Evaporite | 0.14 to 7.7 | [22,23] |
| Salt (Halite) | 0.04 to 0.9 | [24] |
| Shales | 0.11 to 14 | [23,25,26] |
| Mudstone | 2 to 8.6 | [23,27] |
| Unfractured metamorphic and igneous rocks | 0 to 5 | [26] |
| Rock/Mineral | Temperature (°C) | Pressure (MPa) | Equilibrium CA (°) | Advancing CA (°) | Receding CA (°) | Reference |
|---|---|---|---|---|---|---|
| Basalt | 35 | 5 to 20 | - | 32.29 to 59.31 | ~29.4 to 56.9 | [39] |
| 70 | 47.86 to 68.61 | ~43.5 to 65.3 | ||||
| S. Arabia Basalt | 50 | 5 to 20 | 19.3 to 42.1 | 13.2 to 36.3 | [18] | |
| Anhydrate | 30 | 3.44 to 17.23 | 18 to 20.5 | - | - | [11] |
| 75 | 19.5 to 17.5 | |||||
| Quartz | 40 | 1 to 10 | 45 to 51 | [17] | ||
| Calcite | 40 to 59 | |||||
| Basalt | 21 to 27 | |||||
| Granite | 27 to 48 | |||||
| Shale | 39 to 52 | |||||
| Anhydrite | 40 to 43 | |||||
| Gypsum | 48 to 52 | |||||
| Wolf Camp Shale TOC < 0.3% | 50 | 1.37 to 6.89 | 65 to 60 | - | - | [45] |
| Eagle Ford Shale TOC = 3.83% | 90 to 80 | |||||
| Jordanian Oil Shale (TOC = 13%) | 50 | 0.34 to 11.03 | - | 43 to 79 | 43 to 76 | [41] |
| Mica | 50 | 5 to 20 | - | 21.7 to 42.9 | 18.3 to 36.6 | [40] |
| Shale TOC = 0.08% | 25 | 5 to 20 | - | ~26.8 to 47 | ~21.9 to 41.6 | [47] |
| Shale TOC = 0.1% | ~18.3 to 35.1 | ~15.3 to 30.3 | ||||
| Shale TOC = 0.09% | ~14.8 to 21.3 | ~12.1 to 18.2 | ||||
| Evaporite | ~11.7 to 16.3 | ~9.6 to 13.7 | ||||
| Shale TOC = 0.08% | 80 | - | ~17.3 to 30.4 | ~14.3 to 27.9 | ||
| Shale TOC = 0.1% | ~10.6 to 20.2 | ~7.5 to 15.3 | ||||
| Shale TOC = 0.09% | ~10 to 14.6 | ~7.6 to 12.9 | ||||
| Evaporite | ~7.9 to 12.7 | ~5.9 to 9.3 | ||||
| Kaolinite | 60 | 5 to 20 | 13.4 to 26 | - | - | [48] |
| Illite | 16.3 to 31.7 | |||||
| Montmorillonite | 19.8 to 38.6 |
| Rock/Mineral | Temperature (°C) | Pressure (MPa) | Equilibrium CA (°) | Advancing CA (°) | Receding CA (°) | Reference |
|---|---|---|---|---|---|---|
| Basalt | 35 to 70 | 5 | - | 32.29 to 47.86 | ~29.4 to 43.5 | [39] |
| 20 | 59.31 to 68.61 | ~56.9 to 65.3 | ||||
| S. Arabia Basalt | 25 to 50 | 20 | - | 38.5 to 42.1 | 33.2 to 36.3 | [18] |
| Anydrate | 30 to 75 | 3.44 | 18 to 19.5 | - | - | [11] |
| 10.34 | 21 to 19.5 | |||||
| 17.23 | 20.5 to 17.5 | |||||
| Mica | 35 to 70 | 15 | - | 53.1 to 35.4 | 47.3 to 29.2 | [49] |
| Calcite | 25 to 80 | 15 | - | 80.35 to 57.85 | 76.6 to 57.85 | [50] |
| Calcite | 20 to 80 | 10 | 40 to 93 | - | - | [17] |
| Quartz | 40 to 73 | |||||
| Basalt | 17 to 35 | |||||
| Granite | 30 to 67 | |||||
| Shale | 38 to 81 | |||||
| Anhydrite | 38 to 88 | |||||
| Gypsum | 45 to 71 | |||||
| Shale TOC = 0.08% | 25 to 80 | 20 | - | ~47 to 30.4 | ~41.6 to 27.9 | [47] |
| Shale TOC = 0.1% | ~35.1 to 20.2 | ~30.3 to 15.3 | ||||
| Shale TOC = 0.09% | ~21.3 to 14.6 | ~18.2 to 12.9 | ||||
| Evaporite | ~16.3 to 12.7 | ~13.7 to 9.3 |
| Rock/Mineral | Pressure (MPa) | Acid Concentration (Mol/lt) | Acid | Equilibrium CA (°) | Advancing CA (°) | Receding CA (°) | Reference |
|---|---|---|---|---|---|---|---|
| Basalt | 15 | 10−9 to 10−2 | Stearic | - | ~73.09 to 92.29 | 67.09 to 86.29 | [39] |
| S. Arabia Basalt | 5 | 0 to 10−2 | Stearic | - | 19.3 to 78.4 | 13.2 to 72.3 | [18] |
| 5 to 25 | 10−2 | 78.4 to 100.8 | 72.3 to 94.2 | ||||
| Mica | 15 | 0 to 10−9 | Lignoceric | - | ~42.9 to 63.2 | 36.6 to ~56.2 | [49] |
| 10−9 to 10−2 | ~63.2 to 91.8 | ~56.2 to 84 | |||||
| 25 | 10−2 | Hexanoic to lauric | ~67.5 to 89.2 | ~74.5 to 83.8 | |||
| Mica | 15 | 10−9 to 10−2 | Stearic | - | 53.2 to 84.6 | 48.7 to 76.4 | [40] |
| 15 to 25 | 10−2 | Stearic | - | 84.6 to 98.8 | 76.4 to 90.8 | ||
| Calcite | 10 | 10−9 to 10−2 | Stearic | - | 75.85 to 115.85 | 68.7 to 110.85 | [50] |
| Shale(Wolf Camp, and Eagle Ford) | 6.89 | TOC < 0.3% and 3.83% | Not aged | 60 and 90 | - | - | [45] |
| Shale TOC = 0.08% | 15 | 10−9 to 10−2 | Stearic | - | ~39.8 to 76.2 | ~34.6 to 69.3 | [47] |
| Shale TOC = 0.1% | ~31.5 to 57.9 | ~27.8 to 51.8 | |||||
| Shale TOC = 0.09% | ~26.7 to 55.4 | ~22.63 to 51.7 | |||||
| Evaporite | ~16.4to 42.8 | ~11.8 to 40.5 |
| Rock/Mineral | Temperature (°C) | Pressure (MPa) | Untreated | Treated | Reference |
|---|---|---|---|---|---|
| Quartz | 50 | 0.1 | 37.8° | 54.2° | [13] |
| 27 | 5.8° | 14.4° | |||
| Water-wet quartz | 50 | 13 | 85° | 95° | [53] |
| Oil-wet quartz | 105° | 90° | |||
| Basalt | 50 | 27 | 19.5 ° | 69° | [52] |
| Calcite | 50 | 8 | 57 ° | 40° | [54] |
| Rock/Mineral | Temperature and Pressure | Salinity | Advancing CA (°) | Receding CA (°) | Reference |
|---|---|---|---|---|---|
| Calcite | 50 °C and 15 MPa | 0 to 4.9 mol/kg | 69.8 to 80.65 | 63.35 to 73.3 | [50] |
| Calcite | 40 °C and 10 MPa | DI to formation brine | 51 to 59 | - | [17] |
| Quartz | 45 to 51 | ||||
| Basalt | 24 to 27 | ||||
| Granite | 27 to 48 | ||||
| Shale | 46 to 52 | ||||
| Anhydrite | 50 to 43 | ||||
| Gypsum | 51 to 52 |
| Rock/Mineral | Temperature and Pressure | Gas Mixture | Equilibrium CA (°) | Advancing CA (°) | Receding CA (°) | Reference |
|---|---|---|---|---|---|---|
| Jordanian oil shale (TOC 13%) | 50 °C+ 11.03 MPa | Pure H2 | - | 79 | 76 | [41] |
| Pure CH4 | 103 | 88 | ||||
| H2-CH4 | 99 | 88 | ||||
| Anhydrite | 75 °C+ 3.44–17.23 MPa | Pure H2 | 23 (stable) | - | - | [14] |
| H2-CH4 | 23 (stable) | |||||
| H2-CO2 | 24 (stable) | |||||
| Calcite | 75 °C+ 3.44–17.23 MPa | Pure H2 | 24 to 25 | [14] | ||
| H2-CH4 | 26 (stable) | |||||
| H2-CO2 | ~27 to 26 |
| Rock | Temperature (°C) | Pressure (MPa) | Rock–Liquid IFT (mN/m) | Rock–Gas IFT (mN/m) | Reference |
|---|---|---|---|---|---|
| Calcite | 60 | 1 to 10 | 52.66 (stable) | 98.84 to 69.52 | [44] |
| Quartz | 42.04 (stable) | 84.57 to 73.11 | |||
| Basalt | 11.25 (stable) | 68.37 to 64.86 | |||
| Granite | 39.55 (stable) | 85.15 to 74.85 | |||
| Shale | 51.78 (stable) | 98.96 to 72.31 | |||
| Anhydrite | 47.17 (stable) | 91.18 to 74.53 | |||
| Gypsum | 39.95 (stable) | 81.31 to 71.32 | |||
| Montmorillonite | 60 | 5 to 20 | - | 67.26 to 58.15 | [58] |
| Illite | 67.89 to 59.78 | ||||
| Kaolinite | 68.64 to 61.2 | ||||
| Muscovite | 50 | 5 to 20 | ~49 (stable) | 115 to 95 | [57] |
| 70 | ~43 (stable) | 104 to 90 |
| Temperature (°C) | Pressure (MPa) | Mixture or Rock Presence | Liquid–Gas IFT (mN/m) | Reference |
|---|---|---|---|---|
| 25 | 2.76 to 34.47 | H2 + brine | 80.77 to 75 | [60] |
| 150 | 58 to 56 | |||
| 60 | 5 to 20 | Kaolinite + H2 + brine | 67.02 to 65.53 | [58] |
| Montmorillonite + H2 + brine | 61.02 to 65.53 | |||
| Illite + H2 + brine | 67.02 to 65.53 | |||
| 50 | 1.37 to 11.03 | H2 + brine | 55 to 53 | [61] |
| 50 | 0.1 to 20 | 83.48 to 65.15 | ||
| 70 | 78.93 to 62.59 | |||
| 20 | 1 to 10 | H2 + brine | 69.07 to 30.97 | [17] |
| 40 | 77.52 to 36.44 | |||
| 60 | 78.77 to 39.6 | |||
| 80 | 1 to 7 | 82.07 to 58.31 | ||
| 25 | 2 to 40 | H2 + brine | 73 to 69.1 | [59] |
| 50 | 69.3 to 65.8 | |||
| 100 | 59.7 to 57.6 | |||
| 175 | 44.1 to 43.2 |
| Rock/Mineral | Temperature (°C) | Pressure (MPa) | Rock–Liquid IFT (mN/m) | Rock–Gas IFT (mN/m) | Reference |
|---|---|---|---|---|---|
| Calcite | 20 to 80 | 4 | 23.32 to 52.92 | 73.69 to 84.52 | [44] |
| Quartz | 29.64 to 42.84 | 79.66 to 76.28 | |||
| Basalt | 0.01 to 19.47 | 60.35 to 71.75 | |||
| Granite | 31.66 to 43.17 | 88.02 to 78.26 | |||
| Shale | 44.95 to 50.68 | 99.34 to 86.6 | |||
| Anhydrite | 12.92 to 50.45 | 63.8 to 75.75 | |||
| Gypsum | 0.76 to 38.5 | 45.38 to 64.4 | |||
| Muscovite | 35 to 70 | 5 | ~58 to 43 | ~124 to~104 | [57] |
| Temperature (°C) | Pressure (MPa) | Liquid–Gas IFT (mN/m) | Reference |
|---|---|---|---|
| 25 to 150 | 2.76 | 80.77 to 58 | [60] |
| 34.47 | 75 to 56 | ||
| 50 to 70 | 20 | 65.15 to 62.59 | [61] |
| 0.1 | 83.48 to78.93 | ||
| 20 to 40 | 1 | 63.6 to 62.6 | [17] |
| 60 to 80 | 60.3 to 58.5 | ||
| 25 to 175 | 2 | 73 to 44.1 | [59] |
| 40 | 69.1 to 43.2 |
| Temperature (°C) | Pressure (MPa) | Concentration (mol/lt) | Acid | Rock–Liquid IFT (mN/m) | Reference |
|---|---|---|---|---|---|
| 50 | 15 | 10−9 to 10−2 | Lignoceric | 51 to 53 | [57] |
| 50 | 25 | 10−3 | Hexanoic to lignoceric | 53 to 58 | |
| 50 | 10 | 10−9 to 10−2 | Stearic | 51 to 56.1 | [63] |
| 50 | 25 | 10−9 to 10−2 | Stearic | 41 to 54 | [62] |
| Rock/Mineral | Temperature (°C) | Pressure (MPa) | Salinity | Rock–Liquid IFT (mN/m) | Rock–Gas IFT (mN/m) |
|---|---|---|---|---|---|
| Calcite | 60 | 7 | DI to formation water | 48.54 to 52.66 | 56.57 to 78.69 |
| Quartz | 34.54 to 42.04 | 80.88 to 76.82 | |||
| Basalt | 1.98 to 11.25 | 63.01 to 66.02 | |||
| Granite | 16.9 to 39.59 | 70.95 to 78.2 | |||
| Shale | 38.22 to 51.78 | 84.38 to 80.7 | |||
| Anhydrite | 38.72 to 47.17 | 76.89 to 79.88 | |||
| Gypsum | 13.68 to 39.95 | 57.69 to 74.57 |
| Temperature (°C) | Pressure (MPa) | Brine Composition | Salinity (mol/kg) | Liquid–Gas IFT (mN/m) | Reference |
|---|---|---|---|---|---|
| 50 | 10 | H2 + brine (NaCl) | 0.96 to 2.93 | 68.56 to 71.43 | [61] |
| H2 + brine (CaCl2) | 0.93 to 2.4 | 70.57 to 82.02 | |||
| 25 | 2.76 | H2 + brine (NaCl + KCl) | 0 to 4.95 | ~73 to ~81 | [60] |
| 34.47 | ~69 to ~74 | ||||
| 150 | 2.76 | ~48 to ~58 | |||
| 34.47 | ~47 to ~56 | ||||
| 20 | 10 | H2+ brine (NaCl + KCl + CaCl2 + MgCl2) | 0 to brine | 24 to 39.97 | [17] |
| 40 | 35.81 to36.44 | ||||
| 60 | 37.5 to 39.6 |
| Temperature (°C) | Pressure (MPa) | Gas Mixture | Liquid–Gas IFT (mN/m) | Reference |
|---|---|---|---|---|
| 50 | 5 to 40 | 100%H2 | 58.5 to 56.9 | [66] |
| 50%H2 + 50%CO2 | 57.3 to 54.5 | |||
| 3.44 to 20.7 | 50%H2 + 50%CH4 | 57.4 to 56 | [64] | |
| 70%CO2 + 30%H2 | 54.7 to 32.1 | |||
| 50%CO2 + 50%H2 | 60.6 to 38.2 | |||
| 30%CO2 + 70%H2 | 65.4 to 51.7 | |||
| 1.37 to 11.03 | 100%H2 | 55 to 53 | [41] | |
| 50%H2 + 50%CH4 | 54.8 to 50 | |||
| 100%CH4 | 54 to 46.5 | |||
| 50 to 80 | 3.44 | 70%CO2 + 30%H2 | 54.7 to 47.7 | [64] |
| 50%CO2 + 50%H2 | 60.6 to 51.5 | |||
| 30%CO2 + 70%H2 | 65.4 to 58.9 |
| Temperature and Pressure | Salinity (mol/kg) | Gas Mixture | Liquid–Gas IFT (mN/m) | Reference |
|---|---|---|---|---|
| 50 °C + 3.44 MPa | 0 to 3.15 | 70%CO2 + 30%H2 | 51.5 to 60.4 | [64] |
| 50%CO2 + 50%H2 | 57.9 to 64.6 | |||
| 30%CO2 + 70%H2 | 62.4 to 67.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trimi, P.-M.; Bellas, S.; Vakalas, I.; Gholami, R.; Gaganis, V.; Gontikaki, E.; Stamatakis, E.; Yentekakis, I.V. A Review of Caprock Integrity in Underground Hydrogen Storage Sites: Implication of Wettability, Interfacial Tension, and Diffusion. Hydrogen 2025, 6, 91. https://doi.org/10.3390/hydrogen6040091
Trimi P-M, Bellas S, Vakalas I, Gholami R, Gaganis V, Gontikaki E, Stamatakis E, Yentekakis IV. A Review of Caprock Integrity in Underground Hydrogen Storage Sites: Implication of Wettability, Interfacial Tension, and Diffusion. Hydrogen. 2025; 6(4):91. https://doi.org/10.3390/hydrogen6040091
Chicago/Turabian StyleTrimi, Polyanthi-Maria, Spyridon Bellas, Ioannis Vakalas, Raoof Gholami, Vasileios Gaganis, Evangelia Gontikaki, Emmanuel Stamatakis, and Ioannis V. Yentekakis. 2025. "A Review of Caprock Integrity in Underground Hydrogen Storage Sites: Implication of Wettability, Interfacial Tension, and Diffusion" Hydrogen 6, no. 4: 91. https://doi.org/10.3390/hydrogen6040091
APA StyleTrimi, P.-M., Bellas, S., Vakalas, I., Gholami, R., Gaganis, V., Gontikaki, E., Stamatakis, E., & Yentekakis, I. V. (2025). A Review of Caprock Integrity in Underground Hydrogen Storage Sites: Implication of Wettability, Interfacial Tension, and Diffusion. Hydrogen, 6(4), 91. https://doi.org/10.3390/hydrogen6040091










