1. Introduction
Hydrogen energy, with its high combustion enthalpy, versatile utilization pathways, and substantial development potential is recognized as a strategic clean energy resource for mitigating global warming and advancing sustainable development [
1,
2,
3]. However, persistent challenges in storage and transportation continue to hinder the large-scale development and application of the hydrogen economy [
4,
5,
6]. Current hydrogen storage approaches primarily encompass high-pressure compressed gas storage, cryogenic liquid hydrogen (LH) storage, and solid-state hydrogen storage. Cryogenic LH is regarded as the most competitive method for achieving efficient and long-term storage and transportation [
7]. In LH pressure vessels, the extreme temperature difference between LH and ambient environment [
8], coupled with the low latent heat of vaporization and the flammable and explosive nature of hydrogen, implies that even minimal heat ingress can trigger substantial evaporation, leading to economic loss and heightened safety risks [
9]. To address this issue, most LH vessels utilize high vacuum multilayer insulation (HVMLI), which suppresses radiative, conductive, and gaseous heat transfer through the combined use of radiation shields, spacer materials, and vacuum pumping. This configuration achieves an apparent thermal conductivity on the order of 10
−5 W/(m·K) [
10]. During vessel operation, the insulation structure remains largely stable, making the vacuum level of the interlayer the decisive factor governing thermal insulation performance [
11]. HVMLI demonstrates exceptionally low and stable apparent thermal conductivity when the vacuum pressure is below 1 × 10
−2 Pa. However, once the pressure exceeds this threshold, thermal conductivity increases dramatically [
10]. Insulation performance degrades rapidly, resulting in complete failure. Owing to material outgassing and leakage effects, interlayer vacuum inevitably deteriorates over time, leading to diminished insulation performance, reduced vessel lifetime, and increased safety risks. Hydrogen has been identified as the predominant residual gas within the interlayer, rendering its removal a critical challenge in the design, fabrication, and operation of LH pressure vessels [
12,
13,
14].
At present, adsorption is widely recognized as the most effective strategy for removing residual hydrogen from LH storage tanks [
15]. However, a significant gap remains in the current literature regarding a systematic review that comprehensively connects material properties with system-level performance. Existing studies have mostly focused on evaluating the adsorption capacity of candidate materials, while the clarification of the correlation between interlayer gas load, adsorbent dosage, and the overall insulation performance of the system remains insufficient. To address these gaps, this work first analyzes the sources of residual gas in vacuum multi-layer insulation systems and their impacts on thermal performance degradation and adsorbent requirements. It then reviews the adsorption mechanisms, testing methods, and application potential of typical adsorbents, including cryogenic adsorbents, metal oxides, zeolite molecular sieves, and non-volatile compounds. Finally, the key challenges in adsorbent applications are summarized, and potential research directions for the development of novel adsorbents and optimization of system design are proposed. The main component of this paper is shown in
Figure 1.
2. Gas Load and Vacuum Lifetime
2.1. Sources of Interlayer Gas Loads
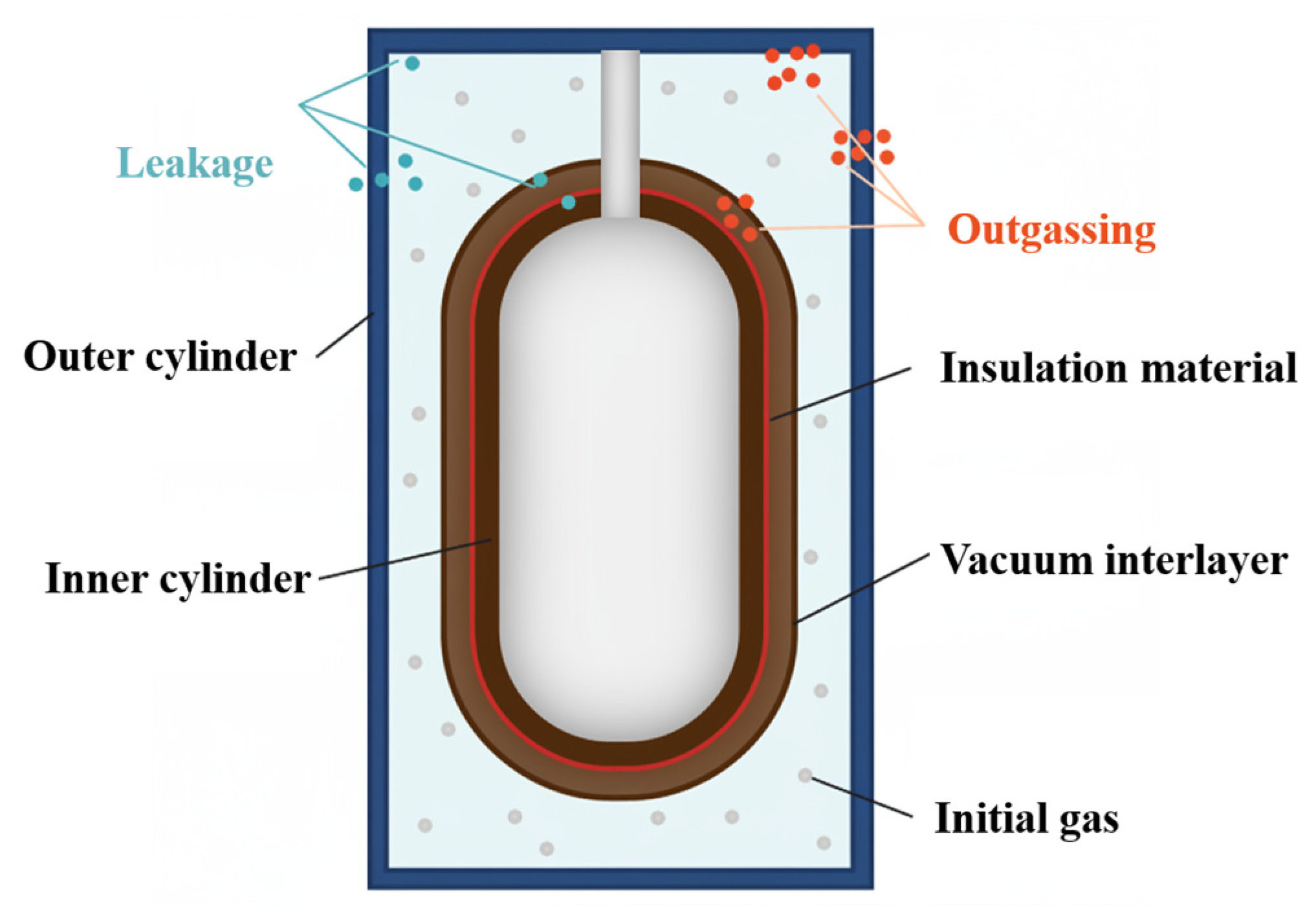
As illustrated in
Figure 2, the primary gas loads in the vacuum-insulated layer of an evacuated and sealed cryogenic container arise from leakage between the inner and outer cylinders, outgassing from the metal walls and insulation materials, and other minor sources. Leakage primarily results from defects in the cavity walls and seals, as well as from trapped gases escaping from dead volumes within the container after evacuation. Outgassing is attributed to the desorption of molecules from material surfaces and the permeation of external gases into the interior [
16,
17].
2.1.1. Air Leakage Load
Leakage between the inner and outer cylinders is mainly caused by residual air or cryogenic gases within the system. The primary gases involved are N
2, O
2, and H
2O, among others. These gases can generally be removed by installing adsorbents such as molecular sieves or activated carbon on the cryogenic side (commonly referred to as cryogenic adsorbents). A helium mass spectrometer leak detector is capable of detecting leak rates as low as 1 × 10
−10 Pa·m
3/s [
19]. At 20–30 K, the equilibrium vapor pressures of all gases except He, H
2, and Ne fall below 10
−2 Pa, while the concentrations of He, H
2, and Ne in the leaks remain extremely low. When cryogenic adsorbents are employed, the influence of leaks on the vacuum can be considered negligible.
2.1.2. Vent Load
The outgassing rate of materials including metal walls and insulation structures is considerably higher than the leakage rate of the cylinder body [
20], with hydrogen constituting the majority of released gas. Extensive studies [
21,
22] have shown that hydrogen is the dominant residual gas in stainless-steel cryogenic sealed tanks. After degassing and related treatments, hydrogen accounts for about 70% of the total residual gas, while H
2O and CO make up the remaining 30%. When the container is filled with liquid nitrogen, the hydrogen partial pressure rises to nearly 97%. Cryogenic adsorbents—molecular sieves and activated carbon—exhibit strong hydrogen adsorption at 20 K; however, even a slight temperature increase reduces their capacity by an order of magnitude, and at 77 K the capacity becomes negligible. Since such temperature shifts are inherent to liquid hydrogen loading procedures, this underscores a fundamental limitation of physical adsorbents. To achieve effective H
2 removal, physical and chemical adsorbents are therefore typically employed on the ambient-temperature side, often referred to as ambient-temperature adsorbents.
2.2. Vacuum Life
Experimental data on the apparent thermal conductivity of HVMLI systems in liquid-nitrogen temperature zones under varying interlayer vacuum pressures have been compiled. The results [
10,
11] indicate that for various common insulation structures, once the vacuum pressure exceeds 1.33 × 10
−2 Pa, the apparent thermal conductivity increases linearly, and insulation performance deteriorates rapidly. Once the pressure reaches the vacuum threshold, tank must be returned to the factory for re-evacuation. The interval between successive evacuations defines the vacuum lifespan of the tank [
23]. Research indicates that a 0.75 L stainless-steel cryogenic gas tank with a sealing pressure of 1 × 10
−2 Pa has a vacuum lifespan of less than one year without hydrogen adsorbents, whereas the incorporation of hydrogen adsorbents extends the lifespan to approximately ten years [
24].
2.3. Calculation of Adsorbent Dosage
Cumulative gas volume from interlayer leakage and material venting exceeds the adsorption capacity of the adsorbent at the vacuum threshold, the vacuum life ends. The formula for calculating the amount of adsorbent required is
In the formula, is mass of the adsorbent, with the unit of g; is gas leakage and outgassing rate of the container, that is, the total amount of gas generated by leakage and outgassing per unit time, with the unit of Pa·m3/s; is the unit adsorption capacity of the adsorbent corresponding to the vacuum threshold, with the unit of Pa·m3/g; is the adsorption efficiency of the adsorbent; is the designed vacuum life of the cryogenic container, with the unit of a.
The dosage of adsorbents must consider the potential volume of escaped gas, the specific gas load within the interlayer, and the effects of cryogenic condensation. Dosage calculations for cryogenic and ambient-temperature adsorbents are conducted separately according to gas type. Variations in adsorption temperature, initial state, system configuration, and adsorption time cause deviations between actual adsorption capacity and theoretical test values. Accurate determination of adsorption efficiency is therefore critically important. At present, the design of hydrogen adsorbent dosage relies mainly on empirical and semi-empirical methods. Establishing the hydrogen adsorption characteristics of these materials under diverse conditions and developing a more unified, precise approach for dosage calculation remain essential.
3. Types of Hydrogen Adsorbents
The selection of hydrogen adsorbents for LH tanks may include both cryogenic and ambient-temperature types.
Table 1 provides a classification and comparison, indicating that cryogenic adsorbents are primarily physical, while ambient-temperature adsorbents comprise metal oxides, ion-exchanged zeolitic molecular sieves, and non-evaporable getters (NEGs).
Figure 3 shows a typical arrangement of hydrogen adsorbents in cryogenic tanks. In practice, placement is not fixed; ambient-temperature adsorbents may also be applied on the cryogenic side. Such use requires careful evaluation of adsorbent performance and filling procedures. Silver molecular sieves (Ag-exchanged zeolites), although classified as ambient-temperature adsorbents, can also function on the cryogenic side. Under these conditions, larger amounts of N
2, O
2, and H
2O are co-adsorbed, reducing the effective adsorption capacity for hydrogen. As a result, greater adsorbent quantities are usually required.
3.1. Cryogenic Adsorbents
Cryogenic adsorbents typically rely on physical adsorption mechanisms, wherein hydrogen molecules interact with the adsorbent surface via van der Waals forces. At cryogenic temperatures, such as liquid hydrogen temperature (approximately 20 K), these adsorbents exhibit significant hydrogen adsorption capacity, with activated carbon and molecular sieves being the most commonly used materials. Mounted on the outer surface of the inner cylinder, their porous structures enable efficient gas capture. Their adsorption capacity varies significantly with temperature in the 77–90 K range, and to mitigate external heat leakage, they are often encapsulated within an insulating layer [
26]. In practical applications, adsorbents should be distributed as uniformly as possible along the cold wall of the inner cylinder and provided with thermal insulation. An alternative approach involves the use of an independent adsorption chamber located at the bottom of the inner vessel, where the adsorbents are enclosed in mesh or cloth bags, and openings are incorporated into the outer insulation layer to facilitate gas ingress [
27].
The performance of adsorbents varies considerably. Activated carbon is generally more effective for achieving higher vacuum levels, whereas molecular sieves offer greater adsorption capacities [
28]. At 77 K, the hydrogen adsorption capacity of 5A molecular sieves, which have a pore size of approximately 5 Å and are particularly effective at adsorbing small molecules like hydrogen, is about twice that of activated carbon and more than five times that of 13X molecular sieves, which have larger pores (approximately 10 Å) and are better suited for adsorbing larger molecules, but offer lower hydrogen adsorption capacity at cryogenic temperatures [
29]. However, exposure to moisture from air significantly diminishes their performance. Ikemoto et al. [
30] showed that residual water vapor affects the hydrogen adsorption capacities of various A-type molecular sieves at 77 K to different degrees, with 5A molecular sieves maintaining superior performance and thus being more suitable for hydrogen adsorption. The physical adsorption of cryogenic adsorbents is reversible but highly sensitive to temperature. During LH depletion or refilling, gas desorption deteriorates interlayer insulation, making it necessary to use ambient-temperature adsorbents to remove the released gas [
31]. Although ambient-temperature adsorbents exhibit high hydrogen adsorption capacities, they are easily affected by impurities such as water vapor and perform best when combined with cryogenic adsorbents. Francis et al. [
32] compared it with using 5A zeolite alone, combining it with barium-based adsorbents improved the vacuum level by two orders of magnitude at room temperature and by 25-fold under cryogenic conditions.
Beyond conventional adsorbents, metal–organic frameworks (MOFs)—novel porous materials constructed through the self-assembly of metal ions and organic ligands—offer the combined advantages of organic and inorganic systems. With tunable microporous structures, large specific surface areas, and low densities, MOFs exhibit considerable potential for applications in gas storage, separation, and catalysis. For example, MOF-177 achieves a hydrogen storage capacity of 7.5 wt% (approximately 851 cm
3(STP)/g) at 77 K and 7 MPa [
33]. Because gases preferentially adsorb onto micropores at low pressures, the advantages of MOFs in terms of specific surface area and pore volume are particularly pronounced. Consequently, the use of MOFs as hydrogen adsorbents in the vacuum interlayers of multilayer-insulated cryogenic vessels holds substantial research and application value. Liu et al. [
34] synthesized MOF-101 as a hydrogen adsorbent for cryogenic vessels and compared its performance with that of conventional cryogenic adsorbents (e.g., activated carbon and molecular sieves) and ambient-temperature adsorbents (e.g., PdO- and Ag-loaded molecular sieves). The results revealed that at 298 K, the cumulative hydrogen adsorption capacity of the MOF adsorbent was low (1 cm
3(STP)/g) and partially irreversible, whereas at 77 K, the capacity was high (173 cm
3(STP)/g) and fully reversible, making it well-suited for deployment on the cryogenic side of the interlayer. Therefore, MOF-based adsorbents demonstrate the potential to replace both cryogenic and ambient-temperature adsorbents.
3.2. Ambient Temperature Adsorbents
Ambient temperature adsorbents are typically positioned on the inner surface of the outer cylinder and function through chemical adsorption or physiochemical synergistic adsorption. The hydrogen adsorption capacity of these adsorbents is relatively low because molecular motion is more vigorous, making it difficult for hydrogen molecules to be adsorbed. However, the hydrogen adsorption capacity of room-temperature adsorbents under low-pressure conditions is generally lower than that of cryogenic adsorbents. Representative materials include metal oxides, ion-exchanged molecular sieves, and NEGs. Palladium oxide (PdO) has long served as a conventional material, offering high hydrogen adsorption capacity, a broad operating pressure range, and no activation requirement. Its high cost and explosion risk in LH tanks restrict large-scale applications. Silver-exchanged molecular sieves provide greater safety and lower cost, leading to widespread adoption worldwide. NEGs are relatively inexpensive and display strong hydrogen adsorption performance. Their use remains limited in cryogenic tanks due to activation temperature requirements and process constraints.
3.2.1. Metal Oxides
Metal oxides are considered efficient hydrogen adsorbents owing to their low-stability metal–oxygen bonds, high oxygen diffusion coefficients, and large specific surface areas [
35]. The adsorption mechanism primarily involves the redox reaction of hydrogen, with the resulting water subsequently removed by molecular sieves. PdO was one of the earliest materials applied in this field, characterized by high hydrogen adsorption capacity, a broad operating pressure range, and the advantage of not requiring activation. Its high cost restricts large-scale application. Hydrogen adsorption on PdO is an exothermic process, and its performance can be further improved by dispersing it in powdered form within a well-ventilated adsorption chamber [
36]. However, in the event of LH leakage, PdO undergoes a violent redox reaction with hydrogen, which may generate sparks or even trigger explosions. Copper oxide (CuO) provides the advantages of rapid hydrogen adsorption, high capacity, and low cost, although external heating is required to initiate adsorption. Xie [
33] designed an external heating device and demonstrated that, at 473 K, the combination of CuO and 5A molecular sieves achieved hydrogen adsorption capacity significantly exceeding that of PdO, particularly within the 1 × 10
−3 Pa pressure range. Wang et al. [
37,
38] proposed the synergistic use of CuO with cryogenic adsorbents to enhance hydrogen adsorption, and investigated composite materials consisting of CuO, Cu, C, and 5A molecular sieves. Their results indicated that when the mass ratio of CuO:Cu:C was 1:6.4:2, the composite adsorbent achieved optimal performance, with a saturated hydrogen adsorption capacity of 415.91 mL (STP)/g.
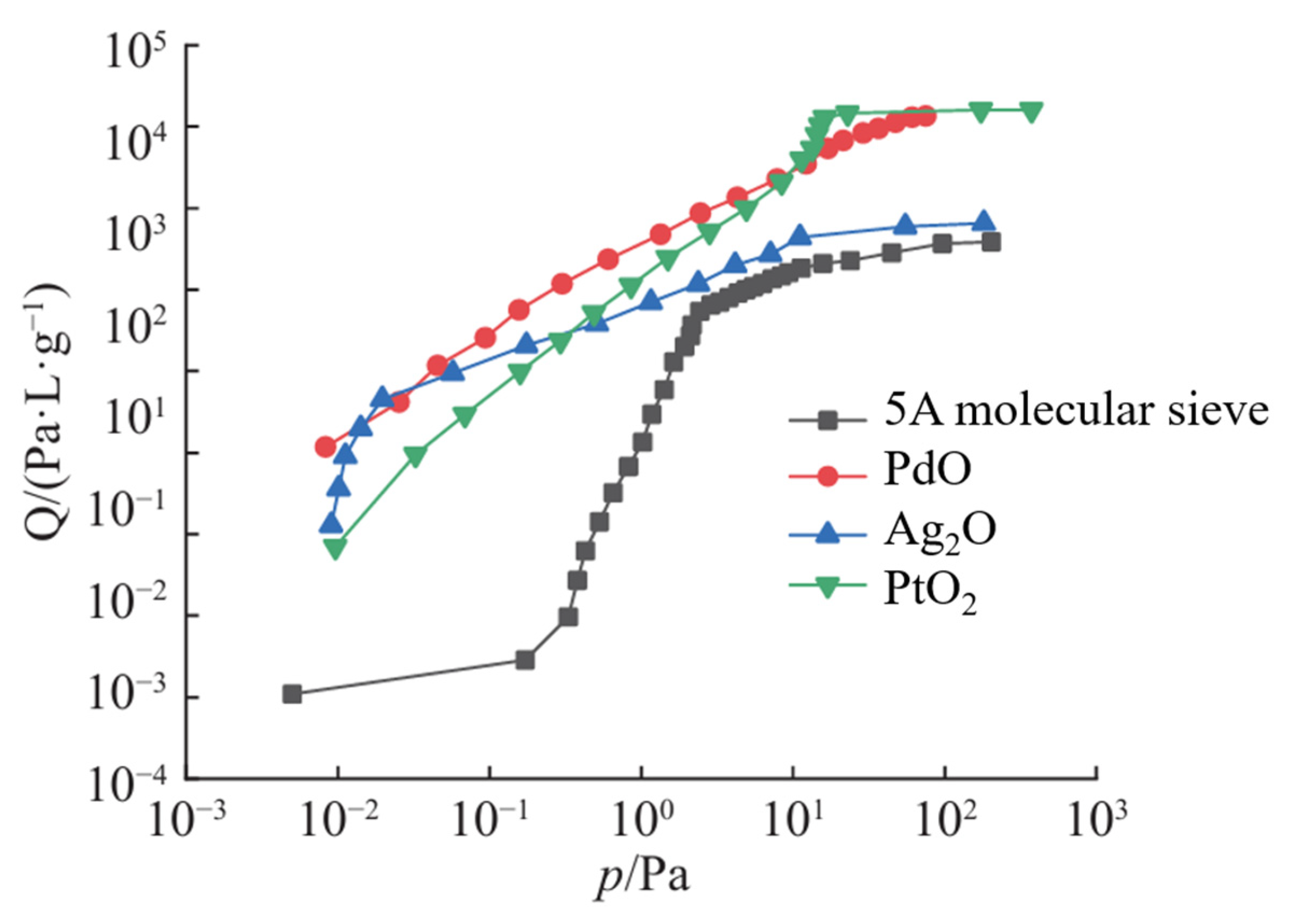
Different metal oxides exhibit varying hydrogen adsorption capacities and applicable pressure ranges (
Figure 4). Within the 10
−2–10
2 Pa range, PdO exhibits the highest hydrogen adsorption capacity; Ag
2O approaches PdO only near 2 × 10
−2 Pa, whereas PtO
2 surpasses PdO above 10 Pa. To balance performance and cost, PdO can be combined with Ag
2O, PtO
2, or other oxides to form composite adsorbents, thereby significantly reducing cost without compromising adsorption performance [
39].
Chen et al. [
40] investigated composite adsorbents with different mass ratios of PdO and Ag
2O, and found that the addition of Ag
2O as a catalyst optimized the oxygen ion mass fraction at 22%. Furthermore, Pd can serve as a catalyst to improve the reduction efficiency of metal oxides, enabling high hydrogen adsorption capacity when incorporated in small amounts. Belousov et al. [
35] reported that, under 0.001–0.700 kPa and 77–320 K, doping metal oxides with 0.1–0.5% Pd reduced their reduction temperature and increased hydrogen uptake by 15–100 times.
Table 2 presents the performance parameters of various metal oxides before and after Pd doping. The results revealed that Pd-doped Co
3O
4, CuO, and MnO
2 exhibited 12-, 16-, and 114-fold improvements in hydrogen adsorption, demonstrating strong potential under cryogenic vacuum conditions. Industry reports indicate that
SAES Getters has adopted Pd-doped Co
3O
4 (St820) as an adsorbent for cryogenic tanks. This adsorbent requires no high-temperature activation, operates efficiently at cryogenic temperatures, achieves about half the hydrogen adsorption capacity of PdO, and offers superior safety, making it suitable for oxygen storage tanks.
3.2.2. Ion Exchange Zeolite Molecular Sieves
Ion-exchanged molecular sieves are generally synthesized by treating zeolitic molecular sieves with metal salt solutions under controlled conditions, followed by separation, washing, and drying. Complexes formed through ion exchange with heavy metals including Cu, Ni, Rh, and Ag (e.g., AgX) have been identified as highly effective hydrogen adsorbents for cryogenic tanks [
32,
36]. Silver molecular sieves (SMSs) are the most widely used, offering stable hydrogen adsorption performance. Their adsorption capacity is approximately one-tenth that of PdO, while their cost is only about one-fortieth [
41]. SMS are aluminosilicate zeolites loaded with silver ions, and their hydrogen adsorption mechanism integrates physical adsorption with catalytic chemical processes: hydrogen molecules first penetrate the micropores via van der Waals forces, then react with oxygen under the catalytic effect of active Ag ions to form stable hydrogen bonds embedded within the zeolite lattice, while the water by-products are absorbed into the intrinsic channels of the sieve. Commercial SMS adsorbents are typically off-white or light-yellow spherical granules that require no pre-activation. Upon hydrogen uptake, they darken to grayish-black due to water absorption. He et al. [
42] compared four commercial SMS hydrogen adsorbents with PdO and found that, at equilibrium pressures of 2 × 10
−2 to 10 Pa, the two best SMS products exhibited hydrogen adsorption capacities nearly two orders of magnitude higher than PdO, attributed to their abundant microporous structure (with
vmic fractions as high as 38–77%). Yu et al. [
41] further compared various Ag molecular sieves, CuO-, and Zr-based composite adsorbents with PdO, demonstrating that SMS outperform others in both hydrogen adsorption capacity and cost-effectiveness. In addition, greater pore volume, higher specific surface area, smaller pore diameter, and higher silver-ion loading were all found to enhance hydrogen adsorption performance. By contrast, the high cost of metal oxides, coupled with their violent redox reactions during hydrogen adsorption, poses serious explosion risks in the event of LH leakage, which significantly limits their large-scale application in cryogenic tanks.
3.2.3. Non-Evaporative Inhalant
Alloys composed of transition metals, refractory metals, and rare earth elements can effectively remove gaseous atoms and molecules via surface chemical reactions, while maintaining low equilibrium pressures under suitable conditions. These materials, which exhibit excellent adsorption and absorption properties for active gases at specific temperatures, are termed getters. NEGs require no evaporation or sublimation and can operate under ambient conditions once activated at a specified temperature. NEGs offer high hydrogen capacity, rapid adsorption kinetics, and low cost. Their adsorption mechanism involves three processes: surface adsorption, near-surface penetration, and bulk diffusion [
43]. Because their performance depends on active surfaces, exposure to air results in rapid passivation as fresh surfaces react with water and hydrocarbons, necessitating pre-use activation by heating above 573 K [
44].
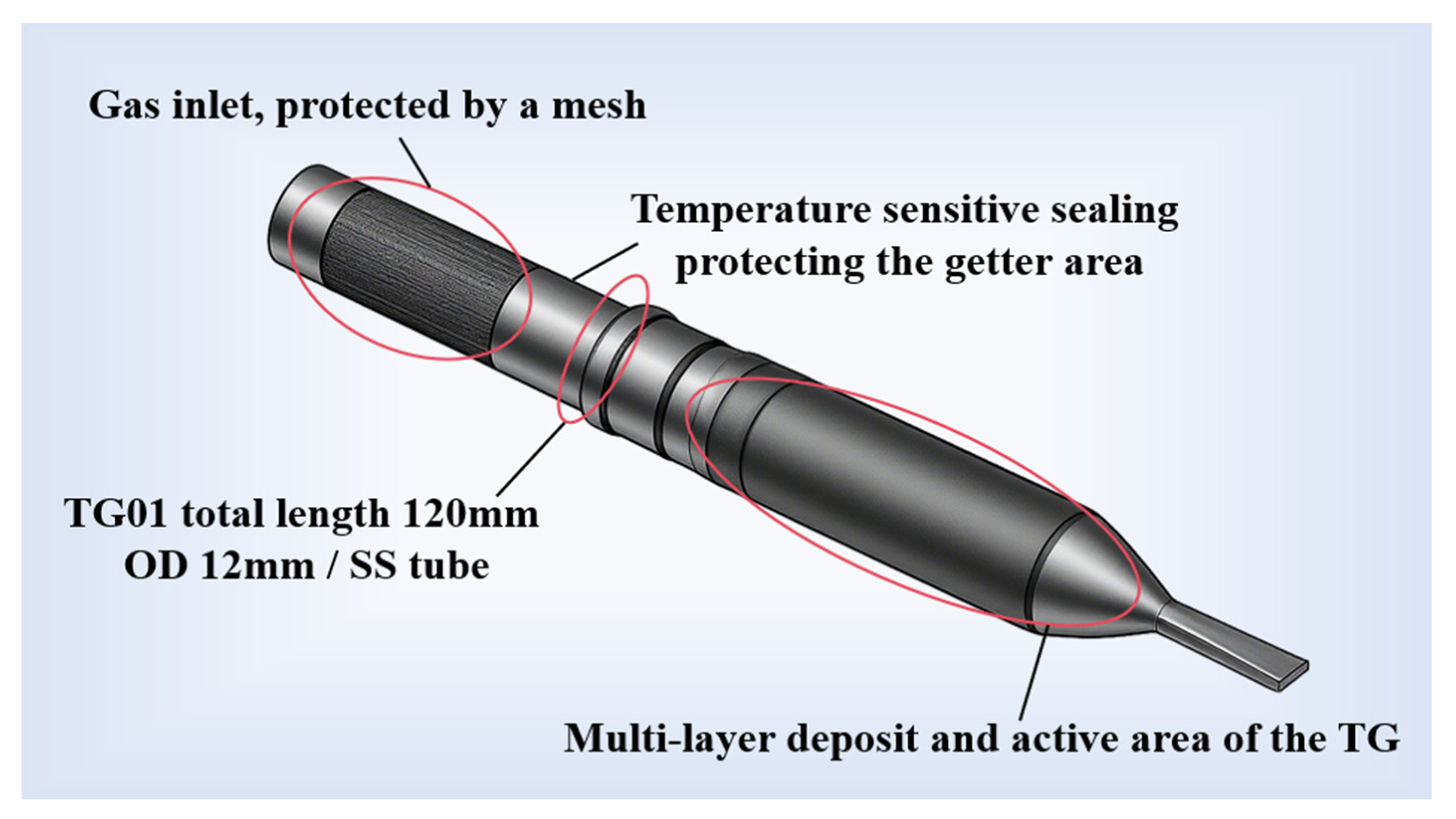
To meet the demands of HVMLI tanks, researchers have sought to develop NEGs with reduced activation temperatures. Francis et al. [
32] proposed that materials such as Ba, Sr, Li, and Ce could operate at room temperature or even lower. Londer et al. [
45] proposed fabricating efficient tubular NEGs by depositing Ba onto the inner wall of a metal tube, demonstrating their potential for application in mobile LH tanks. As shown in
Figure 5, tubular NEGs can maintain vacuum levels of 10
−3–10
−4 Pa for extended periods after activation. Zr–V–Fe ternary alloy has attracted significant attention due to its low activation temperature and high hydrogen adsorption capacity, exhibiting excellent gettering performance for hydrogen and other active gases. Boffito et al. [
46] reported that at elevated hydrogen pressures, this alloy undergoes vigorous hydrogenation reactions, particularly under LH leakage conditions, where the material temperature rises sharply. Doping with Ni, Ti, or similar elements reduces the temperature rise from 993 K to 493 K, well below the autoignition temperature of hydrogen in air (843 K), thereby improving safety. Okseniuk et al. [
47] studied various Zr-based alloys under 10
−2 Pa and 300–1100 K, and found that hydrogen readily undergoes chemisorption on sputter-cleaned alloy surfaces, with adsorption capacity strongly dependent on composition. Hsu et al. [
43] tested the hydrogen adsorption performance of SAES’s commercial NEG product St707 and reported that its hydrogen capacity peaked at 196 K.
4. Application of Hydrogen Adsorbents in Storage Tanks
4.1. Adsorption Performance Test Method
The adsorption capacity of an adsorbent is governed by pressure and temperature and is strongly influenced by the physicochemical properties of both the adsorbent material and the gas. At constant temperature, the relationship between adsorption capacity and pressure is described by an adsorption isotherm. Isothermal data obtained at different temperatures are critical for calculating adsorbent dosage and for guiding the design of HVMLI cryogenic tanks. Experimental determination remains the most reliable method for obtaining adsorption isotherms. Common experimental methods include the static constant-volume method, the gravimetric (weight) method, and the dynamic method. Gravimetric method achieves detection by measuring the mass change in the adsorbent caused by gas adsorption. While this method provides valuable data, it is highly sensitive to temperature fluctuations and buoyancy effects, which can lead to significant errors—particularly under low-temperature conditions and when handling small-molecule gases such as hydrogen. Dynamic method can characterize adsorption kinetics, but equilibrium is difficult to confirm, making both methods unsuitable for hydrogen isotherm testing under cryogenic and low-pressure conditions [
48]. For cryogenic and vacuum environments, the static constant-volume method has become the most widely adopted standard technique. Currently, various automated commercial instruments are available that ensure accuracy while enhancing operational efficiency.
Static constant-volume method determines adsorption capacity by measuring changes in gas volume and pressure. In this method, the adsorption chamber volume is fixed and maintained at a constant temperature. Initial pressure is recorded, after which a quantified amount of gas is introduced into the adsorption chamber from the gas distribution chamber. When the gas comes into contact with the adsorbent and equilibrium is achieved, the equilibrium adsorption pressure and the residual pressure in the gas distribution chamber are measured. The difference in gas volume between the two chambers is then calculated, and the adsorption capacity corresponding to the equilibrium pressure is determined using the principle of mass conservation. By gradually increasing the gas dosage and adjusting the equilibrium pressure, a complete adsorption isotherm can be obtained. The adsorption capacity is calculated according to the following formula:
In the equation: is the amount of gas adsorbed in the i-th adsorption cycle, and are the initial pressures in the gas distribution chamber and adsorption chamber during the i-th adsorption cycle, respectively, and are the volumes of the gas distribution chamber and adsorption chamber, respectively, and is the equilibrium pressure during the i-th adsorption cycle.
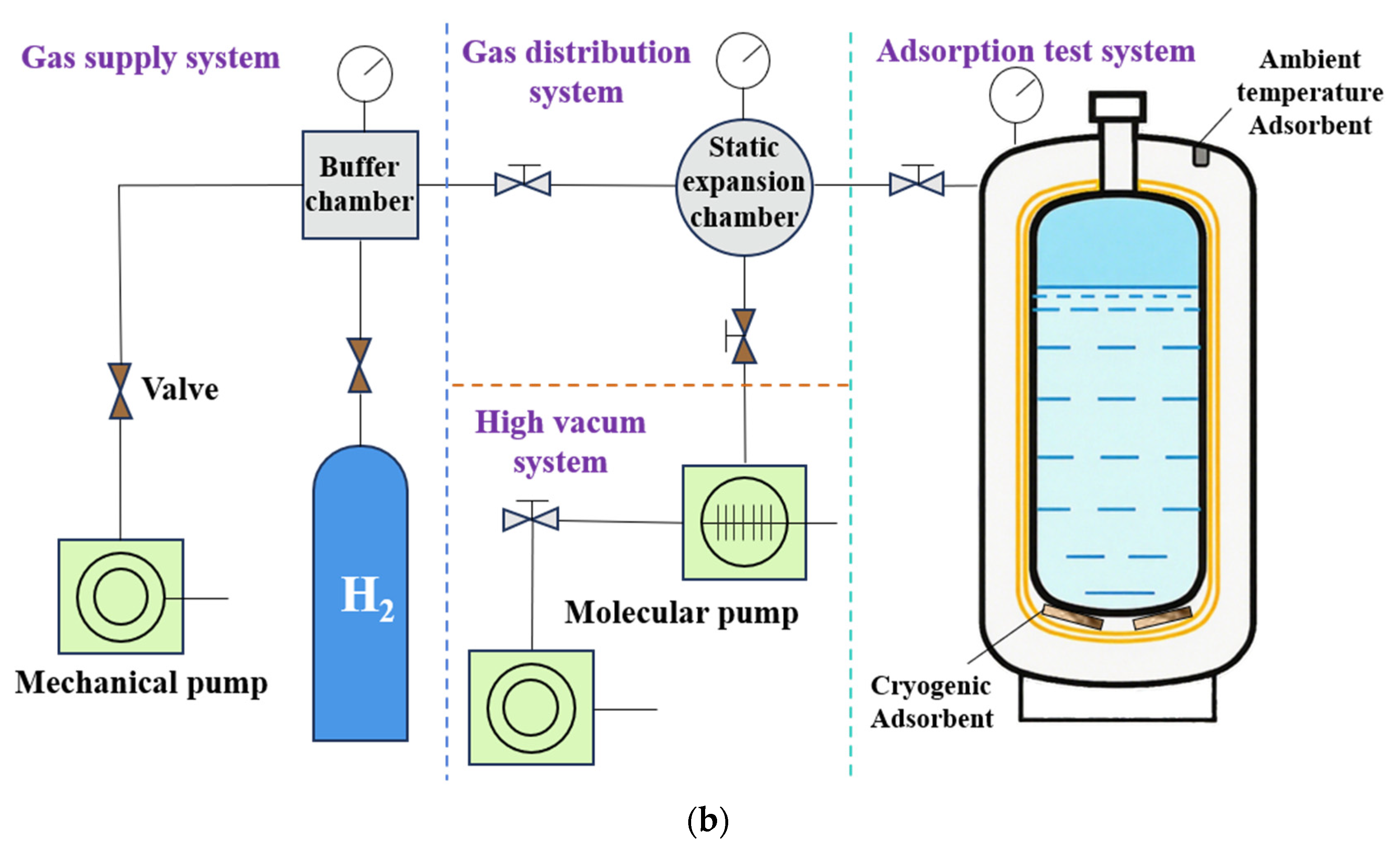
Based on sample chamber design, static volumetric testing systems can be classified into adsorption chamber-type and tank-type configurations (
Figure 6). Adsorption chamber-type systems feature a simple structure and straightforward volume calculations, but they cannot accurately replicate real-world tank conditions. Tank-type systems, by contrast, better simulate actual operating conditions and facilitate the study of adsorbent loading processes. However, calculations involving interlayer and dead volumes are more complex, and the experimental procedures for adsorbent loading and thermal insulation handling are less convenient.
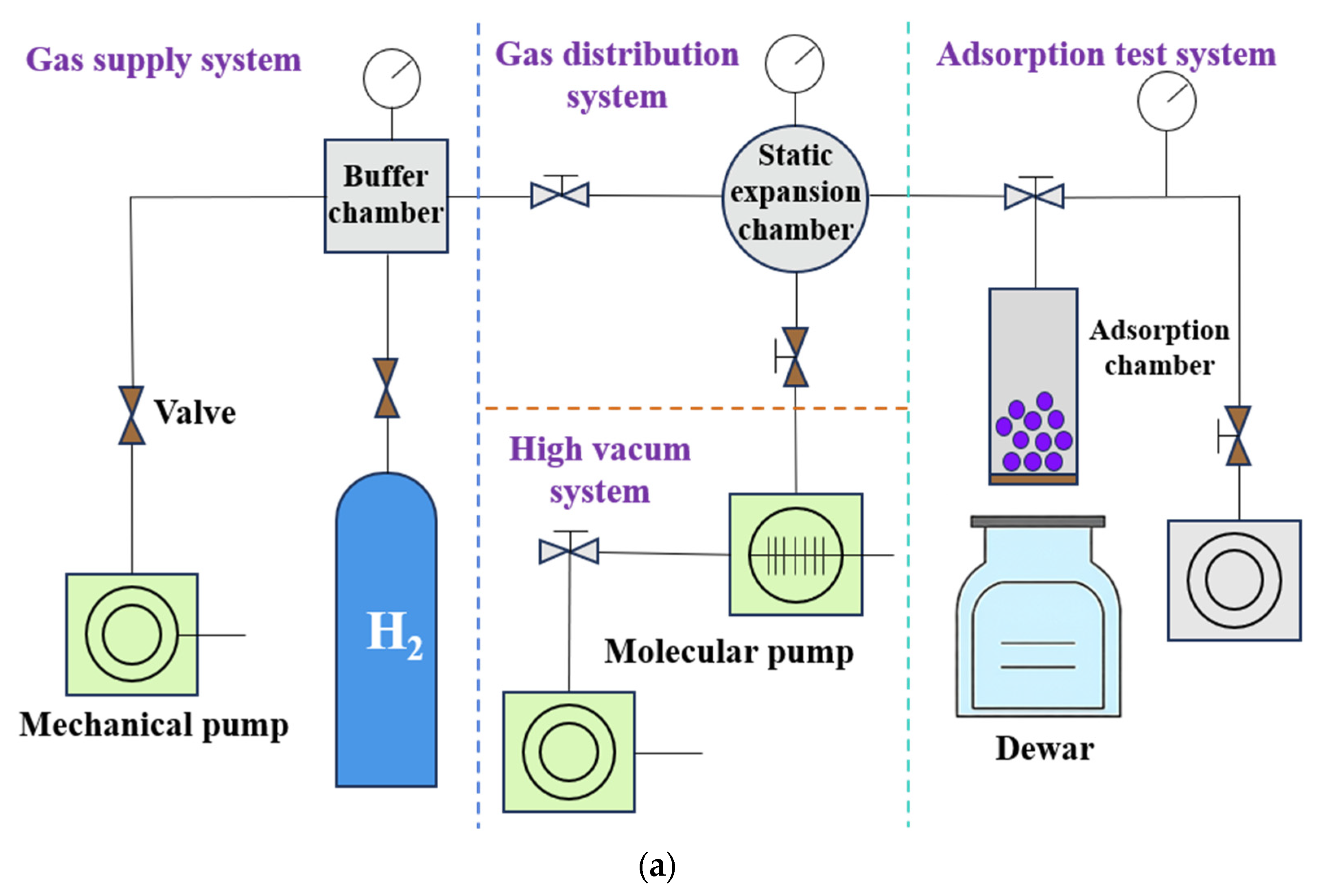
At present, most systems employ cryogenic liquid cooling, with test temperatures typically above 77 K. When equipped with a cryogenic refrigeration unit, however, the temperature range can be extended to that of LH and even lower.
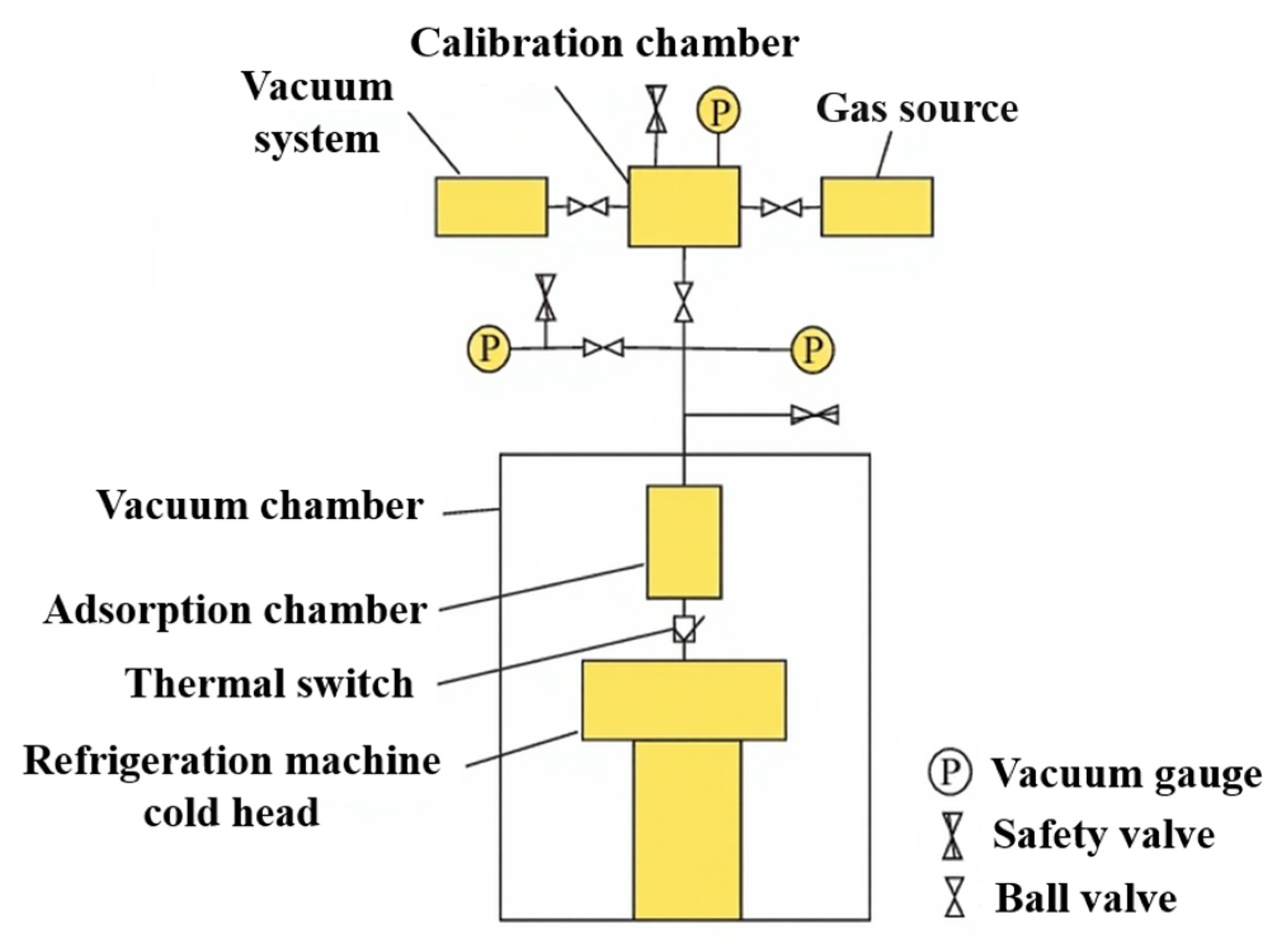
Figure 7 illustrates an example of a test system based on a cryogenic refrigeration machine. To investigate the helium adsorption characteristics of activated carbon below 30 K, the Institute of Physics and Chemistry, Chinese Academy of Sciences, developed an adsorption chamber-type apparatus incorporating a pulse tube refrigerator and a two-stage GM refrigerator, enabling tests at 15–30 K and 4–10 K [
49,
50].
4.2. Application of Adsorbents in Storage Tanks
The vacuum level within the interlayer of cryogenic storage tanks is the decisive factor governing thermal insulation performance. Edward et al. [
51] conducted a two-dimensional thermal analysis of various insulating materials and demonstrated that MLI achieves the lowest thermal conductivity when vacuum pressure remains below 10
−3 Pa, ensuring optimal insulation. Oh et al. [
52] established a numerical simulation model to investigate the relationship between evaporation behavior, heat flux, and vacuum pressure in LH storage tanks, confirming that the optimal vacuum pressure range is 10
−3–10
−4 Torr (0.133–0.013 Pa). Zeng et al. [
53] proposed a method for characterizing vacuum pressure in high-vacuum MLI tanks that functions even during pipeline operation without a dedicated gauge port. By correlating ambient temperature with the outer surface temperature of the external wall, they achieved indirect determination of interlayer vacuum level. Experimental validation confirmed that the outer surface temperature serves as a reliable indicator of internal vacuum pressure.
Wu et al. [
54] examined the effects of incorporating adsorbents into the vacuum interlayers of LH and liquefied natural gas (LNG) storage tanks. Among the candidate materials, SMS is regarded as the most cost-effective choice. As shown in
Table 3, it only requires 29.38% of the PdO cost and 32.06% of the AMG cost, while providing comparable insulation performance. For the pressurization process inside the tank, there is almost no difference among the three adsorbents in their original form. The performance loss of LH tanks and LNG tanks over 20 years is shown in
Figure 8. The performance of LH tanks improves significantly with an increase in adsorbent loading, mainly due to their lower storage temperature, which improves adsorption efficiency. Excessive addition of adsorbents does not yield linear improvements in insulation performance, since the dominant heat leakage mechanism under high-vacuum conditions is largely pressure-independent. Taking both adsorption efficiency and safety margins into account, the optimal SMS loading is 700–800 g for a 250 m
3 LH tank and 900–1000 g for a 250 m
3 LNG tank. Beyond the intrinsic performance and dosage of adsorbents, their spatial arrangement within the vacuum interlayer also affects the vacuum stability and insulation efficiency of cryogenic storage tanks. Furthermore, the interplay among adsorption characteristics, storage tank operating temperature, and hydrogen desorption rates must be comprehensively considered in both design and practical applications.
5. Key Challenges and Analysis
Cryogenic adsorbents, particularly those designed for low temperatures (~20 K), exhibit strong hydrogen adsorption performance; however, their efficiency declines significantly with increasing temperature. This temperature sensitivity results in hydrogen desorption during liquid hydrogen discharge or refueling, which compromises the vacuum insulation performance. This necessitates pairing with ambient temperature adsorbents, thereby increasing system complexity.
Metal oxides (e.g., PdO) offer high adsorption capacity but are costly, posing risks of exothermic reactions or even explosions during liquid hydrogen leaks. SMS are safe and low-cost, yet their adsorption capacity is limited. While non-evaporable getters (NEGs) provide rapid adsorption and are inexpensive, their high-temperature activation requirement and stringent process conditions significantly restrict their broader application in cryogenic environments.
Current adsorption performance testing primarily relies on liquid nitrogen cooling (≥77 K), which differs significantly from actual liquid hydrogen temperatures (~20 K). Cryogenic refrigerators can extend cooling to 4 K but involve complex equipment. Additionally, static volumetric methods used to evaluate adsorption capacity struggle to simulate the tank dead volume and gas distribution, leading to discrepancies between experimental results and real-world applications.
MOFs offer high surface area and structural tunability, which could make them ideal for hydrogen adsorption. However, the adsorption mechanisms at low temperatures, cycle stability, and cost-effectiveness of MOFs require further research. Moreover, the optimization of composite adsorbents, such as the combination of CuO with zeolites, depends on precise material ratios and processing techniques, but long-term operational data is still scarce, making it difficult to determine their practical feasibility.
6. Conclusions
LH, as a key pathway toward achieving the “dual-carbon” strategic goals, requires safe and efficient storage methods to support its large-scale deployment. This study investigates hydrogen adsorbents in the vacuum interlayer of LH tanks, analyzing the influence of residual gas loads on thermal insulation performance and adsorbent dosage, and further summarizing recent advances in adsorbent material types and their adsorption characteristics. Main conclusions are as follows:
Common hydrogen adsorbents used in LH tanks include cryogenic adsorbents (e.g., molecular sieves, activated carbon), metal oxides, ion-exchanged zeolitic molecular sieves, and NEGs. Current research focuses on the combined use of different adsorbents and modification strategies, such as metal doping, to enhance overall performance.
Cryogenic adsorbents are cost-effective, operationally safe, and exhibit excellent hydrogen adsorption capacity at approximately 20 K, making them among the most promising solutions for LH tanks. However, challenges remain in improving heat transfer with the inner vessel and mitigating reversible hydrogen desorption caused by physical adsorption. Metal oxides—PdO, Ag2O, and PtO2—offer high hydrogen adsorption capacity but are limited by high costs, while CuO requires heating for effective hydrogen adsorption. Silver molecular sieves, although lower in adsorption capacity, are inexpensive, display stable performance, and provide greater safety. NEGs demonstrate rapid hydrogen adsorption but require heating activation, and their application is constrained by processing conditions, restricting widespread domestic adoption.
Testing methods for adsorption performance under cryogenic and low-pressure conditions still rely mainly on the static constant-volume method. Adsorption chambers designed to mimic real storage tanks allow more realistic simulations of interlayer gas environments, though the testing process becomes more complex. While most experiments use liquid nitrogen cooling (≥77 K), cryogenic refrigeration-based systems extending to 4 K and below offer more accurate data under actual LH storage conditions.
7. Perspectives
Future research should prioritize the following directions:
Exploring novel hydrogen adsorption materials, particularly efficient and cost-effective candidates suitable for cryogenic vacuum tanks, including composite adsorbents, polymers, polyacetylenes, MOFs, and carbon nanotubes.
Establishing unified adsorption testing standards, developing experimental platforms based on cryogenic refrigeration systems, and constructing systematic databases to enable performance evaluation across diverse products and operating conditions.
Conducting investigations of hydrogen adsorbents under high-pressure conditions following LH leakage, with the aim of guiding the safe design of LH storage and transportation vessels.
Author Contributions
Conceptualization, M.Y., Y.W., J.W. and X.Y.; methodology, M.Y.; software; validation, M.Y., Y.W. and Y.Z.; formal analysis, M.Y., J.W. and X.Y.; investigation, Y.W., J.W., Y.Z. and X.Y.; resources, X.Y.; data curation, M.Y., Y.W., J.W., X.Y. and L.J.; writing—original draft preparation, Y.W.; writing—review and editing, M.Y.; visualization, Y.W.; supervision, L.J.; project administration, L.J.; funding acquisition, L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [the National Key Research and Development Program of China] grant number [No. 2024YFF0620002].
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This research was supported by the National Key Research and Development Program of China (No. 2024YFF0620002), the Science and Technology Project of Special Equipment Safety Supervision and Inspection Institute of Jiangsu Province (KJ(Y)202415) and “the Fundamental Research Funds for the Central Universities” (No. 226-2025-00076). During the preparation of this manuscript, the author utilized GPT-4 for language refinement. The author has reviewed and edited the generated content and assumes full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CuO | Copper oxide |
| LH | Liquid hydrogen |
| LNG | Liquefied natural gas |
| MLI | Multilayer insulation |
| HVMLI | High vacuum multilayer insulation |
| MOFs | Metal–organic frameworks |
| NEGs | Non-evaporable getters |
| PdO | Palladium oxide |
| SMS | Silver molecular sieves |
References
- Ding, Y.; Shao, D.L.; Jin, S.K.; Yu, M.; Wang, Y.B.; Jiang, L. Numerical Study on Composite Multilayer Insulation Material for Liquid Hydrogen Storage. Coatings 2024, 14, 1417. [Google Scholar] [CrossRef]
- An, R.; Luo, S.; Zhou, H.; Zhang, Y.; Zhang, L.; Hu, H.; Li, P. Effects of hydrogen-rich water combined with vacuum precooling on the senescence and antioxidant capacity of pakchoi (Brassica rapa subsp. Chinensis). Sci. Hortic. 2021, 289, 110469. [Google Scholar] [CrossRef]
- Meng, Z.; Jin, S.; Yu, M.; Mehari, A.; Jiang, L. Analysis of the Boss Structure of Type IV Composite Vessel for a High-Pressure Hydrogen Tube Trailer. Sustainability 2024, 16, 5098. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Pasha, A.A.; Khan, H.W.; Imteyaz, B.; Irshad, K. Sustainable and energy efficient hydrogen production via glycerol reforming techniques: A review. Int. J. Hydrogen Energy 2022, 47, 41397–41420. [Google Scholar] [CrossRef]
- Wu, J.K.; Chen, S.; Yu, M.; Zhang, X.J.; Jiang, L. Techno-economic analysis on low-temperature and high-pressure cryo-adsorption hydrogen storage. Fuel 2025, 381, 133532. [Google Scholar] [CrossRef]
- Wang, H.R.; Wang, B.; Pan, Q.W.; Wu, Y.Z.; Jiang, L.; Wang, Z.H.; Gan, Z.H. Modeling and thermodynamic analysis of thermal performance in self-pressurized liquid hydrogen tanks. Int. J. Hydrogen Energy 2022, 47, 30530–30545. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Li, R.; Shen, X.; Wu, Y.; Pan, Q.; He, Y.; Zhou, W.; Gan, Z. Thermodynamic analysis of the effect of initial ortho-hydrogen concentration on thermal behaviors for liquid hydrogen tanks. Int. J. Hydrogen Energy 2024, 55, 243–260. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y. Thermal physical performance in liquid hydrogen tank under constant wall temperature. Renew. Energy 2019, 130, 601–612. [Google Scholar] [CrossRef]
- Wang, B.; Luo, R.; Chen, H.; Zheng, C.; Gao, Y.; Wang, H.; Hashmi, A.R.; Zhao, Q.; Gan, Z. Characterization and monitoring of vacuum pressure of tank containers with multilayer insulation for cryogenic clean fuels storage and transportation. Appl. Therm. Eng. 2021, 187, 116569. [Google Scholar] [CrossRef]
- Fesmire, J.E.; Johnson, W.L. Cylindrical cryogenic calorimeter testing of six types of multilayer insulation systems. Cryogenics 2018, 89, 58–75. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Nemanič, V. An overview of methods to suppress hydrogen outgassing rate from austenitic stainless steel with reference to UHV and EXV. Vacuum 2003, 69, 501–512. [Google Scholar] [CrossRef]
- Long, L.L.; Cao, S.Y.; Jin, B.; Yuan, X.Q.; Han, Y.Y.; Wang, K. Construction of a Novel Fluorescent Probe for On-site Measuring Hydrogen Sulfide Levels in Food Samples. Food Anal. Methods 2019, 12, 852–858. [Google Scholar] [CrossRef]
- Hu, H.; Li, P.; Shen, W. Preharvest application of hydrogen-rich water not only affects daylily bud yield but also contributes to the alleviation of bud browning. Sci. Hortic. 2021, 287, 110267. [Google Scholar] [CrossRef]
- Wu, J.K.; Zhang, Y.X.; Yu, M.; Jiang, L. High-efficient boil-off gas storage using low-temperature activated carbon adsorption. Gas. Sci. Eng. 2025, 144, 205765. [Google Scholar] [CrossRef]
- Grinham, R.; Chew, A. A Review of Outgassing and Methods for its Reduction. Appl. Sci. Converg. Technol. 2017, 26, 95–109. [Google Scholar] [CrossRef]
- Ahmed, S.; Xin, H.; Faheem, M.; Qiu, B.J. Stability Analysis of a Sprayer UAV with a Liquid Tank with Different Outer Shapes and Inner Structures. Agriculture 2022, 12, 379. [Google Scholar] [CrossRef]
- Cha, S.-J.; Hwang, B.-K.; Kim, H.-T.; Lee, G.-H.; Lee, J.-P.; Kim, J.-H.; Lee, J.-M. Evaluating the effectiveness of double-shell vacuum insulation for liquefied hydrogen storage systems. Int. J. Hydrogen Energy 2025, 144, 8–18. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Z.; Hu, R.; Yang, Q.; Wang, S.; Lin, K. Realization of ultramicropore characterization of activated charcoal by cryogenic helium adsorption technique. Measurement 2025, 251, 117243. [Google Scholar] [CrossRef]
- He, C.; Yu, R.; Sun, H.; Chen, Z. Lightweight multilayer composite structure for hydrogen storage tank. Int. J. Hydrogen Energy 2016, 41, 15812–15816. [Google Scholar] [CrossRef]
- Fremerey, J.K. Residual gas: Traditional understanding and new experimental results. Vacuum 1999, 53, 197–201. [Google Scholar] [CrossRef]
- Hong, S.S.; Shin, Y.H.; Kim, J.T. Residual gas survey of stainless steel 304 extreme high vacuum chamber with hot cathode ionization gauge. Measurement 2008, 41, 1026–1031. [Google Scholar] [CrossRef]
- He, W.S.; Liu, Q.; Yu, H.; Si, X.J.; Zhang, J.K. Efficient Synthesis of Octacosanol Linoleate Catalyzed by Ionic Liquid and Its Structure Characterization. J. Am. Oil Chem. Soc. 2016, 93, 509–517. [Google Scholar] [CrossRef]
- Chen, S.; Wang, R.; Li, X. Experimental investigation and theoretical analysis on measurement of hydrogen adsorption in vacuum system. Int. J. Hydrogen Energy 2010, 35, 4347–4353. [Google Scholar] [CrossRef]
- Mata, C.; Hunter, R.; Peterson, A.; Mortensen, M. Study on the evacuation of gas in bulk-fill insulation materials used in large-scale LH2 storage tanks. Int. J. Hydrogen Energy 2025, 97, 1498–1506. [Google Scholar] [CrossRef]
- Xie, S.; Zhu, M.; Wang, R.; Ding, C. Adsorption performance of activated carbon in high vacuum multilayer insulation at liquid nitrogen temperature. Cryog. Supercond. 2012, 40, 12–15. [Google Scholar]
- Petitpas, C.M.; Turner, J.T.; Deeds, J.R.; Keafer, B.A.; McGillicuddy, D.J., Jr.; Milligan, P.J.; Shue, V.; White, K.D.; Anderson, D.M. PSP toxin levels and plankton community composition and abundance in size-fractionated vertical profiles during spring/summer blooms of the toxic dinoflagellate Alexandrium fundyense in the Gulf of Maine and on Georges Bank, 2007, 2008, and 2010: 2. Plankton community composition and abundance. Deep Sea Res. 2 Top. Stud. Ocean. 2014, 103, 350–367. [Google Scholar] [CrossRef]
- Abd, A.A.; Shamsudin, I.K.; Jasim, D.J.; Othman, M.R.; Kim, J. Hydrogen purification to fuel cell quality using pressure swing adsorption for CO2 separation over activated carbon molecular sieve: Experimental and dynamic modelling evaluation under non-isothermal condition. Mater. Today Sustain. 2024, 27, 100918. [Google Scholar] [CrossRef]
- He, X.; Zhu, M.; Chen, G. Study on isotherm distribution of low temperature and low pressure adsorption of low temperature adsorbent. China Spec. Equip. Saf. 2021, 37, 18–23. [Google Scholar]
- Ikemoto, N.; Kawakami, T.; Yonehara, K.; Natori, Y.; Tatenuma, K.; Hara, M. Adsorption of hydrogen and deuterium on A-type zeolites at 77 K after various heat treatments. Fusion Eng. Des. 2020, 158, 111701. [Google Scholar] [CrossRef]
- Boffito, C.; Schiabel, A.; Gallitognotta, A. Thermally Insulating Jacket and Related Process. U.S. Patent No. 3114469, 25 April 1995. [Google Scholar]
- Francis, H.C.A.W. Means for Improving Thermal Insulation Space. U.S. Patent No. 3114469A, 25 December 1963. [Google Scholar]
- Chae, H.K.; Siberio-Perez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef]
- Liu, L. Study on Vacuum Hydrogen Adsorption Performance of MLF-101(Cr) and Its Application in Liquid Hydrogen Tank Container. Master’s Thesis, Zhejiang University, Hangzhou, China, 2024. [Google Scholar]
- Belousov, V.M.; Vasylyev, M.A.; Lyashenko, L.V.; Vilkova, N.Y.; Nieuwenhuys, B.E. The low-temperature reduction of Pd-doped transition metal oxide surfaces with hydrogen. Chem. Eng. J. 2003, 91, 143–150. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, Y.; Huang, Q.; Li, X.; Gu, C.; Zhang, B.; Yao, X.; Li, Z.; Hao, C. Experimental study on the sorption performance of New–Fashioned silver getters compared with the traditional PdO in High–Vacuum multi–layer insulation vessels. Vacuum 2023, 208, 111685. [Google Scholar] [CrossRef]
- Wang, J.; Zhan, Y.; Wei, W.; Chen, S.; Wang, R. A new cost effective composite getter for application in high-vacuum-multilayer-insulation tank. Vacuum 2016, 131, 44–50. [Google Scholar] [CrossRef]
- Wang, J.; Zhan, Y.; Wang, W.; Wang, R. Optimization and performance of highly efficient hydrogen getter applied in high vacuum multilayer insulation cryogenic tank. Vacuum 2018, 149, 87–92. [Google Scholar] [CrossRef]
- Chen, S.P.; Bai, B.K.; Cheng, Y.J. Experimental study on hydrogen absorption characteristics and microstructure of platinum oxide under vacuum. Cryogenics 2020, 6, 26–32. [Google Scholar]
- Chen, S.; Li, X.; Wang, R.; Xie, G.; Zeng, Y. Experimental investigation on hydrogen adsorption performance of composite adsorbent in the tank with high vacuum multilayer insulation. Vacuum 2009, 83, 1184–1190. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, M.; Huang, Q.; Huang, L.; Dong, C.; Chen, S. Sorption properties of novel-fashioned low-cost hydrogen getters in a high-vacuum-multilayer insulation structure. Vacuum 2021, 192, 110452. [Google Scholar] [CrossRef]
- He, Y.X.; Liu, L.; Pan, Q.W.; Xiong, Z.Y.; Lu, H.; Shen, X.; Wang, B.; Zhou, W.M.; Gan, Z.H. Performance characterization and comparison study of silver molecular sieve hydrogen adsorbents in liquid hydrogen vessels. Vacuum 2024, 221, 112917. [Google Scholar] [CrossRef]
- Kambondo, A.; Wang, J.; Yigit, K.; Si, Q.; Qin, Y.; Su, Y.; Zhang, R.; Wu, H.; Liang, C.; Wang, S. Hydrogen adsorption mechanism on non-evaporable getter ternary alloy Ti-V-Nb surface. Vacuum 2025, 238, 114203. [Google Scholar] [CrossRef]
- Feng, T.; Cheng, Y.; Chen, L.; Xi, Z.; Zhu, J.; Li, Y. Hydrogen adsorption characteristics of Zr57V36Fe7 non-evaporable getters at low operating temperatures. Vacuum 2018, 154, 6–10. [Google Scholar] [CrossRef]
- Londer, H.; Myneni, G.R.; Adderley, P.; Bartlok, G.; Knapp, W.; Schleussner, D.; Ogris, E. New high capacity getter for vacuum insulated mobile LH2 storage tank systems. Vacuum 2007, 90, 60. [Google Scholar] [CrossRef]
- Boffito, C.; Doni, F.; Ferrario, B. Getter Materials for the Vacuum Insulation of Liquid Hydrogen Storage Vessels or Transport Lines. U.S. Patent No. 5,678,724, 21 October 1997. [Google Scholar]
- Okseniuk, I.I.; Litvinov, V.O.; Shevchenko, D.I.; Vasilenko, R.L.; Bogatyrenko, S.I.; Bobkov, V.V. Hydrogen interaction with Zr-based getter alloys in high vacuum conditions: In situ SIMS-TPD studies. Vacuum 2022, 197, 110861. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liu, J.Y.; Liu, X.F.; Shang, J.X.; Yu, R.H.; Shui, J.L. Non-classical hydrogen storage mechanisms other than chemisorption and physisorption. Appl. Phys. Rev. 2022, 9, 021315. [Google Scholar] [CrossRef]
- Chen, L.-b.; Kong, C.-h.; Wu, X.-l.; Zhou, Y.; Wang, J.-j. Specific heat capacities and flow resistance of an activated carbon with adsorbed helium as a regenerator material in refrigerators. New Carbon Mater. 2018, 33, 47–52. [Google Scholar] [CrossRef]
- Xi, X.-t.; Wang, J.; Chen, L.-b.; Guo, L.-n.; Yang, B.; Zhou, Y.; Wang, J.-j. Adsorption characteristics of helium on an activated carbon at 4–10 K and its prospective application in 4 K-class regenerative cryocoolers. New Carbon Mater. 2019, 34, 524–532. [Google Scholar] [CrossRef]
- Grądziel, S.; Edward, L.; Filip, L.; Łopata, S.; Sobota, T.; Zima, W. Influence of vacuum level on insulation thermal performance for LNG cryogenic road tankers. MATEC Web Conf. 2018, 240, 4. [Google Scholar] [CrossRef]
- Oh, S.J.; Kwon, J.; Jeon, K.S.; Yoon, J.H. A numerical analysis study on the characteristics of evaporation in liquid hydrogen tank with vacuum layer according to changes in heat flux and vacuum pressure. Int. J. Hydrogen Energy 2024, 50, 542–557. [Google Scholar] [CrossRef]
- Zeng, Q.F.; Jiang, H.; Liu, Q.; Li, G.K. A Method for Measuring Interlayer Vacuum Degree of the Liquified Natural Gas Vacuum Multi-layer Insulation Pipe by Temperatures. Instrum. Exp. Tech. 2022, 65, 818–825. [Google Scholar] [CrossRef]
- Wu, J.K.; Xie, R.Y.; Yu, M.; Luo, C.Y.; Wang, B.; Zhang, X.J.; Jiang, L. Techno-economic analysis on the performance of hydrogen adsorbents in the vacuum layer of cryogenic liquid storage tank. Int. J. Hydrogen Energy 2024, 88, 132–141. [Google Scholar] [CrossRef]
- Liu, Y.n.; Chen, S.; Zhao, B.; Shi, Q.; Liu, J.; Yu, Y. Synthesis of novel graphene-based targeted hydrogen getter nanocomposites and their properties. Vacuum 2023, 213, 112096. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, M.; Zhou, Y.; Dong, X.; Shen, J. Thermodynamics analysis of hydrogen storage based on compressed gaseous hydrogen, liquid hydrogen and cryo-compressed hydrogen. Int. J. Hydrogen Energy 2019, 44, 16833–16840. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).