Undoped Polybenzimidazole Membranes Composited with CeP5O14 for Use in Hydrogen Fuel Cells at 200 °C
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of CUP
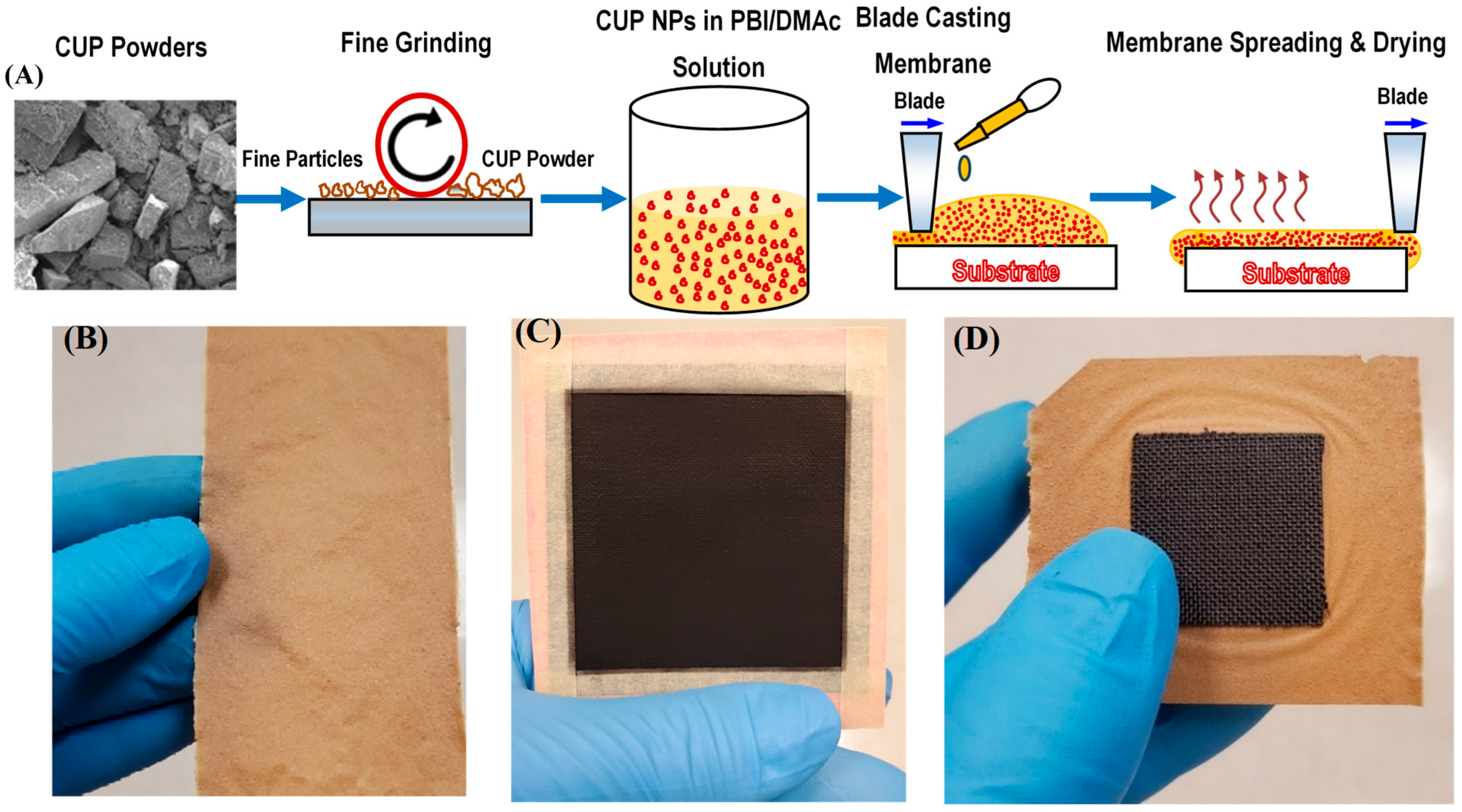
2.3. Solution-Casting of PIC Membranes
2.4. Water Uptake Testing
2.5. Preparation of GDEs and IT-MEAs
2.6. Structural Characterization of GDEs
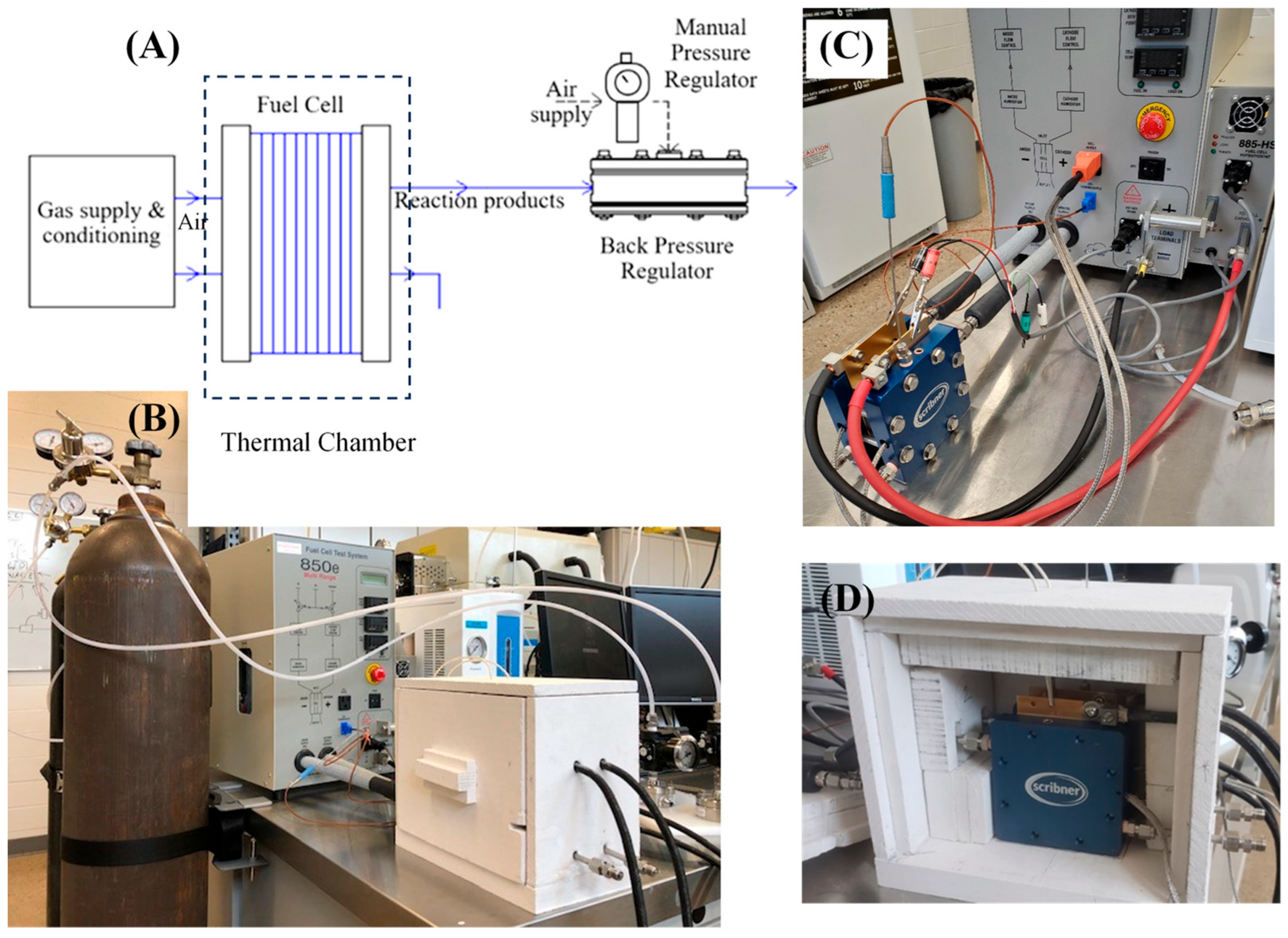
2.7. Single-Stack Hydrogen Fuel Cell Testing
3. Results and Discussion
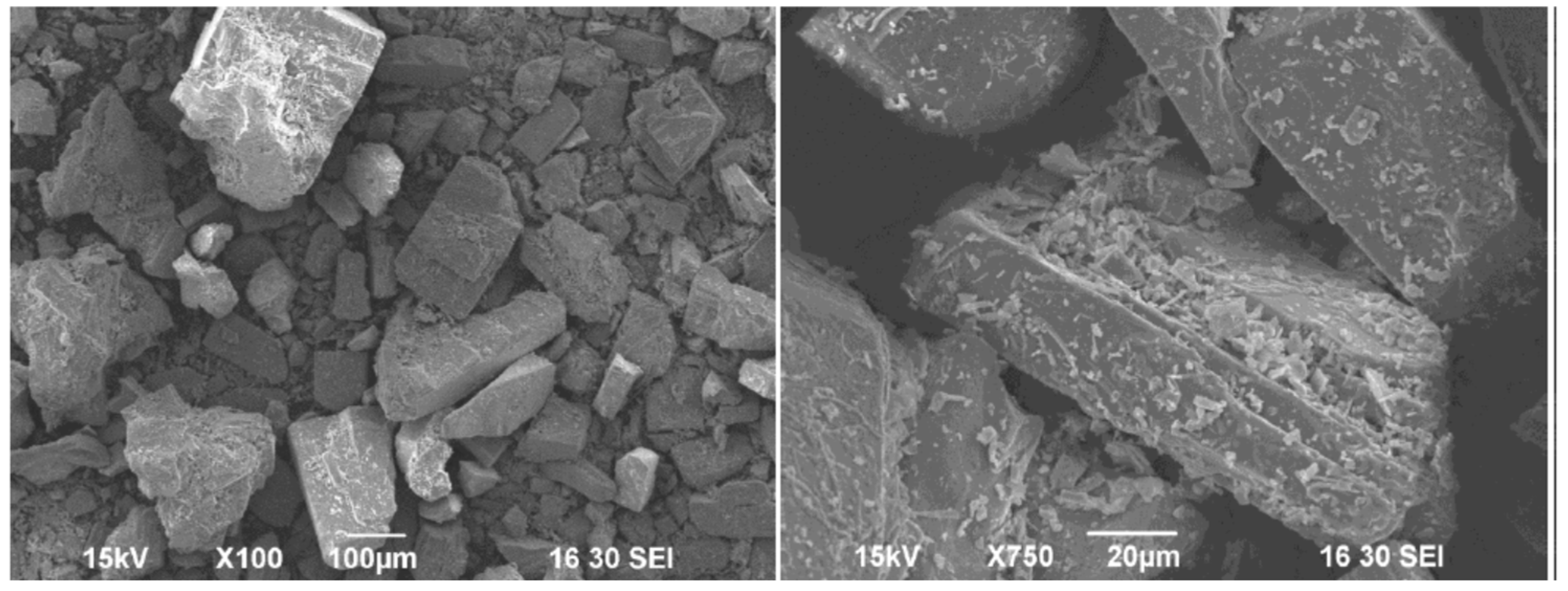
3.1. CUP Particle Size Characterization
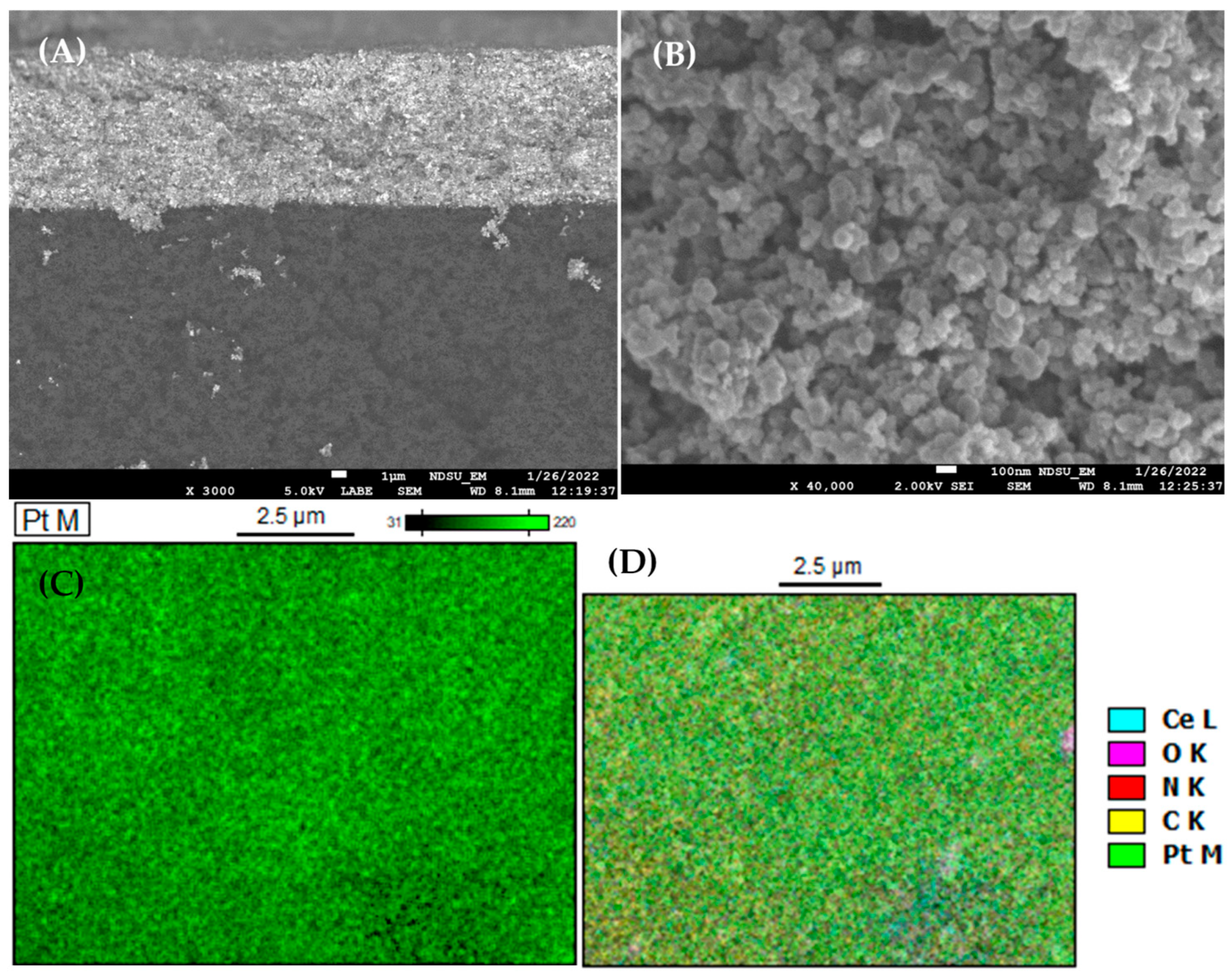
3.2. GDE Characterization
3.3. Proton Conductivity Measurements of IT-MEAs Made of CUP-PBI PIC PEMs
3.4. Water Absorption of the CUP-PBI PIC PEMs
3.5. Fuel Cell Performance Characterization
3.5.1. Membrane Thickness Effect
3.5.2. Effect of Spraying CUP Particles onto Membrane
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors. Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef]
- Singla, M.K.; Nijhawan, P.; Oberoi, A.S. Hydrogen fuel and fuel cell technology for cleaner future: A review. Environ. Sci. Pollut. Res. 2021, 28, 15607–15626. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.P.; Li, Q. Introduction to Fuel Cells: Electrochemistry and Materials; Springer: New York, NY, USA, 2022. [Google Scholar]
- Zeis, R.; Beilstein, J. Materials and characterization techniques for high-temperature polymer electrolyte membrane fuel cells. Nanotechnology 2015, 6, 68–83. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef]
- Ying, J.; Liu, T.; Wang, Y.; Guo, M.; Shen, Q.; Lin, Y.; Yu, J.; Yu, Z. Perspectives on membrane development for high temperature proton exchange membrane fuel cells. Energy Fuels 2024, 38, 6613–6643. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef]
- Sun, W.; Sahin, N.E.; Sun, D.; Wu, X.; Munoz, C.; Thakare, J.; Aulich, T.; Zhang, J.; Hou, X.; Oncel, N.; et al. One-pot synthesis of ruthenium-based nanocatalyst using reduced graphene oxide as matrix for electrochemical synthesis of ammonia. ACS Appl. Mater. Interfaces 2022, 15, 115–1128. [Google Scholar] [CrossRef]
- Kilner, J.A.; Burriel, M. Materials for intermediate-temperature solid-oxide fuel cells. Annu. Rev. Mater. Res. 2014, 44, 365–393. [Google Scholar] [CrossRef]
- Steininger, H.; Schuster, M.; Kreuer, K.D.; Kaltbeitzel, A.; Bingöl, B.; Meyer, W.H.; Schauff, S.; Brunklaus, G.; Spiess, H.W. Intermediate temperature proton conductor for PEM fuel cells based phosphoric acid as protogenic group: A progress report. Phys. Chem. Chem. Phys. 2007, 9, 1764–1773. [Google Scholar] [CrossRef]
- Li, Q.; Jensen, J.Q.; Savinell, R.F.; Bjerrum, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef]
- Authayanun, S.; Im-orb, K.; Arpornwichanop, A. A review of the development of high temperature proton exchange membrane fuel cells. Chin. J. Catal. 2015, 36, 473–483. [Google Scholar] [CrossRef]
- Li, Q.; Aili, D.; Hjuler, H.A.; Jensen, J.O. High Temperature Polymer Electroltye Membrane Fuel Cells: Approaches, Status, and Perspectives; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Zhou, Z.; Zholobko, O.; Wu, X.F.; Aulich, T.; Thakare, J.; Hurley, J. Polybenzimidazole-based polymer electrolyte membranes for high-temperature fuel cells: Current status and prospects. Energies 2021, 14, 135. [Google Scholar] [CrossRef]
- Scott, K.; Xu, C.; Wu, X. Intermediate temeprature proton-exchange memrbane electrolyte for fuel cells. WIREs Energy Environ. 2014, 3, 24–41. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, R.; Chang, Z.; Fang, Z.; Zhu, Z.; Xu, C. Electrolyte membranes for intermediate temeprature proton exchange membrane fuel cell. Prog. Nat. Sci. Mater. Int. 2020, 30, 743–750. [Google Scholar] [CrossRef]
- Hibino, R.T. Intermediate-tempeature proton conductors and their applications to energy and environmental devices. J. Ceram. Soc. Jpn. 2011, 119, 677–686. [Google Scholar] [CrossRef]
- Jin, Y.; Shen, Y.; Hibino, T. Proton conduction in metal pyrophospates (MP2O7) at intermediate tempeatures. J. Mater. Chem. 2010, 20, 6214–6217. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Mo, S.; Du, L.; Huang, Z.; Chen, J.; Zhou, Y.; Wu, P.; Meng, L.; Wang, N.; Xing, L.; Zhao, M.; et al. Recent advances on PEM fuel cells: From key materials to membrane electrode assembly. Electrochem. Energy Rev. 2023, 6, 28. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Wang, M.; Su, C.; Zhu, Z.; Wang, H.; Ge, L. Composite cathodes for protonic ceramic fuel cells: Ratinales and materials. Compos. Part B Eng. 2022, 238, 109881. [Google Scholar] [CrossRef]
- Büchi, F.N.; Inaba, M.; Schimidt, J.J. Polymer Electrolyte Fuel Cell Durability; Springer: New York, NY, USA, 2009. [Google Scholar]
- Lin, K.; Wang, C.; Qiu, Z.; Yan, Y. Enhancement of proton conductivity performance in high temperature polymer electrolyte membrane, processed the adding of pyridobismidazole. Polymers 2022, 14, 1283. [Google Scholar] [CrossRef] [PubMed]
- Anfimova, T. Metal Phosphates as Proton Conducting Materials for Intermediate Temperature Fuel Cell and Electrolyser Applications. Ph.D. Thesis, Technical University of Denmark, Copenhaken, Denmark, 2014. [Google Scholar]
- Salam, A.; Zholobko, O.; Wu, X.F. Roles of functionalized nanoparticles in the performance improvement of proton-exchange membranes used in low- and intermediate-temperature hydrogen fuel cells: A review. Prog. Nat. Sci. Mater. Int. 2024, 34, 437–453. [Google Scholar] [CrossRef]
- Le, M.V.; Tsai, D.S.; Yang, C.Y.; Chung, W.H.; Lee, H.Y. Proton conductors of cerium pyrophosphate for intermeidate temperature fuel cell. Electrochim. Acta 2011, 56, 6654–6660. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Y.; Zhao, X.; Tang, W.; Yang, S.; Zhong, S.; Wei, M. Effects of cerium doping on the performance of LSCF cathodes for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2018, 43, 18946–18954. [Google Scholar] [CrossRef]
- Shirbhate, S.C.; Singh, K.; Acharya, S.A.; Yadav, A.K. Review on local structural properties of ceria-based electrlytes for IT-SOFC. Ionics 2017, 23, 1049–1057. [Google Scholar] [CrossRef]
- Bhabu, K.A.; Theerthagiri, J.; Madhavan, J.; Balu, T.; Rajasekaran, T.R. Superior oxide ion conductivity of novel acceptor doped cerium oxide electrolytes for intermediate-temperature solid oxide fuel cell applications. J. Phys. Chem. C 2016, 120, 18452–18461. [Google Scholar] [CrossRef]
- Huang, J.; Mao, Z.; Liu, Z.; Wang, C. Development of novel low-telperature SOFCs co-ionic conducting SDC-carbonate composite elecrolytes. Electrochem. Commun. 2007, 9, 2601–2605. [Google Scholar] [CrossRef]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602. [Google Scholar] [CrossRef]
- Zhang, J.; Lenser, C.; Menzler, N.H.; Guillon, O. Comparison of solid oxide fuel cell (SOFC) electrolyte materials for operation at 500 °C. Solid State Ionics 2020, 344, 115138. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.H. Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices. Energy Sci. Eng. 2021, 9, 984–1011. [Google Scholar] [CrossRef]
- Ahmed, S.; Kazmi, W.W.; Hussain, A.; Khan, M.Z.; Bibi, S.; Saleem, M.; Song, R.H.; Sajid, Z.; Ullah, A.; Khan, M.K. Facile and low-temperature synthesis approach to fabricate Sm0.5Sr0.5CoO3-δ cathode material for solid oxide fuel cell. J. Korean Ceram. Soc. 2023, 60, 272–282. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Z.; Akbar, M.; Gao, C.; Dong, W.; Meng, Y.; Jin, X.; Xia, C.; Wang, B.; Zhu, B.; et al. Efficiently enhance the proton conductivity of YSZ-based electrolyte for low temperature solid oxide fuel cell. Ceram. Int. 2023, 49, 5637–5645. [Google Scholar] [CrossRef]
- Desta, H.G.; Gebreslassie, G.; Zhang, J.; Lin, B.; Zheng, Y.; Zhang, J. Enhancing performance of low-tempeature solid oxide fuel cell cathodes through surface engineering. Prog. Mater. Sci. 2025, 147, 101353. [Google Scholar] [CrossRef]
- Younis; Chu, D.; Li, D.S. Cerium oxide nanostructures and their applications. In Functionalized Nanomaterials; Farrukh, M.A., Ed.; InTech: Rijeka, Croatia, 2016; pp. 53–68. [Google Scholar]
- Trovarelli, A.; Fornasiero, P. Catalysis by Ceria and Related Mateials, 2nd ed.; Imperial College Press: London, UK, 2013. [Google Scholar]
- Scire, S.; Palmisano, L. Cerium oxide (CeO2): Synthesis, Properties and Applications (Metal Oxides), 1st ed.; Elsevier: New York, NY, USA, 2019. [Google Scholar]
- Das, S.; Bhaskar, R.; Narayanan, K.B. Multifunctional applications of gadolinium-doped cerium oxide (Ce1–xGdxO2–∂) ceramics: A review. J. Rare Earth 2024, 42, 1817–1834. [Google Scholar] [CrossRef]
- Shirpour, M.; Gregori, G.; Merkle, R.; Maier, J. On the proton conductivity in pure and gadolinium doped nanocrystalline cerium oxide. Phys. Chem. Chem. Phys. 2011, 13, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Gregori, G.; Shirpour, M.; Maier, J. Proton conduction in dense and porous nanocrystalline ceria thin films. Adv. Funct. Mater. 2013, 23, 5861–5867. [Google Scholar] [CrossRef]
- Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; del Castillo, L.F.; Compañ, V. Recent progress in the development of composite membranes based on polybenzimidazole for high temperature proton exchange membrane (PEM) fuel cell applications. Polymers 2020, 12, 1861. [Google Scholar] [CrossRef]
- Zholobko, O.; Hurley, J.; Wu, X.F.; Aulich, T.; Thakare, J. Intermediate-temperature proton exchange membranes based on cerium ultraphosphate composited with polybenzimidazole. J. Electrochem. Soc. 2022, 169, 94505. [Google Scholar] [CrossRef]
- Aulich, T.R.; Thakare, J.; Hurey, J.; Wu, X.; Zhou, Z.; Zholobko, O. Proton-Exchange Membrane. U.S. Patent No. 1190582, 19 February 2024. [Google Scholar]
- Cheng, Y.; Zhang, J.; Lu, S.; Kuang, H.; Bradley, J.; De Marco, R.; Aili, D.; Li, Q.; Cui, C.Q.; Jiang, S.P. High CO tolerance of new SiO2 doped phosphoric acid/polybenzimidazole polymer electrolyte membrane fuel cells at high temperatures of 200–250 °C. Int. J. Hydrogen Energy 2018, 43, 22487–22499. [Google Scholar] [CrossRef]
- Zhang, J.; Aili, D.; Lu, S.; Li, Q.; Jiang, S.P. Advancement toward polymer electrolyte membrane fuel cells at elevated temperatures. Research (AAAS SPJ) 2020, 2020, 9089405. [Google Scholar] [CrossRef]
- Cong, S.; Wang, J.; Wang, Z.; Liu, X. Polybenzimidazole (PBI) and benzimidazole-linked polymer (BILP) membranes. Green Chem. Eng. 2021, 2, 44–56. [Google Scholar] [CrossRef]
- Tang, H.; Geng, K.; Aili, D.; Ju, Q.; Pan, J.; Chao, G.; Yin, X.; Guo, X.; Li, Q.; Li, N. Low Pt loading for high-performance fuel cell electrodes enabled by hydrogen-bonding microporous polymer binders. Nat Commun. 2022, 13, 7577. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, A.; Hack, J.; Stocker, R.; Suter, T.A.M.; Rettie, A.J.E.; Brett, D.J.L. Challenges and opportunities for characterisation of high-temperature polymer electrolyte membrane fuel cells: A review. J. Mater. Chem. A 2024, 12, 8014–8064. [Google Scholar] [CrossRef]
- Zholobko, O.; Wu, X.F.; Zhou, Z.; Aulich, T.; Thakare, J.; Hurley, J. A comparativev experimental study of the hydroscopic and mechanical behaviors of electrospun membranes and solution-cast films of polybenzimidazole. J. Appl. Polym. Sci. 2020, 137, 49639. [Google Scholar] [CrossRef]
- Yan, X.; Hou, M.; Sun, L.; Liang, D.; Shen, Q.; Xu, H.; Ming, P.; Yi, B. AC impedance characteristics of a 2 kW PEM fuel cell stack under different operating conditions and load changes. Int. J. Hydrogen Energy 2007, 32, 4358–4364. [Google Scholar] [CrossRef]
- Park, K.T.; Jung, U.H.; Choi, D.W.; Chun, K.; Lee, H.M.; Kim, S.H. ZrO2–SiO2/Nafion® composite membrane for polymer electrolyte membrane fuel cells operation at high temperature and low humidity. J. Power Sources 2008, 177, 247–253. [Google Scholar] [CrossRef]
- Kawahara, M.; Rikukawa, M.; Sanui, K. Relationship between absorbed water and proton conductivity in sulfopropylated poly(benzimidazole). Polym. Adv. Technol. 2000, 11, 544–547. [Google Scholar] [CrossRef]
- He, R.; Li, Q.; Xiao, G.; Bjerrum, N.J. Proton conductivity of phosphoric acid doped polybenzimidazole and its composites with inorganic proton conductors. J. Membr. Sci. 2003, 226, 169–184. [Google Scholar] [CrossRef]
- Baranov, A.I.; Khiznichenko, V.P.; Sandler, V.A.; Shuvalov, L.A. Frequency dielectric dispersion in the ferroelectric and superionic phases of CsH2PO4. Ferroelectrics 1988, 81, 183–186. [Google Scholar] [CrossRef]
- Louie, M.W.C. Electrocatalysis in Solid Acid Fuel Cells. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 2011. [Google Scholar]
- Colomban, P.; Novak, A. Proton conductors: Classification and conductivity. In Proton Conductors: Solids, Membranes and Gels—Materials and Devices; Colomban, P., Ed.; Cambridge University: Cambridge, UK, 1992. [Google Scholar]
- Nagao, M.; Kamiya, T.; Heo, P.; Tomita, A.; Hibino, T.; Sano, M. Proton conduction in In3+-doped SnP2O7 at intermediate temperatures. J. Electrochem. Soc. 2006, 153, A1604–A1609. [Google Scholar] [CrossRef]
- Heo, P.; Nagao, M.; Kamiya, T.; Sano, M.; Tomita, A.; Hibino, T. Sn0.9 In0.1P2O7-based organic/inorganic composite membranes. J. Electrochem. Soc. 2007, 154, B63–B67. [Google Scholar] [CrossRef]
- Sun, X.; Wang, S.; Wang, Z.; Ye, X.; Wen, T.; Huang, F. Proton conductivity of CeP2O7 for intermediate temperature fuel cells. Solid State Ion 2008, 179, 1138–1141. [Google Scholar] [CrossRef]
- Averbuch-Pouchot, M.T.; Durif, A. Topics in Phosphate Chemistry; World Scientific: Singapore, 1996. [Google Scholar]
- Luo, X.; Lau, G.; Tesfaye, M.; Arthurs, C.R.; Cordova, I.; Wang, C.; Yandrasits, M.; Kusoglu, A. Thickness dependence of proton-exchange-membrane properties. J. Electrochem. Soc. 2021, 168, 104517. [Google Scholar] [CrossRef]
- Mohanty, S.; Desai, A.N.; Singh, S.; Ramadesigan, V. Effects of the membrane thickness and ionomer volume fraction on the performance of PEMFC with U-shaped serpentine channel. Int. J. Hydrogen Energy 2021, 46, 20650–20663. [Google Scholar] [CrossRef]
- Oh, Y.; Kim, S.K.; Peck, D.H.; Jung, D.H.; Shul, Y. Effects of membranes thickness on performance of DMFCs under freeze-thaw cycles. Int. J. Hydrogen Energy 2014, 39, 15760–15765. [Google Scholar] [CrossRef]
- Seo, S.H.; Lee, C.S. The effects of membrane thickness on the performance and impedance of the direct methanol fuel cell. Proc. Inst. Mech. Eng. C 2010, 224, 2211–2221. [Google Scholar] [CrossRef]


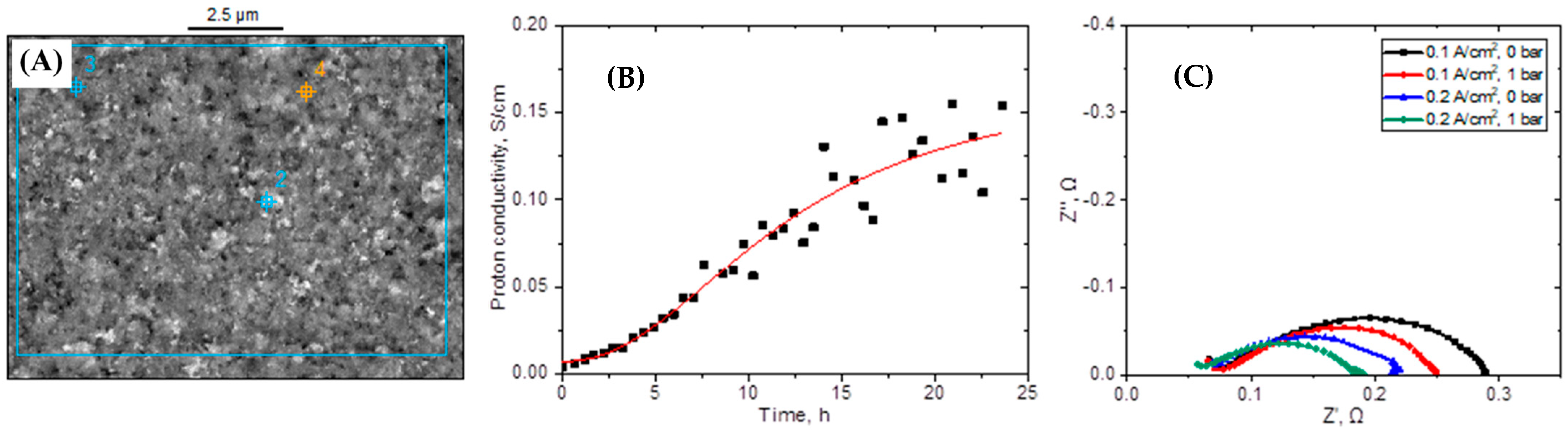

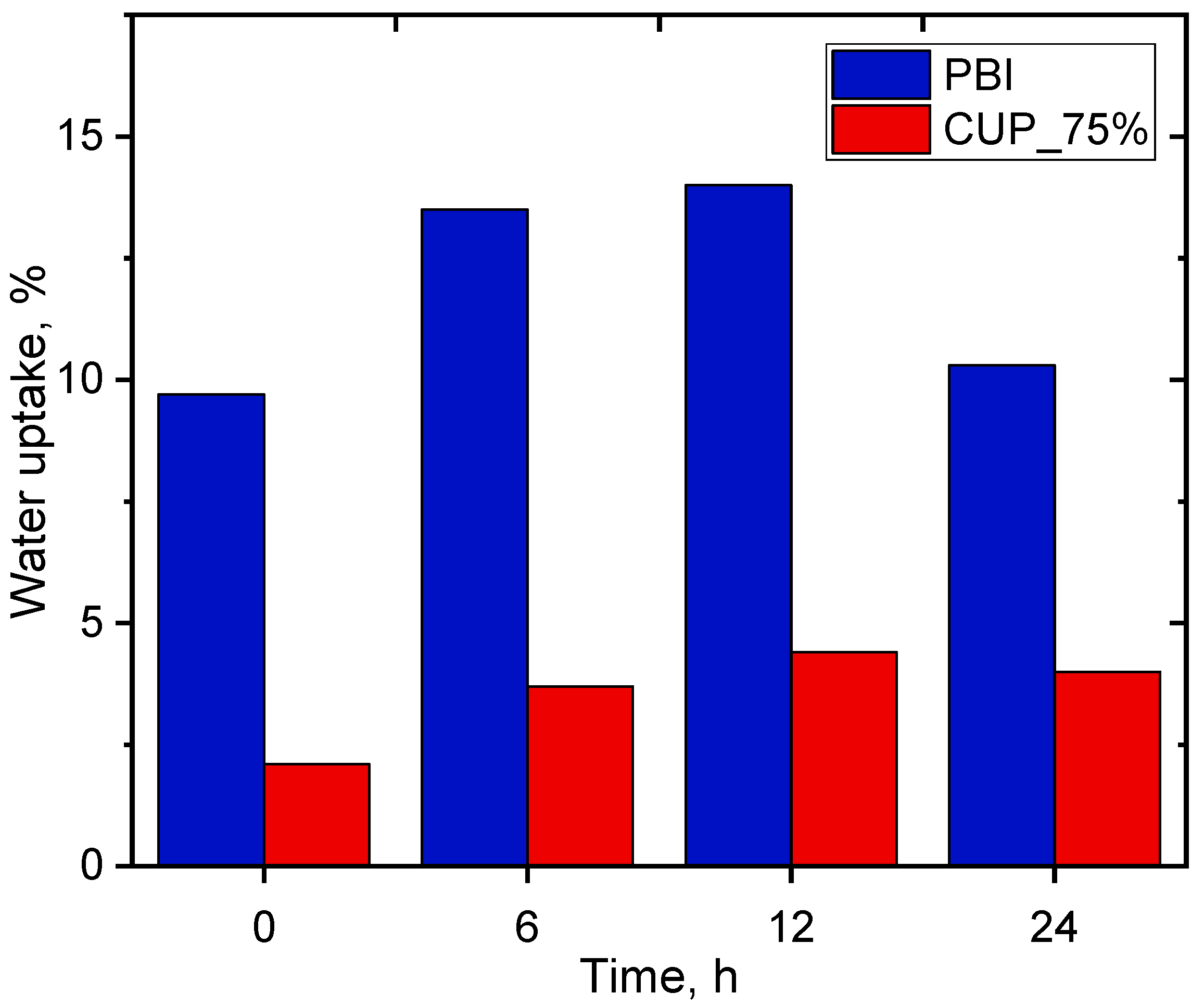
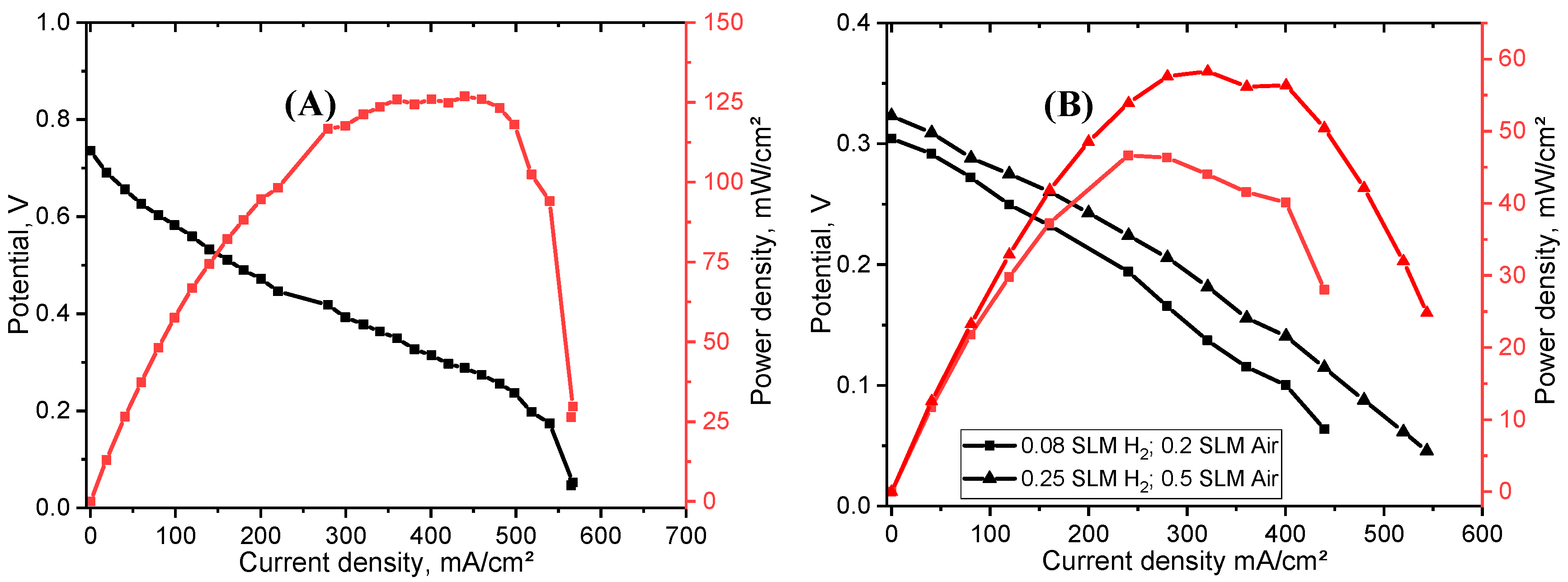
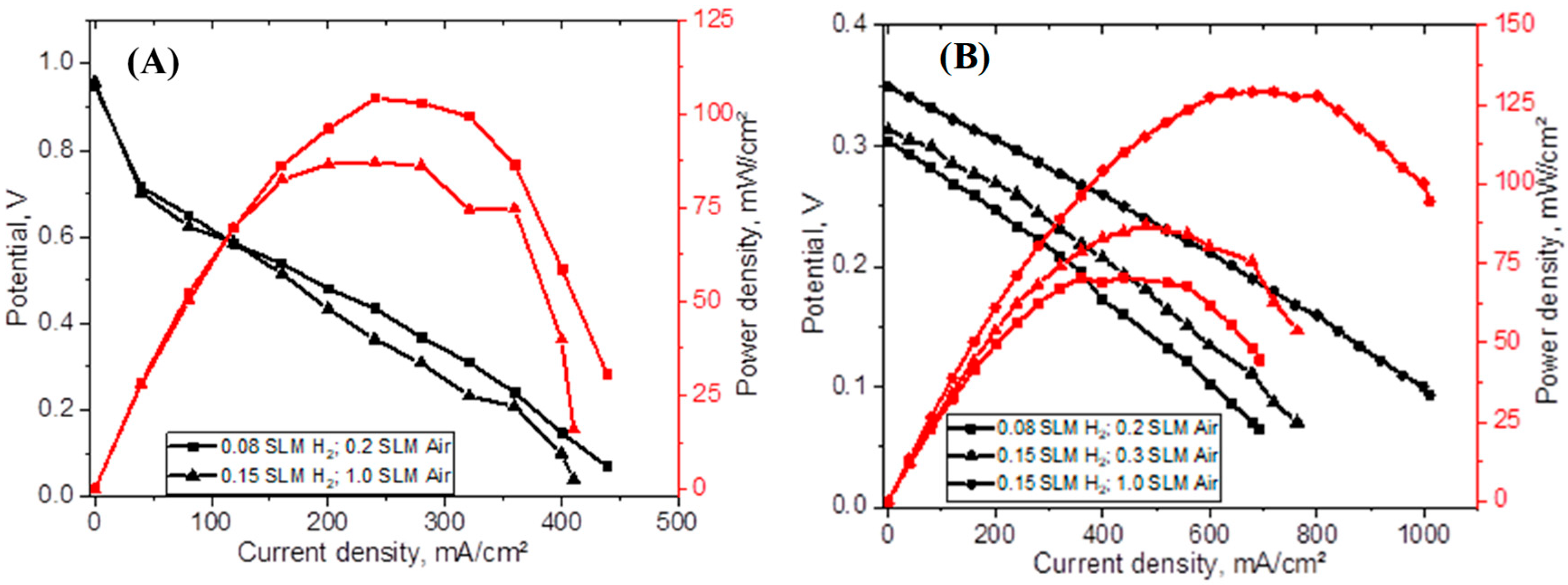
| LT PEMFCs | IT Fuel Cells | HT Fuel Cells | |
|---|---|---|---|
| Work Temperature | 50–100 °C | 150–700 °C (e.g., IT-SOFCs, PAFCs †) | 600–1000 °C (e.g., SOFCs, MCFCs ‡) |
| Electrolytes | ● Polymer electrolyte membranes (e.g., Nafion®). ● Aqueous alkaline solutions (e.g., KOH), etc. | ● H3PO4 for PAFCs. ● Metal phosphates, e.g., CsH2PO4, Zr(HPO4)2.nH2O, metal pyrophosphates (CeP2O7, Ti P2O7, Sn2O7); CsHSO4, etc. ● Heteropolyacids, e.g., H3PW12O40, H4SiW12O40, H4GeW12O40, H6P2W18O62, H5BW12O40, H6CoW12O40, H3PMo12O40, etc. ● Ceramic materials, e.g., doped ceria (CeO2) or stabilized zirconia (ZrO2), etc. | ● Ceramic materials, e.g., yttria (Y2O3)-stabilized zirconia (YSZ). ● Molten carbonate salts, e.g., Li2CO3/K2CO3 mixture. |
| Advantages | ● Fast start-up time and rapid dynamic response, suitable for mobile and portable applications, e.g., vehicles, personal electronics, etc. ● High power density, compact size. ● Zero-emissions. | ● Higher tolerance to fuel impurities, e.g., CO, H2S, etc., compared to LT PEMFCs. ● High energy efficiency via recovering waste heat for combined heat and power (CHP) systems. ● High energy conversion rate due to faster reaction kinetics than LT PEMFCs. ● Simpler water and thermal management than LT PEMFCs. ● Lower degradation rates compared to SOFCs. ● Use of low-loading Pt or non-Pt-group catalysts. ● Suitable for hydrogen and direct liquid fuel cells, e.g., methanol, ethanol, etc. | ● Fuel flexibility, suitable to use a wide variety of fuels, e.g., natural gas, biogas, liquid fuels, etc., through internal reforming. ● Noble metal catalysts are not required, leading to lower material costs. ● Not vulnerable to CO poisoning. ● High electrical efficiency up to 60–65%. ● High-quality waste heat for CHP. |
| Disadvantages | ● Complex and costly water/thermal management. ● High costs due to use of expensive noble metal catalysts, e.g., Pt, and high-purity H2. ● High sensitivity to CO poisoning. | ● Slower start-up than LT PEMFCs. ● Materials degradation at higher temperatures, existing a trade-off between efficiency and component degradation. | ● Very slow start-up. ● Request expensive and heat-resistant materials. ● Significant challenges with thermal expansion and system sealing. |
| Challenges | ● High cost of catalysts. ● Long-term durability. ● Building a costly hydrogen infrastructure of production, delivery, and storage. | ● Developing new IT cathode materials with faster kinetics. ● Degradation mitigation. ● Improvement of long-term stability. | ● Material degradation at HT. ● Thermal stress management. ● Complex system design. |
| CUP Powder Samples | Fractions | Average Size (µm) |
|---|---|---|
| As-synthesized raw CUP powder | Initial | 16.18 ± 5.54 2.4 ± 0.9 † 0.3 ± 0.1 ‡ |
| After 2 h grinding using mortar and pestle | Large | 19.3 ± 5.7 2.1 ± 0.9 |
| Medium | 7.5 ± 2.6 0.3 ± 0.1 | |
| Small | 2.0 ± 0.6 0.3 ± 0.1 |
| Particle Fractions | Large | Medium | Small | Total |
|---|---|---|---|---|
| Mass (g) | 3.1379 | 0.7609 | 0.7996 | 4.6984 |
| Mass Percent (%) | 66.8 | 16.2 | 17.0 | 100 |
| Element | C | N | O | P | Ce | Pt |
|---|---|---|---|---|---|---|
| Wt. % | 11.18 ± 0.82 | 0.62 ± 0.14 | 3.75 ± 0.77 | 1.23 ± 1.20 | 0.79 ± 0.89 | 81.40 ± 1.99 |
| Sample Specification | Membrane Thickness (μm) | Proton Conductivity (S/cm) |
|---|---|---|
| CUP_75%_82 µm † | 82 | 0.048 |
| CUP_75%_134 µm | 134 | 0.063 |
| CUP_75%_sprayed_166 µm ‡ | 166 | 0.046 |
| CUP_75%_sprayed_105 µm | 105 | 0.105 |
| CUP_75%_spr_2 h_94 µm * | 94 | 0.051 |
| CUP_75%_spr_2 h_95 µm (0.5 mg/cm2 Pt/C) | 95 | 0.066 |
| Proton Conductors | Proton Conductivity (S/cm) | Measurement Temperature (°C) | Refs. |
|---|---|---|---|
| Nafion® 112 | 0.092 0.06 ~0.15–0.16 0.0175 | 20 (in liquid water) 30 (100% RH) 80–100 (90–100% RH) 120 (50% RH) | [16] [16] [54] [54] |
| H3PO4-doped PBI | 10−9–10−5 0.04–0.07 | 160 (1.9 H3PO4 per unit) 200 (4–6 H3PO4 per unit) | [55] [56] |
| CsH2PO4 | 10−7 10−2 | Room temperature 232 | [57,58] |
| CaHPO4.2H2O | 10−3 | 327 | [59] |
| In0.1Sn0.9P2O7 (pellet) | 0.01–0.195 | 750–300 | [60,61] |
| CeP2O7 (pellet) | 0.001–0.018 0.002–0.035 | 50–200 (dry) 60–200 (PH2O = 0.15 atm) | [62] [27] |
| CeP5O14 (pellet) | 0.03 | 250 (PH2O = 0.15 atm) | [26] |
| CUP-PBI membrane (CeP5O14, 75 wt.%) | 0.046–0.105 | 200 | this study |
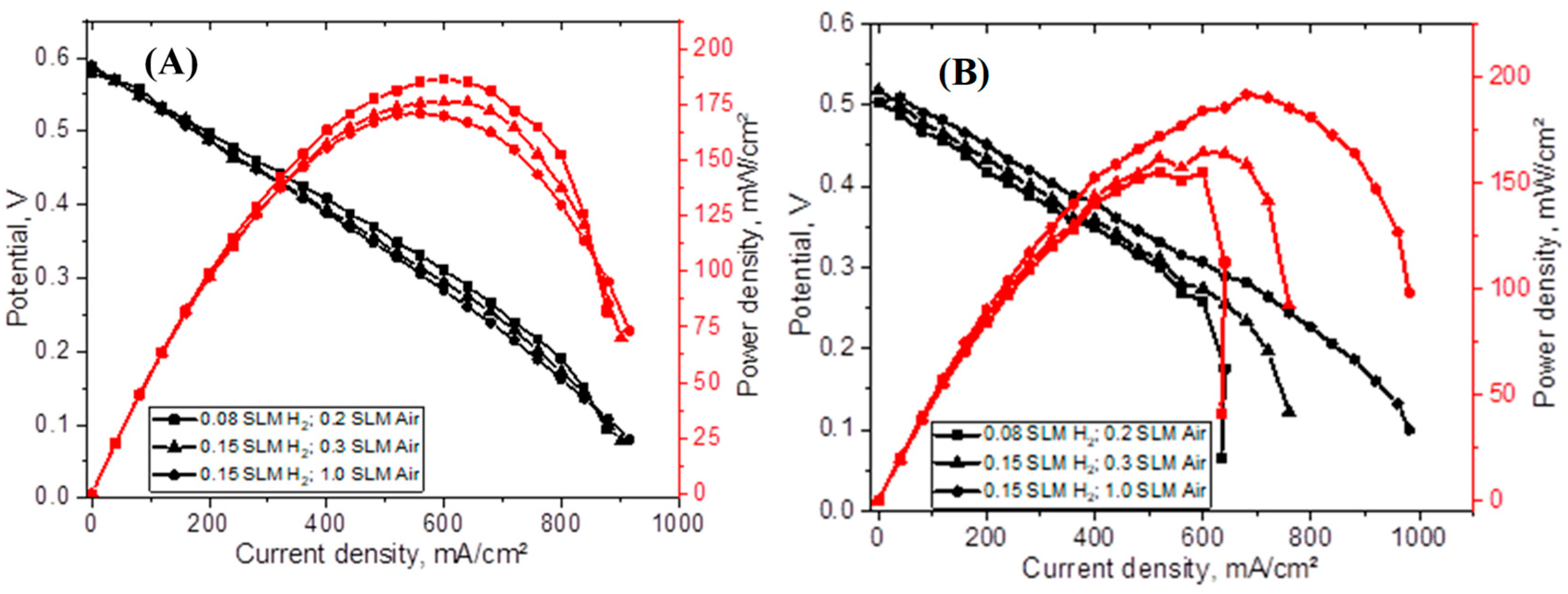
| PIC Membranes | Flow Rates (H2 and Air) (L/min) | OCV (V) | Max. Current Density (A/cm2) | Max. Power Density (mW/cm2) |
|---|---|---|---|---|
| CUP_75%_82 µm (1.0 mg/cm2 Pt/C) | 0.08, 0.2 | 0.31 | 439.6 | 46.6 |
| 0.25, 0.5 | 0.33 | 543.6 | 58.3 | |
| CUP_75%_134 µm (1.0 mg/cm2 Pt/C) | 0.08, 0.2 | 0.58 | 572.9 | 114.3 |
| CUP_75%_sprayed_166 µm (1.0 mg/cm2 Pt/C) | 0.08, 0.2 | 0.95 | 439.3 | 104.2 |
| 0.15, 1.0 | 0.96 | 410.5 | 87.1 | |
| CUP_75%_sprayed_105 µm (1.0 mg/cm2 Pt/C) | 0.08, 0.2 | 0.31 | 693.8 | 70.5 |
| 0.15, 0.3 | 0.32 | 763.3 | 87.1 | |
| 0.15, 1.0 | 0.35 | 1010.1 | 129.1 | |
| CUP_75%_spr_2 h_94 µm (1.0 mg/cm2 Pt/C) | 0.08, 0.2 | 0.58 | 877.9 | 186.4 |
| 0.15, 0.3 | 0.59 | 902.1 | 176.3 | |
| 0.15, 1.0 | 0.59 | 915.9 | 171.1 | |
| CUP_75%_spr_2 h_95 µm (0.5 mg/cm2 Pt/C) | 0.08, 0.2 | 0.51 | 640.4 | 155.3 |
| 0.15, 0.3 | 0.52 | 759.0 | 164.5 | |
| 0.15, 1.0 | 0.51 | 981.3 | 191.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zholobko, O.; Salam, A.; Ashfaq, M.M.; Qi, X.; Wu, X.-F. Undoped Polybenzimidazole Membranes Composited with CeP5O14 for Use in Hydrogen Fuel Cells at 200 °C. Hydrogen 2025, 6, 70. https://doi.org/10.3390/hydrogen6030070
Zholobko O, Salam A, Ashfaq MM, Qi X, Wu X-F. Undoped Polybenzimidazole Membranes Composited with CeP5O14 for Use in Hydrogen Fuel Cells at 200 °C. Hydrogen. 2025; 6(3):70. https://doi.org/10.3390/hydrogen6030070
Chicago/Turabian StyleZholobko, Oksana, Abdul Salam, Muhammad Muzamal. Ashfaq, Xiaoning Qi, and Xiang-Fa Wu. 2025. "Undoped Polybenzimidazole Membranes Composited with CeP5O14 for Use in Hydrogen Fuel Cells at 200 °C" Hydrogen 6, no. 3: 70. https://doi.org/10.3390/hydrogen6030070
APA StyleZholobko, O., Salam, A., Ashfaq, M. M., Qi, X., & Wu, X.-F. (2025). Undoped Polybenzimidazole Membranes Composited with CeP5O14 for Use in Hydrogen Fuel Cells at 200 °C. Hydrogen, 6(3), 70. https://doi.org/10.3390/hydrogen6030070







