Consequence Analysis of Liquid Hydrogen Leakage from Storage Tanks at Urban Hydrogen Refueling Stations: A Case Study
Abstract
1. Introduction
2. Methods
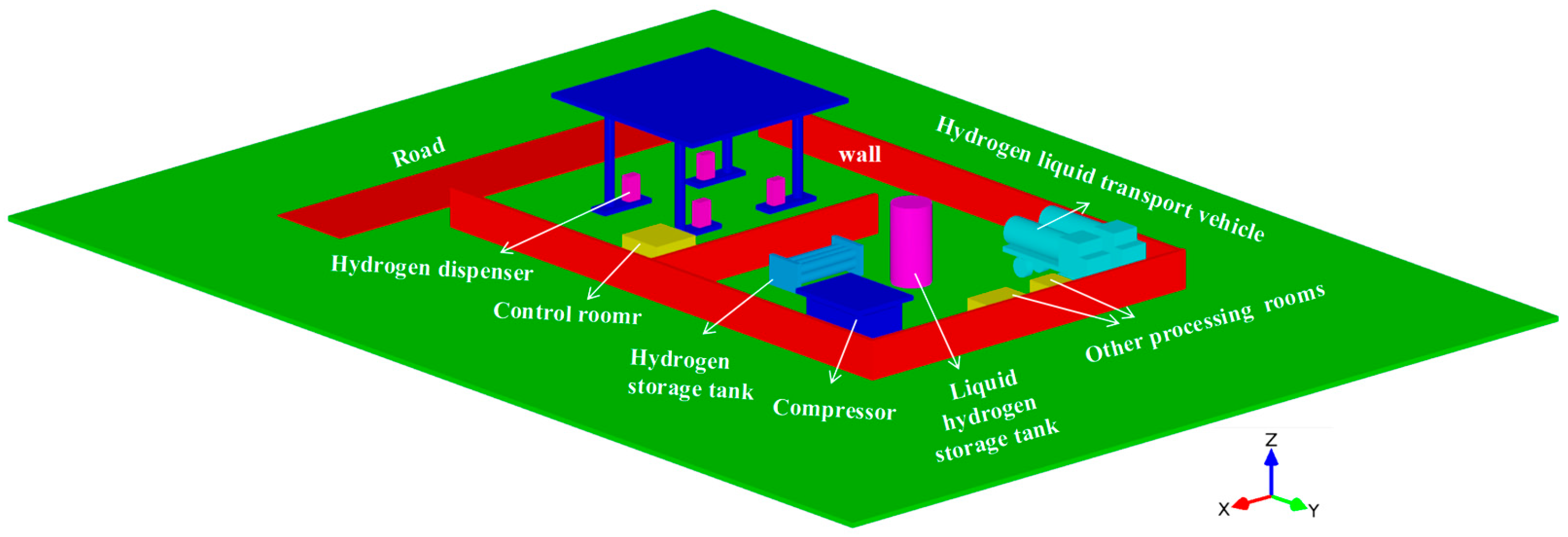
2.1. Physical Model
2.2. Numerical Model
2.2.1. Pseudo-Source Model
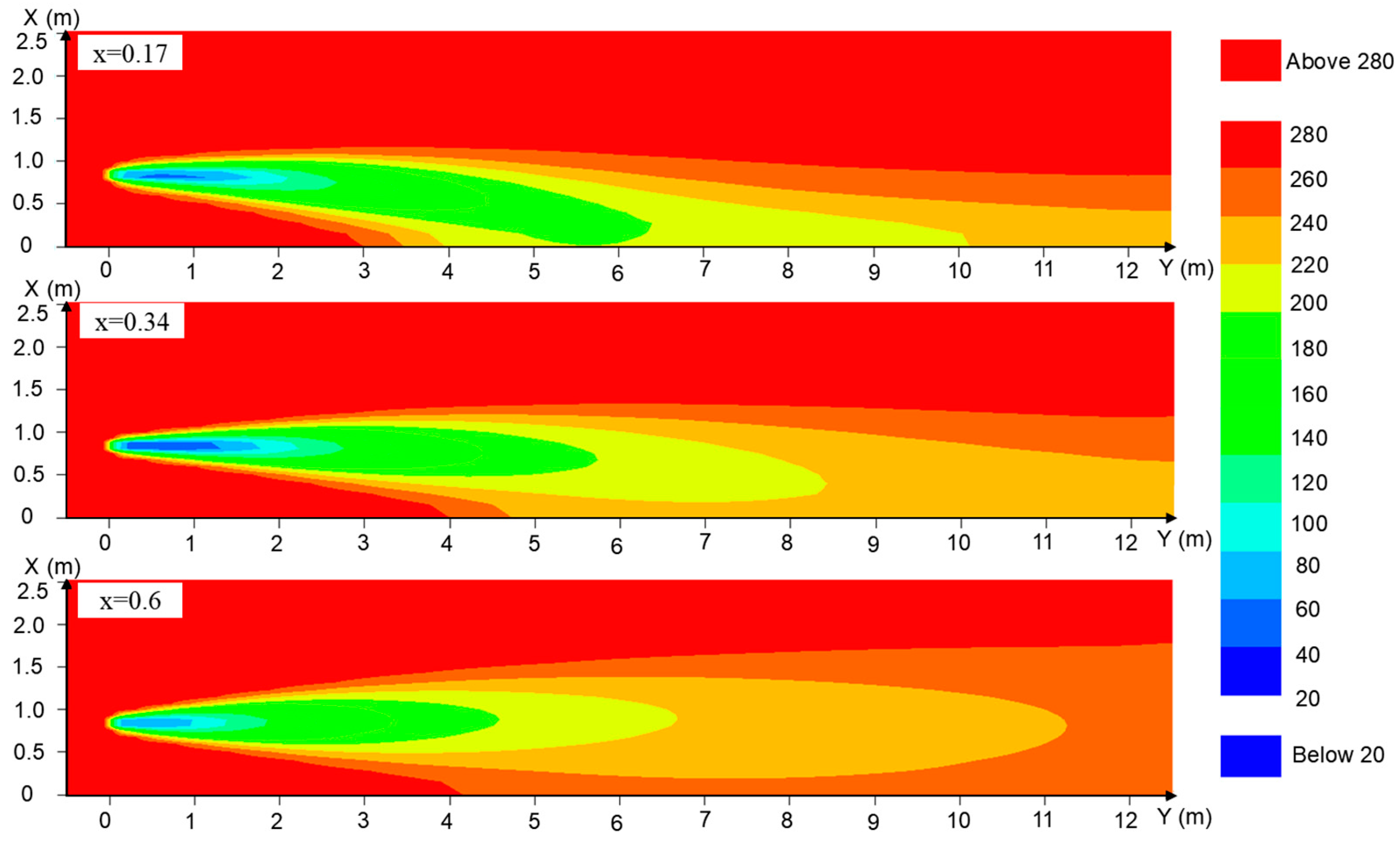
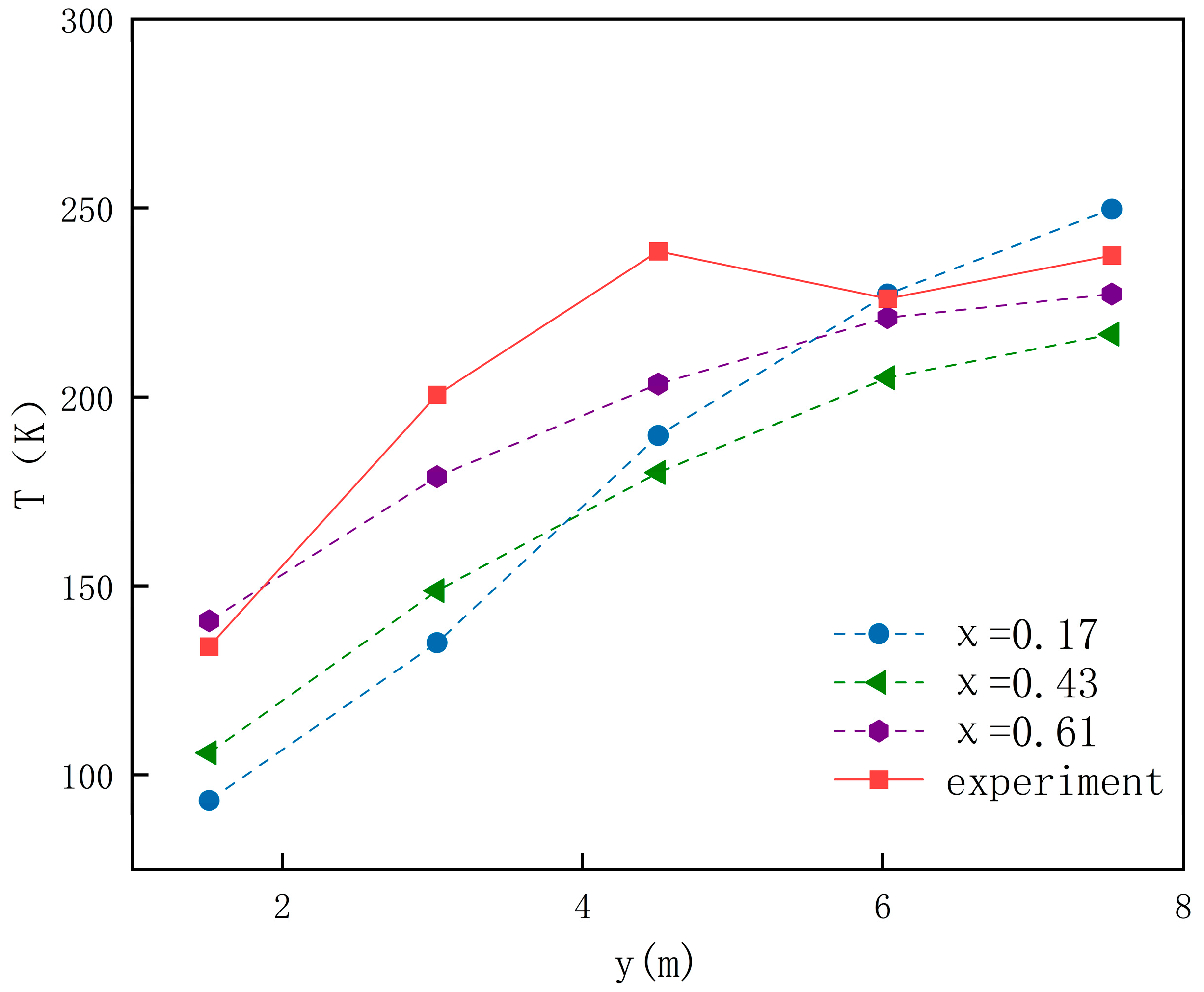
2.2.2. Model Validation
2.3. Model Parameter
2.4. Simulation Condition Parameters
3. Results and Discussion
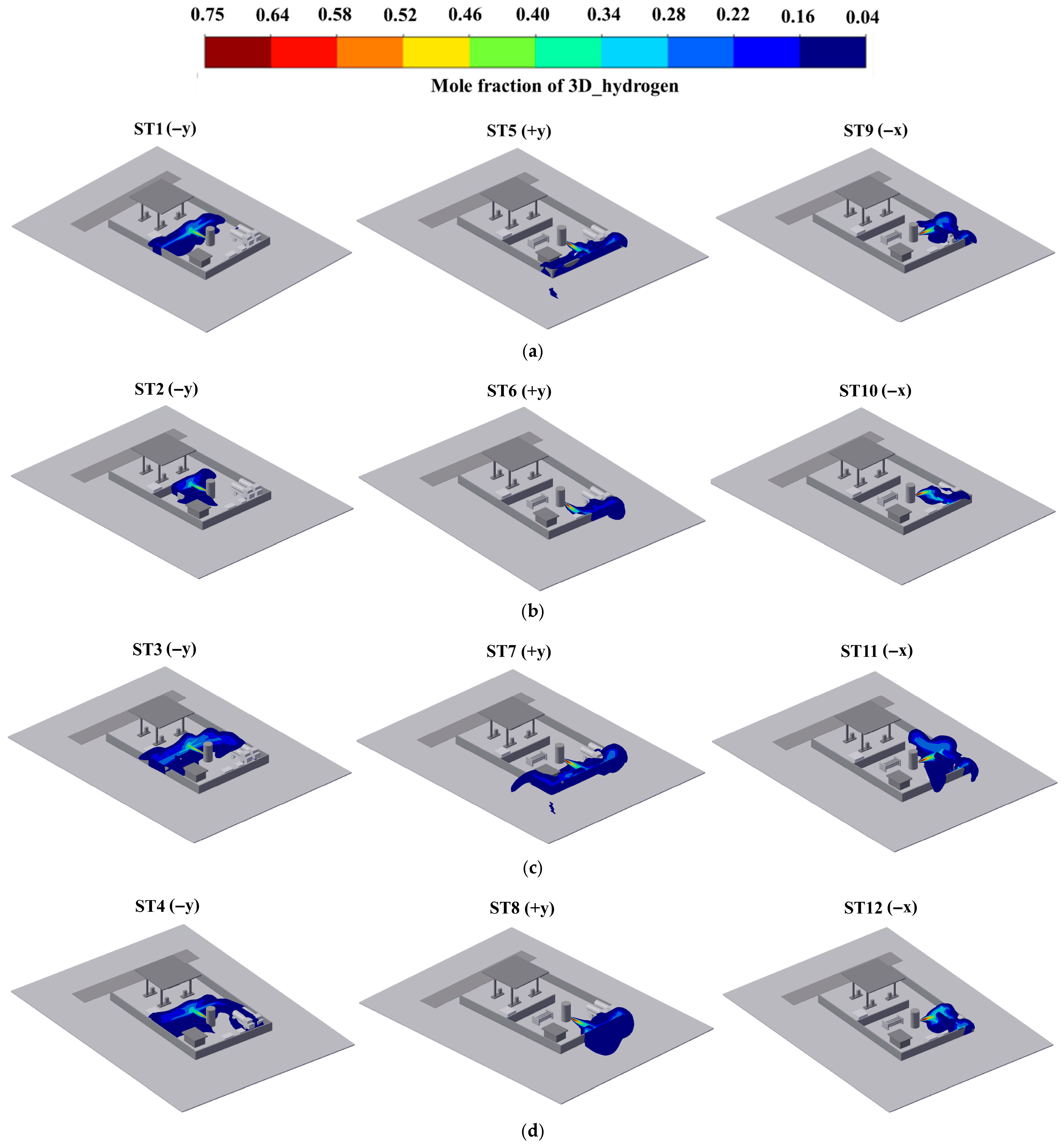
3.1. Dispersion Characteristics of LH2 Leakage
3.1.1. The Diffusion Characteristics of FGCs
3.1.2. Characteristics of Low-Temperature Hazard Area
3.2. Explosion Consequence Analysis
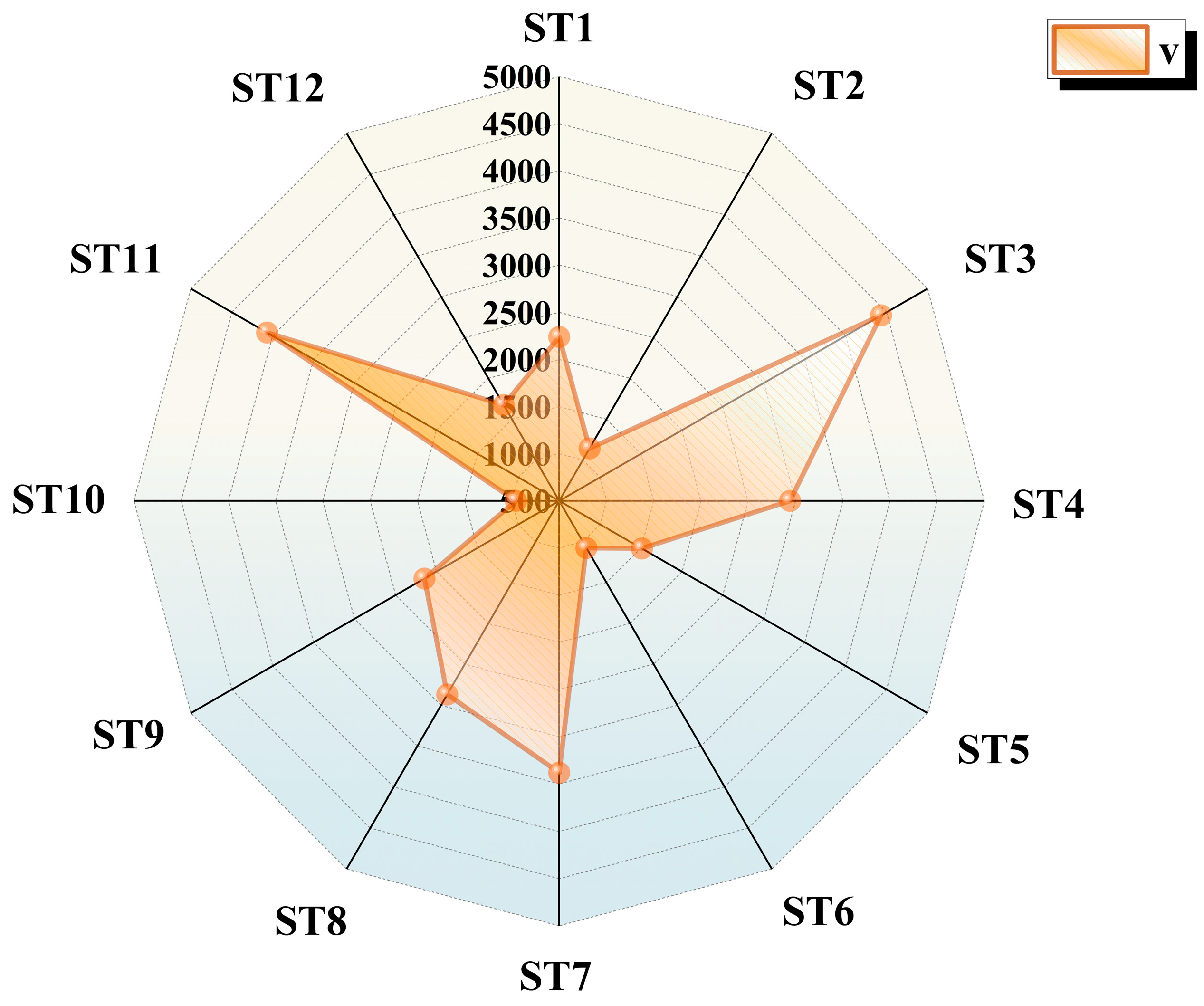
3.2.1. FGC Volume Calculation
3.2.2. Explosion Overpressure Analysis
3.3. Safety Protection Strategies
- To prevent FGC accumulation near walls following an LH2 storage tank leak at an HRS, a directional airflow system should be installed to disrupt accumulation. Additionally, a spoiler should be placed downwind to mitigate wind field–obstacle interaction. Equipment layout in upwind and crosswind areas should be optimized to minimize the wake area, along with implementing other safety measures.
- LH2 leaks at HRSs can easily cause low-temperature damage near the source. To mitigate these hazards, laser hydrogen sensors and infrared low-temperature detectors should be installed to continuously monitor leakage concentration and temperature, particularly within the 30–75% FGC concentration range. An automatic emergency shut-off valve should be configured to close within 0.5 s if leakage exceeds a preset threshold. Operators should be equipped with explosion-proof, self-contained oxygen antifreeze suits capable of withstanding temperatures as low as −196 °C and shock wave overpressures of 0.5 bar. Alternatively, deploying ultra-low-temperature dry powder fire extinguishing robots, capable of operating independently at −200 °C, is recommended for low-temperature disaster zones.
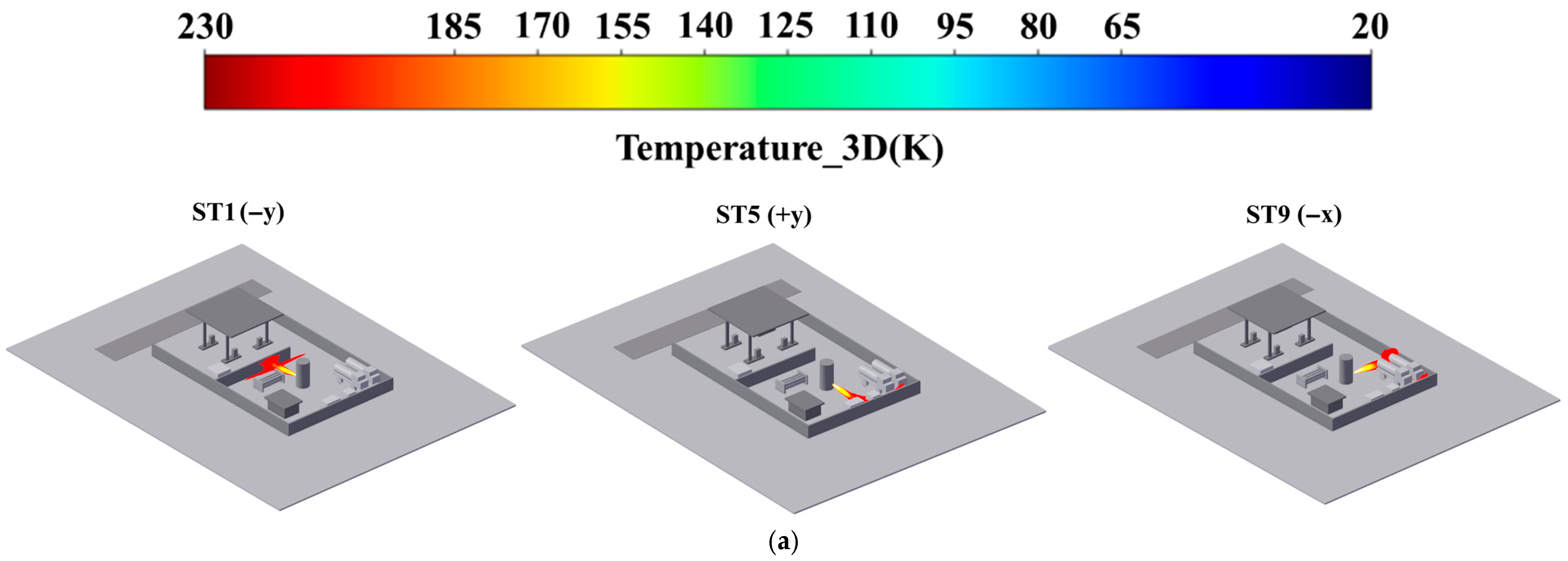
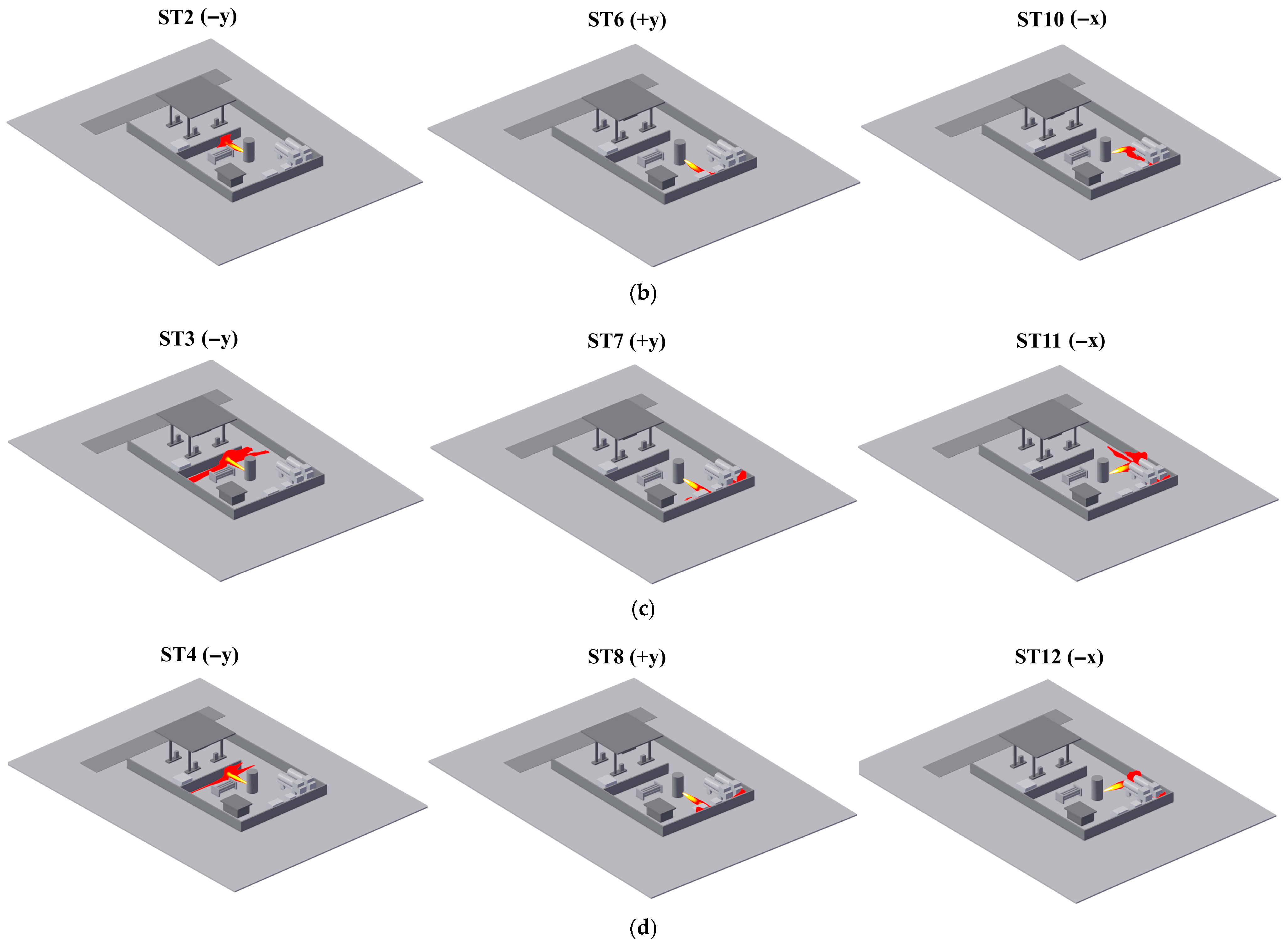
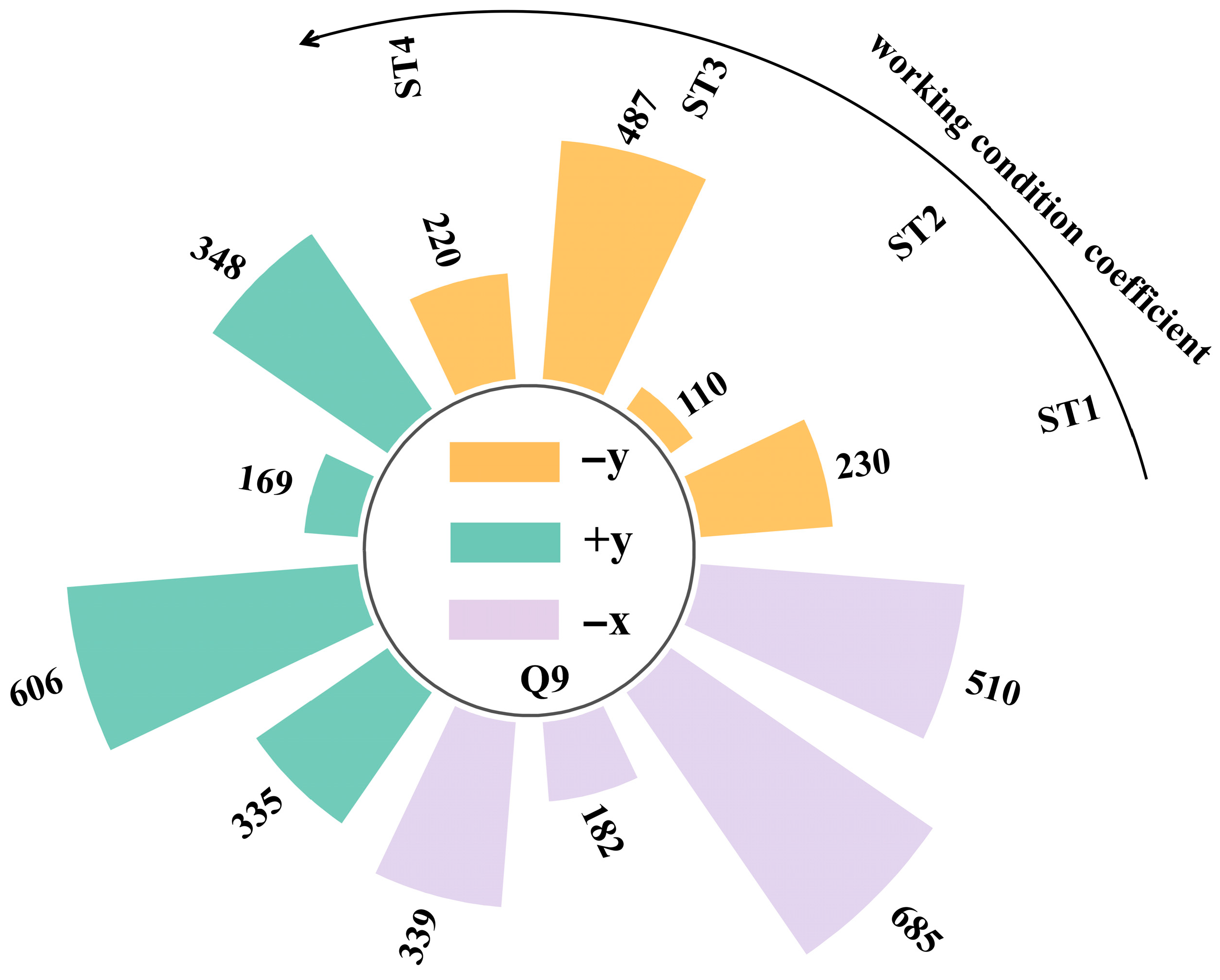
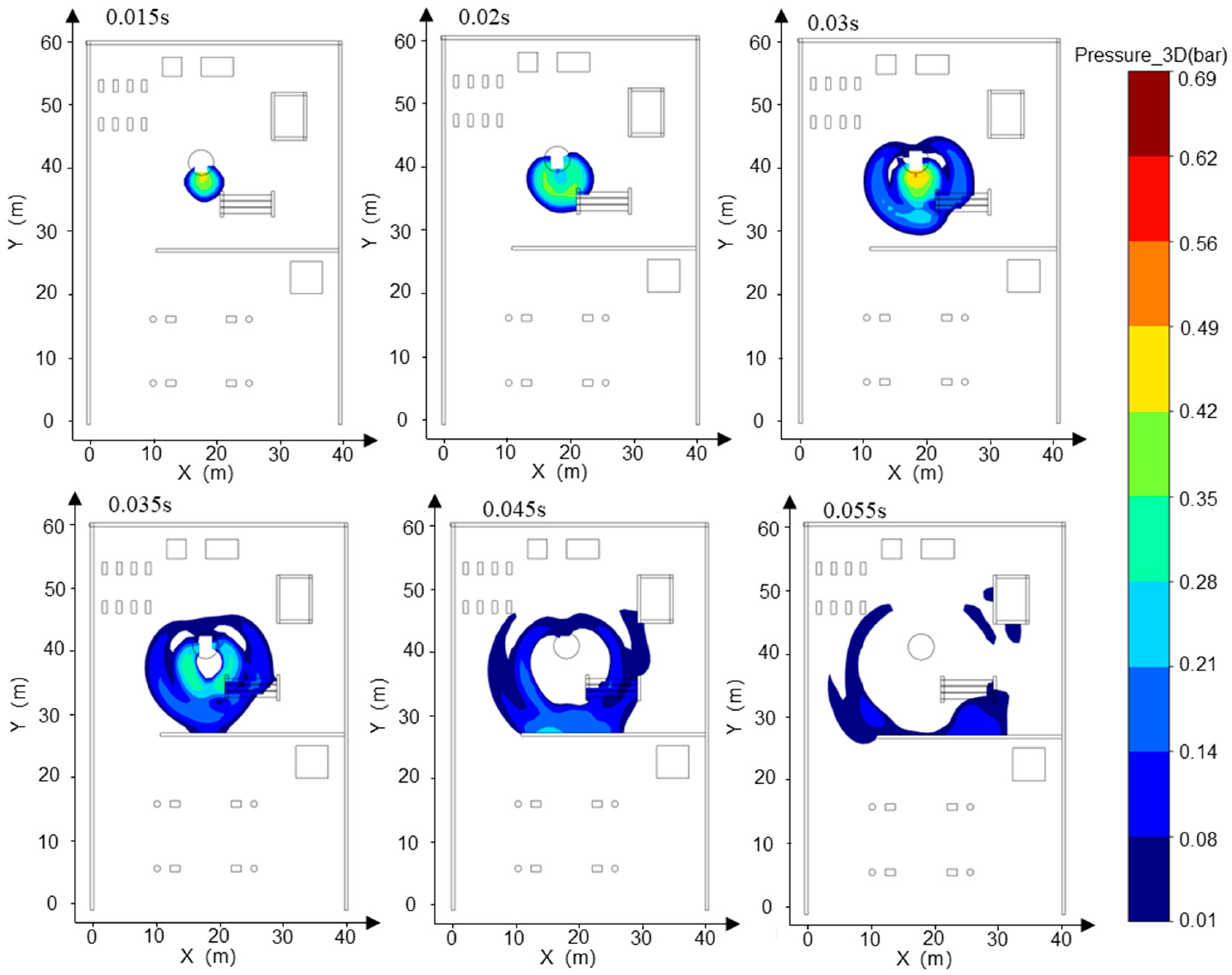
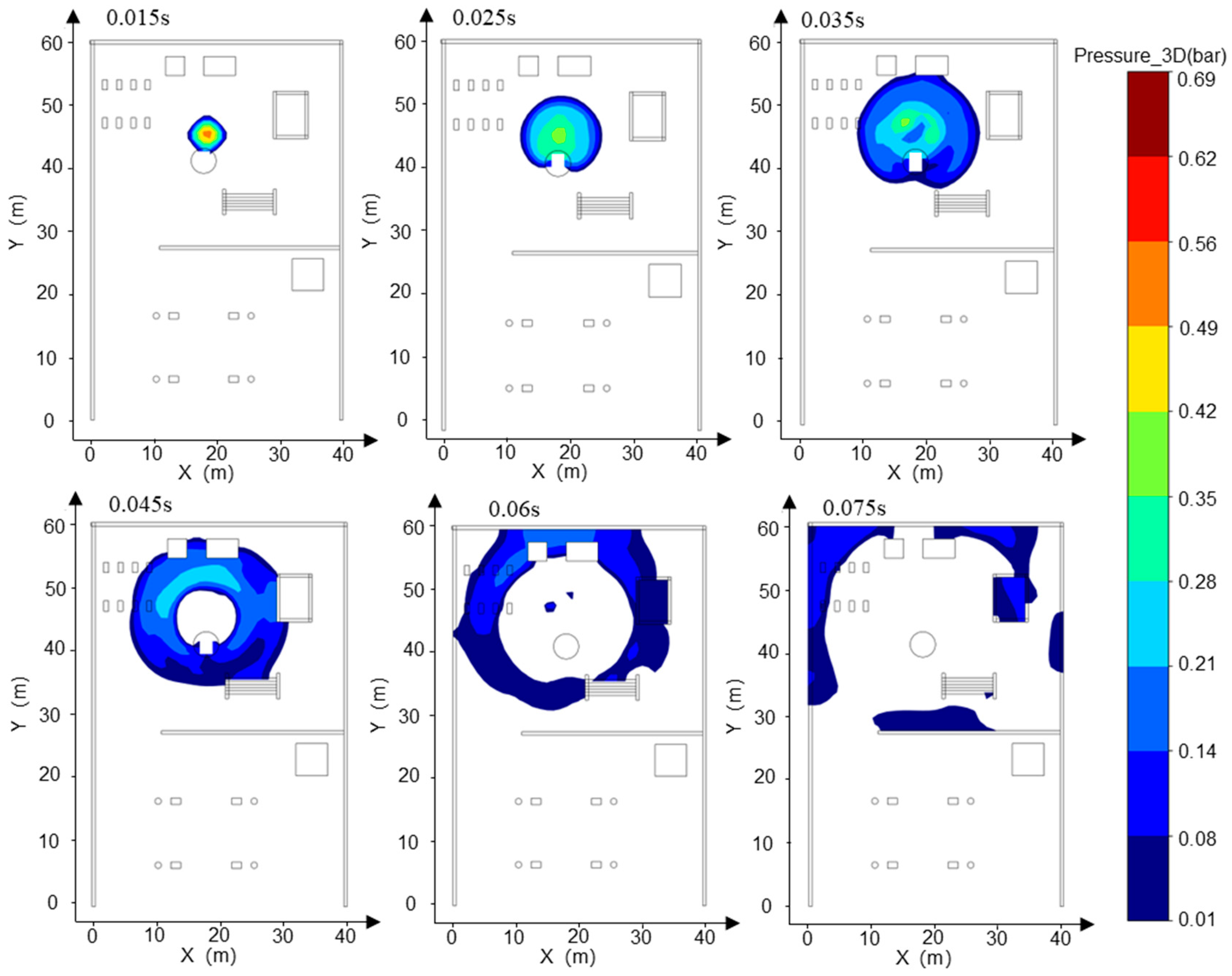
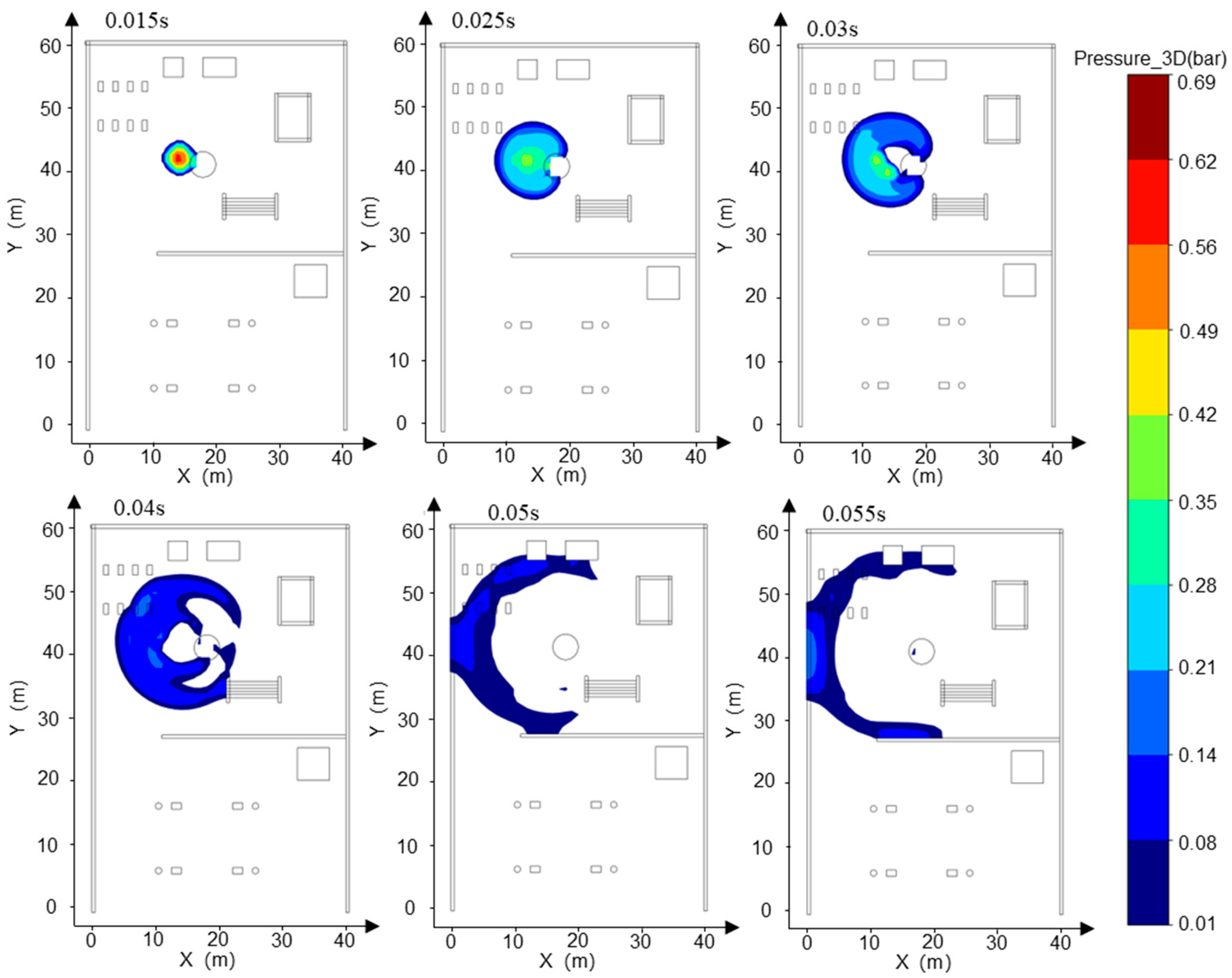
- Addressing the characteristics of vapor cloud explosions occurring when the FGC encounters an ignition source after LH2 storage tank leakage at the HRS, combined with the time-dependent explosion overpressure distribution maps in the X-Y section for each leakage direction (Figure 8, Figure 9 and Figure 10) and the explosion hazard value data in Table 6, it is evident that key HRS facilities are at risk of overpressure from LH2 storage tank leakage and explosion, necessitating safety protection.
- For the compressor room: When the LH2 storage tank leaks and explodes in the −x and −y directions, the explosion overpressure affecting the compressor room is 0 bar and 0.01 bar, respectively, posing no hazard. When delayed ignition leakage occurs in the +y direction, the maximum explosion overpressure affecting the compressor room reaches 0.07 bar. This overpressure can cause damage to the compressor room’s door and window glass and minor injuries from debris. Therefore, during HRS equipment layout, the distance between the LH2/gaseous hydrogen storage tank and the compressor room must be strictly observed. A specified distance exceeding 9 m can effectively reduce the explosion overpressure hazard to the compressor room from leakage explosion [44].
- For the hydrogen storage cylinder group: When the LH2 storage tank leaks and explodes in the −x direction, the explosion overpressure affecting the gaseous hydrogen storage tank is 0 bar, posing no hazard. In the +y direction, an overpressure of 0.08 bar can cause damage to doors and windows and minor injuries from debris. In the −y direction, an overpressure of 0.31 bar is sufficient to collapse the structure of the hydrogen storage cylinder group and may cause serious injury or death within its range. Therefore, it is recommended to clearly define the distance between the gaseous hydrogen storage tank and the LH2 storage tank or to install a firewall between them.
- For the LH2 transport vehicle: When the LH2 storage tank leaks and explodes in the −y and +y directions, the explosion overpressure affecting the LH2 transport vehicle is 0 bar, posing no hazard. In the −x direction, an overpressure of 0.3 bar will cause serious damage to the tank truck, leading to catastrophic leakage and chained secondary accidents, significantly increasing the risk of casualties. Therefore, to effectively mitigate the extreme risk of tank rupture and secondary accidents caused by LH2 storage tank leakage and explosion overpressure in the −x direction, engineering isolation measures based on explosion-proof design must be implemented in the tank truck loading and unloading area, along with establishing an independent reinforced concrete base to provide a necessary physical barrier and impact resistance.
4. Conclusions
- The low leakage rate combined with a weak wind field results in buoyancy-dominated diffusion, creating an isotropic FGC. When paired with a strong wind field, forced convection restricts the FGC’s diffusion in the +y, −y, and −x directions, leading to local aggregation in the crosswind area. A high leakage rate with a weak wind field expands the FGC volume in three directions to twice that of the low leakage rate, forming a high-concentration core with distinct boundary diffusion. In contrast, a high leakage rate with a strong wind field significantly reduces the diffusion range of FGCs in both downwind and crosswind directions. In the upwind direction, a backflow stagnation zone forms due to flow around an obstacle, allowing the FGC volume to reach 290 m3 without notable shrinkage.
- The hazard zone resulting from the phase transition of LH2 at low temperatures overlaps spatially with the cluster of combustible clouds across nearly all operational scenarios, primarily concentrated within the 0.3–0.75 range of FGC concentration. The distribution is primarily influenced by the rate of leakage, with wind speed exerting minimal impact. Mitigation strategies in the overlapping region should concurrently address the risks of combustion and frostbite, necessitating the implementation of tailored protective measures.
- The rapid vaporization and explosion of an LH2 leak at an HRS exhibit transient, high-impact characteristics, with a duration of less than 1 s. The explosion exhibits a homogeneous spatial distribution, with a radius of 16 m. However, the peak overpressure values differ in directionality, reaching up to 0.61 bar in the −x direction, 0.55 bar in the −y direction, and 0.49 bar in the +y direction. The leakage and explosion of the LH2 storage tank can cause varying degrees of damage to other critical components of the HRS. The most severe impact is the 0.30 bar overpressure spread in the −x direction, which may lead to the rupture of the LH2 transport vehicle body and catastrophic secondary leakage.
- To mitigate the risk of FGCs, gradient concentration sensors should be installed on the leeward side of the upwind wall, the downwind wall, and in the crosswind zone. A directional airflow system should be implemented near the wall to disperse FGC accumulation, and a spoiler baffle added downwind to reduce wind field coupling effects. For low-temperature hazards, laser hydrogen sensors and infrared cryogenic detectors should be used within the 30–75% FGC range. Anti-freezing gear, or ultra-low-temperature fire-extinguishing robots should be deployed. To address explosion hazards, the distance between storage tanks and compressor rooms must exceed 9 m. Specifications for LH2/gaseous hydrogen storage tank spacing should be defined, and a firewall should be installed. Additionally, the explosion-proof brackets of hydrogen storage cylinders should be reinforced. An anti-explosion isolation base should be established in the LH2 tank truck loading and unloading area.
- The simplification of the non-equilibrium mass transfer mechanism in the LH2 flash evaporation process relies on the steady-state wind-field assumption and pseudo-source model in this study. It is assumed that the isenthalpic equilibrium flash evaporation changes the droplet phase into an instantaneous process. Further research should focus on multiphase flow numerical simulation to quantify phase-change kinetics effects. Furthermore, the leakage consequences of LH2 storage tanks in HRSs in different leakage scenarios should be analyzed and investigated.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LH2 | Liquid hydrogen |
| HRS | Hydrogen refueling station |
| LHRS | Liquid hydrogen refueling station |
| FGC | Flammable gas cloud |
| CFD | Computational Fluid Dynamics |
| HSL | The Health and Safety Laboratory |
References
- Buckle, N. Some Observations on the History of Electrochemistry in Europe. In Electrochemical Society Meeting Abstracts; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2023; Volume 244, p. 3210. [Google Scholar]
- Smolinka, T.; Bergmann, H.; Garche, J.; Kusnezoff, M. The history of water electrolysis from its beginnings to the present. In Electrochemical Power Sources: Fundamentals, Systems, and Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–164. [Google Scholar]
- Shaposhnik, V.A. Prospects of Membrane Catalysis in Hydrogen Energetics. Mini Review. Condens. Matter Interphases 2024, 26, 37–44. [Google Scholar] [CrossRef]
- Fabbrizzi, L. Strange Case of Signor Volta and Mister Nicholson: How Electrochemistry Developed as a Consequence of an Editorial Misconduct. Angew. Chem. Int. Ed. Engl. 2019, 58, 5810–5822. [Google Scholar] [CrossRef] [PubMed]
- Laghlimi, C.; Moutcine, A.; Ziat, Y.; Belkhanchi, H.; Koufi, A.; Bouyassan, S. Hydrogen: Chronology and electrochemical production. Sol. Energy Sustain. Dev. Spec. Issue 2024, 22–37. [Google Scholar] [CrossRef]
- Stein, A.; Nolte, B.; Kizgin, U.V.; Grünewald, O. Relationship Between Area and Capacity of Hydrogen Refueling Stations and Derivation of Design Recommendations. Hydrogen 2025, 6, 16. [Google Scholar] [CrossRef]
- Matveev, K.I.; Leachman, J.W. The Effect of Liquid Hydrogen Tank Size on Self-Pressurization and Constant-Pressure Venting. Hydrogen 2023, 4, 444–455. [Google Scholar] [CrossRef]
- Sun, Y.Q. Explosions of Hydrogen Storages and the Safety Considerations in Hydrogen-Powered Railway Applications—A Review. Hydrogen 2024, 5, 901–918. [Google Scholar] [CrossRef]
- Genovese, M.; Blekhman, D.; Fragiacomo, P. An Exploration of Safety Measures in Hydrogen Refueling Stations: Delving into Hydrogen Equipment and Technical Performance. Hydrogen 2024, 5, 102–122. [Google Scholar] [CrossRef]
- Deng, X.; Sun, J.; Yang, F. Simulation and Quantitative Assessment of Sensor Placement in a Hydrogen Bus for Risk Mitigation. Hydrogen 2024, 5, 976–986. [Google Scholar] [CrossRef]
- Haoren, W.; Bo, W.; Ruize, L.; Xian, S.; Yingzhe, W.; Quanwen, P.; Yuanxin, H.; Weiming, Z.; Zhihua, G. Theoretical investigation on heat leakage distribution between vapor and liquid in liquid hydrogen tanks. Int. J. Hydrogen Energy 2023, 48, 17187–17201. [Google Scholar] [CrossRef]
- Holborn, P.G.; Benson, C.M.; Ingram, J.M. Modelling hazardous distances for large-scale liquid hydrogen pool releases. Int. J. Hydrogen Energy 2020, 45, 23851–23871. [Google Scholar] [CrossRef]
- Qu, Z.; Cai, Z.; Ma, Z.; Zhang, X.; Shu, Z.; Liang, W.; Cai, L. Co-simulation of Thermal Behavior in Liquid Hydrogen Tanks under Vacuum degradation. Energy 2025, 329, 136841. [Google Scholar] [CrossRef]
- Lv, H.; Chen, L.; Zhang, Z.; Zhang, Z.; Chen, S.; Hou, Y. Investigation of the non-equilibrium heat transfer and self-pressurization behavior of liquid hydrogen tanks. Int. J. Hydrogen Energy 2025, 122, 125–138. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, S.; Xie, Z.; Yang, S.; Bi, M. Experimental study on vaporization behavior of liquid hydrogen on different substrates. Int. J. Hydrogen Energy 2025, 132, 1–9. [Google Scholar] [CrossRef]
- Winters, W.; Houf, W. Simulation of small-scale releases from liquid hydrogen storage systems. Int. J. Hydrogen Energy 2011, 36, 3913–3921. [Google Scholar] [CrossRef]
- Wan, C.; Shi, C.; Zhu, S.; Fang, S.; Qiu, L.; Shi, G.; Li, D.; Shao, S.; Wang, K. Comprehensive design and preliminary experiments of liquid hydrogen storage tank for trucks. Int. J. Refrig. 2025, 169, 279–293. [Google Scholar] [CrossRef]
- Lv, H.; Su, Y.; Huang, G.; Zhang, Q.; Zhang, C. Research on rapid refueling of Type-IV hydrogen storage tanks for hydrogen-powered heavy-duty commercial vehicles. Renew Energy 2025, 6, 123673. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Li, X.-J.; Gao, Z.-X.; Jin, Z.-J. A numerical study of hydrogen leakage and diffusion in a hydrogen refueling station. Int. J. Hydrogen Energy 2020, 45, 14428–14439. [Google Scholar] [CrossRef]
- Wu, J.; Xie, R.; Yu, M.; Luo, C.; Wang, B.; Zhang, X.; Jiang, L. Techno-economic analysis on the performance of hydrogen adsorbents in the vacuum layer of cryogenic liquid storage tank. Int. J. Hydrogen Energy 2024, 88, 132–141. [Google Scholar] [CrossRef]
- Sun, R.; Pu, L.; Yu, H.; Dai, M.; Li, Y. Modeling the diffusion of flammable hydrogen cloud under different liquid hydrogen leakage conditions in a hydrogen refueling station. Int. J. Hydrogen Energy 2022, 47, 25849–25863. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, D.; Lv, H.; Zhang, C. Dispersion characteristics of large-scale liquid hydrogen spills in a real-world liquid hydrogen refueling station with various releasing and environmental conditions. Renew. Energy 2024, 236, 121327. [Google Scholar] [CrossRef]
- Jiang, Y.; Xing, Z.; Xu, Q.; Wu, J.; Peng, M.; Liu, Y. Research on fence protection for liquid hydrogen leakage in the storage tank area. J. Energy Storage 2024, 95, 112481. [Google Scholar] [CrossRef]
- Gao, X.; Huang, L.; Ren, J.; Lan, Y.; Li, M.; Xiao, H. Numerical study of the effect of barrier wall on liquid hydrogen leakage and dispersion. Int. J. Hydrogen Energy 2025, 142, 460–471. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Z.; Fei, Z.; Luan, X.; Duan, Y.; Zhang, B. Numerical study on mitigation effect of fence on the vapor dispersion of liquid hydrogen leak in a dike. Int. J. Hydrogen Energy 2025, 111, 667–680. [Google Scholar] [CrossRef]
- Hansen, O.R. Liquid hydrogen releases show dense gas behavior. Int. J. Hydrogen Energy 2020, 45, 1343–1358. [Google Scholar] [CrossRef]
- Yuan, W.; Li, J.; Zhang, R.; Li, X.; Xie, J.; Chen, J. Numerical investigation of the leakage and explosion scenarios in China’s first liquid hydrogen refueling station. Int. J. Hydrogen Energy 2022, 47, 18786–18798. [Google Scholar] [CrossRef]
- Peng, J.; Li, M.; Huang, X.; Xie, J.; Chen, J. Numerical investigation on evaluation of leakage and explosion overpressure at liquid hydrogen receiving terminal. Cryogenics 2025, 150, 104117. [Google Scholar] [CrossRef]
- Shang, S.; Zhang, J.; Zhang, J.; Luo, T.; Bi, M.; Jiang, H.; Li, Y.; Gao, W. Study on the effect of explosion suppression equipment on hydrogen explosions. J. Loss Prev. Process. Ind. 2023, 83, 105046. [Google Scholar] [CrossRef]
- Xiao, H.; Duan, Q.; Sun, J. Premixed flame propagation in hydrogen explosions. Renew. Sust. Energy Rev. 2018, 81, 1988–2001. [Google Scholar] [CrossRef]
- Ma, X.; Nie, B.; Wang, W.; Zhao, D.; Zhang, Y.; Yang, Y.; Ma, C.; Hu, B.; Chang, L.; Yang, L. Effect of hydrogen concentration, initial pressure and temperature on mechanisms of hydrogen explosion in confined spaces. Combust. Flame 2024, 269, 113696. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, S.; Zhang, X.; Wang, F. Enhancing disaster prevention and structural resilience of tunnels: A study on liquid hydrogen leakage, diffusion, and explosion mitigation. Tunn. Undergr. Space Technol. 2025, 162, 106626. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, D.; Lv, H.; Zhang, C. Numerical study on leakage, diffusion and accident consequences of liquid hydrogen jet and analysis of influencing factors. Process Saf. Environ. Prot. 2025, 194, 14–34. [Google Scholar] [CrossRef]
- Amaral, P.C.S.; Oh, C.B.; Do, K.H.; Choi, B.-I. Risk assessment of hydrogen leakage and explosion in a liquid hydrogen facility using computational analysis. Int. J. Hydrogen Energy 2024, 91, 950–964. [Google Scholar] [CrossRef]
- Liang, Y.; Pan, X.; Zhang, C.; Xie, B.; Liu, S. The simulation and analysis of leakage and explosion at a renewable hydrogen refuelling station. Int. J. Hydrogen Energy 2019, 44, 22608–22619. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Z.; Han, X.; Chen, G.; Wang, X. Numerical and experimental studies on the evolution characteristics of high-pressure hydrogen leakage and explosion accidents in hydrogen refueling stations. Int. J. Hydrogen Energy 2025, 142, 580–595. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wang, T.S.; Zhu, X.W. Location and capacity planning of hydrogen refueling station in highway network based on hydrogen life cycle cost: Modeling, optimization and case verification. Int. J. Hydrogen Energy 2025, 139, 606–620. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Wang, J.; Wu, J.; Wu, H.; Li, W.; Yu, T.; Han, Y.; Lu, W.; Xing, Z.; et al. Liquid hydrogen refueling stations: A review on process layouts, pump technology, and cold energy utilization. Int. J. Hydrogen Energy 2025, 137, 260–280. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Shi, X.; Fan, R. Safety analysis of hydrogen leakage accident with a mobile hydrogen refueling station. Process. Saf. Environ. Prot. 2023, 171, 619–629. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Z.; Chen, G.; Li, X. Optimizing hydrogen refueling station layout based on consequences of leakage and explosion accidents. Int. J. Hydrogen Energy 2024, 54, 817–54836. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Zhao, H.; Liu, M.; Zhang, C. Numerical simulation of hydrogen leakage and accident consequence of hydrogen explosion in compact confined hydrogen-electric coupling systems. Process. Saf. Environ. Prot. 2025, 201, 107444. [Google Scholar] [CrossRef]
- NFPA. NFPA 2: Hydrogen Technologies Code; National Fire Protection Association: Quincy, MA, USA, 2020. [Google Scholar]
- Nolan, D.P. Explosion Protection Engineering Principles for Oil; China Petrochemical Press: Beijing, China, 2020; pp. 154–169. [Google Scholar]
- GB 50516-2010; Technical Code for Hydrogen Fuelling Station. GB Standards: Shenzhen, China, 2021.
| Serial Number | Outlet Mass Flow Rate (kg/s) | Temperature (k) | Hydrogen Mass Fraction | Outlet Velocity (m/s) | Fictitious Source Speed (m/s) | False Source Area (m2) |
|---|---|---|---|---|---|---|
| ST1 | 0.071 | 20.28 | 0.17 | 30.25 | 13.01 | 0.0055 |
| ST2 | 0.071 | 20.28 | 0.34 | 57.44 | 27.97 | 0.002 |
| ST3 | 0.071 | 20.28 | 0.60 | 100.62 | 62.01 | 0.0017 |
| Boundary | Diffusion Process | Explosion Process |
|---|---|---|
| XHI | Nozzle | PLANE_WAVE |
| XLO | Wind | PLANE_WAVE |
| YHI | Nozzle | PLANE_WAVE |
| YLO | Wind | PLANE_WAVE |
| ZHI | Wind | PLANE_WAVE |
| ZLO | Nozzle | EULER |
| Inlet | JET | / |
| Simulation Condition | Leakage Direction | Leakage Point | Leakage Rate (kg/s) | Wind Speed (m/s) |
|---|---|---|---|---|
| ST1 | −y | (19, 40, 2.5) | 0.5182 | 1.0 |
| ST2 | −y | 0.5182 | 3.0 | |
| ST3 | −y | 1.0329 | 1.0 | |
| ST4 | −y | 1.0329 | 3.0 | |
| ST5 | +y | (19, 45, 2.5) | 0.5182 | 1.0 |
| ST6 | +y | 0.5182 | 3.0 | |
| ST7 | +y | 1.0329 | 1.0 | |
| ST8 | +y | 1.0329 | 3.0 | |
| ST9 | −x | (16.5, 42.5, 2.5) | 0.5182 | 1.0 |
| ST10 | −x | 0.5182 | 3.0 | |
| ST11 | −x | 1.0329 | 1.0 | |
| ST12 | −x | 1.0329 | 3.0 |
| Comparison | ST1 | ST2 | ST3 | ST4 | ST5 | ST6 | ST7 | ST8 | ST9 | ST10 | ST11 | ST12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| The farthest combustible distance (m) | 17 | 16 | 17.5 | 16 | 46 | 27 | 41 | 34 | 21 | 25 | 50 | 25 |
| The farthest low-temperature distance (m) | 16 | 16 | 16 | 16 | 16 | 13 | 16 | 16 | 19 | 10 | 19 | 19 |
| Peak Overpressure (bar) | Losses to Buildings | Injury to Persons |
|---|---|---|
| 0.06895 | Door and window glass damage | Fragments cause minor damage |
| 0.1379 | Causes moderate damage to houses (e.g., doors and windows blown off, roofs severely damaged) | Smashed by debris and falling objects |
| 0.2068 | The collapse of residential structure | May cause serious injury or death |
| 0.3447 | Most buildings collapsed | Increased probability of death |
| 0.6895 | Serious damage to reinforced concrete buildings | Most deaths |
| 1.37 | The reinforced concrete building was toppled down | The mortality rate is close to 100%. |
| Parameter | −y Direction | +y Direction | −x Direction | |
|---|---|---|---|---|
| Maximum hazard radius (m) | 16 | 16 | 16 | |
| Peak overpressure | overpressure (bar) | 0.55 | 0.49 | 0.61 |
| detriment | The probability of most buildings collapsing and the number of casualties increasing. | The probability of most buildings collapsing and the number of casualties increasing. | The probability of most buildings collapsing and the number of casualties increasing. | |
| Maximum overpressure in compressor room | overpressure (bar) | 0.01 | 0.07 | 0 |
| detriment | Almost nothing happened. | The windows and doors’ glass were damaged and the fragments caused minor injuries to the personnel. | Nothing | |
| Maximum overpressure of hydrogen storage cylinder group | overpressure (bar) | 0.31 | 0.08 | 0 |
| detriment | Most buildings collapsed and the probability of casualties increased. | The windows’ and doors’ glass were damaged and the fragments caused minor injuries to the personnel. | Nothing | |
| Maximum overpressure of liquid hydrogen tanker | overpressure (bar) | 0 | 0.24 | 0.3 |
| detriment | Nothing | The collapse of the residential structure and it may result in serious injuries or deaths to the people. | Most buildings collapsed and the probability of casualties increased. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, W.; Song, H.; Kuang, T.; Li, Y.; Guang, Y. Consequence Analysis of Liquid Hydrogen Leakage from Storage Tanks at Urban Hydrogen Refueling Stations: A Case Study. Hydrogen 2025, 6, 58. https://doi.org/10.3390/hydrogen6030058
Liu H, Wang W, Song H, Kuang T, Li Y, Guang Y. Consequence Analysis of Liquid Hydrogen Leakage from Storage Tanks at Urban Hydrogen Refueling Stations: A Case Study. Hydrogen. 2025; 6(3):58. https://doi.org/10.3390/hydrogen6030058
Chicago/Turabian StyleLiu, Hongxi, Wenhe Wang, Hongwei Song, Tingting Kuang, Yuanyang Li, and Yu Guang. 2025. "Consequence Analysis of Liquid Hydrogen Leakage from Storage Tanks at Urban Hydrogen Refueling Stations: A Case Study" Hydrogen 6, no. 3: 58. https://doi.org/10.3390/hydrogen6030058
APA StyleLiu, H., Wang, W., Song, H., Kuang, T., Li, Y., & Guang, Y. (2025). Consequence Analysis of Liquid Hydrogen Leakage from Storage Tanks at Urban Hydrogen Refueling Stations: A Case Study. Hydrogen, 6(3), 58. https://doi.org/10.3390/hydrogen6030058






