Effect of Applied DC Electric Fields on H2–Air Axisymmetric Laminar Co-Flow Diffusion Flames with Low Carbon Impurities
Abstract
1. Introduction
2. Experimental Design and Image Analysis
2.1. Experimental Setup
2.1.1. Co-Flow Burner and Gas System
2.1.2. Electrode System
2.1.3. Optical System
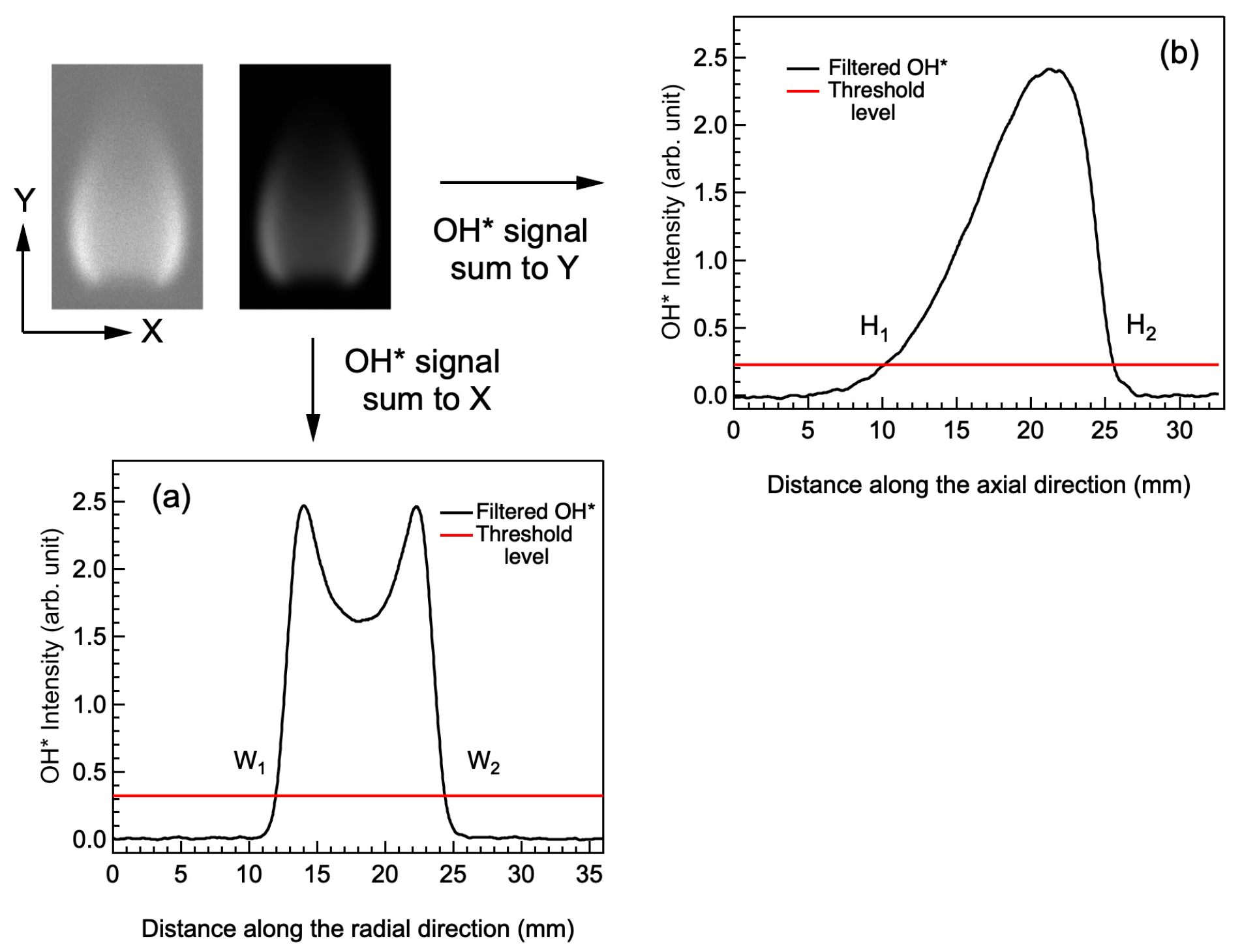
2.2. Image Analysis
3. Modeling
3.1. Electrode Modeling
3.2. CHEMKIN Modeling
4. Results and Discussion
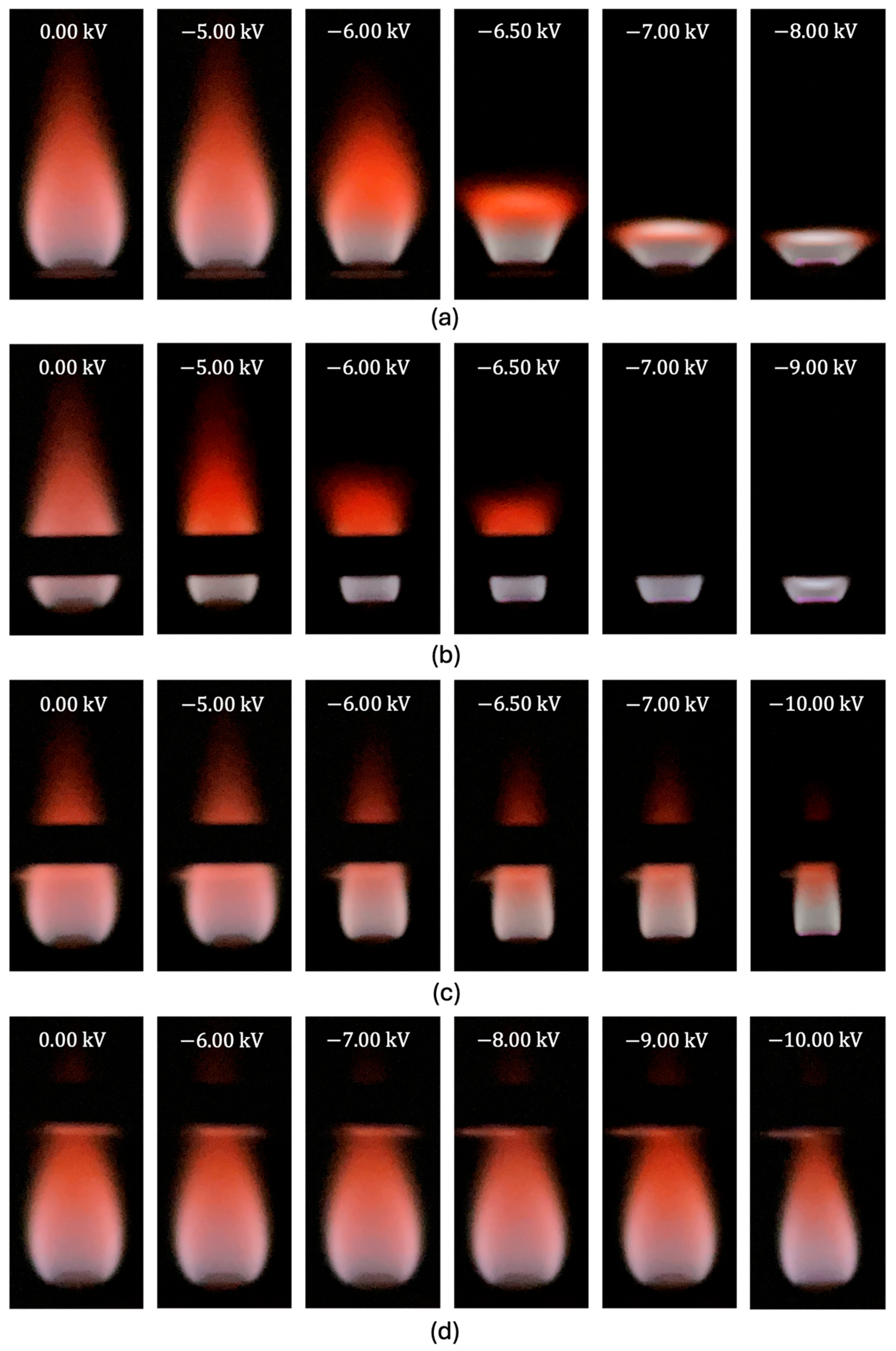
4.1. Effect of Electric Field on OH* Flame Structure
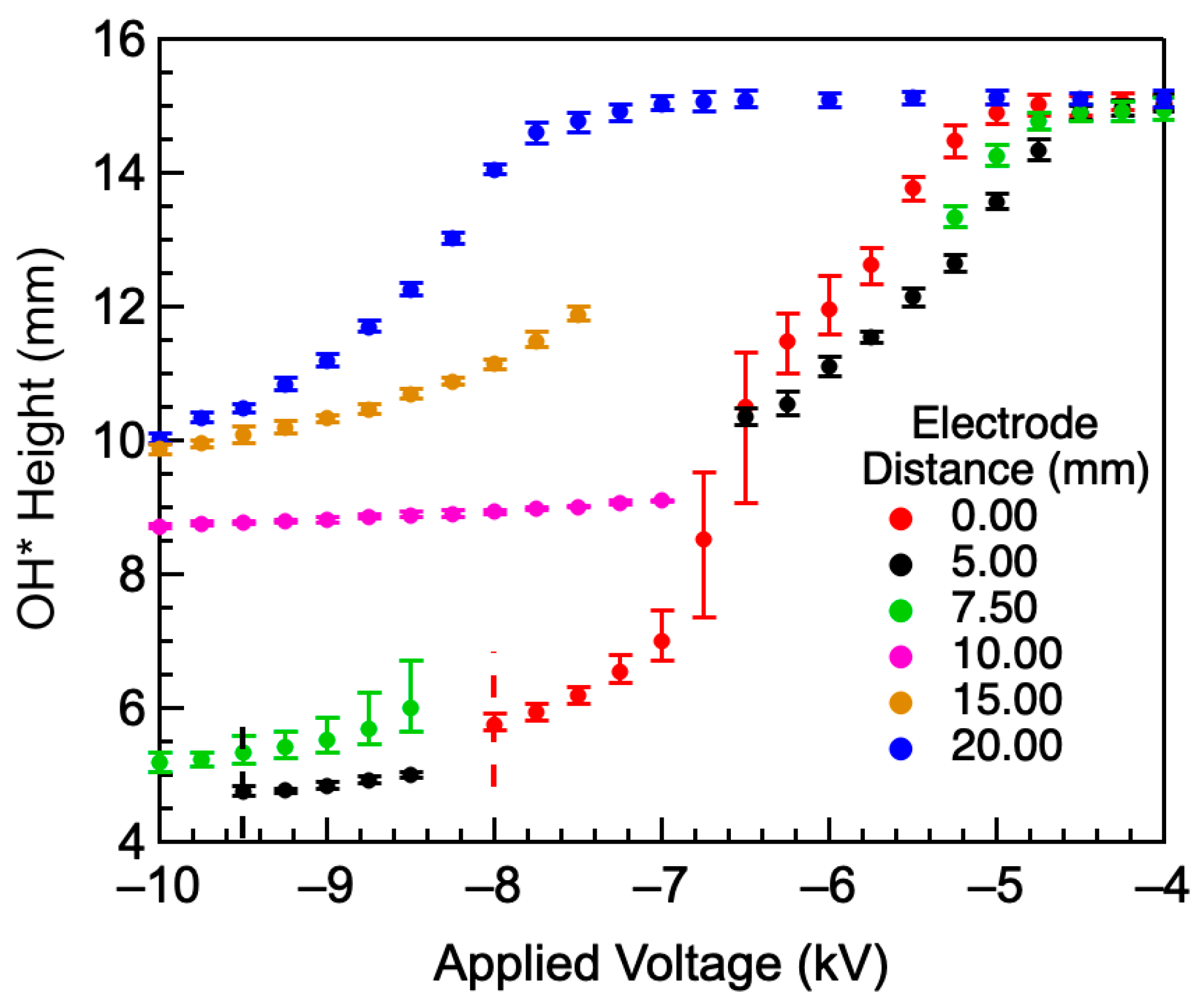
4.2. Effect of Airflow Rate on OH* Height
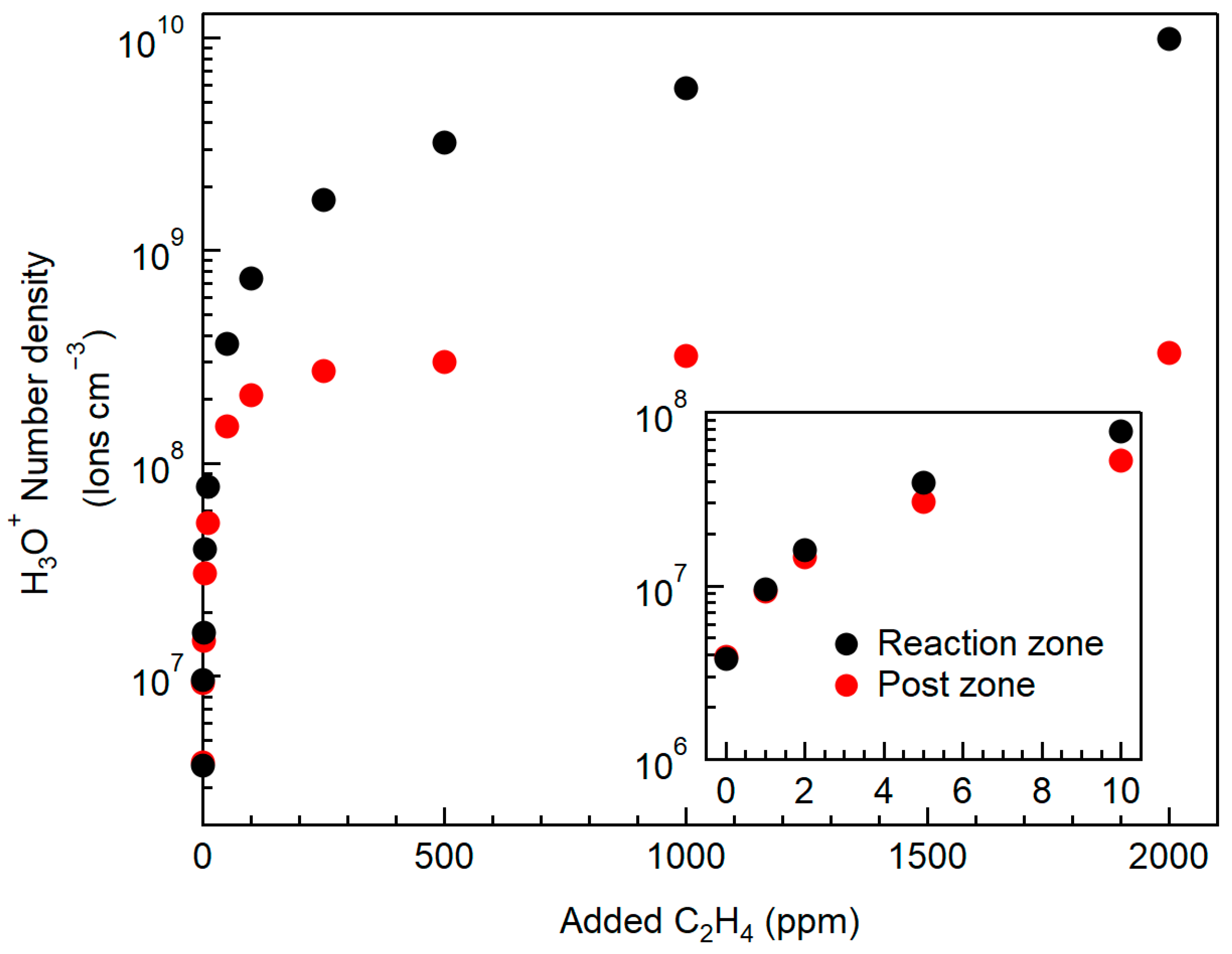
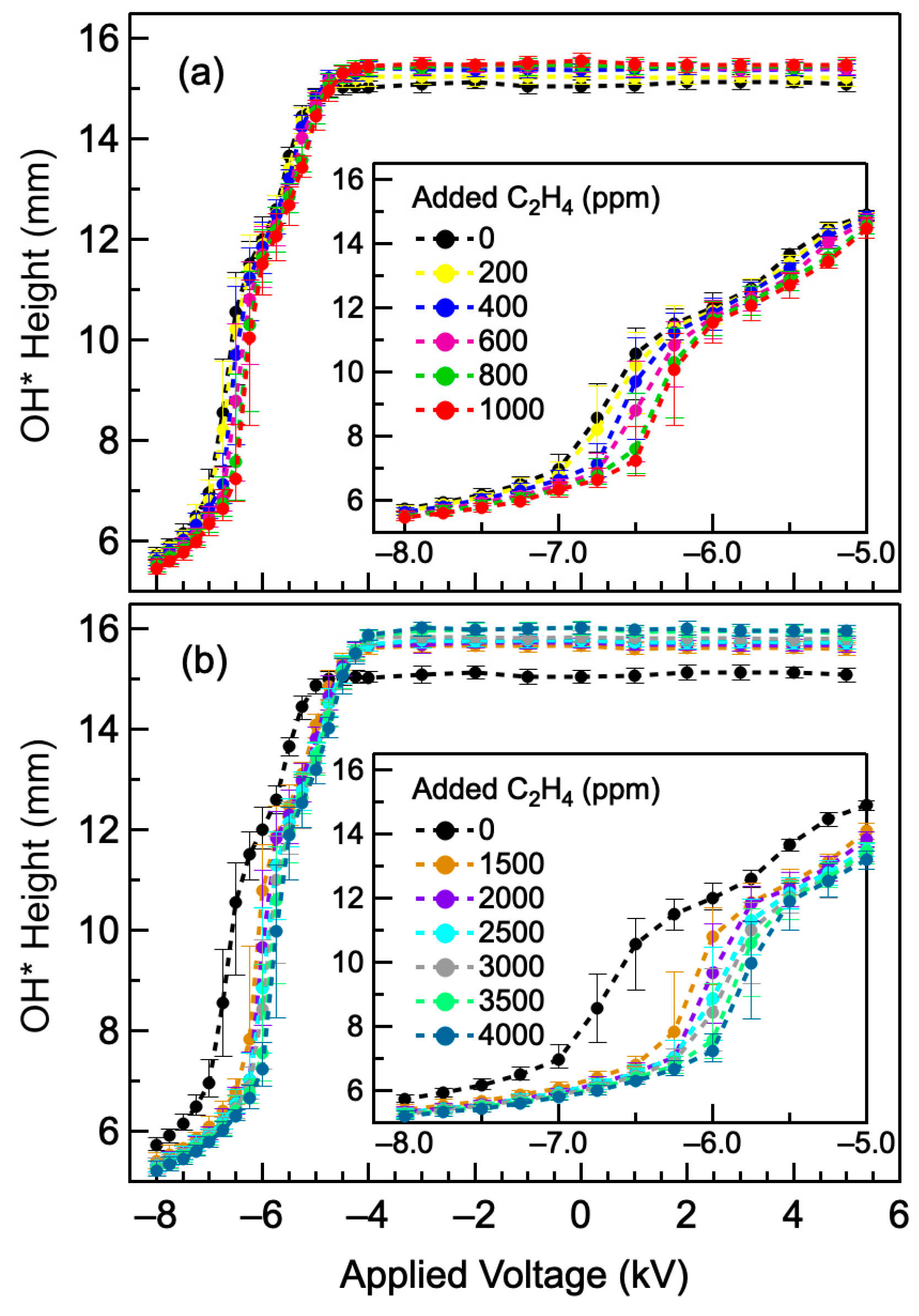
4.3. Effect of Added Hydrocarbon Impurities
5. Conclusions
- Hydrocarbon impurities as low as 10 ppm may generate sufficient charge carriers to observe changes in H2–air flame properties under externally applied electric fields. Above 100 ppm, adding more carbon impurities only had a small effect on the flame interaction with the electric field.
- The dimensions of the reaction zone, as defined by OH* emission, decreased for applied voltages below −5 kV. The OH* flame height decreased up to 67% at the lowest negative voltage. No changes in flame dimensions were observed for positive voltages.
- The most significant effects were observed when the ring electrode was near or within the reaction zone. Electrodes placed above the reaction zone only affected the flame tip, where the cation number density was low.
- For an electrode near or within the reaction zone, the flame transitioned from a teardrop shape to a flat flame at a voltage corresponding to an inflection point in the height–voltage profiles. The inflection point was independent of the electrode position within the reaction zone but moved to more negative values as the airflow rate increased. This voltage likely corresponds to an electric field for which ionic forces are equal to flow forces.
- The minimum achievable flame height depended only upon the electrode height above the burner. Once the cations were confined to a small volume, ionic forces were able to further contract the flame, regardless of flow rate and added impurity concentrations.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Victor, N.; Nichols, C. Future of hydrogen in the U.S. energy sector: MARKAL modeling results. Appl. Energy Combust. Sci. 2024, 18, 10. [Google Scholar] [CrossRef]
- Sun, M.J.; Duan, L.Q.; Zhou, Y.F.; Zhang, H.F.; Huang, L.C.; Zheng, N. Study on a novel hydrogen purification approach base on methane steam reforming process with CO-preferential oxidation and CO2 removal. Appl. Energy 2025, 377, 20. [Google Scholar] [CrossRef]
- Hayhurst, A.N. Mass spectrometric sampling of flames: How ionic equilibria in flames produce sampling falsifications and “fake” ions, but provide kinetic and thermodynamic data on the reaction occurring. Prog. Energy Combust. Sci. 2022, 88, 56. [Google Scholar] [CrossRef]
- Barmina, I.; Purmalis, M.; Valdmanis, R.; Zake, M. Electrodynamic Control of the Combustion Characteristics and Heat Energy Production. Combust. Sci. Technol. 2016, 188, 190–206. [Google Scholar] [CrossRef]
- Sánchez-Sanz, M.; Murphy, D.C.; Fernandez-Pello, C. Effect of an external electric field on the propagation velocity of premixed flames. Proc. Combust. Inst. 2015, 35, 3463–3470. [Google Scholar] [CrossRef]
- Fang, J.F.; Wu, X.M.; Duan, H.; Li, C.; Gao, Z.Q. Effects of Electric Fields on the Combustion Characteristics of Lean Burn Methane-Air Mixtures. Energies 2015, 8, 2587–2605. [Google Scholar] [CrossRef]
- Park, D.G.; Choi, B.C.; Cha, M.S.; Chung, S.H. Soot reduction under dc electric fields in counterflow non-premixed laminar ethylene flames. Combust. Sci. Technol. 2014, 186, 644–656. [Google Scholar] [CrossRef]
- Belhi, M.; Domingo, P.; Vervisch, P. Modelling of the effect of DC and AC electric fields on the stability of a lifted diffusion methane/air flame. Combust. Theory Model. 2013, 17, 749–787. [Google Scholar] [CrossRef]
- Wang, Y.; Nathan, G.J.; Alwahabi, Z.T.; King, K.D.; Ho, K.; Yao, Q. Effect of a uniform electric field on soot in laminar premixed ethylene/air flames. Combust. Flame 2010, 157, 1308–1315. [Google Scholar] [CrossRef]
- van den Boom, J.; Konnov, A.A.; Verhasselt, A.; Kornilov, V.N.; de Goey, L.P.H.; Nijmeijer, H. The effect of a DC electric field on the laminar burning velocity of premixed methane/air flames. Proc. Combust. Inst. 2009, 32, 1237–1244. [Google Scholar] [CrossRef]
- Payne, K.G.; Weinberg, F.J. A preliminary investigation of field-induced ion movement in flame gases and its applications. Proc. R. Soc. Lond. A 1959, 250, 316–336. [Google Scholar]
- Hu, J.; Rivin, B.; Sher, E. The effect of an electric field on the shape of co-flowing and candle-type methane-air flames. Exp. Therm. Fluid Sci. 2000, 21, 124–133. [Google Scholar] [CrossRef]
- Belhi, M.; Lee, B.J.; Cha, M.S.; Im, H.G. Three-dimensional simulation of ionic wind in a laminar premixed Bunsen flame subjected to a transverse DC electric field. Combust. Flame 2019, 202, 90–106. [Google Scholar] [CrossRef]
- Ghabi, A.; Boushaki, T.; Bocanegra, P.; Robert, E. Laminar Burning Velocity, Adiabatic Flame Temperature, and Pollutants of Biogas/Air Mixture at Various CO2 Concentrations and Plasma Assist. Combust. Sci. Technol. 2023, 195, 1599–1621. [Google Scholar] [CrossRef]
- Ghabi, A.; Darny, T.; Dozias, S.; Bocanegra, P.E.; Pouvesle, J.M.; Sarh, B.; Robert, E.; Boushaki, T. Effects of pulsed gliding arc plasma on non-premixed CH4/CO2-air flame stability. Therm. Sci. Eng. Prog. 2023, 40, 11. [Google Scholar] [CrossRef]
- Guan, W.L.; Fu, C.; Chen, J.L.; Liao, Y.H. Effects of electric field waveforms on a lifted non-premixed jet flame. Exp. Therm. Fluid Sci. 2024, 159, 15. [Google Scholar] [CrossRef]
- Saridede, E.; Kritikos, E.M.; Giusti, A. Investigation of the effect of electrostatic fields and iron nanoparticles on hydrogen-oxygen combustion. Proc. Combust. Inst. 2024, 40, 105769. [Google Scholar] [CrossRef]
- Shahsavari, M.; Konnov, A.A.; Bai, X.S.; Valera-Medina, A.; Li, T.; Jangi, M. Synergistic effects of nanosecond plasma discharge and hydrogen on ammonia combustion. Fuel 2023, 348, 128475. [Google Scholar] [CrossRef]
- Law, C.K. Combustion Physics; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Burke, M.P.; Chaos, M.; Ju, Y.G.; Dryer, F.L.; Klippenstein, S.J. Comprehensive H2/O2 kinetic model for high-pressure combustion. Int. J. Chem. Kinet. 2012, 44, 444–474. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.W.; Kazakov, A.; Dryer, F.L. An updated comprehensive kinetic model of hydrogen combustion. Int. J. Chem. Kinet. 2004, 36, 566–575. [Google Scholar] [CrossRef]
- Mueller, M.A.; Kim, T.J.; Yetter, R.A.; Dryer, F.L. Flow reactor studies and kinetic modeling of the H2/O2 reaction. Int. J. Chem. Kinet. 1999, 31, 113–125. [Google Scholar] [CrossRef]
- Davis, S.G.; Joshi, A.V.; Wang, H.; Egolfopoulos, F. An optimized kinetic model of H2/CO combustion. Proc. Combust. Inst. 2005, 30, 1283–1292. [Google Scholar] [CrossRef]
- Sun, H.Y.; Yang, S.I.; Jomaas, G.; Law, C.K. High-pressure laminar flame speeds and kinetic modeling of carbon monoxide/hydrogen combustion. Proc. Combust. Inst. 2007, 31, 439–446. [Google Scholar] [CrossRef]
- Knewstubb, P.F.; Sugden, T.M. Mass-spectrometric studies of ionization in flames. 1. the spectrometer and its application to ionization in hydrogen flames. Proc. R. Soc. Lond. A 1960, 255, 520–537. [Google Scholar]
- Green, J.A.; Sugden, T.M. Some observations on the mechanism of ionization in flames containing hydrocarbons. Proc. Combust. Inst. 1963, 9, 607–621. [Google Scholar] [CrossRef]
- Lawton, J.; Weinberg, F.J. Electrical Aspects of Combustion; Clarendon Press: Oxford, UK, 1969. [Google Scholar]
- Hayhurst, A.N.; Telford, N.R. Kinetics of dissociative recombination of free-electrons with hydronium ions in premixed flame. J. Chem. Soc. Faraday 1974, 70, 1999–2010. [Google Scholar] [CrossRef]
- Hayhurst, A.N.; Telford, N.R. Proton affinity of water and mechanism and kinetics of production of H3O+ in flames of H2, O2 and N2. J. Chem. Soc. Faraday 1975, 71, 1352–1362. [Google Scholar] [CrossRef]
- Goodings, J.M.; Hayhurst, A.N. Kinetics of electron-attachment to oxygen and water in flames. Nature 1979, 281, 204–206. [Google Scholar] [CrossRef]
- Axford, S.D.T.; Hayhurst, A.N. Ionization in premixed fuel-lean flames of H2, O2 and N2. 1. naturally-occurring positive-ions. J. Chem. Soc. Faraday 1995, 91, 827–833. [Google Scholar] [CrossRef]
- Axford, S.D.T.; Hayhurst, A.N. Ionization in premixed fuel-lean flames of H2, O2 and N2. 2. naturally-occurring positive-ions. J. Chem. Soc. Faraday 1995, 91, 835–841. [Google Scholar] [CrossRef]
- Butler, C.J.; Hayhurst, A.N. Kinetics of dissociative recombination of H3O+ ions with free electrons in premixed flames. J. Chem. Soc. Faraday 1996, 92, 707–714. [Google Scholar] [CrossRef]
- Goodings, J.M.; Guo, J.Z.; Hayhurst, A.N.; Taylor, S.G. Current-voltage characteristics in a flame plasma: Analysis for positive and negative ions, with applications. Int. J. Mass Spectrom. Ion. Proc. 2001, 206, 137–151. [Google Scholar] [CrossRef]
- Calcote, H.F. Ions in Flames. In Ion-Molecule Reactions; Springer: Berlin/Heidelberg, Germany, 1972; pp. 673–706. [Google Scholar]
- Zhang, M.; Wang, J.H.; Chang, M.; Huang, Z.H. Turbulent flame topology and the wrinkled structure characteristics of high pressure syngas flames up to 1.0 MPa. Int. J. Hydrogen Energy 2019, 44, 15973–15984. [Google Scholar] [CrossRef]
- Hayhurst, A.N.; Telford, N.R. Charge exchange reactions of H3O+ with metals in flame. Trans. Faraday Soc. 1970, 66, 2784–2793. [Google Scholar] [CrossRef]
- Jones, H.R.N.; Hayhurst, A.N. Measurements of the concentrations of positive and negative ions along premixed fuel-rich flames of methane and oxygen. Combust. Flame 2016, 166, 86–97. [Google Scholar] [CrossRef]
- Sugden, T.M. Excited species in flames. Annu. Rev. Phys. Chem. 1962, 13, 369–390. [Google Scholar] [CrossRef]
- Calcote, H.F. Mechanisms for the formation of ions in flames. Combust. Flame 1957, 1, 385–403. [Google Scholar] [CrossRef]
- Casey, T.A. Electric Field Effects in Combustion with Non-Thermal Plasma. Doctoral Dissertation, University of California, Berkeley, CA, USA, 2016. [Google Scholar]
- Bascombe, K.N.; Green, J.A.; Sugden, T.M. The Ionization Produced by Addition of Acetylene to a Hydrogen-Oxygen-Nitrogen Flame. In Advances in Mass Spectrometry; Elsevier: Amsterdam, The Netherlands, 1963; pp. 66–86. [Google Scholar]
- Knyazkov, D.A.; Gerasimov, I.E.; Shmakov, A.G.; Bolshova, T.A.; Paletsky, A.A. Positively charged species in premixed flames fuelled by H2/CO mixture: Experimental and numerical study. In Proceedings of the COMPHYSCHEM’20-21, Adelaide, Australia, 27–31 July 2020. [Google Scholar]
- Fialkov, A.B. Investigations on ions in flames. Prog. Energy Combust. Sci. 1997, 23, 399–528. [Google Scholar] [CrossRef]
- Prager, J.; Riedel, U.; Warnatz, J. Modeling ion chemistry and charged species diffusion in lean methane-oxygen flames. Proc. Combust. Inst. 2007, 31, 1129–1137. [Google Scholar] [CrossRef]
- Rodrigues, J.M.; Agneray, A.; Jaffrézic, X.; Bellenoue, M.; Labuda, S.; Leys, C.; Chernukho, A.P.; Migoun, A.N.; Cenian, A.; Savel’ev, A.M.; et al. Evolution of charged species in propane/air flames: Mass-spectrometric analysis and modelling. Plasma Sources Sci. Technol. 2007, 16, 161–172. [Google Scholar] [CrossRef]
- Knyazkov, D.A.; Cherepanov, A.V.; Kiselev, V.G.; Gerasimov, I.E.; Kasper, T.; Shmakov, A.G. Experimental and kinetic modeling study of the positive ions in premixed ethylene flames over a range of equivalence ratios. Proc. Combust. Inst. 2023, 39, 1753–1761. [Google Scholar] [CrossRef]
- Hayhurst, A.; Kittelson, D. The positive and negative ions in oxy-acetylene flames. Combust. Flame 1978, 31, 37–51. [Google Scholar] [CrossRef]
- Goodings, J.M.; Bohme, D.K.; Ng, C.W. Detailed ion chemistry in methane-oxygen flames. 1. positive-ions. Combust. Flame 1979, 36, 27–43. [Google Scholar] [CrossRef]
- Goodings, J.M.; Bohme, D.K.; Ng, C.W. Detailed ion chemistry in methane-oxygen flames. 2. negative-ions. Combust. Flame 1979, 36, 45–62. [Google Scholar] [CrossRef]
- Axford, S.D.T.; Hayhurst, A.N. Mass spectrometric sampling of negative ions from flames of hydrogen and oxygen: The kinetics of electron attachment and detachment in hot mixtures of H2O, O2, OH and HO2. Proc. R. Soc. A-Math. Phys. Eng. Sci. 1996, 452, 1007–1033. [Google Scholar]
- Axford, S.D.T.; Hayhurst, A.N. The stabilities of the gas-phase ions CO3− and HCO3−, together with the kinetics of both their production and removal in O2-rich flames of H2 + O2 + N2. Proc. R. Soc. A-Math. Phys. Eng. Sci. 1996, 452, 1035–1054. [Google Scholar]
- Calcote, H.F. Ion and electron profiles in flames. Symp. Int. Combust. 1963, 9, 622–637. [Google Scholar] [CrossRef]
- Belhi, M.; Domingo, P.; Vervisch, P. Direct numerical simulation of the effect of an electric field on flame stability. Combust. Flame 2010, 157, 2286–2297. [Google Scholar] [CrossRef]
- Liu, H.C.; Cai, W.W. Recent progress in electric-field assisted combustion: A brief review. Front. Energy 2022, 16, 883–899. [Google Scholar] [CrossRef]
- Park, D.G.; Chung, S.H.; Cha, M.S. Bidirectional ionic wind in nonpremixed counterflow flames with DC electric fields. Combust. Flame 2016, 168, 138–146. [Google Scholar] [CrossRef]
- Xia, H.; Wang, J.H.; Ju, R.Y.; Li, Y.M.; Mu, H.B.; Huang, Z.H. Effect of Ionic Wind Induced by DC Electric Field on Biogas/Air Turbulent Premixed Flame Structure. Combust. Sci. Technol. 2023, 195, 294–312. [Google Scholar] [CrossRef]
- Yamashita, K.; Karnani, S.; Dunn-Rankin, D. Numerical prediction of ion current from a small methane jet flame. Combust. Flame 2009, 156, 1227–1233. [Google Scholar] [CrossRef]
- Di Renzo, M.; Urzay, J.; De Palma, P.; de Tullio, M.D.; Pascazio, G. The effects of incident electric fields on counterflow diffusion flames. Combust. Flame 2018, 193, 177–191. [Google Scholar] [CrossRef]
- Seo, B.H.; Kim, K.T.; Sapkal, N.P.; Chung, S.H. Effect of AC Electric Field on Decreasing Liftoff Height in Laminar Lifted Jet Flames. J. Korean Soc. Combust. 2017, 22, 17–22. [Google Scholar]
- Xiong, Y.; Park, D.G.; Lee, B.J.; Chung, S.H.; Cha, M.S. DC field response of one-dimensional flames using an ionized layer model. Combust. Flame 2016, 163, 317–325. [Google Scholar] [CrossRef]
- Pedersen, T.; Brown, R. Simulation of electric field effects in premixed methane flames. Combust. Flame 1993, 94, 433–448. [Google Scholar] [CrossRef]
- Dunn-Rankin, D.; Weinberg, F.J. Using large electric fields to control transport in microgravity. In Interdisciplinary Transport Phenomena in the Space Sciences; Sadhal, S.S., Ed.; Annals of the New York Academy of Sciences; Wiley-Blackwell: Malden, MA, USA, 2006; Volume 1077, pp. 570–584. [Google Scholar]
- Chien, Y.C.; Stocker, D.P.; Hegde, U.G.; Dunn-Rankin, D. Electric-field effects on methane coflow flames aboard the international space station (ISS): ACME E-FIELD flames. Combust. Flame 2022, 246, 11. [Google Scholar] [CrossRef]
- Xiong, Y.; Lacoste, D.A.; Chung, S.H.; Cha, M.S. Effects of DC Electric Fields on Flickering and Acoustic Oscillations of an M-shape Premixed Flame. Flow Turbul. Combust. 2022, 109, 459–475. [Google Scholar] [CrossRef]
- Kuhl, J.; Seeger, T.; Zigan, L.; Will, S.; Leipertz, A. On the effect of ionic wind on structure and temperature of laminar premixed flames influenced by electric fields. Combust. Flame 2017, 176, 391–399. [Google Scholar] [CrossRef]
- Cha, M.S.; Lee, S.M.; Kim, K.T.; Chung, S.H. Soot suppression by nonthermal plasma in coflow jet diffusion flames using a dielectric barrier discharge. Combust. Flame 2005, 141, 438–447. [Google Scholar] [CrossRef]
- Weng, W.G.; Kamikawa, D.; Fukuda, Y.; Hasemi, Y.; Kagiya, K. Study on flame height of merged flame from multiple fire sources. Combust. Sci. Technol. 2004, 176, 2105–2123. [Google Scholar] [CrossRef]
- Hamins, A.; Konishi, K.; Borthwick, P.; Kashiwagi, T. Global properties of gaseous pool fires. Proc. Combust. Inst. 1996, 26, 1429–1436. [Google Scholar] [CrossRef]
- Mikofski, M.A.; Williams, T.C.; Shaddix, C.R.; Blevins, L.G. Flame height measurement of laminar inverse diffusion flames. Combust. Flame 2006, 146, 63–72. [Google Scholar] [CrossRef]
- Luo, Y.L.; Jiang, Z.W.; Gan, Y.H.; Liang, J.L.; Ao, W. Evaporation and combustion characteristics of an ethanol fuel droplet in a DC electric field. J. Energy Inst. 2021, 98, 216–222. [Google Scholar] [CrossRef]
- He, L.; Guo, Q.H.; Gong, Y.; Wang, F.C.; Yu, G.S. Investigation of OH* chemiluminescence and heat release in laminar methane-oxygen co-flow diffusion flames. Combust. Flame 2019, 201, 12–22. [Google Scholar] [CrossRef]
- Li, Z.S.; Li, B.; Sun, Z.W.; Bai, X.S.; Aldén, M. Turbulence and combustion interaction: High resolution local flame front structure visualization using simultaneous single-shot PLIF imaging of CH, OH, and CH2O in a piloted premixed jet flame. Combust. Flame 2010, 157, 1087–1096. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.G.; Wan, M.G.; Zhang, L.; Yao, X. Quantitative Measurement of OH* and CH* Chemiluminescence in Jet Diffusion Flames. ACS Omega 2020, 5, 15922–15930. [Google Scholar] [CrossRef]
- Walsh, K.T.; Long, M.; Tanoff, M.A.; Smooke, M.D. Experimental and computational study of CH, CH*, and OH* in an axisymmetric laminar diffusion flame. Proc. Combust. Inst. 1998, 27, 615–623. [Google Scholar] [CrossRef]
- López-Cámara, C.F.; Saggese, C.; Pitz, W.J.; Shao, X.; Imc, H.G.; Dunn-Rankin, D. Reduced chemical kinetic model for CH4-air non-premixed flames including excited and charged species. Combust. Flame 2023, 253, 14. [Google Scholar] [CrossRef]
- Higgins, B.; McQuay, M.Q.; Lacas, F.; Rolon, J.C.; Darabiha, N.; Candel, S. Systematic measurements of OH chemiluminescence for fuel-lean, high-pressure, premixed, laminar flames. Fuel 2001, 80, 67–74. [Google Scholar] [CrossRef]
- Xie, F.; Zhou, Y.; Song, X.D.; Bai, Y.H.; Wu, R.M.; Yao, M.; Yu, G.S. Investigation of OH* chemiluminescence with lift-off characteristic in methane-oxygen inverse diffusion flame. Int. J. Hydrogen Energy 2021, 46, 1461–1472. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Weller, L.; Hochgreb, S. Instantaneous flame front identification by Mie scattering vs. OH PLIF in low turbulence Bunsen flame. Exp. Fluids 2022, 63, 16. [Google Scholar] [CrossRef]
- Xiong, Y.; Cha, M.S.; Chung, S.H. AC electric field induced vortex in laminar coflow diffusion flames. Proc. Combust Inst. 2015, 35, 3513–3520. [Google Scholar] [CrossRef]
- Zhao, M.M.; Buttsworth, D.; Choudhury, R. Experimental and numerical study of OH* chemiluminescence in hydrogen diffusion flames. Combust. Flame 2018, 197, 369–377. [Google Scholar] [CrossRef]
- Kwak, S.; Choi, J.; Ahn, M.; Yoon, Y. Effects of hydrogen addition on the forced response of H2/CH4 flames in a dual-nozzle swirl-stabilized combustor. Int. J. Hydrogen Energy 2022, 47, 28139–28151. [Google Scholar] [CrossRef]
- Altendorfner, F.; Kuhl, J.; Zigan, L.; Leipertz, A. Study of the influence of electric fields on flames using planar LIF and PIV techniques. Proc. Combust. Inst. 2011, 33, 3195–3201. [Google Scholar] [CrossRef]
- Cessou, A.; Varea, E.; Criner, K.; Godard, G.; Vervisch, P. Simultaneous measurements of OH, mixture fraction and velocity fields to investigate flame stabilization enhancement by electric field. Exp. Fluids 2012, 52, 905–917. [Google Scholar] [CrossRef]
- Kojima, J.; Ikeda, Y.; Nakajima, T. Basic aspects of OH(A), CH(A), and C2(d) chemiluminescence in the reaction zone of laminar methane-air premixed flames. Combust. Flame 2005, 140, 34–45. [Google Scholar] [CrossRef]
- Ayoolan, B.O.; Balachandran, R.; Frank, J.H.; Mastorakos, E.; Kaminski, C.F. Spatially resolved heat release rate measurements in turbulent premixed flames. Combust. Flame 2006, 144, 1–16. [Google Scholar] [CrossRef]
- Paul, P.H.; Najm, H.N. Planar laser-induced fluorescence imaging of flame heat release rate. Proc. Combust. Inst. 1998, 27, 43–50. [Google Scholar] [CrossRef]
- Santoro, R.J.; Semerjian, H.G.; Dobbins, R.A. Soot particle measurements in diffusion flames. Combust. Flame 1983, 51, 203–218. [Google Scholar] [CrossRef]
- Park, J.; Son, J.; Butterworth, T.D.; Cha, M.S. Electric fields in a counterflow nonpremixed flame: Measurement and simulation. Sci. Rep. 2023, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Abboud, J.; Schobing, J.; Legros, G.; Matynia, A.; Bonnety, J.; Tschamber, V.; Brillard, A.; Leyssens, G.; Da Costa, P. Impacts of ester’s carbon chain length and concentration on sooting propensities and soot oxidative reactivity: Application to Diesel and Biodiesel surrogates. Fuel 2018, 222, 586–598. [Google Scholar] [CrossRef]
- Lacoste, D.A.; Xiong, Y.; Moeck, J.P.; Chung, S.H.; Roberts, W.L.; Cha, M.S. Transfer functions of laminar premixed flames subjected to forcing by acoustic waves, AC electric fields, and non-thermal plasma discharges. Proc. Combust. Inst. 2017, 36, 4183–4192. [Google Scholar] [CrossRef]
- Simeni, M.S.; Tang, Y.; Hung, Y.C.; Eckert, Z.; Frederickson, K.; Adamovich, I.V. Electric field in Ns pulse and AC electric discharges in a hydrogen diffusion flame. Combust. Flame 2018, 197, 254–264. [Google Scholar] [CrossRef]
- ANSYS. ANSYS FLUENT; ANSYS, Inc.: Canonsburg, PA, USA, 2011. [Google Scholar]
- Sayed-Kassem, A.; Elorf, A.; Gillon, P.; Idir, M.; Sarh, B.; Gilard, V. Numerical modelling to study the effect of DC electric field on a laminar ethylene diffusion flame. Int. Commun. Heat Mass Transf. 2021, 122, 13. [Google Scholar] [CrossRef]
- Luo, Y.L.; Gan, Y.H.; Xu, J.L.; Yan, Y.Y.; Shi, Y.L. Effects of electric field intensity and frequency of AC electric field on the small-scale ethanol diffusion flame behaviors. Appl. Therm. Eng. 2017, 115, 1330–1336. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, B.Y.; Zhao, B.; Li, T.W.; Shu, Q. Numerical Simulation of Ethanol Air Diffusion Flame Quenching under Transverse AC Electric Field. Fire 2022, 5, 196. [Google Scholar] [CrossRef]
- Sakhrieh, A.; Lins, G.; Dinkelacker, F.; Hammer, T.; Leipertz, A.; Branston, D.W. The influence of pressure on the control of premixed turbulent flames using an electric field. Combust. Flame 2005, 143, 313–322. [Google Scholar] [CrossRef]
- Chemical-Kinetic Mechanisms for Combustion Applications, San Diego Mechanism Web Page, Mechanical and Aerospace Engineering (Combustion Research), University of California at San Diego. Available online: http://combustion.ucsd.edu (accessed on 1 June 2024).
- Schefer, R.W.; Kulatilaka, W.D.; Patterson, B.D.; Settersten, T.B. Visible emission of hydrogen flames. Combust. Flame 2009, 156, 1234–1241. [Google Scholar] [CrossRef]
- Vinogradov, J.; Sher, E.; Rutkevich, I.; Mond, M. Voltage-current characteristics of a flame-assisted unipolar corona. Combust. Flame 2001, 127, 2041–2050. [Google Scholar] [CrossRef]



| Electrode Distance (mm) | Maximum % Decrease | Initial Threshold (kV) | Inflection Point (kV) | |
|---|---|---|---|---|
| OH* Height | OH* Width | |||
| 0.00 | 62 (−8.00 kV) | 17 (−8.00 kV) | −5.25 | −6.75 |
| 5.00 | 67 (−9.50 kV) | - | −4.75 | - |
| 7.50 | 65 (−10.00 kV) | - | −5.00 | - |
| 10.00 | 42 (−10.00 kV) | 39 (−10.00 kV) | - | - |
| 15.00 | 35 (−10.00 kV) | 30 (−10.00 kV) | - | - |
| 20.00 | 33 (−10.00 kV) | 26 (−10.00 kV) | −7.75 | −8.25 |
| Airflow | Initial Threshold (kV) | Inflection Point (kV) | Maximum % Decrease in OH* Height | |
|---|---|---|---|---|
| Rate (slm) | Velocity (cm s−1) | |||
| 10 | 2.44 | −5.25 | −6.75 | 63(−8.00) |
| 25 | 6.10 | −5.25 | −6.75 | 62(−8.00) |
| 79 | 19.34 | −5.50 | −7.25 | 60(−8.50) |
| 85 | 20.72 | −5.50 | −7.50 | 61(−9.00) |
| 10 * | 13.37 | −5.00 | −6.50 | 62(−8.00) |
| 40 * | 53.01 | −5.50 | −8.00 | 60(−9.25) |
| 56 * | 75.72 | −5.75 | −8.75 | 52(−10.00) |
| 90 * | 121.16 | −6.25 | – | 21(−10.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halowitage, S.D.P.G.; Perera, H.E.; Elmore, N.M.; Goulay, F. Effect of Applied DC Electric Fields on H2–Air Axisymmetric Laminar Co-Flow Diffusion Flames with Low Carbon Impurities. Hydrogen 2025, 6, 38. https://doi.org/10.3390/hydrogen6020038
Halowitage SDPG, Perera HE, Elmore NM, Goulay F. Effect of Applied DC Electric Fields on H2–Air Axisymmetric Laminar Co-Flow Diffusion Flames with Low Carbon Impurities. Hydrogen. 2025; 6(2):38. https://doi.org/10.3390/hydrogen6020038
Chicago/Turabian StyleHalowitage, Susith D. P. G., Hasith E. Perera, Nicholas M. Elmore, and Fabien Goulay. 2025. "Effect of Applied DC Electric Fields on H2–Air Axisymmetric Laminar Co-Flow Diffusion Flames with Low Carbon Impurities" Hydrogen 6, no. 2: 38. https://doi.org/10.3390/hydrogen6020038
APA StyleHalowitage, S. D. P. G., Perera, H. E., Elmore, N. M., & Goulay, F. (2025). Effect of Applied DC Electric Fields on H2–Air Axisymmetric Laminar Co-Flow Diffusion Flames with Low Carbon Impurities. Hydrogen, 6(2), 38. https://doi.org/10.3390/hydrogen6020038







