Abstract
Hydrogen plays a leading role in achieving a future with net zero greenhouse gas emissions. The present challenge is producing green hydrogen to cover the fuel demands of transportation and industry to gain independence from fossil fuels. This review’s goal is to critically demonstrate the existing methods of biomass treatment and assess their ability to scale up. Biomass is an excellent hydrogen carrier and biomass-derived processes are the main target for hydrogen production as they provide an innovative pathway to green hydrogen production. Comparing the existing processes, thermochemical treatment is found to be far more evolved than biological or electrochemical treatment, especially with regard to scaling prospects.
1. Introduction
1.1. Why Hydrogen?
The increasing population has increased the energy demand and, as per the International Energy Agency (IEA), by 2030, the energy demand may be increased by 50% globally []. The world’s most exploited energy sources are fossil fuels and their derivatives [,,]. The excessive use of these fuels increases greenhouse gases (GHGs) such as CO2, which, in turn, have a noteworthy effect on global warming and climate change [,]. The climate goals outlined within the Paris Agreement (COP21) aim to establish strategies for the mitigation of GHG emissions to prevent the global average temperature from rising by more than 2 °C above the temperature levels in the pre-industrial era [].
To tackle this issue, the replacement of conventional energy sources with environmentally friendly energy sources is crucial []. The long-term replacement of fossil fuels can be accomplished through the enhanced use of sustainable energy options in the energy mix. In this review, particular focus is given to hydrogen (H2) as an energy carrier generated from renewable energy sources and solutions that could obtain zero-emission levels in transport, industry, buildings, the energy sector, etc.
Hydrogen is considered to be a green fuel as the product of H2 combustion is water vapor. Thus, it has zero CO2 emissions when used to produce energy (e.g., via fuel cells or ICEs) [,,,]. Its heating value (on a mass basis) surpasses that of methane, gasoline, and coal by 2.4, 2.8, and 4 times, respectively, and it has a 100 times higher energy density than a conventional lithium–ion battery. When compared to other known fuels, hydrogen has the highest energy content per unit weight. It also has many other characteristics, such as a better storage capability than electricity, that make it an attractive and probable candidate to play a significant role as a fuel for the future [,,,,]. Concerning H2 production, it is predicted that 50–82 Mt of H2 is generated, with a rate of growth of 5–10% per year estimated by the year 2050 [,]. Its transportation can be achieved through the conventional means of domestic/industrial consumption. H2 safety for transportation and handling is comparable to that of domestic natural gas []. However, hydrogen demonstrates an extremely low energy density per unit of volume, primarily due to it being the lightest element of the periodic table, even when compressed at high pressures [].
In Table 1, the lower heating value and energy density per volume of hydrogen are presented, for a better understanding of hydrogen’s features, in comparison to other well-known and widely used fuels.

Table 1.
Energy contents of different fuels per weight [,] and per volume [,].
Considering all these characteristics, hydrogen is worth exploring as a renewable energy carrier and is particularly interesting in applications such as heavy-duty applications, transportation, and other industrial cases, where electricity can be hard to use [].
1.2. Hydrogen Production Methods
To date, 48% of hydrogen production derives from natural gas, 30% from heavy oils and naphtha, and 18% from coal, with a total of 96% deriving from non-renewable energy sources [,,,,].
The most common approaches to hydrogen production relying on fossil fuels are as follows:
- Steam reforming of natural gas (SR): This method involves the catalytic conversion of hydrocarbon and steam to hydrogen and carbon oxides. It consists of the main steps of reforming or synthesis gas (syngas) generation, a water–gas shift (WGS), and methanation or gas purification [,]. This method is the most common way of producing hydrogen and has a TRL of nine [].
- Partial oxidation process (POX): This method involves the conversion of steam, oxygen, and hydrocarbons to hydrogen and carbon oxides. The catalytic process occurs at 950 °C with feedstock changing from methane to naphtha. The non-catalytic process occurs at 1150–1315 °C with feedstock that includes methane, heavy oil, and coal. After sulfur removal, pure O2 is used to partially oxidize the hydrocarbon feedstock. The syngas that is produced is further treated in the same way as the product gas of the SR process [,].
Hydrogen is to be used as a sustainable alternative to fossil fuels. Therefore, it is essential for it to be produced without net emissions of GHG []. The above, currently employed, methods are accompanied by considerable GHG emissions; therefore, they should be replaced by alternative ones that are primarily based on renewables.
- Water Electrolysis: This method uses an electrical current in order to separate water into oxygen and hydrogen. This approach results in the production of green hydrogen without any direct release of carbon dioxide emissions. The process is exceptionally endothermic. Thus, renewable energy sources can provide the required energy input [,,,,,].
Water electrolysis stands as one of the most promising and eco-friendly alternatives for the production of hydrogen. It is a technology with a technology readiness level (TRL) of nine and is already producing around 2–3% of the world’s hydrogen [].
In summary, green hydrogen production is still in its infancy when it comes to industrial-scale production. Using biomass as an energy source for green hydrogen production offers the possibility of boosting green hydrogen production rates. In this review, the main biomass processes used for hydrogen production will be presented and compared. More specifically, the structure is based on three categories of treatment: biological, electrochemical, and thermochemical.
The primary purpose of this review is to present an all-round view of alternative methods utilizing biomass to generate green hydrogen and to demonstrate the most advantageous method. A complementary aim of this review is for it to consist as a first step in further research. Research on green hydrogen production has attracted significant attention and this review summarizes numerous studies and showcases the most promising and viable path for large-scale hydrogen production so that it can be implemented in a full-scale plant. Notably, we present an extended analysis of each method’s characteristics, challenges, yields, and costs, as well as gaps in the literature and other obstacles.
2. Alternative Processes from Biomass
Biomass is a source of energy that derives from plant and animal material. It is renewable and it can consist of energy crops, crop residues, forest wood, forest residues, grass, industrial residues, animal and municipal waste, etc. Biomass mainly consists of organic matter, in which the energy of sunlight is stored in chemical bonds through photosynthesis. When biomass is utilized to produce energy, CO2 is released. However, the quantity of CO2 emitted is equivalent to the amount absorbed by the organisms during their lifetime [,,,]. Therefore, there is no contribution to the carbon cycle; it is only accelerated [,].
In spite of the numerous benefits of producing hydrogen from biomass, as mentioned before, it is not being used commercially at present. Biomass-derived feedstock has limitations, especially when it is highly distributed, for example in agricultural residues, forest residues, and organic waste.
2.1. Biological Treatment
2.1.1. Dark Fermentation
Dark fermentation is based on anaerobic bacteria growing in the dark that decompose biomass []. Bacteria, such as Enterobacter, Bacillus, and Clostridium, are known to produce hydrogen [,]. Most known bacteria used in dark fermentation belong to Clostridium sp. including, C. buytricum, C. thermolacticum, C. pasteurianum, and C. bifermentants [,]. The bacteria or micro-algae are sustained in dark conditions at 25–80 °C. Depending on the strains, they can be sustained even at hyperthermophilic temperatures (>80 °C) [].
Among other carbohydrates, glucose serves as the most favored carbon source regarding the fermentation process. With glucose used as the main model substrate, acetic and butyric acids are produced and cover more than 80% of the total products. In theory, when 1 mol of glucose undergoes bioconversion, 12 mol of hydrogen are produced [,,].
The reaction is displayed below [,]:
C6H12O + 2H2O → 2CH3COOH + 4H2 + 2CO2 (acetate fermentation)
C6H12O + 2H2O → CH3CH2CH2COOH + 2H2 + 2CO2 (butyrate fermentation)
Numerous carbon sources are able to be treated with anaerobic dark fermentative bacteria to produce hydrogen, e.g., simple sugars (glucose, sucrose, lactose), waste containing starch or cellulose, food industry waste, and wastewaters [,]. Using different substrates results in different yields and studies show that sucrose and acetate can achieve high hydrogen yields [].
The conditions in which the process is occurring, e.g., the pH (between 5 and 6) [,], hydraulic retention time (HΡΤ), and gas partial pressure, act on the metabolism balance of the bacteria used in fermentation. The H2 partial pressure is one of the most critical factors because hydrogen synthesis pathways are sensitive to H2 concentration. As H2 concentration increases, the H2 synthesis decreases [,]. Thus, the H2 gas must be removed as it is generated [,].
2.1.2. Photo Fermentation
Photo fermentation (PF) takes place in nitrogen-deficient conditions and with the implementation of purple non-sulfur bacteria []. Some examples include Rhodobacter sphaeroides, Rhodobacter capsulatus, Rhodobacter sulfidophilus, Rhodopseudomonas palustris, and Rhodospirillum rubrum. These bacteria present a variable metabolism regulated by the carbon source, light intensity, and degree of anaerobiosis []. The reaction is catalyzed by nitrogenases enabling the conversion of organic acids, such as acetic, lactic, and butyric acids, to hydrogen and carbon dioxide through photosynthetic bacteria [].
The reaction is displayed below:
CH3COOH +2H2O + light energy → 4H2 + 2CO2
These photosynthetic bacteria are suitable for converting light energy to hydrogen through the utilization of organic wastes as a substrate [,,] in batch processes [] and continuous cultures []. It is essential to achieve and control the proper ratio of carbon to nitrogen nutrients to increase the nitrogenase activity and decrease the energy demand [,]. Moreover, the light intensity is a factor that contributes to hydrogen production rate and yield but has a negative effect on the light conversion efficiency [].
PF has been studied as a waste-prevention process to produce H2 from industrial and agricultural wastes [].
2.1.3. Biocatalyzed Electrolysis
This process is another way of oxidizing organic matter for hydrogen production. The different aspect of this method is that the external energy required is in the form of electrical energy [].
Τhis method takes place in a microbial electrolysis cell (MEC) [], often referred to as a bio-electrochemically assisted microbial reactor (BEAMR) [,], a biocatalyzed electrolysis cell (BEC) [], or electrohydrogenesis [,,]. Microorganisms that are electrochemically active are able to utilize an electrode as an electron acceptor under anaerobic conditions, as they release the electrons at a high energy level. As a result, the electrode turns into a bioanode. The organic matter is electrolyzed and then hydrogen is generated. The applied voltage required for this method of electrolysis is about 10–12 mV [,,]. The anode materials are essential in influencing the bacterial adherence to the anode, the electrode’s biocompatibility, and the electron transmission [,]. The schematic illustration (Scheme 1) appears below [].
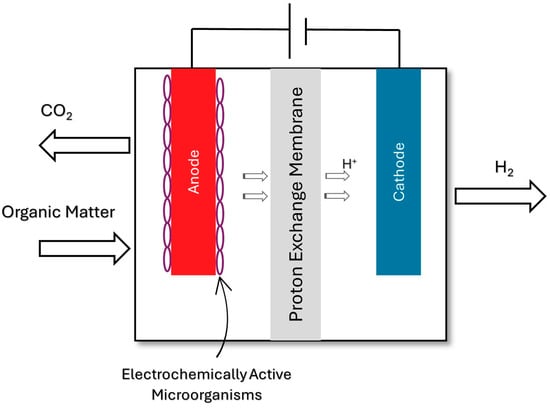
Scheme 1.
Schematic illustration of hydrogen production through biocatalyzed electrolysis [].
Biocatalyzed electrolysis is also capable of converting dissolved organic compounds that might be produced from dark fermentation, addressing the endothermic tendency of these reactions. It is also very flexible as a method, as it is designed to produce hydrogen from wastewater [].
2.2. Electrochemical Treatment
Electrooxidation
Electrosynthesis has seen a rebirth in the last decade due to research on a dual production platform for both molecules and energy carriers. It is referred to as a “Power-to-X” approach, where X refers to the fuel or chemical. “X-to-Power” refers to a strategy in which clean energy is produced from an energy carrier, as an inverse approach. A fuel cell, in the broadest sense, is an electrochemical device made up of two electrodes separated by a spacer that turns chemical energy directly into electrical energy (while releasing heat). Thermodynamic and faradaic contributions will not alter during a fuel cell’s normal operation if the reaction selectivity does not change significantly. Thus, the efficiency is solely influenced by the experimentally measured cell voltage U [].
The research on fuel cells and electrolysis cells is closely linked with the study of organic selective electrooxidation processes. Their slow electrochemical kinetics have been overcome by a variety of (bio)catalytic interfaces. The key electrocatalytic reaction characteristics that have been established allow for the proposal of new materials that could maximize the activity, selectivity, and durability of anode materials [].
The oxygen reduction reaction (ORR), which is carried out by a cathode, is thermodynamically anticipated to begin at about 1.2 V when compared to the reversible hydrogen electrode (RHE). The best activity is attained in the potential range of 1.0–0.7 V versus RHE due to ORR’s sluggishness. The primary criterion for choosing anode electrocatalysts for fuel cells (to achieve voltage optimization) is their ability to achieve electrooxidation at the lowest electrode potential (E), ideally E < 0.5 V vs. RHE, with the lowest overpotential [].
The electro-oxidation of bio-derived feedstocks may provide compounds with a significant economic value that can increase income and represent a more cost-competitive option. According to studies, most electrocatalysts used for the electro-oxidation of organic compounds have an increased activity and stability under alkaline conditions [,,,]. The schematic illustration (Scheme 2) is presented below [].
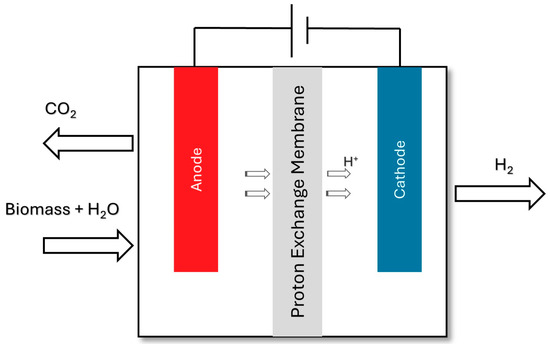
Scheme 2.
Schematic illustration of hydrogen production through electrooxidation [].
The main drawback of alkaline electro-oxidation is its lower H2 selectivity. H2 accounts for no more than 6% of the total product share in the processes. The demand for hydrogen, however, is projected to experience substantial growth over time in comparison to the coproducts []. The lack of a sizable enough market for the coproducts may limit the total deployment potential of this technology to small-scale applications [].
2.3. Thermochemical Treatment
The most mature process for producing hydrogen from biomass is thermochemical conversion []. Aqueous phase reforming, pyrolysis, and gasification are the three main thermochemical methods [,]. In these conversion methods, CH4 and CO are produced and can be further processed to increase hydrogen production through steam reforming and the WGS reaction [].
2.3.1. Gasification
Gasification constitutes a thermochemical treatment according to which biomass is decomposed at elevated temperatures in an environment with limited oxygen [,]. It is highly endothermic and takes place at temperatures between 500 and 1400 °C and at operating pressures, depending on the plant scale, ranging from atmospheric to 33 bar []. The process can be classified as air gasification, oxygen gasification, or steam gasification, as determined by the oxidizing agent utilized [].
The gasifier is the most significant element of a gasification plant. It offers enough space to mix biomass and the gasification agent to a certain level, sometimes in the presence of primary catalysts and/or additives []. Gasification can generally be categorized into three groups, depending on how the process is structured: dual-fluidized bed (DFB) steam-blown gasification [], also referred to as indirect gasification, direct-blown, steam/oxygen or air, fluidized-bed gasification [], and entrained flow gasification [,].
The feedstock normally undergoes pre-treatment before being introduced into the gasification reactor. This frequently entails drying the biomass to increase conversion rates in the gasification reactor, but it can also entail the use of other processes, including milling, to produce a uniform feedstock in terms of size [,].
Syngas, a gas mixture composed of hydrogen, methane, carbon monoxide, nitrogen, and carbon dioxide, is produced when it consumes an oxidizing agent []. Minor organic and inorganic contaminants have also been discovered in syngas. The inorganic molecules include H2S, HCl, NH3, and alkali metals, while the organic compounds include light hydrocarbons (LHC), such as CH4, and tar, a viscous liquid made up of condensable organic compounds [,]. Depending on the desired end-product of the gasification process, a range of product gas upgrading and conditioning sequences may be included in the process design [].
The general air gasification reaction of biomass is displayed below [,]:
Biomass + Air → N2 + CO + H2 + CO2 + CH4 + H2O + LHC + Tar + Char
Regarding feedstock, air gasification requires a dry raw material []. The main challenge in air gasification is the removal of nitrogen from the products, as it is a non-combustible gas that results in a reduction in the heat value of the fuel.
The product of oxygen gasification is a gas of greater purity, containing a higher level of H2, less tar and char, and no nitrogen.
Steam gasification serves as a middle ground between air and oxygen gasification. This process can take place with wet biomass (moisture between 5 and 35 wt%) [].
The general reaction of biomass’ steam gasification is presented below []:
Biomass + Steam → H2 + CO + CO2 + CH4 + HC + Tar + Char
Gasification also produces CO and CH4, which can be further treated to make more hydrogen if they undergo steam reforming and the WGS reaction [].
2.3.2. Pyrolysis
Pyrolysis is another thermochemical method which does not require an oxidizing agent and can be achieved at lower temperatures of 400–800 °C [] at 0.1–0.5 MPa []. The method is categorized according to the rising reaction temperature into three primary classifications: slow pyrolysis, fast pyrolysis, and flash pyrolysis []. This reaction is also highly endothermic [].
The three key components of a biomass pyrolysis feedstock are cellulose, hemicellulose, and lignin. These three constituents comprise up to 90% of the lignocellulosic biomass, with ash and extracts making up the remaining 10% or so [,]. The type of feedstock, the type of catalyst that is utilized, the temperature, and the time of residence affect the yield of hydrogen production from biomass pyrolysis [,,].
Biomass pyrolysis typically occurs in four steps []: pre-heating and drying, pre-pyrolysis, solid decomposition, and the residual char decomposition process, which is a complex chemical process that includes numerous simultaneous reactions [].
The general pyrolysis reaction is presented below []:
Biomass + Heat → H2 + CO + CO2 + CH4 + H2O + Bio-Oil + Charcoal
Methane and other produced hydrocarbon gases, similar to gasification, can be steam-reformed, and the WGS process is utilized to produce even more hydrogen. After pyrolysis, condensed oxygenated molecules (aldehydes, ketones, alcohols, phenolic compounds, and carboxylic acids), water, and ash can create bio-oil, a complex liquid fraction. The water-insoluble fraction and water-soluble fraction are the two categories into which bio-oils can be separated. For usage in adhesive applications, the insoluble fraction can be broken down into platform molecules like BTX or olefins []. The integration of a steam gasification device can enhance the yield of H2 produced from the soluble fraction [].
2.3.3. Biogas Reforming
Biogas may be produced from biomass and agricultural residue, such as rice straw and wheat straw, via anaerobic digestion. It may also be obtained from landfills; in such cases, it is referred to as landfill gas. The composition of biogas differs according to the feedstock and anaerobic digesters utilized []. Biogas is a practical and sustainable energy resource due to the abundant supply of low-cost feedstocks []. In most studies, the biogas composition is simulated at 60% CH4 and 40% CO2. In Europe, biogas is mostly produced by the anaerobic digestion of agricultural or industrial wastes, as well as biowaste, municipal organic waste, and sewage sludge [,]. Table 2 [,,,,,] and Table 3 [] demonstrate the chemical composition of biogas and the differences in the composition when biogas is produced form landfill and anaerobic digestion, respectively. Also, natural gas composition is being displayed for further comparison of the two fuels.

Table 2.
Chemical composition of biogas [,,,,,].

Table 3.
Chemical composition of biogas from landfill and anaerobic digestion compared to natural gas [].
Steam reforming (SR) and autothermal reforming (ATR) are methods that have been established as prototypes for biogas-to-hydrogen generation due to their significant similarity to natural gas []. Biogas steam reforming (BSR) produces H2 at temperatures ranging from 600 to 1000 °C through endothermic and reversible reactions, typically coupled with catalytic methods []. Two reforming techniques are combined in the ATR process: SR and catalytic partial oxidation (CPOX) [,]. Both of these processes may be carried out at low pressure (usually at atmospheric pressure) in tubular fixed-bed or fluidized reactors [,,,,]. The gas mixture produced by the conversion process has a high concentration of hydrogen; thus, CO2 and other contaminants must be separated from the gas output [].
The reforming reactions are displayed below []:
Steam Reforming (SR): CH4 + H2O → CO + 3H2, ΔH = 206.2 kJ/mol;
Catalytic Partial Oxidation (POX): CH4 + ½ O2 → CO + 2H2, ΔH = −36 kJ/mol;
Autothermal Reforming (ATR): CH4 + x½O2 + yCO2 + (1 − x − y)H2O
→(y + 1)CO + (3 − x − y)H2.
→(y + 1)CO + (3 − x − y)H2.
Biogas reforming takes place in several steps in order to obtain purity. The process includes the following units, presented in order: a high-temperature reformer, a high-temperature shift reactor (HT), a low-temperature shift reactor (LT), and a separation unit []. At present, several gas separation technologies are able to separate hydrogen from the synthesis gas. The membrane gas separation system is simple to use, requires less energy, has a compact footprint, can operate continuously, and is easy to scale up, among other advantages. [,]. Steam methane reforming demands a feedstock stream with high purity. Thus, we make the assumption that biogas is upgraded with 99.5% purity [].
Another interesting method to process biogas is dry reforming (DR) because it eliminates the need to separate CO2, in contrast to BSR, Pox, and ATR, as both CH4 and CO2 are converted into H2 and CO []. Other contaminants, such as siloxanes, chlorides, most often CH3CL, and benzene derivatives, can lead to catalyst deactivation. Sulfur is especially likely to do so, since it is highly absorbent on the metal catalysts’ surfaces. However, current technologies can significantly remove impurities, particularly sulfur and siloxanes; thus, impurities do not cause concern during this process [].
Dry reforming is highly endothermic and takes place at temperatures between 700 and 900 °C. The reaction also requires a CO2/CH4 ratio between 1 and 1.5. Typically, the reverse water–gas shift reaction also occurs as a secondary reaction in order to obtain an increased conversion of CO2 compared to CH4, lowering the H2/CO ratio in the syngas. Water or oxygen can also be added to the biogas to achieve total CH4 conversion. The steps of the reaction are described below [,]:
Dry reforming of methane (DRM): CH4 + CO2 ⇔ 2H2 + 2CO, ΔH0298Κ = 248 kJmol−1;
Reverse water–gas shift (RWGS): CO2 + H2 ⇔ CO + H2O, ΔH0298Κ = 41 kJmol−1;
Steam reforming (SR);
or partial oxidation (POX);
Similar to SR, syngas production is closely linked to the catalyst used in the reaction. Ni-based catalysts are inexpensive and thus have attracted a lot of attention. The major drawback of this process is that carbon deposition results in the deactivation of the catalyst []. Coke formation can occur through methane decomposition, CO disproportionation (also called the Boudouard reaction), and the hydrogenation of carbon oxides. However, these reactions are favored at temperatures lower than 527 °C, except methane decomposition, which occurs at temperatures higher than 553 °C [,]. Hence, coke deposition occurs at temperatures between 553 and 700 °C. Another factor that leads to coke deposition is the ratios of C, H, and O. It is shown that, in dry reforming, it is necessary to modify the CO2/CH4 feed ratio to under 1 to cope with coke deposition [].
3. Prospects of Hydrogen Production from Biomass at Scale
To assess the previously mentioned processes, it is a necessity to examine the type of feedstock and pre-treatment that may be needed, the production materials/energy requirements, and the purity of the gas output.
There are two forms of feedstock that can be utilized to provide the amount of biomass that is required to create hydrogen: lignocellulosic residue and dedicated crops. Because they are produced towards the end of the harvest season, for crops like cereal wheat, or during the transformation process, lignocellulosic waste is easily accessible and inexpensive. Obtaining dedicated energy from crops like sorghum, however, requires land utilization and growth time, which might be difficult. Clustering different types of biomasses based on their chemical components (carbohydrates, lignin, and other components) can make the necessary pre-treatment easier [,]. When using industrial waste and wastewater as feedstock, it is essential to remove unwanted components. The pretreatment of feedstock prevents hydrogenotrophic methanogens and enhances acidogenic bacterial growth [,,]. It also removes lignin from lignocellulosic biomass wastes [,]. There are four commonly used pretreatment techniques and they are categorized as follows: physico-mechanical, physicochemical, chemical, and enzymatic pretreatments [].
When comparing the biological processes of biomass, dark fermentation seems to be the most competitive when considering the scale-up possibilities. It has an efficiency of 60–80% [] and a yield between 0.004 and 0.044 kg H2/kg biomass [], and has a TRL of five [,,]. It is a simple method that can produce H2 without light, contributing to waste recycling. It is also CO2-neutral and has no O2 limitation []. The main disadvantages of this process are the fatty acid removal, low H2 rates and yields, low conversion efficiency, and the requirement of a large reactor volume []. In addition, dark fermentation is limited by the poor catalyst durability and product contaminants []. The production cost is around 0.332–2.63 EUR/kg H2 [,] but these numbers are indicative because there are few full plant cost estimations []. There are no commercial-scale plants at present. Also, enzymatically fermentable feedstock pretreatment is significantly expensive. Thus, the industrial scale of the process development is limited [].
Photo fermentation is also a CO2-neutral process that contributes to waste recycling and can use different organic wastes and wastewaters []. Nonetheless, the light conversion efficiency is only 1–5% [], the production cost is 0.362–3.66 EUR/kg H2 [,,], and the estimated yield is 0.004–0.049 kg H2/kg biomass []. The method also requires sunlight and a large reactor volume, is sensitive to O2, and has a TRL of four [,,,]. Hydrogenases’ oxygen sensitivity is a major issue in biohydrogen generation. Although it has been established that [FeFe]-hydrogenases may generate hydrogen more efficiently, they are exceptionally sensitive to O2, and even at 2% O2 partial pressure they become inactive []. [NiFe]-hydrogenases are not as susceptible to O2, but have a significantly lower hydrogen generation activity, as much as 10–100 times lower than [FeFe]-hydrogenases []. Problems that might also occur with the use of industrial effluents for H2 production are the color of the wastewaters, which will prevent light penetration, and heavy metals or other toxic compounds that might be carried in the wastewaters [,]. Lastly, there are difficulties in controlling the various bacteria []. Compared to dark fermentation, this method is thought to be less financially competitive because it achieves approximately the same yield while requiring a higher production cost [].
The two fermentation methods can be incorporated in two ways: sequential (consecutive operation) and combined (simultaneous operation) []. Considering sequential operation, during dark fermentation, acids and alcohols are produced by bacteria breaking down more complex carbohydrate substances and they are later used as substrates for the photosynthetic bacteria in photo fermentation []. In the combined system, hydrogen is produced by dark- and photo fermentation at the same time in a single bioreactor. Dark fermentation produces volatile fatty acids that are processed in site by photo fermentative organisms into hydrogen [].
The last biological process, biocatalyzed electrolysis, has an electrical requirement that remains far below that of commercial water electrolysis. Even when microbial metabolic and other energy losses increase the energy demand, it is expected to remain below 1 kWh/m3 H2. Biological anodes can easily be operated under non-sterile conditions because electrochemically active consortia can be naturally selected from a wide range of inocula. This process can theoretically produce hydrogen as a pure gas in the cathode chamber instead of having a mixed-gas output []. The most significant barrier to these techniques in terms of viability is the low hydrogen production rate []. In addition, enzymatic biocathodes are relatively unstable and they are not self-regenerating []. There has not been thorough recent research on this method, especially from a techno-economic standpoint. Another challenge that this method faces is that the lignocellulosic biomass, a plentiful natural resource, cannot be directly processed by the microorganisms in the microbial electrolysis cell. In order to transform it into monosaccharides or low-molecular-weight molecules, it must first undergo fermentation []. Therefore, biocatalyzed electrolysis emerges as a supplementary technology to fermentative processes. Their combination enables the retrieval of up to 90% of the energy content in the substrate [,].
Dark fermentation and biocatalyzed electrolysis can be performed in a microbial fuel cell (MFC). Acids and carbon dioxide derived from the fermentation of waste and wastewater through anaerobic microbes can produce protons and electrons in an anodic chamber. Electrons contribute to generating electricity and protons pass through the proton exchange membrane into the chamber of the cathode. The fermented effluent is proven to be a helpful substrate for electricity production in the MFC. Hydrogen is produced when these protons combine with electrons through a reduction process. Water is also produced when protons combine with oxygen molecules. Studies show that the combination of dark fermentation with MFC or MEC systems has achieved yields 41% higher than the use of solely dark fermentation [].
Biomass electrolysis offers a higher hydrogen production efficiency and lower ΔEeq than water electrolysis because the oxidation of the biomass-derived material has lower thermodynamic requirements. The production cost when applying the current density range of 0.2–1.0 A/cm2 in biomass-based organic molecules is approximately 8–10 EUR/kg H2, but this can be significantly reduced considering the value-added chemical(s) that are co-produced []. This process’s disadvantage is its slow kinetics due to the numerous electron transfer mechanisms []. Electro-oxidation has a TRL of 2–4 [] and there has not been extended recent research on this method.
For thermochemical processes, the industrial design has already been defined []. The approach was developed using similar techniques, while the necessary adaptations from steam methane reforming (SMR) were made []. Gasification has an efficiency between 35 and 50% [], a TRL of seven, a production cost between 1.14 and 3.29 EUR/kg H2, and produces the highest yields [,,,]. Steam gasification has a 40% H2 percentage in the gas, a higher H2/CO ratio (1.6), reduced impurities compared to air gasification, and produces 0.040 kg H2/kg biomass without a catalyst and 0.070 kg H2/kg biomass with a catalyst [,]. This process is thought to be the most suitable process for hydrogen production. Concerning the feedstock, steam gasification is feasible for wet biomass (moisture from 5 to 35 wt%), while air gasification requires a dry raw material []. However, because of the production of tar and char, the processes are more vulnerable to catalyst deactivation and the gas products need to be separated and purified [,]. It was also shown that the gasification process is economically non-viable due to ash-related issues such as corrosion, erosion, agglomeration, and sintering [,].
Pyrolysis has shown an efficiency of up to 65% using high-density polyethylene (HDPE) as a feedstock, yielding 0.100 kg H2/kg biomass and HDPE and 0.373 kg H2/kg HDPE [] with a TRL of seven [,]. The production cost of this process is around 1.14–2.41 EUR/kg H2 [,,]. Studies show that, at the same temperature, the fast pyrolysis of biomass releases more volatiles than slow pyrolysis []. It has been observed that the presence of a catalyst increased the H2 gas yield while reducing the CO, C2–C4, and CH4 yield []. However, because of the production of tar and char, the processes are more vulnerable to catalyst deactivation. There is fewer data on the biomass-to-hydrogen yields and production costs in the scientific literature containing techno-economic analyses of pyrolysis compared to gasification. The gasification and pyrolysis of biomass use similar procedures to those used to treat fossil fuels. They are projected to develop and reach a TRL of up to nine in the following two decades. However, to produce negative emissions, the thermochemical processes that release CO2 must be combined with carbon capture systems (CCS) [].
Comparing biogas from landfill and the anaerobic digestion of biomass, the performing efficiency is slightly higher for biogas from anaerobic digestion. When undergoing SR and ATR at 20 bar, it is shown to achieve a maximum of 51.7% and 27.8%, respectively []. As the temperature of the reforming process increases, the yield of H2 increases correspondingly.
It reaches a peak before slightly decreasing []. Although this approach produces significant hydrogen yields, achieving the required high-purity hydrogen, it involves complicated energy integration, costly heat exchangers (high temperature), and numerous process units. The number of distinct process stages affects system efficiency, making scaling down uneconomical []. The approximate production cost is between 4.21–4.29 EUR/kg H2 for SR and 6.41–6.60 EUR/kg H2 for ATR when both processes are performed at 20 bar using biogas from an anaerobic digestor and landfill, respectively. SMR reaches a TRL of nine, and ATR reaches a TRL of eight when processing natural gas []. The process’ model generates 0.29 kg H2 per kilogram of bio-methane [,].
Antonini et al. [] have conducted research that compares natural gas to biomethane and investigates CCS and storage systems. The results show that natural gas- and biowaste-based biomethane have minor differences in performance when undergoing SR and ATR, despite their different compositions. However, when addressing the life cycle impacts on climate change, biomethane is clearly more sustainable as the CO2 that is released from biomethane does not contribute to the carbon cycle, in contrast with the contribution of natural gas to GHG.
Regarding the dry reforming of biogas, noble catalysts, such as Ru-, Rh-, and Pt-based catalysts, are found to provide more control of carbon deposition and achieve higher conversion rates. However, they seem to be costly, which limits their prospects. More studies have been conducted for Ni-based catalysts and they are more commonly used on an industrial scale. Surely, there is more room for research focused on the creation of bimetallic catalysts (Ni-noble metals, Ni-Co, Ni-Fe, and Ni-Cu) with high and steady activity, especially the utilization of non-noble metals to lower expenses and suit industrial applications []. The effect of gas hourly space velocity (GHSV) is a common issue in biogas dry reforming research, since it is a critical operational parameter that affects catalytic performance. A higher GHSV reduces catalytic activity as the contact period of the gas and solid phases lessens; thus, many reactants stay intact after passing the catalyst [,,,]. In terms of cost, an economic analysis of dry reforming for hydrogen production indicates that the production cost is closely linked to the production rate. The production cost lessens when the production rate increases. This results in a range of 1.34–6.60 EUR/kg H2 [,,,]. In general, biogas is a great fit for this process, mainly because it uses CO2 contained in the biogas and its high conversion efficiency. The dry reforming of biogas, however, has limitations due to the carbon formation, the operation conditions such as high temperatures, and the expensive catalyst, which makes the process financially unappealing. In addition, hydrogen production from biogas is favored in smaller reformers while dry reforming involves larger reformers [].
The following table, Table 4, displays a summary of the most important features of each process from various literature references, which are mentioned in the previous paragraphs. The table highlights each method’s efficiency (%), yield (kg H2/kg biomass), production cost (EUR/kg H2), and TRL. In order to compare them, the necessary unit conversions were made. In addition, data from water electrolysis are shown in Table 3 to compare the previously mentioned methods of green hydrogen production to water electrolysis, another method of green hydrogen production. The most advanced methods of water electrolysis are alkaline electrolysis (AEL), proton-exchange membrane electrolysis (PEMEL), and solid-oxide electrolysis (SOEL) [].

Table 4.
Important features of each method.
The following figures (Figure 1, Figure 2 and Figure 3) are based on Table 4. The two colors of the bars represent the range of values that are mentioned in Table 4. The orange and yellow colors depict the minimum and the maximum value, respectively.
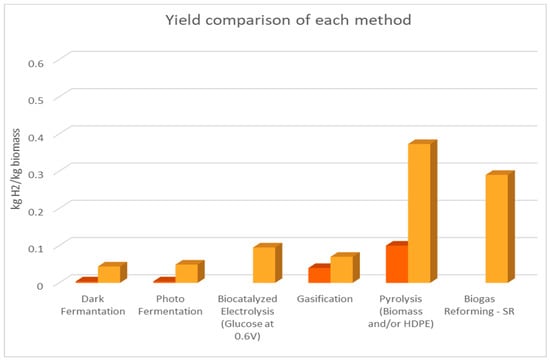
Figure 1.
Comparison of each method’s yield.
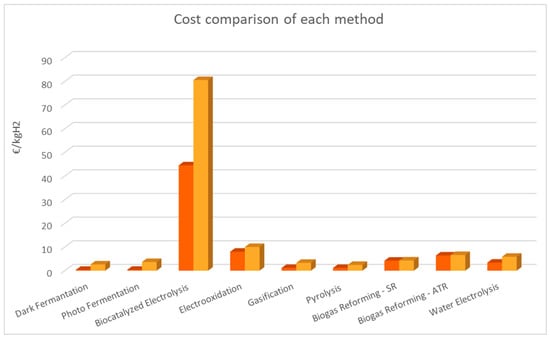
Figure 2.
Comparison of each method’s cost.
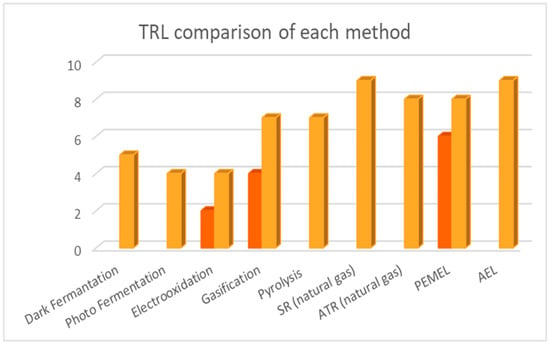
Figure 3.
Comparison of each method’s TRL.
In Figure 1, a comparison of the methods’ yields is presented. The chart clearly shows the advantages of thermochemical methods regarding yield. This conclusion is justified because of the extensive research that was conducted and the implementation of these technologies in fields other than hydrogen production. Biocatalyzed electrolysis has a lead among the biological methods, even though their performance does not differ much. In general, two obstacles are preventing biological processes from evolving and expanding on a global scale. Even while dark fermentation has made biological methods more competitive, the H2 yield and production pace are significantly lower compared to thermochemical methods [,]. Lastly, pilot-scale processes are limited by the need for pre-treatment throughout the synthesis of a complex biomass [].
Figure 2 was created by comparing the cost of each method. This chart also indicates that the thermochemical methods are quite advantageous but not far more inviting than the fermentation processes in terms of production cost. Electrooxidation is more expensive than the previously mentioned methods but not as unviable as biocatalyzed electrolysis, which is far more expensive than any other method, even though the data we obtained are from laboratory materials. Biogas reforming methods are costly compared to the other thermochemical and biological methods but have a similar cost to water electrolysis. In general, we can conclude that thermochemical and biological methods are the most advantageous financially.
In Figure 3, the TRL of each method is depicted. This figure aims to compare the TRL of hydrogen production methods based on biomass to other established methods, such as SR and ATR of natural gas and water electrolysis via PEM and AE. It is clearly shown in Figure 3 that the methods that are competitive with the dominant methods of SR and water electrolysis are gasification and pyrolysis. Biological and electrochemical methods do not present a TRL above five, which means that they are still under development, while thermochemical methods are in a state of pre-commercial demonstration.
Thermochemical methods have shown an advantage in terms of both the yield and TRL aspects. However, the CO2 emissions’ intensity and the incorporation of CCS need to be taken into account to assess these methods’ potential to meet our goal to mitigate GHG emissions. The life cycle assessment conducted by Antonini et al. [], which was previously mentioned, also indicates that hydrogen production from bio-methane combined with CCS demonstrates net-negative CO2 emissions results. ATR has a higher rate of CO2 capture than SMR and the addition of a low-temperature WGS generally improves the life cycle performance. It is safe to conclude that, in general, adding CCS leads to clear benefits considering the implications of climate change. However, the results show that these methods perform worse with CCS than without it because the integration of CCS increases the energy demand and consumption, which leads to other environmental pressures.
Table 5 [] displays the CO2 emissions that each thermochemical method produces when used for hydrogen production. In addition, Figure 4 is presented, based on Table 5. The two colors of the bars represent the range of values that are mentioned in Table 5. The orange and yellow colors depict the minimum and the maximum value, respectively.

Table 5.
Hydrogen production methods and CO2 emission intensity.
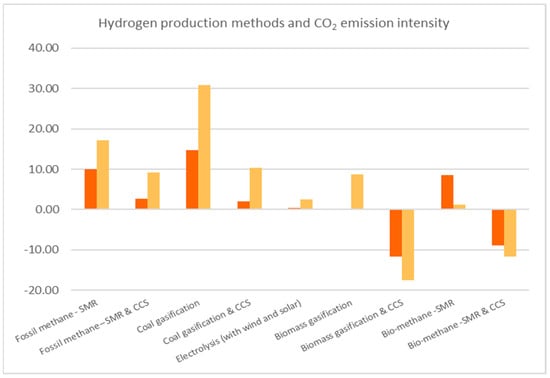
Figure 4.
Comparison of CO2 emission intensity.
From Table 5 [], we conclude that, in every case, the TRL is lower when the process is combined with CCS. Nevertheless, biomass gasification and bio-methane SMR show significant results when CCS is included in the system. Comparing these two methods as the two most inviting methods of green hydrogen production with their non-green equivalent, it is shown that SMR with CCS from fossil methane and SMR with CCS from bio-methane present with the same TRL but a significant difference in CO2 emissions. The latter demonstrates net-negative CO2 emissions, which is a very important factor considering the desire of a net-zero economy, which requires carbon absorption and negative CO2 emissions into the atmosphere, by 2030.
4. Conclusions
This review has comparatively shown that, among the biological processes, dark fermentation has the most potential for large-scale development. Bio-catalyzed electrolysis, as well as electrochemical methods, are advantageous compared to water electrolysis concerning the electrical demand. However, the current status of these methods reveals that they are not yet financially viable, and the obstacles totheir implementation are numerous. Also, there has not been an extended techno-economic analysis of full-scale plants for any biological processes, nor for electrochemical methods. Examining the methods with a lower TRL, the combination of dark fermentation and biocatalyzed electrolysis as two consecutive treating methods has more potential than the use of the two methods separately and may be subject to further exploration.
Thermochemical methods are significantly more advanced because they have already been applied in studies concerning the production of hydrogen from fossil fuels. Dry reforming is a very interesting process when it comes to biogas usage as feedstock, but it is probably more suitable for syngas production, with prospects of direct usage in energy generation. Steam gasification, as well as bio-methane reforming, are considered the most suitable processes for use in a scaled-up plant in the near future. Some of their distinct advantages compared to other methods are the high purity of the gas output, the high ratio of H2/CO2, the low production cost, and the high yield. The prospect of a scaled plant is much more realistic for these methods due to the extended research that has already been conducted. However, it is important to consider that, to produce negative emissions, thermochemical processes that release CO2 must be combined with carbon-capture systems (CCS), in which case the TRL comparison shows that the systems are still under development and require further optimization to be fully efficient. However, they still demonstrate very promising potential for large-scale development.
The need to switch to renewable energy sources is urgent and hydrogen is a very promising energy carrier that may provide the solution to this problem. Biomass has been studied through various processes since it is a significant hydrogen carrier, and it seems like an encouraging alternative for hydrogen production. In the hope of achieving a fully independent plant, there is still room for further research, specifically a complete techno-economic analysis, research on the sustainability of these methods, a life cycle analysis, and research focusing on their impact on the environment.
Author Contributions
Conceptualization, A.P.D., E.S., A.S. and T.T., Formal analysis, A.P.D., E.S., A.S. and T.T., Investigation, A.P.D., E.S., S.B., M.Z. and G.M., Anastasiadis A.G.A., S.K. and G.T., Writing–original draft preparation, A.P.D., Writing–review and editing, A.P.D., E.S., A.S. and T.T., Review and editing, S.B., M.Z. and G.M., Anastasiadis A.G.A., S.K. and G.T., Visualization, A.P.D., Supervision, E.S., A.S. and T.T., Project administration E.S., Funding acquisition, E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Clean Hydrogen Partnership and its members Hydrogen Europe and Hydrogen Europe Research under Grant Agreement No. 101112056 (TRIERES).
Data Availability Statement
The data presented in this study are openly available in article.
Acknowledgments
The authors would like to acknowledge the funding received within the framework of the TRIERES research project, funded by the Clean Hydrogen Partnership and its members Hydrogen Europe and Hydrogen Europe Research under Grant Agreement No. 101112056 (TRIERES).
Conflicts of Interest
Anestis G. Anastasiadis was employed by Power Public Corporation (PPC S.A.). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Sridevi, V.; Surya, D.V.; Reddy, B.R.; Shah, M.; Gautam, R.; Kumar, T.H.; Puppala, H.; Pritam, K.S.; Basak, T. Challenges and opportunities in the production of sustainable hydrogen from lignocellulosic biomass using microwave-assisted pyrolysis: A review. Int. J. Hydrog. Energy 2024, 52 Pt A, 507–531. [Google Scholar] [CrossRef]
- Nguyen, V.G.; Nguyen-Thi, T.X.; Nguyen, P.Q.P.; Tran, V.D.; Ağbulut, Ü.; Nguyen, L.H.; Balasubramanian, D.; Tarelko, W.; Bandh, S.A.; Pham, N.D.K. Recent advances in hydrogen production from biomass waste with a focus on pyrolysis and gasification. Int. J. Hydrog. Energy 2024, 54, 127–160. [Google Scholar] [CrossRef]
- Akkoli, K.; Banapurmath, N.; Shivashimpi, M.; Soudagar, M.E.M.; Badruddin, I.A.; Alazwari, M.A.; Yaliwal, V.; Mujtaba, M.; Akram, N.; Goodarzi, M.; et al. Effect of injection parameters and producer gas derived from redgram stalk on the performance and emission characteristics of a diesel engine. Alex. Eng. J. 2021, 60, 3133–3142. [Google Scholar] [CrossRef]
- Nagarajan, J.; Balasubramanian, D.; Khalife, E.; Usman, K.M. Optimization of compression ignition engine fuelled with cotton seed biodiesel using diglyme and injection pressure. J. Technol. Innov. 2022, 2, 52–61. [Google Scholar] [CrossRef]
- Laleh, S.S.; Zeinali, M.; Mahmoudi, S.; Soltani, S.; Rosen, M.A. Biomass co-fired combined cycle with hydrogen production via proton exchange membrane electrolysis and waste heat recovery: Thermodynamic assessment. Int. J. Hydrog. Energy 2023, 48, 33795–33809. [Google Scholar] [CrossRef]
- Nemmour, A.; Inayat, A.; Janajreh, I.; Ghenai, C. Green hydrogen-based E-fuels (E-methane, E-methanol, E-ammonia) to support clean energy transition: A literature review. Int. J. Hydrog. Energy 2023, 48, 29011–29033. [Google Scholar] [CrossRef]
- Rosa, L.; Mazzotti, M. Potential for hydrogen production from sustainable biomass with carbon capture and storage. Renew. Sustain. Energy Rev. 2022, 157, 112123. [Google Scholar] [CrossRef]
- Liu, W.; Qi, Y.; Zhang, R.; Zhang, Q.; Wang, Z. Hydrogen production from ammonia-rich combustion for fuel reforming under high temperature and high pressure conditions. Fuel 2022, 327, 124830. [Google Scholar] [CrossRef]
- Johan, M.A. Renewable Hydrogen Production from Biomass. Available online: https://www.etipbioenergy.eu/images/Renewable_Hydrogen_Production_from_Biomass.pdf (accessed on 26 April 2024).
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment. Biomass-Bioenergy 2020, 144, 105920. [Google Scholar] [CrossRef]
- Engin GÜRTEKİN, Faculty of Engineering, Department of Environmental Engineering Firat University, Turkey, Biological Hydrogen Production Methods. Available online: https://i-sem.info/PastConferences/ISEM2014/ISEM2014/papers/A10-ISEM2014ID80.pdf (accessed on 26 April 2024).
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F. Hydrogen energy. Philos. Trans. R. Soc. A 2007, 365, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Environmental and Renewable Energy Systems Division, Gifu University, Gifu City, Japan Hydrogen Production Technologies Overview. Available online: https://www.scirp.org/pdf/JPEE_2019012515395175.pdf (accessed on 26 April 2024).
- Satyapal, S.; Petrovic, J.; Read, C.; Thomas, G.; Ordaz, G. The U.S. Department of Energy’s National Hydrogen Storage Project: Progress towards meeting hydrogen-powered vehicle requirements. Catal. Today 2007, 120, 246–256. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Verhelst, S.; Turner, J.W.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Rumayor, M.; Corredor, J.; Rivero, M.; Ortiz, I. Prospective life cycle assessment of hydrogen production by waste photoreforming. J. Clean. Prod. 2022, 336, 130430. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R. Comparison of environmental and economic aspects of various hydrogen production methods. Renew. Sustain. Energy Rev. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Balat, H.; Kırtay, E. Hydrogen from biomass—Present scenario and future prospects. Int. J. Hydrog. Energy 2010, 35, 7416–7426. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrog. Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Elangovan, D. A Brief Review of Hydrogen Production Methods and Their Challenges. Energies 2023, 16, 1141. [Google Scholar] [CrossRef]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Flamos, A.; Georgallis, P.G.; Doukas, H.; Psarras, J. Using Biomass to Achieve European Union Energy Targets—A Review of Biomass Status, Potential, and Supporting Policies. Int. J. Green Energy 2011, 8, 411–428. [Google Scholar] [CrossRef]
- Parkinson, B.; Balcombe, P.; Speirs, J.F.; Hawkes, A.D.; Hellgardt, K. Levelized cost of CO2 mitigation from hydrogen production routes. Energy Environ. Sci. 2019, 12, 19–40. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrog. Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Bao, M.; Su, H.; Tan, T. Biohydrogen Production by Dark Fermentation of Starch Using Mixed Bacterial Cultures of Bacillus sp and Brevumdimonas sp. Energy Fuels 2012, 26, 5872–5878. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrog. Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Moia, I.C.; Kanaropoulou, A.; Ghanotakis, D.F.; Carlozzi, P.; Touloupakis, E. Photofermentative hydrogen production by immobilized Rhodopseudomonas sp. S16-VOGS3 cells in photobioreactors. Energy Rev. 2024, 3, 100055. [Google Scholar] [CrossRef]
- Fedorov, A.; Tsygankov, A.; Rao, K.; Hall, D. Hydrogen photoproduction by Rhodobacter sphaeroides immobilised on polyurethane foam. Biotechnol. Lett. 1998, 20, 1007–1009. [Google Scholar] [CrossRef]
- Tsygankov, A.A.; Federov, A.S.; Laurinavichene, T.V.; Gogotov, I.N.; Rao, K.K.; Hall, D.O. Actual and potential rates of hydrogen photoproduction by continuous culture of the purple nonsulphur bacteria Rhodobacter capsulatus. Appl. Environ. Microbiol. 1998, 49, 102–107. [Google Scholar] [CrossRef]
- Zürrer, H.; Bachofen, R. Hydrogen Production by the Photosynthetic Bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 1979, 37, 789–793. [Google Scholar] [CrossRef]
- Das, D.; Veziroglu, T.N. Advances in biological hydrogen production processes. Int. J. Hydrog. Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically Assisted Microbial Production of Hydrogen from Acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Ditzig, J.; Liu, H.; Logan, B.E. Production of hydrogen from domestic wastewater using a bioelectrochemically assisted microbial reactor (BEAMR). Int. J. Hydrog. Energy 2007, 32, 2296–2304. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.; Euverink, G.J.; Metz, S.J.; Buisman, C.J. Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int. J. Hydrog. Energy 2006, 31, 1632–1640. [Google Scholar] [CrossRef]
- Lalaurette, E.; Thammannagowda, S.; Mohagheghi, A.; Maness, P.-C.; Logan, B.E. Hydrogen production from cellulose in a two-stage process combining fermentation and electrohydrogenesis. Int. J. Hydrog. Energy 2009, 34, 6201–6210. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Sustainable and efficient biohydrogen production via electrohydrogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18871–18873. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xing, D.; Xie, T.; Ren, N.; Logan, B.E. Hydrogen production from proteins via electrohydrogenesis in microbial electrolysis cells. Biosens. Bioelectron. 2010, 25, 2690–2695. [Google Scholar] [CrossRef] [PubMed]
- Fascetti, E.; Todini, O. Rhodobacter sphaeroides RV cultivation and hydrogen production in a one- and two-stage chemostat. Appl. Microbiol. Biotechnol. 1995, 44, 300–305. [Google Scholar] [CrossRef]
- Rozendal, R.A. Hydrogen production through biocatalyzed electrolysis. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2007. [Google Scholar]
- Caizán-Juanarena, L.; Servin-Balderas, I.; Chen, X.; Buisman, C.J.; ter Heijne, A. Electrochemical and microbiological characterization of single carbon granules in a multi-anode microbial fuel cell. J. Power Sources 2019, 435, 126514. [Google Scholar] [CrossRef]
- Kalyani, P.; Anitha, A. Biomass carbon & its prospects in electrochemical energy systems. Int. J. Hydrog. Energy 2013, 38, 4034–4045. [Google Scholar] [CrossRef]
- Holade, Y.; Tuleushova, N.; Tingry, S.; Servat, K.; Napporn, T.W.; Guesmi, H.; Cornu, D.; Kokoh, K.B. Recent advances in the electrooxidation of biomass-based organic molecules for energy, chemicals and hydrogen production. Catal. Sci. Technol. 2020, 10, 3071–3112. [Google Scholar] [CrossRef]
- Luo, H.; Barrio, J.; Sunny, N.; Li, A.; Steier, L.; Shah, N.; Stephens, I.E.L.; Titirici, M. Progress and Perspectives in Photo- and Electrochemical-Oxidation of Biomass for Sustainable Chemicals and Hydrogen Production. Adv. Energy Mater. 2021, 11, 2101180. [Google Scholar] [CrossRef]
- Kwon, Y.; Schouten, K.J.P.; Koper, M.T.M. Mechanism of the Catalytic Oxidation of Glycerol on Polycrystalline Gold and Platinum Electrodes. ChemCatChem 2011, 3, 1176–1185. [Google Scholar] [CrossRef]
- Bambagioni, V.; Bevilacqua, M.; Bianchini, C.; Filippi, J.; Lavacchi, A.; Marchionni, A.; Vizza, F.; Shen, P.K. Self-Sustainable Production of Hydrogen, Chemicals, and Energy from Renewable Alcohols by Electrocatalysis. ChemSusChem 2010, 3, 851–855. [Google Scholar] [CrossRef]
- Gomes, J.F.; Tremiliosi-Filho, G. Spectroscopic Studies of the Glycerol Electro-Oxidation on Polycrystalline Au and Pt Surfaces in Acidic and Alkaline Media. Electrocatalysis 2011, 2, 96–105. [Google Scholar] [CrossRef]
- IRENA. Hydrogen: A Renewable Energy Perspective; Report Prepared for the 2nd Hydrogen Energy Ministerial Meeting in Tokyo, Japan; IRENA: Abu Dhabi, United Arab Emirates, 2019; ISBN 9789292601515. [Google Scholar]
- Stenberg, V.; Rydén, M.; Mattisson, T.; Lyngfelt, A. Exploring novel hydrogen production processes by integration of steam methane reforming with chemical-looping combustion (CLC-SMR) and oxygen carrier aided combustion (OCAC-SMR). Int. J. Greenh. Gas Control. 2018, 74, 28–39. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Cortazar, M.; Santamaria, L.; Lopez, G.; Alvarez, J.; Zhang, L.; Wang, R.; Bi, X.; Olazar, M. A comprehensive review of primary strategies for tar removal in biomass gasification. Energy Convers. Manag. 2023, 276, 116496. [Google Scholar] [CrossRef]
- Hannula, I.; Kurkela, E. A semi-empirical model for pressurised air-blown fluidised-bed gasification of biomass. Bioresour. Technol. 2010, 101, 4608–4615. [Google Scholar] [CrossRef]
- Weiland, F.; Hedman, H.; Marklund, M.; Wiinikka, H.; Öhrman, O.; Gebart, R. Pressurized Oxygen Blown Entrained-Flow Gasification of Wood Powder. Energy Fuels 2013, 27, 932–941. [Google Scholar] [CrossRef]
- Ahlström, J.M.; Alamia, A.; Larsson, A.; Breitholtz, C.; Harvey, S.; Thunman, H. Bark as feedstock for dual fluidized bed gasifiers-Operability, efficiency, and economics. Int. J. Energy Res. 2019, 43, 1171–1190. [Google Scholar] [CrossRef]
- Srinivasakannan, C.; Balasubramanian, N. Variations in the Design of Dual Fluidized Bed Gasifiers and the Quality of Syngas from Biomass. Energy Sources Part A Recovery Util. Environ. Eff. 2010, 33, 349–359. [Google Scholar] [CrossRef]
- Xu, C.; Chen, S.; Soomro, A.; Sun, Z.; Xiang, W. Hydrogen rich syngas production from biomass gasification using synthesized Fe/CaO active catalysts. J. Energy Inst. 2018, 91, 805–816. [Google Scholar] [CrossRef]
- de Lasa, H.; Salaices, E.; Mazumder, J.; Lucky, R. Catalytic Steam Gasification of Biomass: Catalysts, Thermodynamics and Kinetics. Chem. Rev. 2011, 111, 5404–5433. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Li, S.; Yuan, Y.; Zhang, D.; Wu, Y.; Xie, H.; Brindhadevi, K.; Pugazhendhi, A.; Xia, C. A review of biomass pyrolysis gas: Forming mechanisms, influencing parameters, and product application upgrades. Fuel 2023, 347, 128461. [Google Scholar] [CrossRef]
- Rezaei, P.S.; Shafaghat, H.; Daud, W.M.A.W. Production of green aromatics and olefins by catalytic cracking of oxygenate compounds derived from biomass pyrolysis: A review. Appl. Catal. A Gen. 2014, 469, 490–511. [Google Scholar] [CrossRef]
- Demirbaş, A. Yields of hydrogen-rich gaseous products via pyrolysis from selected biomass samples. Fuel 2001, 80, 1885–1891. [Google Scholar] [CrossRef]
- Duman, G.; Uddin, A.; Yanik, J. Hydrogen production from algal biomass via steam gasification. Bioresour. Technol. 2014, 166, 24–30. [Google Scholar] [CrossRef]
- Bi, P.; Wang, J.; Zhang, Y.; Jiang, P.; Wu, X.; Liu, J.; Xue, H.; Wang, T.; Li, Q. From lignin to cycloparaffins and aromatics: Directional synthesis of jet and diesel fuel range biofuels using biomass. Bioresour. Technol. 2015, 183, 10–17. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. Overview of hydrogen production from biogas reforming: Technological advancement. Int. J. Hydrog. Energy 2022, 47, 34831–34855. [Google Scholar] [CrossRef]
- Antonini, C.; Treyer, K.; Streb, A.; van der Spek, M.; Bauer, C.; Mazzotti, M. Hydrogen production from natural gas and biomethane with carbon capture and storage—A techno-environmental analysis. Sustain. Energy Fuels 2020, 4, 2967–2986. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Alves, H.J.; Junior, C.B.; Niklevicz, R.R.; Frigo, E.P.; Frigo, M.S.; Coimbra-Araújo, C.H. Overview of hydrogen production technologies from biogas and the applications in fuel cells. Int. J. Hydrog. Energy 2013, 38, 5215–5225. [Google Scholar] [CrossRef]
- Lau, C.; Tsolakis, A.; Wyszynski, M. Biogas upgrade to syn-gas (H2–CO) via dry and oxidative reforming. Int. J. Hydrog. Energy 2011, 36, 397–404. [Google Scholar] [CrossRef]
- Effendi, A.; Hellgardt, K.; Zhang, Z.-G.; Yoshida, T. Optimising H2 production from model biogas via combined steam reforming and CO shift reactions. Fuel 2005, 84, 869–874. [Google Scholar] [CrossRef]
- Kolbitsch, P.; Pfeifer, C.; Hofbauer, H. Catalytic steam reforming of model biogas. Fuel 2008, 87, 701–706. [Google Scholar] [CrossRef]
- Nahar, G.; Mote, D.; Dupont, V. Hydrogen production from reforming of biogas: Review of technological advances and an Indian perspective. Renew. Sustain. Energy Rev. 2017, 76, 1032–1052. [Google Scholar] [CrossRef]
- Di Marcoberardino, G.; Foresti, S.; Binotti, M.; Manzolini, G. Potentiality of a biogas membrane reformer for decentralized hydrogen production. Chem. Eng. Process.-Process. Intensif. 2018, 129, 131–141. [Google Scholar] [CrossRef]
- Galvagno, A.; Chiodo, V.; Urbani, F.; Freni, F. Biogas as hydrogen source for fuel cell applications. Int. J. Hydrog. Energy 2013, 38, 3913–3920. [Google Scholar] [CrossRef]
- Camacho, Y.S.M.; Bensaid, S.; Piras, G.; Antonini, M.; Fino, D. Techno-economic analysis of green hydrogen production from biogas autothermal reforming. Clean Technol. Environ. Policy 2017, 19, 1437–1447. [Google Scholar] [CrossRef]
- Göransson, K.; Söderlind, U.; He, J.; Zhang, W. Review of syngas production via biomass DFBGs. Renew. Sustain. Energy Rev. 2011, 15, 482–492. [Google Scholar] [CrossRef]
- Ugarte, P.; Durán, P.; Lasobras, J.; Soler, J.; Menéndez, M.; Herguido, J. Dry reforming of biogas in fluidized bed: Process intensification. Int. J. Hydrog. Energy 2017, 42, 13589–13597. [Google Scholar] [CrossRef][Green Version]
- Minutillo, M.; Perna, A.; Sorce, A. Green hydrogen production plants via biogas steam and autothermal reforming processes: Energy and exergy analyses. Appl. Energy 2020, 277, 115452. [Google Scholar] [CrossRef]
- Yin, H.; Yip, A.C. A Review on the Production and Purification of Biomass-Derived Hydrogen Using Emerging Membrane Technologies. Catalysts 2017, 7, 297. [Google Scholar] [CrossRef]
- Manfro, R.L.; Souza, M.M.V.M. Overview of Ni-Based Catalysts for Hydrogen Production from Biogas Reforming. Catalysts 2023, 13, 1296. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Moon, D.H.; Kim, K.-H.; Kwon, E.E. Upgrading biogas into syngas through dry reforming. Renew. Sustain. Energy Rev. 2021, 143, 110949. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Kammoun, M.; Ayeb, H.; Bettaieb, T.; Richel, A. Chemical characterisation and technical assessment of agri-food residues, marine matrices, and wild grasses in the South Mediterranean area: A considerable inflow for biorefineries. Waste Manag. 2020, 118, 247–257. [Google Scholar] [CrossRef]
- Ramprakash, B.; Lindblad, P.; Eaton-Rye, J.J.; Incharoensakdi, A. Current strategies and future perspectives in biological hydrogen production: A review. Renew. Sustain. Energy Rev. 2022, 168, 112773. [Google Scholar] [CrossRef]
- Kumar, G.; Saratale, R.G.; Kadier, A.; Sivagurunathan, P.; Zhen, G.; Kim, S.-H.; Saratale, G.D. A review on bio-electrochemical systems (BESs) for the syngas and value added biochemicals production. Chemosphere 2017, 177, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Bakonyi, P.; Kobayashi, T.; Xu, K.-Q.; Sivagurunathan, P.; Kim, S.-H.; Buitrón, G.; Nemestóthy, N.; Bélafi-Bakó, K. Enhancement of biofuel production via microbial augmentation: The case of dark fermentative hydrogen. Renew. Sustain. Energy Rev. 2016, 57, 879–891. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Q.; Zhang, Z.; Jing, Y.; Hu, J.; He, C.; Lu, C. A review on biological recycling in agricultural waste-based biohydrogen production: Recent developments. Bioresour. Technol. 2021, 347, 126595. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.; Sun, Z.; Zhang, Y.; Xu, D. Review of renewable energy-based hydrogen production processes for sustainable energy innovation. Glob. Energy Interconnect. 2019, 2, 436–443. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield—A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Salam, M.A.; Ahmed, K.; Akter, N.; Hossain, T.; Abdullah, B. A review of hydrogen production via biomass gasification and its prospect in Bangladesh. Int. J. Hydrog. Energy 2018, 43, 14944–14973. [Google Scholar] [CrossRef]
- Martinez-Merino, V.; José Gil, M.; Cornejo, A. Biomass sources for hydrogen production. In Renewable Hydrogen Technologies: Production, Purification, Storage, Applications and Safety; Newnes: Oxford, UK, 2013; p. 87. [Google Scholar]
- Rupprecht, J.; Hankamer, B.; Mussgnug, J.H.; Ananyev, G.; Dismukes, C.; Kruse, O. Perspectives and advances of biological H2 production in microorganisms. Appl. Microbiol. Biotechnol. 2006, 72, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, T.; Ren, Y.; Wang, J.; Chen, H.; Wang, Q. Biological hydrogen with industrial potential: Improvement and prospection in biohydrogen production. J. Clean. Prod. 2023, 387, 135777. [Google Scholar] [CrossRef]
- Azwar, M.; Hussain, M.; Abdul-Wahab, A. Development of biohydrogen production by photobiological, fermentation and electrochemical processes: A review. Renew. Sustain. Energy Rev. 2013, 31, 158–173. [Google Scholar] [CrossRef]
- Aiken, D.C.; Curtis, T.P.; Heidrich, E.S. Avenues to the financial viability of microbial electrolysis cells [MEC] for domestic wastewater treatment and hydrogen production. Int. J. Hydrog. Energy 2019, 44, 2426–2434. [Google Scholar] [CrossRef]
- Liu, W.; Cui, Y.; Du, X.; Zhang, Z.; Chao, Z.; Deng, Y. High efficiency hydrogen evolution from native biomass electrolysis. Energy Environ. Sci. 2016, 9, 467–472. [Google Scholar] [CrossRef]
- Moreno, R.; Escapa, A.; Cara, J.; Carracedo, B.; Gómez, X. A two-stage process for hydrogen production from cheese whey: Integration of dark fermentation and biocatalyzed electrolysis. Int. J. Hydrog. Energy 2014, 40, 168–175. [Google Scholar] [CrossRef]
- Yao, J.; Kraussler, M.; Benedikt, F.; Hofbauer, H. Techno-economic assessment of hydrogen production based on dual fluidized bed biomass steam gasification, biogas steam reforming, and alkaline water electrolysis processes. Energy Convers. Manag. 2017, 145, 278–292. [Google Scholar] [CrossRef]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A comparative analysis of hydrogen production from the thermochemical conversion of algal biomass. Int. J. Hydrog. Energy 2019, 44, 10384–10397. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.; Xiong, X.; Tsang, D.C.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable hydrogen production through biomass gasification: A review and future prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef]
- Hajjaji, N.; Martinez, S.; Trably, E.; Steyer, J.-P.; Helias, A. Life cycle assessment of hydrogen production from biogas reforming. Int. J. Hydrog. Energy 2016, 41, 6064–6075. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, S.; Chhabra, T.; Tariq, S.; Javed, M.S.; Li, H.; Naqvi, S.R.; Rajendran, S.; Luque, R.; Ahmad, I. Synergic impact of renewable resources and advanced technologies for green hydrogen production: Trends and perspectives. Int. J. Hydrog. Energy 2024, in press. [Google Scholar] [CrossRef]
- Meshkani, F.; Golesorkh, S.F.; Rezaei, M.; Andache, M. Nickel catalyst supported on mesoporous MgAl2O4 nanopowders synthesized via a homogenous precipitation method for dry reforming reaction. Res. Chem. Intermed. 2016, 43, 545–559. [Google Scholar] [CrossRef]
- Han, J.; Zhan, Y.; Street, J.; To, F.; Yu, F. Natural gas reforming of carbon dioxide for syngas over Ni–Ce–Al catalysts. Int. J. Hydrog. Energy 2017, 42, 18364–18374. [Google Scholar] [CrossRef]
- Shiraz, M.H.A.; Rezaei, M.; Meshkani, F. Preparation of nanocrystalline Ni/Al2O3catalysts with the microemulsion method for dry reforming of methane. Can. J. Chem. Eng. 2016, 94, 1177–1183. [Google Scholar] [CrossRef]
- Hajizadeh, A.; Mohamadi-Baghmolaei, M.; Saady, N.M.C.; Zendehboudi, S. Hydrogen production from biomass through integration of anaerobic digestion and biogas dry reforming. Appl. Energy 2022, 309, 118442. [Google Scholar] [CrossRef]
- da Silva, H.N.C.; Prata, D.M.; Lima, G.B.A.; Zotes, L.P.; Mattos, L.V. A techno-economic evaluation of the energy generation by proton exchange membrane fuel cell using biogas reforming. J. Clean. Prod. 2018, 200, 598–608. [Google Scholar] [CrossRef]
- Di Marcoberardino, G.; Vitali, D.; Spinelli, F.; Binotti, M.; Manzolini, G. Green Hydrogen Production from Raw Biogas: A Techno-Economic Investigation of Conventional Processes Using Pressure Swing Adsorption Unit. Processes 2018, 6, 19. [Google Scholar] [CrossRef]
- Panigrahy, B.; Narayan, K.; Rao, B.R. Green hydrogen production by water electrolysis: A renewable energy perspective. Mater. Today Proc. 2022, 67 Pt 8, 1310–1314. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. High hydrogen production rate of microbial electrolysis cell (MEC) with reduced electrode spacing. Bioresour. Technol. 2011, 102, 3571–3574. [Google Scholar] [CrossRef]
- Rasul, M.; Hazrat, M.; Sattar, M.; Jahirul, M.; Shearer, M. The future of hydrogen: Challenges on production, storage and applications. Energy Convers. Manag. 2022, 272, 116326. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of thermochemical routes for hydrogen production from biomass: A review. Energy Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas Reforming to Syngas: A Review. iScience 2020, 23, 101082. [Google Scholar] [CrossRef]
- Madeira, J.G.F.; Delgado, A.R.S.; Boloy, R.A.M.; Coutinho, E.R.; Loures, C.C.A. Exergetic and economic evaluation of incorporation of hydrogen production in a cassava wastewater plant. Appl. Therm. Eng. 2017, 123, 1072–1078. [Google Scholar] [CrossRef]
- Lachén, J.; Durán, P.; Menéndez, M.; Peña, J.; Herguido, J. Biogas to high purity hydrogen by methane dry reforming in TZFBR+MB and exhaustion by Steam-Iron Process. Techno–economic assessment. Int. J. Hydrog. Energy 2018, 43, 11663–11675. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Lee, B.; Heo, J.; Kim, S.; Sung, C.; Moon, C.; Moon, S.; Lim, H. Economic feasibility studies of high pressure PEM water electrolysis for distributed H2 refueling stations. Energy Convers. Manag. 2018, 162, 139–144. [Google Scholar] [CrossRef]
- Hassan, N.; Jalil, A.; Rajendran, S.; Khusnun, N.; Bahari, M.; Johari, A.; Kamaruddin, M.; Ismail, M. Recent review and evaluation of green hydrogen production via water electrolysis for a sustainable and clean energy society. Int. J. Hydrog. Energy 2024, 52 Pt B, 420–441. [Google Scholar] [CrossRef]
- Grigoriev, S.; Fateev, V.; Bessarabov, D.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrog. Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from different biomass gasification processes. Int. J. Hydrog. Energy 2018, 43, 9514–9528. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from natural gas using current and emerging technologies. Int. J. Hydrog. Energy 2017, 42, 18894–18909. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).